Peptides Used for Heavy Metal Remediation: A Promising Approach
Abstract
:1. Introduction
1.1. Remediation Methods for Heavy Metal Pollution
1.1.1. Physical Rehabilitation
1.1.2. Chemical Remediation
1.1.3. Bioremediation
1.1.4. Combined Restoration
Plant Combinatorial Restoration
Electrical Energy Combinatorial Restoration
Nano-Combinatorial Restoration
1.2. Peptide
2. Treatment of Heavy Metal Pollution by Peptides
2.1. Amino Acids Can Remove Heavy Metals
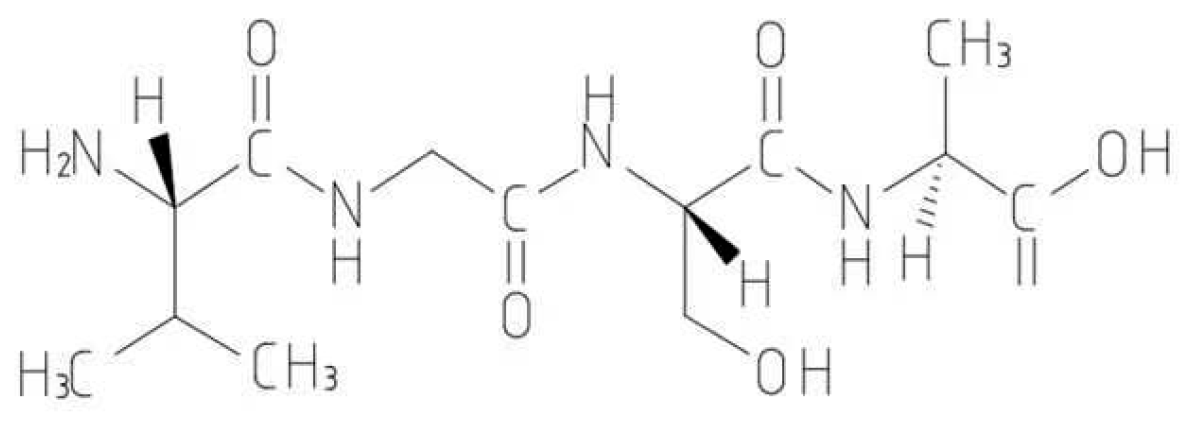
2.2. Peptide Removal of Heavy Metals
3. Sources of Peptides
3.1. Animal and Plant Peptides
3.2. Microbial Fermentation
3.3. Chemical Synthesis
3.3.1. Solid-Phase Synthesis Method
3.3.2. Solution-Phase Synthesis Method
4. Mechanism of Peptide Remediation of Heavy Metal Pollution
4.1. Peptide–Metal Ion Chelate
4.1.1. Preparation Method
4.1.2. Chelation Reaction Mechanism
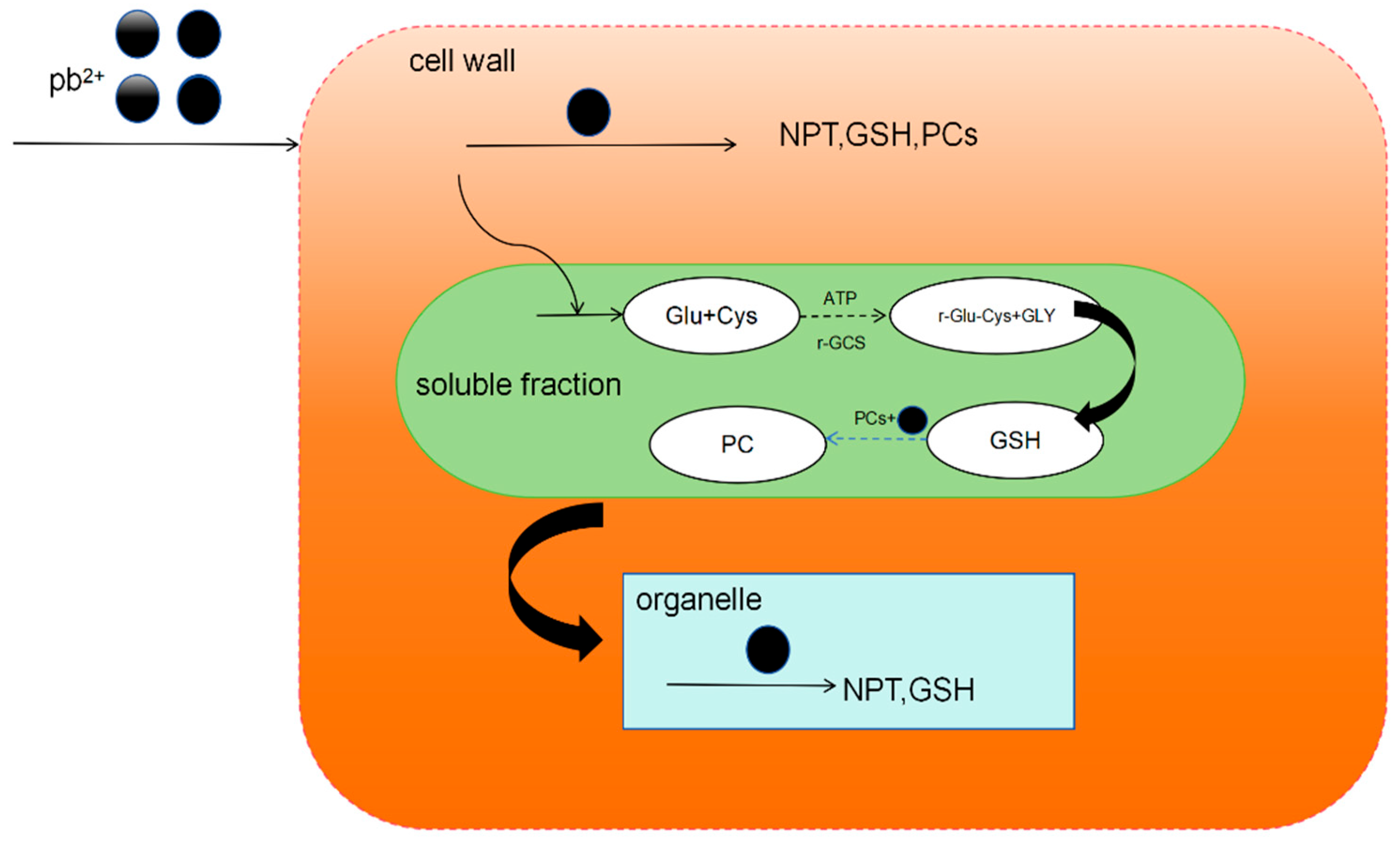
4.2. Phytochelins
| PC Family | Peptide Structure | Identification |
|---|---|---|
| Phytochelatins PCn-Gly | (γGlu-Cys)n-Gly | PCn |
| Iso-phytochelatins | ||
| PCn-Ser | (γGlu-Cys)n-Ser | iso-PCn(Ser) |
| PCn-Ala | (γGlu-Cys)n-Ala | iso-PCn(Ala) |
| PCn-βAla | (γGlu-Cys)n-βAla | iso-PCn(βAla) |
| PCn-Glu | (γGlu-Cys)n-Glu | iso-PCn(Glu) |
| PCn-Gln | (γGlu-Cys)n-Gln | iso-PCn(Gln) |
| PCn-Asn | (γGlu-Cys)n-Asn | iso-PCn(Asn) |
| PCn-Cys | (γGlu-Cys)n-Cys | iso-PCn(Cys) |
| des-Gly-PCn | (γGlu-Cys)n | des-Gly-PCn |
| des-γGlu-PCn-Gly | Cys-(γGlu-Cys)n−1-Gly | des-γGlu-iso-PCn(Gly) |
| des-γGlu-PCn-Ser | Cys-(γGlu-Cys)n−1-Ser | des-γGlu-iso-PCn(Ser) |
| des-Cys-PCn-Glu | Glu-(γGlu-Cys)n−1-Glu | des-Cys-iso-PCn(Glu) |
4.3. Peptide Reduction of Metals
5. Summary and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jiang, H.H.; Cai, L.M.; Wen, H.H.; Hu, G.C.; Chen, L.G.; Luo, J. An integrated approach to quantifying ecological and human health risks from different sources of soil heavy metals. Sci. Total Environ. 2020, 701, 134466. [Google Scholar] [CrossRef] [PubMed]
- Fergusson, J.E. The Heavy Elements: Chemistry, Environmental Impact and Health Effects. 1990. Available online: https://cir.nii.ac.jp/crid/1130282270393822464 (accessed on 20 December 2023).
- He, Z.L.; Yang, X.E.; Stoffella, P.J. Trace elements in agroecosystems and impacts on the environment. J. Trace Elem. Med. Biol. Organ Soc. Miner. Trace Elem. (GMS) 2005, 19, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Herawati, N.; Suzuki, S.; Hayashi, K.; Rivai, I.F.; Koyama, H. Cadmium, copper, and zinc levels in rice and soil of Japan, Indonesia, and China by soil type. Bull. Environ. Contam. Toxicol. 2000, 64, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Shallari, S.; Schwartz, C.; Hasko, A.; Morel, J.L. Heavy metals in soils and plants of serpentine and industrial sites of Albania. Sci. Total Environ. 1998, 209, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Jan, A.T.; Azam, M.; Siddiqui, K.; Ali, A.; Choi, I.; Haq, Q.M. Heavy Metals and Human Health: Mechanistic Insight into Toxicity and Counter Defense System of Antioxidants. Int. J. Mol. Sci. 2015, 16, 29592–29630. [Google Scholar] [CrossRef] [PubMed]
- Flora, S.J.; Mittal, M.; Mehta, A. Heavy metal induced oxidative stress & its possible reversal by chelation therapy. Indian J. Med. Res. 2008, 128, 501–523. [Google Scholar] [PubMed]
- Rahman, Z.; Singh, V.P. The relative impact of toxic heavy metals (THMs) (arsenic (As), cadmium (Cd), chromium (Cr)(VI), mercury (Hg), and lead (Pb)) on the total environment: An overview. Environ. Monit. Assess. 2019, 191, 419. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Dong, Y.; Feng, Y.; Li, Y.; Dong, Y. Thermal desorption for remediation of contaminated soil: A review. Chemosphere 2019, 221, 841–855. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Xu, D.; Yue, J.; Ma, Y.; Dong, S.; Feng, J. Recent advances in soil remediation technology for heavy metal contaminated sites: A critical review. Sci. Total Environ. 2022, 838 Pt 3, 156417. [Google Scholar] [CrossRef]
- Hu, W.; Niu, Y.; Zhu, H.; Dong, K.; Wang, D.; Liu, F. Remediation of zinc-contaminated soils by using the two-step washing with citric acid and water-soluble chitosan. Chemosphere 2021, 282, 131092. [Google Scholar] [CrossRef]
- Dermont, G.; Bergeron, M.; Mercier, G.; Richer-Laflèche, M. Soil washing for metal removal: A review of physical/chemical technologies and field applications. J. Hazard. Mater. 2008, 152, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Wang, J.; Liu, X.; Yuan, L.; Liu, X.; Deng, H. Comparative study on washing effects of different washing agents and conditions on heavy metal contaminated soil. Surf. Interfaces 2021, 27, 101563. [Google Scholar] [CrossRef]
