Microcystis viridis NIES-102 Cyanobacteria Lectin (MVL) Interacts with SARS-CoV-2 Spike Protein Receptor Binding Domains (RBDs) via Protein–Protein Interaction
Abstract
:1. Introduction
2. Results
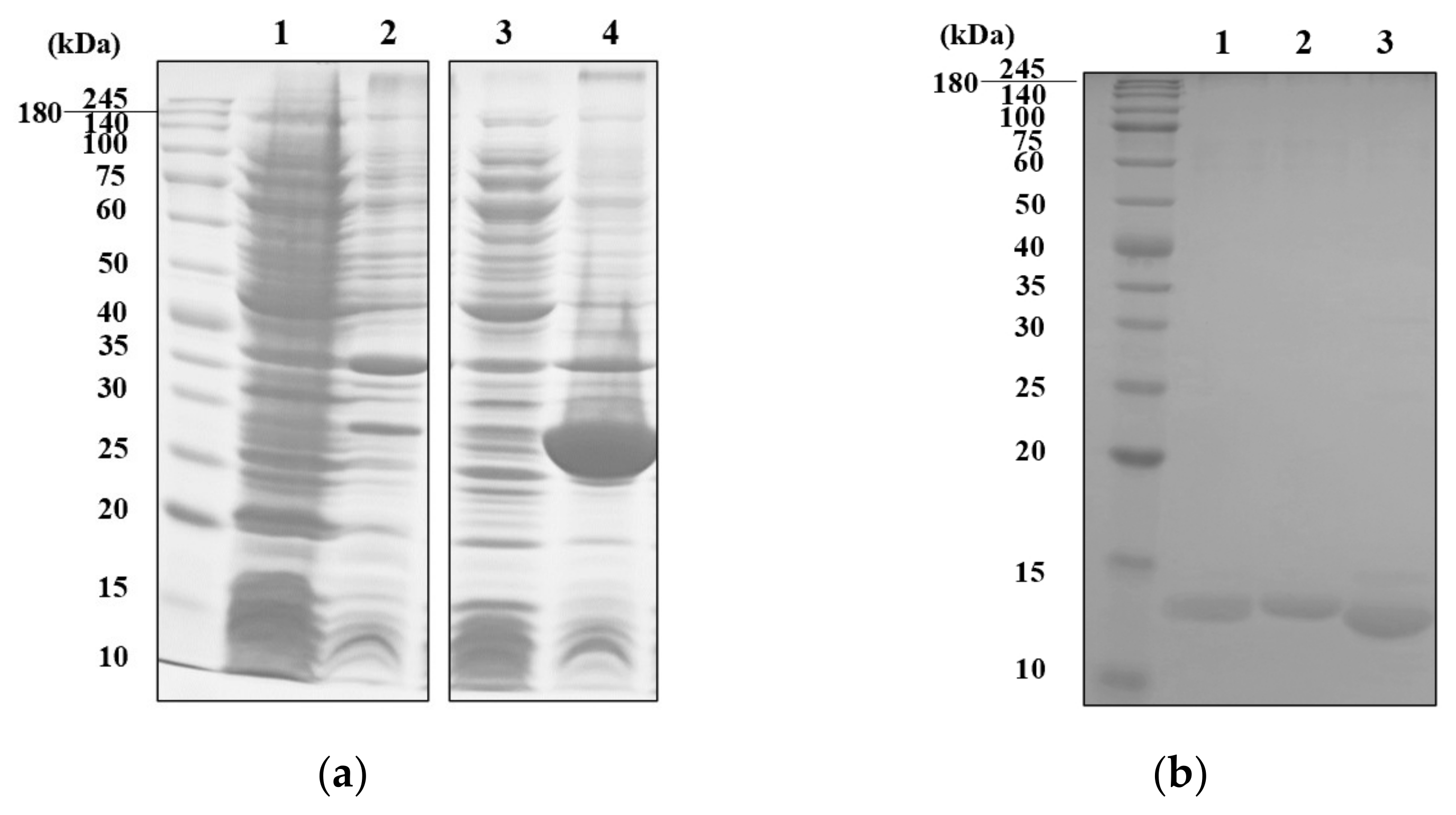
2.1. Expression, Purification and Characterization of Recombinant MVL
2.2. Interaction and Binding Properties of rMVL with SARS-CoV-2 Spike Protein RBDs
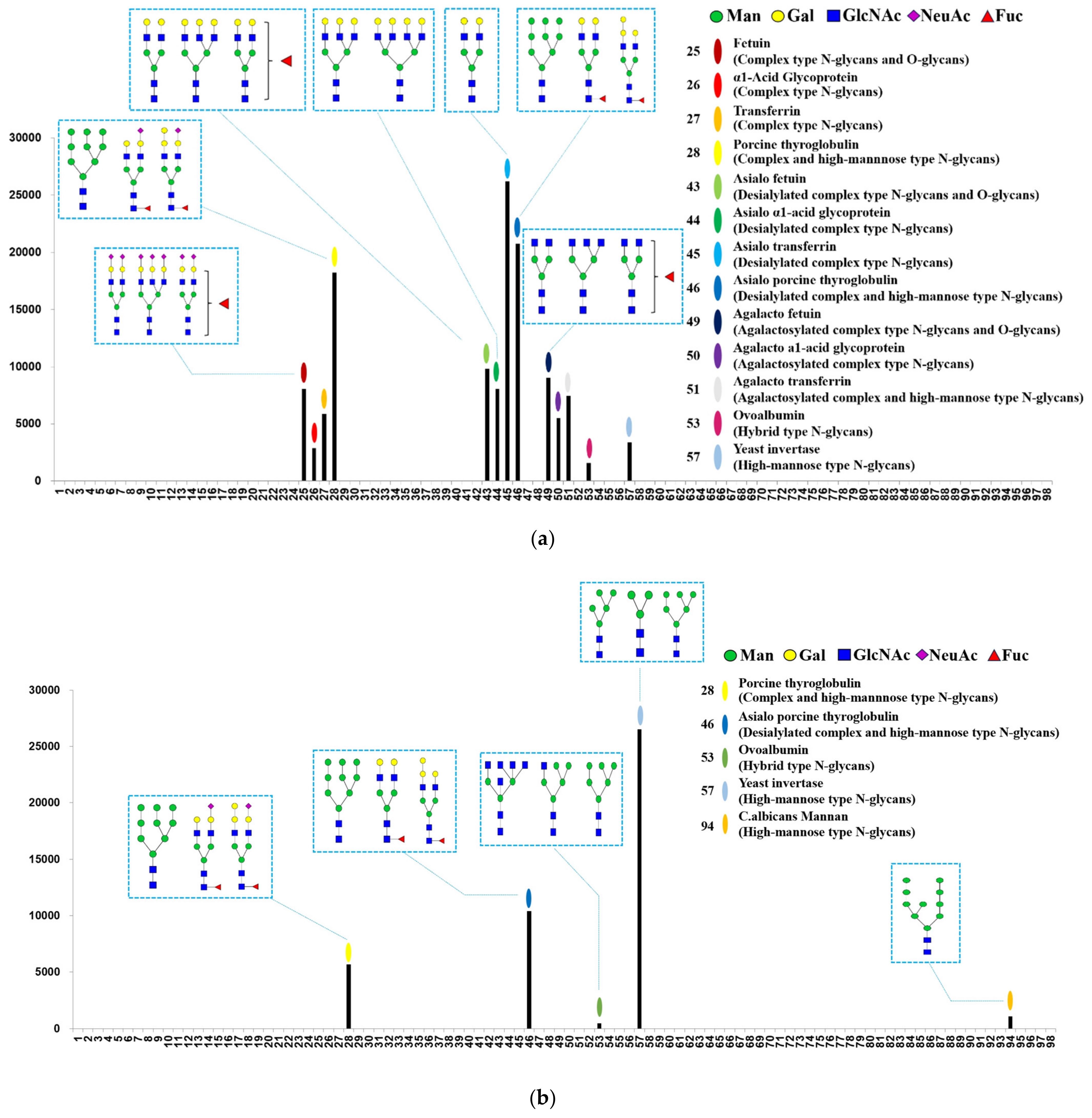
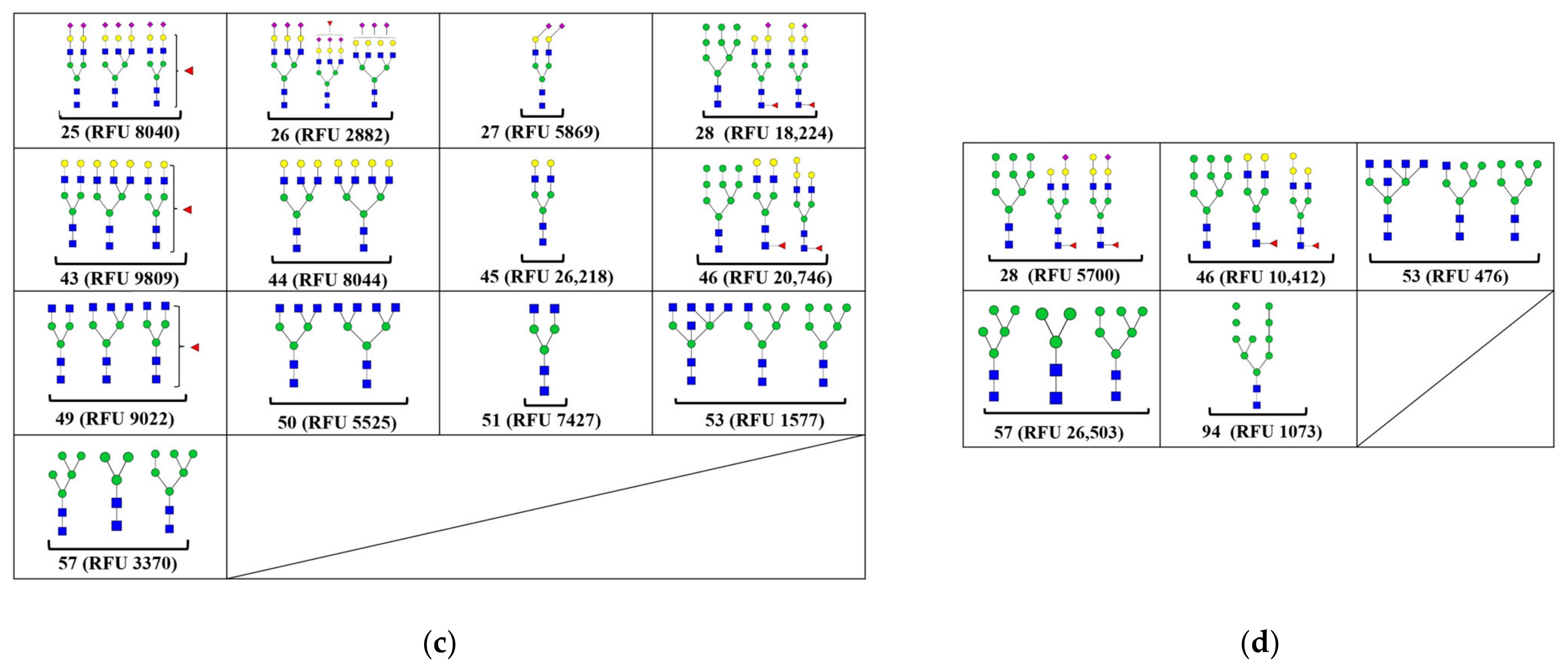
2.3. Carbohydrate Binding Specificity of MVL
2.4. Galactose Inhibits the Interaction between Lectins and RBDs
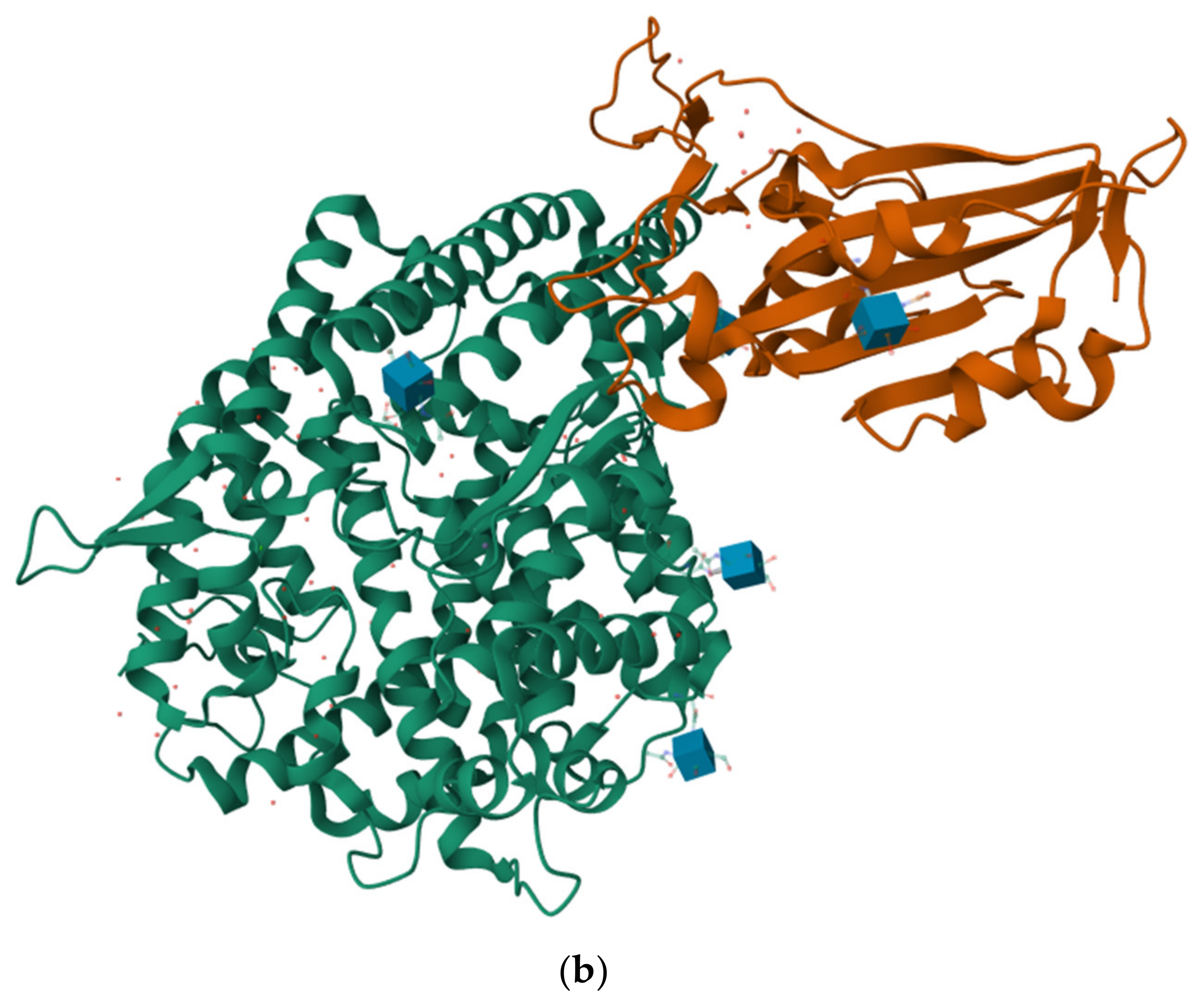
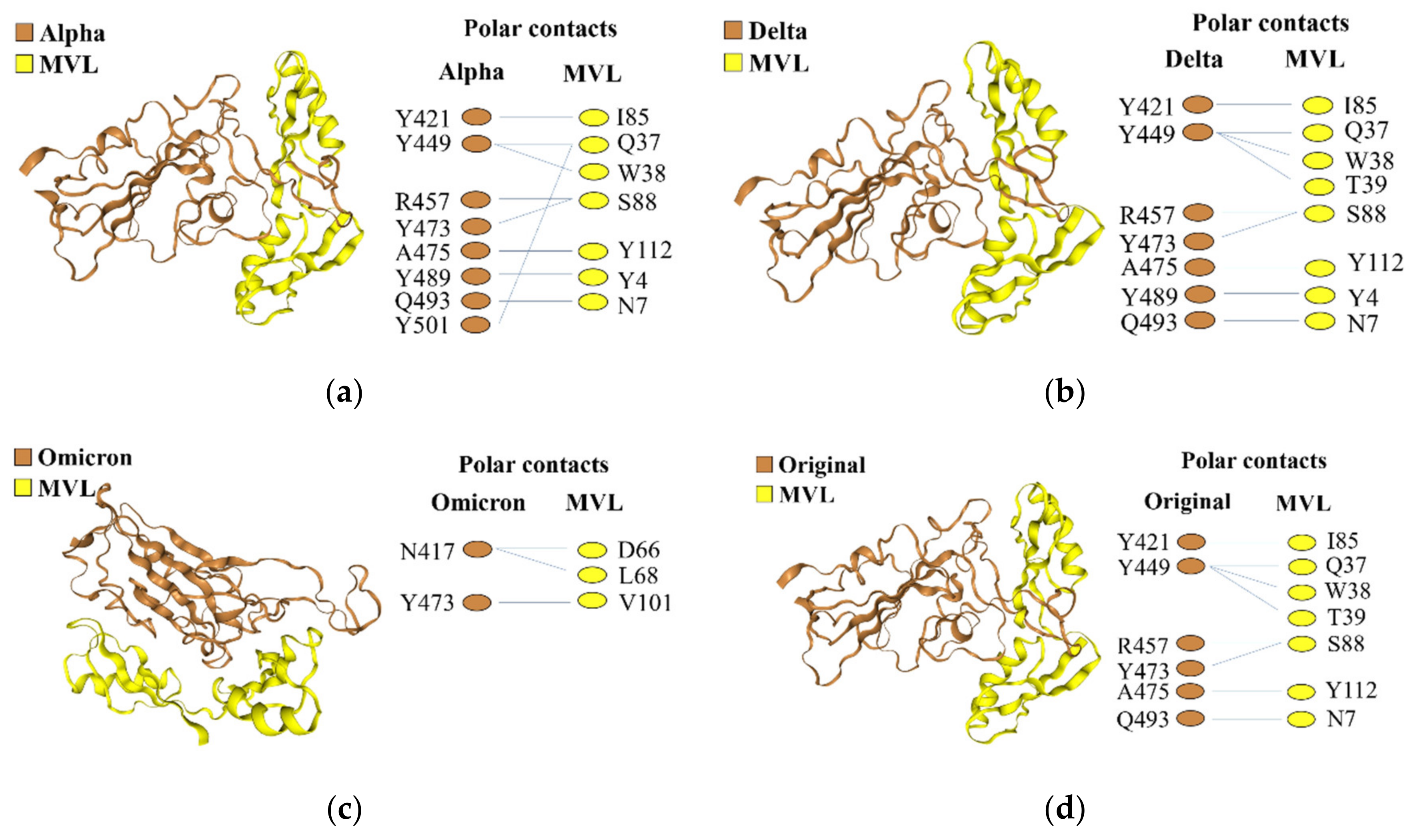
2.5. The Protein–Protein Interaction between MVL and RBDs
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Expression and Purification of Recombinant MVL
4.3. Construction of the SARS-CoV-2 Mutant Spike Protein RBD Expression System
4.4. SDS-PAGE and Western Blotting
4.5. Hemagglutination Assays
4.6. Surface Plasmon Resonance (SPR) Detection of the Interaction between Lectins and SARS-CoV-2 Spike Protein RBDs
4.7. Carbohydrate Specificity Analysis of MVL and DB1 Lectins
4.8. In Silico Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stephen, C. Harrison, Viral membrane fusion. Virology 2015, 479–480, 498–507. [Google Scholar]
- Fenouillet, E.; Barbouche, R.; Jones, I. Cell entry by enveloped viruses: Redox considerations for HIV and SARS-coronavirus. Antioxid. Redox Signal. 2007, 9, 1009–1034. [Google Scholar] [CrossRef] [PubMed]
- Mas, V.; Melero, J. Entry of Enveloped Viruses into Host Cells: Membrane Fusion. Struct. Phys. Viruses 2013, 68, 467–487. [Google Scholar]
