Evaluation of Multiple RNA Extraction Protocols for Chikungunya Virus Screening in Aedes aegypti Mosquitoes
Abstract
:1. Introduction
2. Results
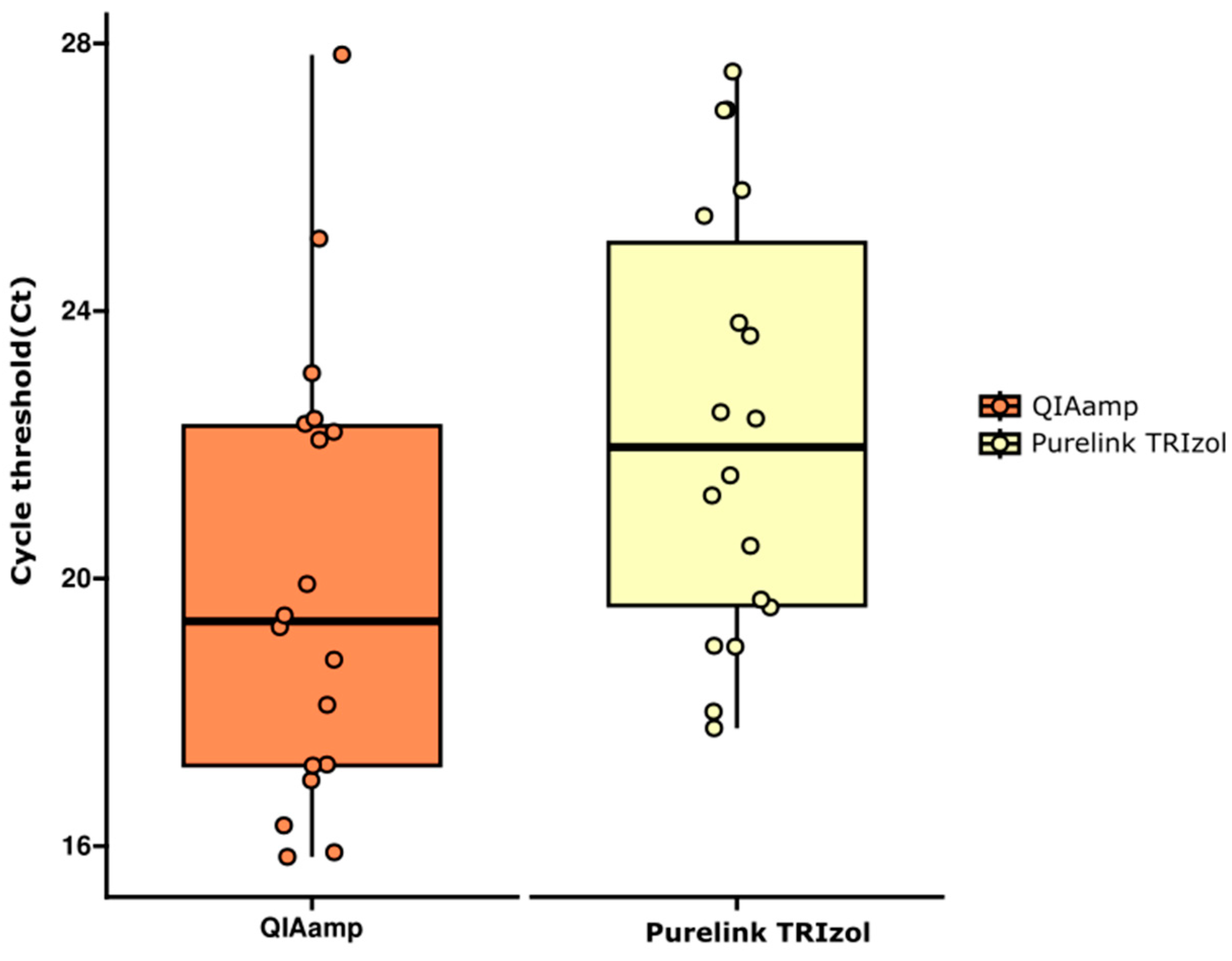
2.1. RNA Extraction Protocols Performance Comparison by RT-qPCR
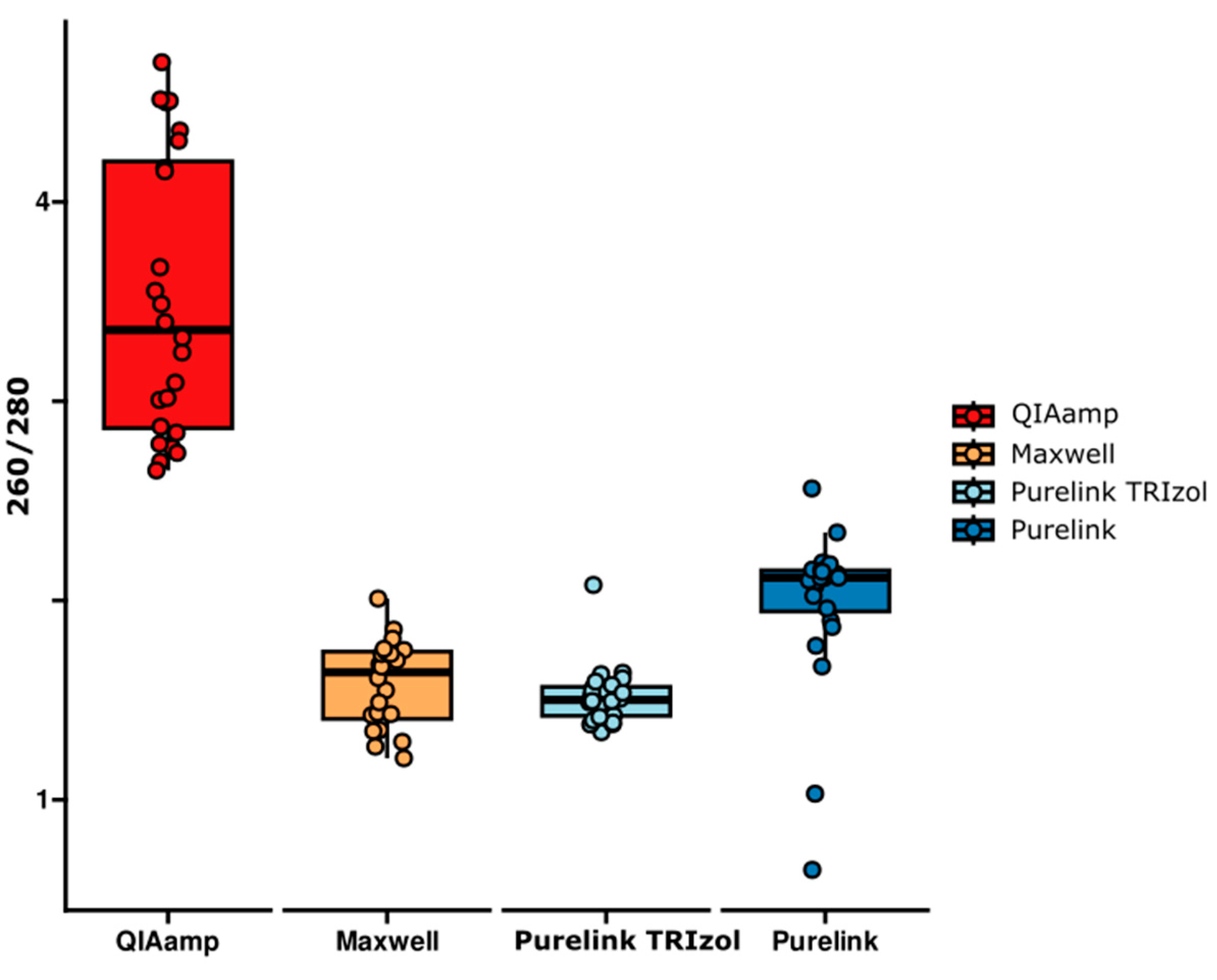
2.2. RNA Purity Comparison
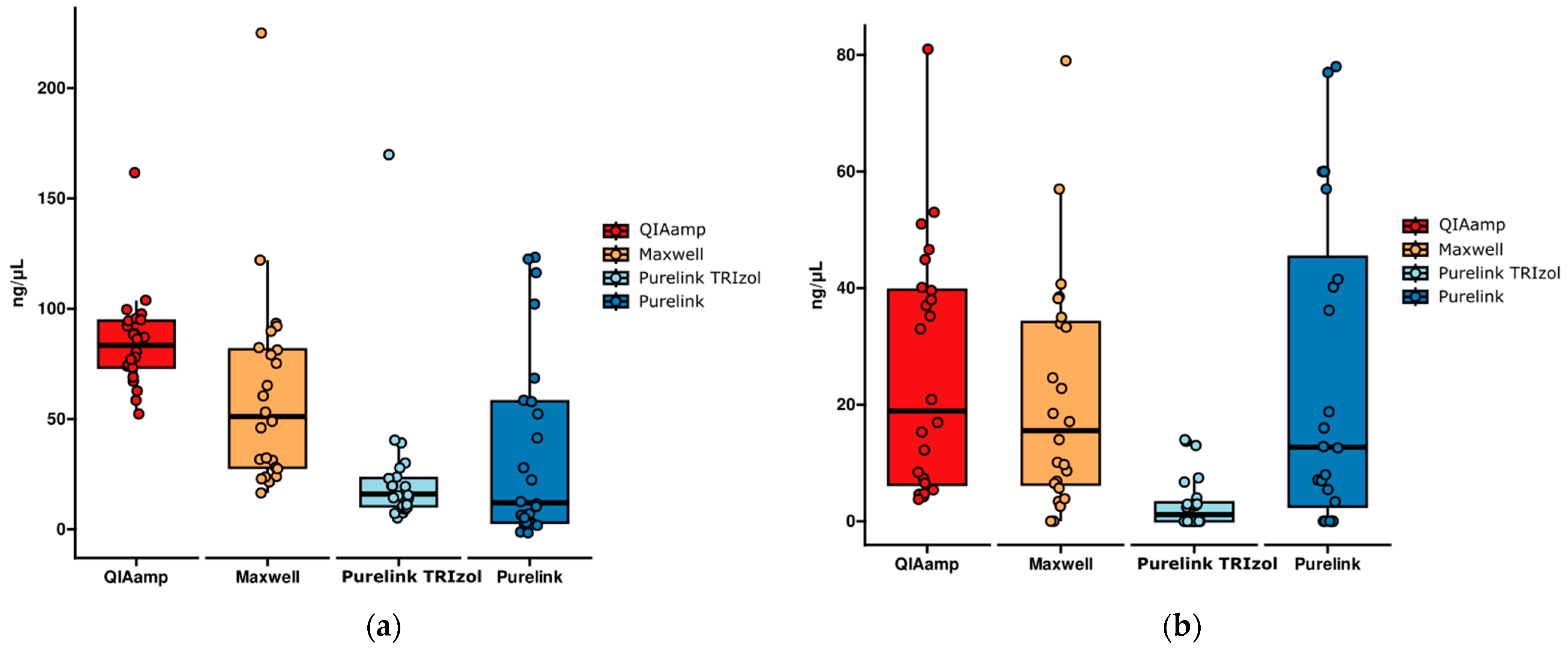
2.3. RNA Quantification Comparison
3. Discussion
4. Materials and Methods
4.1. Viral Titration
4.2. Rearing and Maintenance of Ae. aegypti Colonies
4.3. Experimental Infection
4.4. Mosquitoes Maceration
4.5. RNA Extraction
4.6. RT-qPCR
4.7. RNA Quantification and Purity
4.8. Statistical Analysis
4.9. Ethical Approval
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lima-Camara, T. Emerging Arboviruses and Public Health Challenges in Brazil. Rev. Saúde Pública 2016, 50, 36. [Google Scholar] [CrossRef]
- Carneiro, F.F.; Pessoa, V.M.; Teixeira, A.C.D.A.; Barbosa, M.I.S.; Holanda Lavor, A.C.; Silva, J.F. Experiência Bem-Sucedida No Controle Do Aedes aegypti Sem Uso de Venenos No Sertão Cearense. Vigilância Sanitária Debate 2016, 4, 126–131. [Google Scholar] [CrossRef]
- Smith, S.A.; Silva, L.A.; Fox, J.M.; Flyak, A.I.; Kose, N.; Sapparapu, G.; Khomandiak, S.; Ashbrook, A.W.; Kahle, K.M.; Fong, R.H.; et al. Isolation and Characterization of Broad and Ultrapotent Human Monoclonal Antibodies with Therapeutic Activity against Chikungunya virus. Cell Host Microbe 2015, 18, 86–95. [Google Scholar] [CrossRef]