- Khan, F.I.; Husain, T.; Hejazi, R. An overview and analysis of site remediation technologies. J. Environ. Manag. 2004, 71, 95–122. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Liang, Y.; Xu, X.; Zhao, L.; Cao, X. An integrated approach for simultaneous immobilization of lead in both contaminated soil and groundwater: Laboratory test and numerical modeling. J. Hazard. Mater. 2018, 342, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.-M.; Fu, R.-B.; Wang, J.-X.; Shi, Y.-X.; Guo, X.-P. Chemical stabilization remediation for heavy metals in contaminated soils on the latest decade: Available stabilizing materials and associated evaluation methods—A critical review. J. Clean. Prod. 2021, 321, 128730. [Google Scholar] [CrossRef]
- Ashraf, A.; Bibi, I.; Niazi, N.K.; Ok, Y.S.; Murtaza, G.; Shahid, M.; Kunhikrishnan, A.; Li, D.; Mahmood, T. Chromium(VI) sorption efficiency of acid-activated banana peel over organo-montmorillonite in aqueous solutions. Int. J. Phytoremediat. 2017, 19, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Austruy, A.; Shahid, M.; Xiong, T.; Castrec, M.; Payre, V.; Niazi, N.K.; Sabir, M.; Dumat, C. Mechanisms of metal-phosphates formation in the rhizosphere soils of pea and tomato: Environmental and sanitary consequences. J. Soils Sediments 2014, 14, 666–678. [Google Scholar] [CrossRef]
- Khalid, S.; Shahid, M.; Niazi, N.K.; Murtaza, B.; Bibi, I.; Dumat, C. A comparison of technologies for remediation of heavy metal contaminated soils. J. Geochem. Explor. 2017, 182, 247–268. [Google Scholar] [CrossRef]
- Shahid, M.; Xiong, T.; Masood, N.; Leveque, T.; Quenea, K.; Austruy, A.; Foucault, Y.; Dumat, C. Influence of plant species and phosphorus amendments on metal speciation and bioavailability in a smelter impacted soil: A case study of food-chain contamination. J. Soils Sediments 2014, 14, 655–665. [Google Scholar] [CrossRef]
- Wang, J.; Hou, L.A.; Yao, Z.; Jiang, Y.; Xi, B.; Ni, S.; Zhang, L. Aminated electrospun nanofiber membrane as permeable reactive barrier material for effective in-situ Cr(VI) contaminated soil remediation. Chem. Eng. J. 2021, 406, 126822. [Google Scholar] [CrossRef]
- Robles, I.; Lozano, M.J.; Solís, S.; Hernández, G.; Paz, M.V.; Olvera, M.G.; Bustos, E. Electrokinetic Treatment Of Mercury-Polluted Soil Facilitated By Ethylenediaminetetraacetic Acid Coupled With A Reactor With A Permeable Reactive Barrier Of Iron To Recover Mercury (II) From Water. Electrochim. Acta 2015, 181, 68–72. [Google Scholar] [CrossRef]
- Wen, D.; Fu, R.; Li, Q. Removal of inorganic contaminants in soil by electrokinetic remediation technologies: A review. J. Hazard. Mater. 2021, 401, 123345. [Google Scholar] [CrossRef]
- Rahman, Z.; Mohan, A.; Priya, S. Electrokinetic remediation: An innovation for heavy metal contamination in the soil environment. Mater. Today Proc. 2021, 37, 2730–2734. [Google Scholar] [CrossRef]
- Liang, W.; Wang, G.; Peng, C.; Tan, J.; Wan, J.; Sun, P.; Li, Q.; Ji, X.; Zhang, Q.; Wu, Y.; et al. Recent advances of carbon-based nano zero valent iron for heavy metals remediation in soil and water: A critical review. J. Hazard. Mater. 2022, 426, 127993. [Google Scholar] [CrossRef]
- Leong, Y.K.; Chang, J.S. Bioremediation of heavy metals using microalgae: Recent advances and mechanisms. Bioresour. Technol. 2020, 303, 122886. [Google Scholar] [CrossRef] [PubMed]
- Yaashikaa, P.R.; Kumar, P.S.; Jeevanantham, S.; Saravanan, R. A review on bioremediation approach for heavy metal detoxification and accumulation in plants. Environ. Pollut. 2022, 301, 119035. [Google Scholar] [CrossRef]
- Li, F.; Li, Z.; Mao, P.; Li, Y.; Li, Y.; McBride, M.B.; Wu, J.; Zhuang, P. Heavy metal availability, bioaccessibility, and leachability in contaminated soil: Effects of pig manure and earthworms. Environ. Sci. Pollut. Res. Int. 2019, 26, 20030–20039. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yang, B.; Liang, Y.; Xiao, Y.; Fang, J. Prospect of phytoremediation combined with other approaches for remediation of heavy metal-polluted soils. Environ. Sci. Pollut. Res. Int. 2020, 27, 16069–16085. [Google Scholar] [CrossRef]
- Ashraf, S.; Ali, Q.; Zahir, Z.A.; Ashraf, S.; Asghar, H.N. Phytoremediation: Environmentally sustainable way for reclamation of heavy metal polluted soils. Ecotoxicol. Environ. Saf. 2019, 174, 714–727. [Google Scholar] [CrossRef]