- Cross, K.; Burleigh, L.; Steinhauer, D. Mechanisms of cell entry by influenza virus. Expert Rev. Mol. Med. 2001, 3, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Pal, M.; Berhanu, G.; Desalegn, C.; Kandi, V. Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2): An Update. Cureus 2020, 12, e7423. [Google Scholar] [CrossRef] [PubMed]
- Noman, A.; Aqeel, M.; Khalid, N.; Hashem, M.; Alamari, S.; Zafar, S.; Qasim, M.; Irshad, M.K.; Qari, S.H. Spike glycoproteins: Their significance for corona viruses and receptor binding activities for pathogenesis and viral survival. Microb. Pathog. 2021, 150, 104719. [Google Scholar] [CrossRef] [PubMed]
- Boson, B.; Legros, V.; Zhou, B.; Siret, E.; Mathieu, C.; Cosset, F.; Lavillette, D.; Denolly, S. The SARS-CoV-2 Envelope and Membrane proteins modulate maturation and retention of the Spike protein, allowing assembly of virus-like particles. J. Biol. Chem. 2020, 296, 100111. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xiang, Y. Spike Glycoprotein-Mediated Entry of SARS Coronaviruses. Viruses 2020, 12, 1289. [Google Scholar] [CrossRef] [PubMed]
- Gadanec, L.K.; McSweeney, K.R.; Qaradakhi, T.; Ali, B.; Zulli, A.; Apostolopoulos, V. Can SARS-CoV-2 Virus Use Multiple Receptors to Enter Host Cells? Int. J. Mol. Sci. 2021, 22, 992. [Google Scholar] [CrossRef] [PubMed]
- Nassar, A.; Ibrahim, I.M.; Amin, F.G.; Magdy, M.; Elgharib, A.M.; Azzam, E.B.; Nasser, F.; Yousry, K.; Shamkh, I.M.; Mahdy, S.M.; et al. A Review of Human Coronaviruses’ Receptors: The Host-Cell Targets for the Crown Bearing Viruses. Molecules 2021, 26, 6455. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xiao, T.; Cai, Y.; Chen, B. Structure of SARS-CoV-2 spike protein. Curr. Opin. Virol. 