- de Lima Cavalcanti, T.Y.V.; Pereira, M.R.; de Paula, S.O.; de Franca, R.F.O. A Review on Chikungunya virus Epidemiology, Pathogenesis and Current Vaccine Development. Viruses 2022, 14, 969. [Google Scholar] [CrossRef]
- Vu, D.M.; Jungkind, D.; Labeaud, A.D. Chikungunya virus. Clin. Lab. Med. 2017, 37, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Consoli, R.A.G.B.; Oliveira, R.L.D. Principais Mosquitos de Importância Sanitária No Brasil; FIOCRUZ: Rio de Janeiro, RJ, Brazil, 1994; p. 228. ISBN 85-85676-03-5. [Google Scholar]
- Lwande, O.W.; Obanda, V.; Lindström, A.; Ahlm, C.; Evander, M.; Näslund, J.; Bucht, G. Globe-Trotting Aedes aegypti and Aedes albopictus: Risk Factors for Arbovirus Pandemics. Vector-Borne Zoonotic Dis. 2019, 20, 71–81. [Google Scholar] [CrossRef]
- Powell, J.R.; Gloria-Soria, A.; Kotsakiozi, P. Recent History of Aedes aegypti: Vector Genomics and Epidemiology Records. BioScience 2018, 68, 854–860. [Google Scholar] [CrossRef]
- Conway, M.J.; Colpitts, T.M.; Fikrig, E. Role of the Vector in Arbovirus Transmission. Annu. Rev. Virol. 2014, 1, 71–88. [Google Scholar] [CrossRef]