- Pochodylo, A.L.; Aristilde, L.J.E.C.L. Molecular dynamics of stability and structures in phytochelatin complexes with Zn, Cu, Fe, Mg, and Ca: Implications for metal detoxification. Environ. Chem. Lett. 2017, 15, 495–500. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, F.; Liu, Q.; Chen, M.; Liu, X.; Wang, Y.; Sun, Y.; Zhang, L. Nanomaterials and plants: Positive effects, toxicity and the remediation of metal and metalloid pollution in soil. Sci. Total Environ. 2019, 662, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, M.T.; Najam-Ul-Haq, M.; Idrees, M.; Ullah, I.; Afzal, M. Bacterial endophytes enhance phytostabilization in soils contaminated with uranium and lead. Int. J. Phytoremediat. 2017, 19, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Cameselle, C.; Chirakkara, R.A.; Reddy, K.R. Electrokinetic-enhanced phytoremediation of soils: Status and opportunities. Chemosphere 2013, 93, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Dai, H.; Skuza, L.; Wei, S. The effects of different electric fields and electrodes on Solanum nigrum L. Cd hyperaccumulation in soil. Chemosphere 2020, 246, 125666. [Google Scholar] [CrossRef] [PubMed]
- He, J.; He, C.; Chen, X.; Liang, X.; Huang, T.; Yang, X.; Shang, H. Comparative study of remediation of Cr(VI)-contaminated soil using electrokinetics combined with bioremediation. Environ. Sci. Pollut. Res. Int. 2018, 25, 17682–17689. [Google Scholar] [CrossRef] [PubMed]
- Masud, M.A.A.; Shin, W.S.; Septian, A.; Samaraweera, H.; Khan, I.J.; Mohamed, M.M.; Billah, M.M.; López-Maldonado, E.A.; Rahman, M.M.; Islam, A.R.M.T.; et al. Exploring the environmental pathways and challenges of fluoroquinolone antibiotics: A state-of-the-art review. Sci. Total Environ. 2024, 926, 171944. [Google Scholar] [CrossRef] [PubMed]
- Samaraweera, H.; Khan, A.H. 3-Recent trends in graphene-based materials for pharmaceuticals wastewater treatment. In The Treatment of Pharmaceutical Wastewater; Khan, A.H., Khan, N.A., Naushad, M., Aziz, H.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 53–68. [Google Scholar] [CrossRef]
- Zhao, J.; Qin, Y.; Liu, Y.; Shi, Y.; Lin, Q.; Cai, M.; Jia, Z.; Yu, C.; Shang, A.; Fei, Y.; et al. Cobalt/Iron Bimetallic Biochar Composites for Lead(II) Adsorption: Mechanism and Remediation Performance. Molecules 2024, 29, 1595. [Google Scholar] [CrossRef] [PubMed]
- Samaraweera, H.; Nawalage, S.; Nayanathara, R.M.O.; Peiris, C.; Karunaratne, T.N.; Gunatilake, S.R.; Thirumalai, R.V.K.G.; Zhang, J.; Zhang, X.; Mlsna, T. In Situ Synthesis of Zero-Valent Iron-Decorated Lignite Carbon for Aqueous Heavy Metal Remediation. Processes 2022, 10, 1659. [Google Scholar] [CrossRef]
- Cheng, P.; Zhang, S.; Wang, Q.; Feng, X.; Zhang, S.; Sun, Y.; Wang, F. Contribution of Nano-Zero-Valent Iron and Arbuscular Mycorrhizal Fungi to Phytoremediation of Heavy Metal-Contaminated Soil. Nanomaterials 2021, 11, 1264. [Google Scholar] [CrossRef]
- Boente, C.; Sierra, C.; Martínez-Blanco, D.; Menéndez-Aguado, J.M.; Gallego, J.R. Nanoscale zero-valent iron-assisted soil washing for the removal of potentially toxic elements. J. Hazard. Mater. 2018, 350, 55–65. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, T. Understanding Protein Functions in the Biological Context. Protein Pept. Lett. 2023, 30, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Carter, D.A.; Fai, C.K.; Murphy, D. Neurohypophyseal peptides as regulators of growth and development—A review. J. Mol. Neurosci. 1993, 4, 11–19. [Google Scholar] [CrossRef]
- Lach, G.; Schellekens, H.; Dinan, T.G.; Cryan, J.F. Anxiety, Depression, and the Microbiome: A Role for Gut Peptides. Neurotherapeutics 2018, 15, 36–59. [Google Scholar] [CrossRef]
- Gong, W.; Pan, C.; Cheng, P.; Wang, J.; Zhao, G.; Wu, X. Peptide-Based Vaccines for Tuberculosis. Front. Immunol. 2022, 13, 830497. [Google Scholar] [CrossRef]
- Romanova, E.V.; Sweedler, J.V. Peptidomics for the discovery and characterization of neuropeptides and hormones. Trends Pharmacol. Sci. 2015, 36, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, Y.; Wang, F.; Luo, Z.; Guo, S.; Strähle, U. Toxicity of mercury: Molecular evidence. Chemosphere 2020, 245, 125586. [Google Scholar] [CrossRef]
- Palaniyandi, K.; Wang, S.-H.; Chen, F. Chinese Medicinal Herbs as Source of Rational Anticancer Therapy. In Medicinal Plants-recent Advances in Research and Development; Springer: Singapore, 2016. [Google Scholar]
- Fitzsimmons, J.; Foley, B.; Torre, B.; Wilken, M.; Cutler, C.S.; Mausner, L.; Medvedev, D. Optimization of Cation Exchange for the Separation of Actinium-225 from Radioactive Thorium, Radium-223 and Other Metals. Molecules 2019, 24, 1921. [Google Scholar] [CrossRef]
- Efferth, T.; Kaina, B. Toxicities by herbal medicines with emphasis to traditional Chinese medicine. Curr. Drug Metab. 2011, 12, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, R.; Ban, S.; Devkota, S.; Sharma, S.; Joshi, R.; Tiwari, A.P.; Kim, H.Y.; Joshi, M.K. Technological trends in heavy metals removal from industrial wastewater: A review. J. Environ. Chem. Eng. 2021, 9, 105688. [Google Scholar] [CrossRef]
- Samaraweera, H.; Pittman, C.U.; Thirumalai, R.V.K.G.; Hassan, E.B.; Perez, F.; Mlsna, T. Characterization of graphene/pine wood biochar hybrids: Potential to remove aqueous Cu2+. Environ. Res. 2021, 192, 110283. [Google Scholar] [CrossRef]