2021, 50, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yang, C.; Xu, X.; Xu, W.; Liu, S. Structural and functional properties of SARS-CoV-2 spike protein: Potential antivirus drug development for COVID-19. Acta Pharmacol. Sin. 2020, 41, 1141–1149. [Google Scholar] [CrossRef]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Valdés-Balbín, Y.; Santana-Mederos, D.; Paquet, F.; Fernandez, S.; Climent, Y.; Chiodo, F.; Rodriguez, L.; Ramírez, B.; Leon, K.; Hernández, T.; et al. Molecular Aspects Concerning the Use of the SARS-CoV-2 Receptor Binding Domain as a Target for Preventive Vaccines. ACS Cent. Sci. 2021, 7, 757–767. [Google Scholar] [CrossRef]
- Sharon, N. Lectin-carbohydrate complexes of plants and animals: An atomic view. Trends Biochem. Sci. 1993, 18, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, J.; Pang, X.; Liu, T.; Ning, Z.; Cheng, G. The Roles of Direct Recognition by Animal Lectins in Antiviral Immunity and Viral Pathogenesis. Molecules 2015, 20, 2272–2295. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Hirayama, M.; Morimoto, K.; Hori, K. The OAAH Family: Anti-Influenza Virus Lectins. Methods Mol. Biol. 2020, 2132, 683–693. [Google Scholar] [PubMed]
- Micewicz, E.; Cole, A.; Jung, C.; Luong, H.; Phillips, M.; Pratikhya, P.; Sharma, S.; Waring, A.; Cole, A.; Ruchala, P. Grifonin-1: A Small HIV-1 Entry Inhibitor Derived from the Algal Lectin, Griffithsin. PLoS ONE 2010, 5, e14360. [Google Scholar] [CrossRef] [PubMed]
- Millet, J.; Séron, K.; Labitt, R.; Danneels, A.; Palmer, K.; Whittaker, G.; Dubuisson, J.; Belouzard, S. Middle East respiratory syndrome coronavirus infection is inhibited by griffithsin. Antivir. Res. 2016, 133, 1–8. [Google Scholar] [CrossRef]
- Férir, G.; Palmer, K.; Schols, D. Synergistic activity profile of griffithsin in combination with tenofovir, maraviroc and enfuvirtide against HIV-1 clade C. Virology 2011, 417, 253–258. [Google Scholar] [CrossRef]