- Dias Lemes de Vargas, L.; Miranda de Freitas, D.; Rosa dos Santos, B.; Rose de Oliveira Silva, M.; Dias de Souza, M.; Shimoya-Bittencourt, W. O Aedes aegypti E a Dengue: Aspectos Gerais e Panorama da Dengue no Brasil e no Mundo. UNICIÊNCIAS 2021, 24, 78–85. [Google Scholar] [CrossRef]
- Dettogni, R.S.; Louro, I.D. Desafios da extração do RNA do vírus da dengue (ligação e extração ao DNA: Métodos, aplicações e limitações). In Biotecnologia Aplicada à Agro&Indústria; Blucher: São Paulo, Brazil, 2017; Volume 4, pp. 937–966. [Google Scholar] [CrossRef]
- Tan, S.C.; Yiap, B.C. DNA, RNA, and Protein Extraction: The Past and the Present. J. Biomed. Biotechnol. 2009, 2009, 574398. [Google Scholar] [CrossRef]
- Licínio, C.O.L.; Ayres, F.M. The Use of Real Time PCR for Arboviruses Diagnostics: Integrative Review. J. Bras. Patol. Med. Lab. 2021, 57, e2882021. [Google Scholar] [CrossRef]
- Lanciotti, R.S. Molecular Amplification Assays for the Detection of Flaviviruses. Adv. Virus Res. 2003, 61, 67–99. [Google Scholar] [CrossRef]
- Fleige, S.; Pfaffl, M.W. RNA Integrity and the Effect on the Real-Time qRT-PCR Performance. Mol. Asp. Med. 2006, 27, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.H. Nucleic Acid Extraction Techniques. In Advanced Techniques in Diagnostic Microbiology; Springer: Berlin/Heidelberg, Germany, 2012; pp. 209–225. [Google Scholar] [CrossRef]