- Mahdi, Z.; El Hanandeh, A.; Jimmy, Y.Q. Electro-assisted adsorption of heavy metals from aqueous solutions by biochar. Water Sci. Technol. A J. Int. Assoc. Water Pollut. Res. 2020, 81, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, J.; Xie, H.; Pan, Y.; Liu, J.; Huang, Z.; Long, X.; Xiao, H. Cellulose-based adsorbents loaded with zero-valent iron for removal of metal ions from contaminated water. Environ. Sci. Pollut. Res. Int. 2020, 27, 33234–33247. [Google Scholar] [CrossRef] [PubMed]
- Nejadshafiee, V.; Islami, M.R. Adsorption capacity of heavy metal ions using sultone-modified magnetic activated carbon as a bio-adsorbent. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 101, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Li, K.; He, Y.; Wang, Y.; Qian, X.; Jia, J. Evaluation of magnetic chitosan beads for adsorption of heavy metal ions. Sci. Total Environ. 2018, 627, 1396–1403. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Sharma, A.; Kaur, R.; Thukral, A.K.; Bhardwaj, R.; Ahmad, P. Differential distribution of amino acids in plants. Amino Acids 2017, 49, 821–869. [Google Scholar] [CrossRef]
- Ettiyagounder, P.; Tamilselvan, I.; Veeraswamy, D.; Periasami, K.; Selvaraj Paul, S. Metallothioneins: Diverse Protein Family to Bind Metallic Ions. In Heavy Metals; Mazen Khaled, N., Hongbo, Z., Eds.; IntechOpen: Rijeka, Croatia, 2021; Chapter 9. [Google Scholar] [CrossRef]
- Wu, G.; Fang, Y.Z.; Yang, S.; Lupton, J.R.; Turner, N.D. Glutathione metabolism and its implications for health. J. Nutr. 2004, 134, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Grill, E.; Winnacker, E.-L.; Zenk, M.H. [39] Phytochelatins. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1991; Volume 205, pp. 333–341. [Google Scholar]
- Padhi, A.; Sengupta, M.; Sengupta, S.; Roehm, K.H.; Sonawane, A. Antimicrobial peptides and proteins in mycobacterial therapy: Current status and future prospects. Tuberculosis 2014, 94, 363–373. [Google Scholar] [CrossRef]
- Fosgerau, K.; Hoffmann, T. Peptide therapeutics: Current status and future directions. Drug Discov. Today 2015, 20, 122–128. [Google Scholar] [CrossRef]
- Buchwald, H.; Dorman, R.B.; Rasmus, N.F.; Michalek, V.N.; Landvik, N.M.; Ikramuddin, S. Effects on GLP-1, PYY, and leptin by direct stimulation of terminal ileum and cecum in humans: Implications for ileal transposition. Surg. Obes. Relat. Dis. Off. J. Am. Soc. Bariatr. Surg. 2014, 10, 780–786. [Google Scholar] [CrossRef]
- Ryan, J.T.; Ross, R.P.; Bolton, D.; Fitzgerald, G.F.; Stanton, C. Bioactive peptides from muscle sources: Meat and fish. Nutrients 2011, 3, 765–791. [Google Scholar] [CrossRef]
- Zhu, C.F.; Li, G.Z.; Peng, H.B.; Zhang, F.; Chen, Y.; Li, Y. Therapeutic effects of marine collagen peptides on Chinese patients with type 2 diabetes mellitus and primary hypertension. Am. J. Med. Sci. 2010, 340, 360–366. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; FitzGerald, R.J. Enhancing bioactive peptide release and identification using targeted enzymatic hydrolysis of milk proteins. Anal. Bioanal. Chem. 2018, 410, 3407–3423. [Google Scholar] [CrossRef]
- Wu, J.; Wang, Y.; Liu, H.; Yang, H.; Ma, D.; Li, J.; Li, D.; Lai, R.; Yu, H. Two immunoregulatory peptides with antioxidant activity from tick salivary glands. J. Biol. Chem. 2010, 285, 16606–16613. [Google Scholar] [CrossRef]
- Escudero, E.; Sentandreu, M.A.; Arihara, K.; Toldrá, F. Angiotensin I-converting enzyme inhibitory peptides generated from in vitro gastrointestinal digestion of pork meat. J. Agric. Food Chem. 2010, 58, 2895–2901. [Google Scholar] [CrossRef]
- Harada, K.; Maeda, T.; Hasegawa, Y.; Tokunaga, T.; Tamura, Y.; Koizumi, T. Antioxidant activity of fish sauces including puffer (Lagocephalus wheeleri) fish sauce measured by the oxygen radical absorbance capacity method. Mol. Med. Rep. 2010, 3, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Najafian, L.; Babji, A.S. A review of fish-derived antioxidant and antimicrobial peptides: Their production, assessment, and applications. Peptides 2012, 33, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Kalina, R.; Gladkikh, I.; Dmitrenok, P.; Chernikov, O.; Koshelev, S.; Kvetkina, A.; Kozlov, S.; Kozlovskaya, E.; Monastyrnaya, M. New APETx-like peptides from sea anemone Heteractis crispa modulate ASIC1a channels. Peptides 2018, 104, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ding, X. Characterization of inhibition and stability of soy-protein-derived angiotensin I-converting enzyme inhibitory peptides. Food Res. Int. 2002, 35, 367–375. [Google Scholar] [CrossRef]
- Tang, X.; He, Z.; Dai, Y.; Xiong, Y.L.; Xie, M.; Chen, J. Peptide fractionation and free radical scavenging activity of zein hydrolysate. J. Agric. Food Chem. 2010, 58, 587–593. [Google Scholar] [CrossRef]