- Barrientos, L.; Gronenborn, A. The highly specific carbohydrate-binding protein cyanovirin-N: Structure, anti-HIV/Ebola activity and possibilities for therapy. Mini Rev. Med. Chem. 2005, 5, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Ogawa, T.; Muramoto, K.; Kamio, Y.; Jimbo, M.; Kamiya, H. Isolation and characterization of a mannan-binding lectin from the freshwater cyanobacterium (blue-green algae) Microcystis viridis. Biochem. Biophys. Res. Commun. 1999, 265, 703–708. [Google Scholar] [CrossRef]
- Williams, D.C., Jr.; Lee, J.Y.; Cai, M.; Bewley, C.A.; Clore, G.M. Crystal Structures of the HIV-1 Inhibitory Cyanobacterial Protein MVL Free and Bound to Man3GlcNAc2. J. Biol. Chem. 2005, 280, 29269–29276. [Google Scholar] [CrossRef] [PubMed]
- Bewley, C.A.; Cai, M.; Ray, S.; Ghirlando, R.; Yamaguchi, M.; Muramoto, K. New carbohydrate specificity and HIV-1 fusion blocking activity of the cyanobacterial protein MVL: NMR, ITC and sedimentation equilibrium studies. J. Mol. Biol. 2004, 339, 901–914. [Google Scholar] [CrossRef] [PubMed]
- Kabir, S. Jacalin: A jackfruit (Artocarpus heterophyllus) seed-derived lectin of versatile applications in immunobiological research. J. Immunol. Methods 1998, 212, 193–211. [Google Scholar] [CrossRef] [PubMed]
- Gaidamashvili, M.; Ohizumi, Y.; Iijima, S.; Takayama, T.; Ogawa, T.; Muramoto, K. Characterization of the yam tuber storage proteins from Dioscorea batatas exhibiting unique lectin activities. J. Biol. Chem. 2004, 279, 26028–26035. [Google Scholar] [CrossRef]
- Derewenda, Z.; Yariv, J.; Helliwell, J.; Kalb, A.; Dodson, E.; Papiz, M.; Wan, T.; Campbell, J. The structure of the saccharide-binding site of concanavalin A. EMBO J. 1989, 8, 2189–2193. [Google Scholar] [CrossRef]