- Widen, R.H.; Silbert, S. Nucleic Acid Extraction in Diagnostic Virology. In Clinical Virology Manual; ASM Press: Washington, DC, USA, 2016; pp. 117–128. [Google Scholar] [CrossRef]
- Ahmed, M.; Pollak, N.M.; Hugo, L.E.; van den Hurk, A.F.; Hobson-Peters, J.; Macdonald, J. Rapid Molecular Assays for the Detection of the Four Dengue Viruses in Infected Mosquitoes. Gates Open Res. 2022, 6, 81. [Google Scholar] [CrossRef]
- Kirstein, O.D.; Ayora-Talavera, G.; Koyoc-Cardeña, E.; Chan Espinoza, D.; Che-Mendoza, A.; Cohuo-Rodriguez, A.; Granja-Pérez, P.; Puerta-Guardo, H.; Pavia-Ruz, N.; Dunbar, M.W.; et al. Natural Arbovirus Infection Rate and Detectability of Indoor Female Aedes aegypti from Mérida, Yucatán, Mexico. PLoS Neglected Trop. Dis. 2021, 15, e0008972. [Google Scholar] [CrossRef]
- Ribeiro Cruz, A.C.; Pinto Nunes Neto, J.; Patroca da Silva, S.; Vieira Pinto da Silva, E.; Juscely Galvão Pereira, G.; Maia Santos, M.; Antônio de Oliveira Monteiro, H.; Barreto dos Santos, F.; José de Paula Souza e Guimarães, R.; Fortes Aragão, C.; et al. Chikungunya virus Detection in Aedes aegypti and Culex quinquefasciatus during an Outbreak in the Amazon Region. Viruses 2020, 12, 853. [Google Scholar] [CrossRef]