- Ma, M.-S.; Bae, I.Y.; Lee, H.G.; Yang, C.-B. Purification and identification of angiotensin I-converting enzyme inhibitory peptide from buckwheat (Fagopyrum esculentum Moench). Food Chem. 2006, 96, 36–42. [Google Scholar] [CrossRef]
- Zhang, T.; Li, Y.; Miao, M.; Jiang, B. Purification and characterisation of a new antioxidant peptide from chickpea (Cicer arietium L.) protein hydrolysates. Food Chem. 2011, 128, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Fleming, A. On the antibacterial action of cultures of a penicillium, with special reference to their use in the isolation of B. influenzae. 1929. Bull. World Health Organ. 2001, 79, 780–790. [Google Scholar] [PubMed]
- Waksman, S.A.; Schatz, A.; Reynolds, D.M. Production of antibiotic substances by actinomycetes. Ann. N. Y. Acad. Sci. 2010, 1213, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Chopra, I.; Roberts, M. Tetracycline antibiotics: Mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol. Mol. Biol. Rev. 2001, 65, 232–260. [Google Scholar] [CrossRef] [PubMed]
- Krishna, C. Solid-state fermentation systems—An overview. Crit. Rev. Biotechnol. 2005, 25, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.; Kumar, D. Production of l-methionine by submerged fermentation: A review. J. Enzym. Microb. Technol. 2005, 37, 3–18. [Google Scholar] [CrossRef]
- Wu, Z.; Li, Y.; Zhang, L.; Ding, Z.; Shi, G. Microbial production of small peptide: Pathway engineering and synthetic biology. Microb. Biotechnol. 2021, 14, 2257–2278. [Google Scholar] [CrossRef]
- Mazorra-Manzano, M.A.; Ramírez-Suarez, J.C.; Yada, R.Y. Plant proteases for bioactive peptides release: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 2147–2163. [Google Scholar] [CrossRef] [PubMed]
- Okoth, D.A.; Hug, J.J.; Garcia, R.; Müller, R. Discovery, Biosynthesis and Biological Activity of a Succinylated Myxochelin from the Myxobacterial Strain MSr12020. Microorganisms 2022, 10, 1959. [Google Scholar] [CrossRef]
- Chai, K.F.; Adzahan, N.M.; Karim, R.; Rukayadi, Y.; Ghazali, H.M. Characterization of rambutan (Nephelium lappaceum L.) seed fat and anti-nutrient content of the seed during the fruit fermentation: Effect of turning intervals. LWT 2019, 103, 199–204. [Google Scholar] [CrossRef]
- Chai, K.F.; Adzahan, N.M.; Karim, R.; Rukayadi, Y.; Ghazali, H.M. Fat properties and antinutrient content of rambutan (Nephelium lappaceum L.) seed during solid-state fermentation of rambutan fruit. Food Chem. 2019, 274, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Tamang, J.P.; Cotter, P.D.; Endo, A.; Han, N.S.; Kort, R.; Liu, S.Q.; Mayo, B.; Westerik, N.; Hutkins, R. Fermented foods in a global age: East meets West. Compr. Rev. Food Sci. Food Saf. 2020, 19, 184–217. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Gong, L.; Zhang, X.; Wang, Y.; Wang, Y.; Wang, B.; Li, Y.; Li, W. Effect of Saccharomyces boulardii and Bacillus subtilis B10 on gut microbiota modulation in broilers. Anim. Nutr. 2018, 4, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Oikawa, T. Whole-Genome Sequence of Lactobacillus sakei LT-13 Isolated from Moto Starter of Sake. Genome Announc. 2017, 5, e00651-17. [Google Scholar] [CrossRef] [PubMed]
- Castellano, P.; Aristoy, M.C.; Sentandreu, M.; Vignolo, G.; Toldrá, F. Peptides with angiotensin I converting enzyme (ACE) inhibitory activity generated from porcine skeletal muscle proteins by the action of meat-borne Lactobacillus. J. Proteom. 2013, 89, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Lu, Z.; Wang, X.; Selvaraj, J.N.; Zhang, G. Genetic engineering modification and fermentation optimization for extracellular production of recombinant proteins using Escherichia coli. Appl. Microbiol. Biotechnol. 2018, 102, 1545–1556. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.; Chi, Y. In Vitro and In Vivo Assessment of Angiotensin-Converting Enzyme (ACE) Inhibitory Activity of Fermented Soybean Milk by Lactobacillus casei Strains. Curr. Microbiol. 2016, 73, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, S.; Sun, Y.; Dai, Y. ACE-Inhibitory Peptide Isolated from Fermented Soybean Meal as Functional Food. Int. J. Food Eng. 2013, 9, 1–8. [Google Scholar] [CrossRef]
- Elfahri, K.R.; Donkor, O.N.; Vasiljevic, T. Potential of novel Lactobacillus helveticus strains and their cell wall bound proteases to release physiologically active peptides from milk proteins. Int. Dairy J. 2014, 38, 37–46. [Google Scholar] [CrossRef]
- Behrendt, R.; White, P.; Offer, J. Advances in Fmoc solid-phase peptide synthesis. J. Pept. Sci. 2016, 22, 4–27. [Google Scholar] [CrossRef]
- Al Musaimi, O.; de la Torre, B.G.; Albericio, F.J.G.C. Greening Fmoc/tBu solid-phase peptide synthesis. Green Chem. 2020, 22, 996–1018. [Google Scholar] [CrossRef]