- Kaneda, Y.; Whittier, R.F.; Yamanaka, H.; Carredano, E.; Gotoh, M.; Sota, H.; Hasegawa, Y.; Shinohara, Y. The high specificities of Phaseolus vulgaris erythro-and leukoagglutinating lectins for bisecting GlcNAc or β1–6-linked branch structures, respectively, are attributable to loop B. J. Biol. Chem. 2002, 277, 16928–16935. [Google Scholar] [CrossRef] [PubMed]
- Shiina, N.; Tateno, H.; Ogawa, T.; Muramoto, K.; Saneyoshi, M.; Kamiya, H. Isolation and characterization of L-rhamnose-binding lectins from chum salmon (Oncorhynchus keta) eggs. Fish. Sci. 2002, 68, 1352–1366. [Google Scholar] [CrossRef]
- Lan, J.; Ge, J.; Yu, J.; Shan, S.; Zhou, H.; Fan, S.; Zhang, Q.; Shi, X.; Wang, Q.; Zhang, L.; et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 2020, 581, 215–220. [Google Scholar] [CrossRef]
- Barre, A.; Damme, E.; Simplicien, M.; Poder, S.; Klonjkowski, B.; Benoist, H.; Peyrade, D.; Rougé, P. Man-Specific Lectins from Plants, Fungi, Algae and Cyanobacteria, as Potential Blockers for SARS-CoV, MERS-CoV and SARS-CoV-2 (COVID-19) Coronaviruses: Biomedical Perspectives. Cells 2021, 10, 1619. [Google Scholar] [CrossRef] [PubMed]
- Ziolkowska, N.E.; O’Keefe, B.R.; Mori, T.; Zhu, C.; Giomarelli, B.; Vojdani, F.; Palmer, K.E.; McMahon, J.B.; Wlodawer, A. Domain-swapped structure of the potent antiviral protein griffithsin and its mode of carbohydrate binding. Structure 2006, 14, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- Swanson, M.D.; Boudreaux, D.M.; Salmon, L.; Chugh, J.; Winter, H.C.; Meagher, J.L.; André, S.; Murphy, P.V.; Oscarson, S.; Roy, R.; et al. Engineering a therapeutic lectin by uncoupling mitogenicity from antiviral activity. Cell 2015, 163, 746–758. [Google Scholar] [CrossRef] [PubMed]