- Aragão, C.F.; Pinheiro, V.C.S.; Nunes Neto, J.P.; da Silva, E.V.P.; Pereira, G.J.G.; do Nascimento, B.L.S.; da Castro, K.S.; Maia, A.M.; Catete, C.P.; Martins, L.C.; et al. Natural Infection of Aedes aegypti by Chikungunya and Dengue Type 2 Virus in a Transition Area of North-Northeast Brazil. Viruses 2019, 11, 1126. [Google Scholar] [CrossRef]
- Cevallos, V.; Ponce, P.; Waggoner, J.J.; Pinsky, B.A.; Coloma, J.; Quiroga, C.; Morales, D.; Cárdenas, M.J. Zika and Chikungunya virus Detection in Naturally Infected Aedes aegypti in Ecuador. Acta Trop. 2018, 177, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Promega. Maxwell® 16 Viral Total Nucleic Acid Purification Kit. Available online: https://www.promega.com.br/ (accessed on 20 April 2024).
- Loens, K.; Bergs, K.; Ursi, D.; Goossens, H.; Ieven, M. Evaluation of NucliSens EasyMAG for Automated Nucleic Acid Extraction from Various Clinical Specimens. J. Clin. Microbiol. 2007, 45, 421–425. [Google Scholar] [CrossRef]
- Rossa, W.K.C.; Jin, Y.; Grace, T.Y.C.; Lui, W.B.; Anthony, T.C.C.; Lim, W.; Dennis Lo, Y.M. Automated Extraction Protocol for Quantification of SARS-Coronavirus RNA in Serum: An Evaluation Study. BMC Infect. Dis. 2006, 6, 20. [Google Scholar] [CrossRef]
- Thermo Fisher. TRIzol® Plus RNA Purification Kit. Available online: https://www.thermofisher.cn/cn/zh/home/references/protocols/nucleic-acid-purification-and-analysis/mrna-protocols/trizol-plus-rna-purification-kit.html (accessed on 20 April 2024).