- Bayer, E.; Eckstein, H.; Hägele, K.; König, W.A.; Brüning, W.; Hagenmaier, H.; Parr, W. Failure sequences in the solid phase synthesis of polypeptides. J. Am. Chem. Soc. 1970, 92, 1735–1738. [Google Scholar] [CrossRef] [PubMed]
- Bayer, E.; Mutter, M. Liquid phase synthesis of peptides. Nature 1972, 237, 512–513. [Google Scholar] [CrossRef] [PubMed]
- Bayer, E.; Mutter, M.; Uhmann, R.; Polster, J.; Mauser, H. Kinetic studies of the liquid phase peptide synthesis. J. Am. Chem. Soc. 1974, 96, 7333–7336. [Google Scholar] [CrossRef] [PubMed]
- Mutter, M.; Hagenmaier, H.; Bayer, E. New method of polypeptide synthesis. Angew. Chem. 1971, 10, 811–812. [Google Scholar] [CrossRef] [PubMed]
- Mutter, M.; Bayer, E.J.A.C. Rapid Procedure for Liquid-Phase Peptide Synthesis: The Crystallization Method. Angew. Chem. Int. Ed. Engl. 1974, 13, 88–89. [Google Scholar] [CrossRef]
- Bayer, E.; Mutter, M.J.C.B. Synthese des biologisch aktiven Undecapeptids Substanz P nach der Flüssig-Phasen-Methode. Chem. Berichte 1974, 107, 1344–1352. [Google Scholar] [CrossRef]
- Marchetti, P.; Jimenez Solomon, M.F.; Szekely, G.; Livingston, A.G. Molecular separation with organic solvent nanofiltration: A critical review. Chem. Rev. 2014, 114, 10735–10806. [Google Scholar] [CrossRef]
- Livingston Andrew, G.U.Y.; Peeva Ludmila, G.; So, S. Degradable Supports for Tide Synthesis. CA2743677A1, 12 November 2009. [Google Scholar]
- Livingston, A.G.; Peeva, L.G.; So, S.W.J.; Vasconceles, R.C.; Leatherbarrow, R.J.; Tate, E.W.; Gaffney, P.R.J. Solvent Resistant Diafiltration of Peptides, PNA or Oligonucleotides. U.S. Patent US20110245460A1, 7 August 2009. [Google Scholar]
- Castro, V.; Noti, C.; Chen, W.; Cristau, M.; Livignston, A.; Rodríguez, H.; Albericio, F. Novel Globular Polymeric Supports for Membrane-Enhanced Peptide Synthesis. Macromolecules 2017, 50, 1626–1634. [Google Scholar] [CrossRef]
- So, S.; Peeva, L.G.; Tate, E.W.; Leatherbarrow, R.J.; Livingston, A.G. Organic Solvent Nanofiltration: A New Paradigm in Peptide Synthesis. Org. Process Res. Dev. 2010, 14, 1313–1325. [Google Scholar] [CrossRef]
- So, S.; Peeva, L.G.; Tate, E.W.; Leatherbarrow, R.J.; Livingston, A.G. Membrane enhanced peptide synthesis. Chem. Commun. 2010, 46, 2808–2810. [Google Scholar] [CrossRef] [PubMed]
- Curran, D.P.; Hadida, S. Tris(2-(perfluorohexyl)ethyl)tin Hydride: A New Fluorous Reagent for Use in Traditional Organic Synthesis and Liquid Phase Combinatorial Synthesis. J. Am. Chem. Soc. 1996, 118, 2531–2532. [Google Scholar] [CrossRef]
- Studer, A.; Curran, D.P. A strategic alternative to solid phase synthesis: Preparation of a small isoxazoline library by “fluorous synthesis”. Tetrahedron 1997, 53, 6681–6696. [Google Scholar] [CrossRef]
- Curran, D.P. Chemistry. Fluorous tags unstick messy chemical biology problems. Science 2008, 321, 1645–1646. [Google Scholar] [CrossRef] [PubMed]
- Curran, D.P.; Luo, Z. Fluorous Synthesis with Fewer Fluorines (Light Fluorous Synthesis): Separation of Tagged from Untagged Products by Solid-Phase Extraction with Fluorous Reverse-Phase Silica Gel. J. Am. Chem. Soc. 1999, 121, 9069–9072. [Google Scholar] [CrossRef]
- Zhang, Q.; Luo, Z.; Curran, D.P. Separation of “light fluorous” reagents and catalysts by fluorous solid-phase extraction: Synthesis and study of a family of triarylphosphines bearing linear and branched fluorous tags. J. Org. Chem. 2000, 65, 8866–8873. [Google Scholar] [CrossRef] [PubMed]
- Plaquevent, J.C.; Levillain, J.; Guillen, F.; Malhiac, C.; Gaumont, A.C. Ionic liquids: New targets and media for alpha-amino acid and peptide chemistry. Chem. Rev. 2008, 108, 5035–5060. [Google Scholar] [CrossRef] [PubMed]
- Ghandi, K.J.G.; Chemistry, S. A Review of Ionic Liquids, Their Limits and Applications. Green Sustain. Chem. 2014, 4, 44–53. [Google Scholar] [CrossRef]
- Miao, W.; Chan, T.H. Ionic-liquid-supported peptide synthesis demonstrated by the synthesis of Leu(5)-enkephalin. J. Org. Chem. 2005, 70, 3251–3255. [Google Scholar] [CrossRef]
- Konwar, M.; Khupse, N.D.; Saikia, P.J.; Sarma, D. A potential greener protocol for peptide coupling reactions using recyclable/reusable ionic liquid [C4-DABCO] [N(CN)2]. J. Chem. Sci. 2018, 130, 53. [Google Scholar] [CrossRef]
- Fukumoto, K.; Yoshizawa, M.; Ohno, H. Room temperature ionic liquids from 20 natural amino acids. J. Am. Chem. Soc. 2005, 127, 2398–2399. [Google Scholar] [CrossRef] [PubMed]
- Albericio, F. Developments in peptide and amide synthesis. Curr. Opin. Chem. Biol. 2004, 8, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Fukuyama, T.; Matsui, A.; Kuratsu, M.; Nakaya, R.; Ineyama, T.; Ueda, H.; Ryu, I. Coupling-Reagent-Free Synthesis of Dipeptides and Tripeptides Using Amino Acid Ionic Liquids. Chem. A Eur. J. 2015, 21, 11980–11983. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, Q.; Kong, D.; Xu, P. Production and Functionality of Food-derived Bioactive Peptides: A Review. Mini Rev. Med. Chem. 2018, 18, 1524–1535. [Google Scholar] [CrossRef]