- Gstottner, C.; Zhang, T.; Resemann, A.; Ruben, S.; Pengelley, S.; Suckau, D.; Welsink, T.; Wuhrer, M.; Domínguez-Vega, E. Structural and functional characterization of SARS-CoV-2 RBD domains produced in mammalian cells. Anal. Chem. 2021, 93, 6839–6847. [Google Scholar] [CrossRef] [PubMed]
- Lenza, M.P.; Oyenarte, I.; Diercks, T.; Quintana, J.I.; Gimeno, A.; Coelho, H.; Diniz, A.; Peccati, F.; Delgado, S.; Ereño-Orbea, J.; et al. Structural characterization of N-linked glycans in the receptor binding domain of the SARS-CoV-2 spike protein and their interactions with human lectins. Angew. Chem. 2020, 132, 23971–23979. [Google Scholar] [CrossRef]
- Cai, Y.; Xu, W.; Gu, C.; Cai, X.; Qu, D.; Lu, L.; Xie, Y.; Jiang, S. Griffithsin with a broad-spectrum antiviral activity by binding glycans in viral glycoprotein exhibits strong synergistic effect in combination with a pan-coronavirus fusion inhibitor targeting SARS-CoV-2 spike S2 subunit. Virol. Sin. 2020, 35, 857–860. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.F.; Oh, Y.J.; Yuan, S.; Chu, H.; Yeung, M.L.; Canena, D.; Chan, C.C.; Poon, V.K.; Chan, C.C.; Zhang, A.J.; et al. A molecularly engineered, broad-spectrum anti-coronavirus lectin inhibits SARS-CoV-2 and MERS-CoV infection in vivo. Cell Rep. Med. 2022, 3, 100774. [Google Scholar] [CrossRef] [PubMed]
- Shajahan, A.; Supekar, N.T.; Gleinich, A.S.; Azadi, P. Deducing the N-and O-glycosylation profile of the spike protein of novel coronavirus SARS-CoV-2. Glycobiology 2020, 30, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.M.; Shahed-Al-Mahmud, M.; Chen, X.; Chen, T.H.; Liao, K.S.; Lo, J.M.; Wu, Y.M.; Ho, M.C.; Wu, C.Y.; Wong, C.H. A carbohydrate-binding protein from the edible lablab beans effectively blocks the infections of influenza viruses and SARS-CoV-2. Cell Rep. 2020, 32, 108016. [Google Scholar] [CrossRef] [PubMed]