- Kumar, M.; Mazur, S.; Ork, B.L.; Postnikova, E.; Hensley, L.E.; Jahrling, P.B.; Johnson, R.; Holbrook, M.R. Inactivation and Safety Testing of Middle East Respiratory Syndrome Coronavirus. J. Virol. Methods 2015, 223, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Retterer, C.; Kenny, T.; Zamani, R.; Altamura, L.A.; Kearney, B.; Jaissle, J.; Coyne, S.; Olschner, S.; Harbourt, D. Strategies for Validation of Inactivation of Viruses with Trizol® LS and Formalin Solutions. Appl. Biosaf. 2020, 25, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Gullett, J.C.; Nolte, F.S. Quantitative Nucleic Acid Amplification Methods for Viral Infections. Clin. Chem. 2014, 61, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Valasek, M.A.; Repa, J.J. The Power of Real-Time PCR. Adv. Physiol. Educ. 2005, 29, 151–159. [Google Scholar] [CrossRef]
- Melo, M.R.; Martins, A.R.; Barbosa, I.V.; Romano, P.; Shcolnik, W. Coleta, Transporte e Armazenamento de Amostras para Diagnóstico Molecular. J. Bras. Patol. Med. Lab. 2010, 46, 375–381. [Google Scholar] [CrossRef]
- Fabre, A.-L.; Colotte, M.; Luis, A.; Tuffet, S.; Bonnet, J. An Efficient Method for Long-Term Room Temperature Storage of RNA. Eur. J. Hum. Genet. 2014, 22, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Green, M.R.; Sambrook, J.E. Molecular Cloning: A Laboratory Manual, 4th ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2012; ISBN 978-1-936113-41-5. [Google Scholar]
- Thermo Fisher. NanoDrop Micro-UV/Vis Spectrophotometers NanoDrop One User Guide. Available online: https://assets.thermofisher.com/TFS-Assets/MSD/manuals/nanodrop-one-user-guide-EN-309102-REV-A.pdf (accessed on 20 April 2024).
- Kildemo, H. RNA Expression in Sperm as Markers of Sperm-Quality. Master Thesis, Department of Toxicology Institute of Biology, University of Oslo, Oslo, Norway, 2012. [Google Scholar]
- Moret, I.; Sánchez-Izquierdo, D.; Iborra, M.; Tortosa, L.; Navarro-Puche, A.; Nos, P.; Cervera, J.; Beltrán, B. Assessing an Improved Protocol for Plasma MicroRNA Extraction. PLoS ONE 2013, 8, e82753. [Google Scholar] [CrossRef]
- Tesh, R.B. A Method for the Isolation and Identification of Dengue Viruses, Using Mosquito Cell Cultures. Am. J. Trop. Med. Hyg. 1979, 28, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Panec, M.; Katz, S. Plaque Assay Protocols, American Society for Microbiology. 2006. Available online: https://asm.org/ASM/media/Protocol-Images/Plaque-Assay-Protocols.pdf?ext=.pdf#:~:text=URL%3A%20https%3A%2F%2Fasm.org%2FASM%2Fmedia%2FProtocol (accessed on 21 April 2024).
- Guerra, A.F. Microbiologia de Alimentos: Métodos de Contagem Microbiana; Editora Valença: Rio de Janeiro, RJ, Brazil, 2016; p. 28. [Google Scholar]
- Salazar, M.I.; Richardson, J.H.; Sánchez-Vargas, I.; Olson, K.E.; Beaty, B.J. Dengue Virus Type 2: Replication and Tropisms in Orally Infected Aedes aegypti Mosquitoes. BMC Microbiol. 2007, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Valle, D.; José, E.; Matos, E.R.; Silveira, F.T.; dos Santos, T.V.; Póvoa, M. Artificial Blood-Feeding of Phlebotomines (Diptera: Psychodidae: Phlebotominae): Is It Time to Repurpose Biological Membranes in Light of Ethical Concerns? Parasites Vectors 2022, 15, 399. [Google Scholar] [CrossRef]
- Auguste, A.J.; Volk, S.M.; Arrigo, N.C.; Martinez, R.; Ramkissoon, V.; Adams, A.P.; Thompson, N.N.; Adesiyun, A.A.; Chadee, D.D.; Foster, J.E.; et al. Isolation and Phylogenetic Analysis of Mucambo virus (Venezuelan Equine Encephalitis Complex Subtype IIIA) in Trinidad. Virology 2009, 392, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Lanciotti, R.S.; Kosoy, O.L.; Laven, J.J.; Panella, A.J.; Velez, J.O.; Lambert, A.J.; Campbell, G.L. Chikungunya virus in US Travelers Returning from India, 2006. Emerg. Infect. Dis. 2007, 13, 764–767. [Google Scholar] [CrossRef] [PubMed]
- Lan, Q.; Fallon, A.M. Sequence Analysis of a Mosquito Ribosomal Protein RpL8 Gene and Its Upstream Regulatory Region. Insect Mol. Biol. 1992, 1, 71–80. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria; Available online: https://www.r-project.org/ (accessed on 20 April 2024).