- Bottecchia, C.; Noël, T. Photocatalytic Modification of Amino Acids, Peptides, and Proteins. Chemistry 2019, 25, 26–42. [Google Scholar] [CrossRef]
- Caetano-Silva, M.E.; Netto, F.M.; Bertoldo-Pacheco, M.T.; Alegría, A.; Cilla, A. Peptide-metal complexes: Obtention and role in increasing bioavailability and decreasing the pro-oxidant effect of minerals. Crit. Rev. Food Sci. Nutr. 2021, 61, 1470–1489. [Google Scholar] [CrossRef]
- Rahman, M.-U.; Khalid, H.S.; Akhtar, M.F.; Ijaz, M.; Iqbal, M.; Bukhari, S.A.; Mustafa, G.; Shaukat, K. Chapter 27-Role of metal-binding proteins and peptides in bioremediation of toxic metals. In Handbook of Bioremediation; Hasanuzzaman, M., Prasad, M.N.V., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 437–444. [Google Scholar] [CrossRef]
- Shalev, D.E. Studying Peptide-Metal Ion Complex Structures by Solution-State NMR. Int. J. Mol. Sci. 2022, 23, 5957. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Wang, S.; Zhu, X.; Li, Q.; Fan, Y.; Cheng, D.; Li, B. A novel calcium-binding peptide from Antarctic krill protein hydrolysates and identification of binding sites of calcium-peptide complex. Food Chem. 2018, 243, 389–395. [Google Scholar] [CrossRef]
- Bing-na, C. Optimization of Preparation Process for Cod Skin Collagen Peptide-Iron (II) Chelate via Response Surface Methodology. Food Sci. 2012, 33, 48–52. [Google Scholar]
- Lee, S.H.; Song, K.B. Article isolation of a calcium-binding peptide from enzymatic hydrolysates of porcine blood plasma protein. J. Korean Soc. Appl. Biol. Chem. 2009, 52, 290–294. [Google Scholar] [CrossRef]
- Bao, X.L.; Lv, Y.; Yang, B.C.; Ren, C.G.; Guo, S.T. A study of the soluble complexes formed during calcium binding by soybean protein hydrolysates. J. Food Sci. 2008, 73, C117–C121. [Google Scholar] [CrossRef] [PubMed]
- Kahlen, J.; Peter, C.; Donadio, D. Molecular simulation of oligo-glutamates in a calcium-rich aqueous solution: Insights into peptide-induced polymorph selection. CrystEngComm 2015, 17, 6863–6867. [Google Scholar] [CrossRef]
- Xia, Y.; Bamdad, F.; Gänzle, M.; Chen, L. Fractionation and characterization of antioxidant peptides derived from barley glutelin by enzymatic hydrolysis. Food Chem. 2012, 134, 1509–1518. [Google Scholar] [CrossRef] [PubMed]
- De la Hoz, L.; Ponezi, A.N.; Milani, R.F.; Nunes da Silva, V.S.; Sonia de Souza, A.; Bertoldo-Pacheco, M.T. Iron-binding properties of sugar cane yeast peptides. Food Chem. 2014, 142, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.G.; Fu, W.W.; Ma, M.H. Preparation and structure characterization of soluble bone collagen peptide chelating calcium. Afr. J. Biotechnol. 2011, 10, 10204–10211. [Google Scholar]
- Wang, J.; Green, K.; McGibbon, G.; McCarry, B. Analysis of effect of casein phosphopeptides on zinc binding using mass spectrometry. Rapid Commun. Mass. Spectrom. 2007, 21, 1546–1554. [Google Scholar] [CrossRef] [PubMed]
- Kahlen, J.; Salimi, L.; Sulpizi, M.; Peter, C.; Donadio, D. Interaction of charged amino-acid side chains with ions: An optimization strategy for classical force fields. J. Phys. Chem. B 2014, 118, 3960–3972. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Hu, X.; Yang, X.; Chen, S.; Wu, Y.; Hao, S.; Huang, H.; Li, L. GLPGPSGEEGKR: Fe2+ chelating characterization and potential transport pathways for improving Fe2+ bioavailability in Caco-2 cells. Food Biosci. 2022, 48, 101806. [Google Scholar] [CrossRef]
- Zhang, K.; Li, J.; Hou, H.; Zhang, H.; Li, B. Purification and characterization of a novel calcium-biding decapeptide from Pacific cod (Gadus Macrocephalus) bone: Molecular properties and calcium chelating modes. J. Funct. Foods 2019, 52, 670–679. [Google Scholar] [CrossRef]
- Raskin, I.; Ensley, B.D. Phytoremediation of Toxic Metals: Using Plants to Clean up the Environment; Wiley: Hoboken, NY, USA, 2000. [Google Scholar]
- Guerinot, M.L.; Salt, D.E. Fortified foods and phytoremediation. Two sides of the same coin. Plant Physiol. 2001, 125, 164–167. [Google Scholar] [CrossRef]
- Kumar, P.B.A.N.; Dushenkov, V.; Motto, H.; Raskin, I. Phytoextraction: The Use of Plants To Remove Heavy Metals from Soils. Environ. Sci. Technol. 1995, 29, 1232–1238. [Google Scholar] [CrossRef] [PubMed]
- Navaza, A.P.; Montes-Bayón, M.; LeDuc, D.L.; Terry, N.; Sanz-Medel, A. Study of phytochelatins and other related thiols as complexing biomolecules of As and Cd in wild type and genetically modified Brassica juncea plants. J. Mass. Spectrom. 2006, 41, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Guo, Y.; Yang, L.; Wang, Q. Synergistic defensive mechanism of phytochelatins and antioxidative enzymes in Brassica chinensis L. against Cd stress. Chin. Sci. Bull. 2008, 53, 1503–1511. [Google Scholar] [CrossRef]