- Garrison, A.; Giomarelli, B.; Lear-Rooney, C.; Saucedo, C.; Yellayi, S.; Krumpe, L.; Rose, M.; Paragas, J.; Bray, M.; Olinger, G.; et al. The cyanobacterial lectin scytovirin displays potent in vitro and in vivo activity against Zaire Ebola virus. Antivir. Res. 2014, 112, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Shahzad-ul-Hussan, S.; Gustchina, E.; Ghirlando, R.; Clore, G.; Bewley, C. Solution Structure of the Monovalent Lectin Microvirin in Complex with Manα(1–2)Man Provides a Basis for Anti-HIV Activity with Low Toxicity. J. Biol. Chem. 2011, 286, 20788–20796. [Google Scholar] [CrossRef] [PubMed]
- Barre, A.; Damme, E.; Simplicien, M.; Benoist, H.; Rougé, P. Man-Specific, GalNAc/T/Tn-Specific and Neu5Ac-Specific Seaweed Lectins as Glycan Probes for the SARS-CoV-2 (COVID-19) Coronavirus. Marine Drugs 2020, 18, 543. [Google Scholar] [CrossRef] [PubMed]
- Dohn’alek, J. Tereza Sk’alova´C-type lectin-(like) fold–Protein-protein interaction patterns and utilization. Biotechnol. Adv. 2022, 58, 107944. [Google Scholar]
- Chan, K.K.; Dorosky, D.; Sharma, P.; Abbasi, S.A.; Dye, J.M.; Kranz, D.M.; Herbert, A.S.; Procko, E. Engineering human ACE2 to optimize binding to the spike protein of SARS coronavirus 2. Science 2020, 369, 1261–1265. [Google Scholar] [CrossRef] [PubMed]


| Lectin Name | Minimum Agglutination Concentration (mg.mL−1) | Protein Fold/ Family | Carbohydrate Specificity |
|---|---|---|---|
| rMVL | 0.008 | α/β-fold/MVL | D-mannose/D-galactose [23], this study |
| Jacalin | 0.005 | β-prism/Jacalin-related | D-galactose/GalNAc [25] |