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open-Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Kassambara, A. Rstatix: Pipe-Friendly Framework for Basic Statistical Tests. R Package Version 0.7.0. Available online: https://cran.r-project.org/web/packages/rstatix/index.html (accessed on 20 April 2024).
- Harrell, F.E., Jr. Package ‘Hmisc’. Available online: https://hbiostat.org/R/Hmisc/ (accessed on 20 April 2024).
| Pool | QIAamp® | Maxwell® | PureLink® with TRIzol® | PureLink® |
|---|---|---|---|---|
| 1A | + | + | + | + |
| 1B | + | + | + | - |
| 1C | + | - | + | + |
| 5A | + | - | + | + |
| 5B | + | - | + | + |
| 5C | + | - | + | + |
| 10A | + | + | + | + |
| 10B | + | - | + | + |
| 10C | + | - | + | + |
| 20A | + | - | + | + |
| 20B | + | - | + | + |
| 20C | + | + | + | + |
| 30A | + | - | + | - |
| 30B | + | + | + | - |
| 30C | + | + | + | + |
| 40A | + | + | + | - |
| 40B | + | + | + | + |
| 40C | + | - | + | - |
| Pair of Variables | Correlation Coefficient (ρ) | p-Value | Correlation Level | Statistical Significance |
|---|---|---|---|---|
| n and QIAamp® | −0.7492752 | 0.0003448 | strong | *** |
| n and Maxwell® | −0.003441734 | 0.9892 | very weak | non-significant |
| n and PureLink® with TRIzol® | −0.5423624 | 0.02005 | moderate | * |
| n and PureLink® | −0.2566004 | 0.304 | weak | non-significant |
| Factor | QIAamp® | Maxwell® | PureLink® with TRIzol® | PureLink® |
|---|---|---|---|---|
| Cost per sample | USD 7.30 | USD 14.90 | USD 8.75 | USD 7.04 |
| Efficiency | Very efficient | Not very efficient | Efficient | Not very efficient |
| Sensitivity | High | Very Low | High | Low |
| Time per sample | 20 min | 65 min | 28 min | 8 min |
| Type of extraction | Manual | Manual and automated | Manual | Manual |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freitas, B.C.G.; Dias, D.D.; Reis, L.A.M.; Hernández, L.H.A.; Cereja, G.J.G.P.; Aragão, C.F.; da Silva, S.P.; Nunes Neto, J.P.; Elias, C.N.; Cruz, A.C.R. Evaluation of Multiple RNA Extraction Protocols for Chikungunya Virus Screening in Aedes aegypti Mosquitoes. Int. J. Mol. Sci. 2024, 25, 6700. https://doi.org/10.3390/ijms25126700
Freitas BCG, Dias DD, Reis LAM, Hernández LHA, Cereja GJGP, Aragão CF, da Silva SP, Nunes Neto JP, Elias CN, Cruz ACR. Evaluation of Multiple RNA Extraction Protocols for Chikungunya Virus Screening in Aedes aegypti Mosquitoes. International Journal of Molecular Sciences. 2024; 25(12):6700. https://doi.org/10.3390/ijms25126700
Chicago/Turabian StyleFreitas, Bárbara Caroline Garcia, Daniel Damous Dias, Lúcia Aline Moura Reis, Leonardo Henrique Almeida Hernández, Glennda Juscely Galvão Pereira Cereja, Carine Fortes Aragão, Sandro Patroca da Silva, Joaquim Pinto Nunes Neto, Carmeci Natalina Elias, and Ana Cecília Ribeiro Cruz. 2024. "Evaluation of Multiple RNA Extraction Protocols for Chikungunya Virus Screening in Aedes aegypti Mosquitoes" International Journal of Molecular Sciences 25, no. 12: 6700. https://doi.org/10.3390/ijms25126700