| Con A | 0.008 | β-sandwich/L-type | D-mannose [27] |
| PHA-M | 0.002 | β-sandwich/L-type | complex-type N-glycans [28] |
| DB1 | 0.03 | β-prism II/M-type | D-mannose [26] |
| CSL3 | 0.0002 | α/β-fold/SUEL-related | L-rhamnose [29] |
| Name | Interaction with RBDs | Affinity with RBD [KD (M)] | |||
|---|---|---|---|---|---|
| Alpha | Delta | Omicron | Original | ||
| MVL | Yes | 1.29 × 10−6 | 1.65 × 10−6 | 2.73 × 10−6 | 2.08 × 10−6 |
| Jacalin | Yes | 3.20 × 10−7 | 3.39 × 10−7 | 4.78 × 10−7 | 4.28 × 10−7 |
| DB1 | No | ― | ― | ― | ― |
| Con.A | No | ― | ― | ― | ― |
| PHA-M | No | ― | ― | ― | ― |
| CSL3 | No | ― | ― | ― | ― |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Yang, Z.; Shishido, M.; Daoudi, K.; Hidaka, M.; Tateno, H.; Futai, E.; Ogawa, T. Microcystis viridis NIES-102 Cyanobacteria Lectin (MVL) Interacts with SARS-CoV-2 Spike Protein Receptor Binding Domains (RBDs) via Protein–Protein Interaction. Int. J. Mol. Sci. 2024, 25, 6696. https://doi.org/10.3390/ijms25126696
Wang Z, Yang Z, Shishido M, Daoudi K, Hidaka M, Tateno H, Futai E, Ogawa T. Microcystis viridis NIES-102 Cyanobacteria Lectin (MVL) Interacts with SARS-CoV-2 Spike Protein Receptor Binding Domains (RBDs) via Protein–Protein Interaction. International Journal of Molecular Sciences. 2024; 25(12):6696. https://doi.org/10.3390/ijms25126696
Chicago/Turabian StyleWang, Zhengguang, Zhihan Yang, Mami Shishido, Khadija Daoudi, Masafumi Hidaka, Hiroaki Tateno, Eugene Futai, and Tomohisa Ogawa. 2024. "Microcystis viridis NIES-102 Cyanobacteria Lectin (MVL) Interacts with SARS-CoV-2 Spike Protein Receptor Binding Domains (RBDs) via Protein–Protein Interaction" International Journal of Molecular Sciences 25, no. 12: 6696. https://doi.org/10.3390/ijms25126696