- Salt, D.E.; Prince, R.C.; Pickering, I.J.; Raskin, I. Mechanisms of Cadmium Mobility and Accumulation in Indian Mustard. Plant Physiol. 1995, 109, 1427–1433. [Google Scholar] [CrossRef]
- Persans, M.W.; Salt, D.E. Possible molecular mechanisms involved in nickel, zinc and selenium hyperaccumulation in plants. Biotechnol. Genet. Eng. Rev. 2000, 17, 389–413. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, F.-J.; Meharg, A.A.; Raab, A.; Feldmann, J.; McGrath, S.P. Mechanisms of Arsenic Hyperaccumulation in Pteris vittata. Uptake Kinetics, Interactions with Phosphate, and Arsenic Speciation. Plant Physiol. 2002, 130, 1552–1561. [Google Scholar] [CrossRef]
- Sousa, A.I.; Caçador, I.; Lillebø, A.I.; Pardal, M.A. Heavy metal accumulation in Halimione portulacoides: Intra- and extra-cellular metal binding sites. Chemosphere 2008, 70, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.S.; Lu, Y.P.; Zhen, R.G.; Szczypka, M.; Thiele, D.J.; Rea, P.A. A new pathway for vacuolar cadmium sequestration in Saccharomyces cerevisiae: YCF1-catalyzed transport of bis(glutathionato)cadmium. Proc. Natl. Acad. Sci. USA 1997, 94, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, I.; Kennedy, T.D.; Chino, M.; Lane, B.G. Wheat Ec metallothionein genes. Like mammalian Zn2+ metallothionein genes, wheat Zn2+ metallothionein genes are conspicuously expressed during embryogenesis. Eur. J. Biochem. 1992, 209, 971–976. [Google Scholar] [CrossRef]
- Cobbett, C.S. Phytochelatins and their roles in heavy metal detoxification. Plant Physiol. 2000, 123, 825–832. [Google Scholar] [CrossRef]
- Kondo, N.; Imai, K.; Isobe, M.; Goto, T.; Murasugi, A.; Wada-Nakagawa, C.; Hayashi, Y. Cadystin a and b, major unit peptides comprising cadmium binding peptides induced in a fission yeast-----separation, revision of structures and synthesis. Tetrahedron Lett. 1984, 25, 3869–3872. [Google Scholar] [CrossRef]
- Grill, E.; Winnacker, E.L.; Zenk, M.H. Phytochelatins: The principal heavy-metal complexing peptides of higher plants. Science 1985, 230, 674–676. [Google Scholar] [CrossRef]
- Sharma, R.; Bhardwaj, R.; Handa, N.; Gautam, V.; Kohli, S.K.; Bali, S.; Kaur, P.; Thukral, A.K.; Arora, S.; Ohri, P.; et al. Chapter 10-Responses of Phytochelatins and Metallothioneins in Alleviation of Heavy Metal Stress in Plants: An Overview. In Plant Metal Interaction; Ahmad, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 263–283. [Google Scholar] [CrossRef]
- Yadav, S.K. Heavy metals toxicity in plants: An overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. S. Afr. J. Bot. 2010, 76, 167–179. [Google Scholar] [CrossRef]
- Rauser, W.E. Phytochelatins and related peptides. Structure, biosynthesis, and function. Plant Physiol. 1995, 109, 1141–1149. [Google Scholar] [CrossRef]
- Zenk, M.H. Heavy metal detoxification in higher plants—A review. Gene 1996, 179, 21–30. [Google Scholar] [CrossRef]
- Thakur, M.; Praveen, S.; Divte, P.R.; Mitra, R.; Kumar, M.; Gupta, C.K.; Kalidindi, U.; Bansal, R.; Roy, S.; Anand, A.; et al. Metal tolerance in plants: Molecular and physicochemical interface determines the “not so heavy effect” of heavy metals. Chemosphere 2022, 287, 131957. [Google Scholar] [CrossRef] [PubMed]
- Mirza, N.; Mahmood, Q.; Maroof Shah, M.; Pervez, A.; Sultan, S. Plants as useful vectors to reduce environmental toxic arsenic content. Sci. World J. 2014, 2014, 921581. [Google Scholar] [CrossRef]
- Rauser, W.E. Structure and function of metal chelators produced by plants. Cell Biochem. Biophys. 1999, 31, 19–48. [Google Scholar] [CrossRef] [PubMed]
- Pal, R.; Rai, J.P. Phytochelatins: Peptides involved in heavy metal detoxification. Appl. Biochem. Biotechnol. 2010, 160, 945–963. [Google Scholar] [CrossRef]
- Wątły, J.; Łuczkowski, M.; Padjasek, M.; Krężel, A. Phytochelatins as a Dynamic System for Cd(II) Buffering from the Micro- to Femtomolar Range. Inorg. Chem. 2021, 60, 4657–4675. [Google Scholar] [CrossRef]
- Batista, B.L.; Nigar, M.; Mestrot, A.; Rocha, B.A.; Barbosa Júnior, F.; Price, A.H.; Raab, A.; Feldmann, J. Identification and quantification of phytochelatins in roots of rice to long-term exposure: Evidence of individual role on arsenic accumulation and translocation. J. Exp. Bot. 2014, 65, 1467–1479. [Google Scholar] [CrossRef]
- Cobbett, C.; Goldsbrough, P. Phytochelatins and metallothioneins: Roles in heavy metal detoxification and homeostasis. Annu. Rev. Plant Biol. 2002, 53, 159–182. [Google Scholar] [CrossRef]
- Grill, E.; Winnacker, E.L.; Zenk, M.H. Phytochelatins, a class of heavy-metal-binding peptides from plants, are functionally analogous to metallothioneins. Proc. Natl. Acad. Sci. USA 1987, 84, 439–443. [Google Scholar] [CrossRef]
- Hendrix, S.; Jozefczak, M.; Wójcik, M.; Deckers, J.; Vangronsveld, J.; Cuypers, A. Glutathione: A key player in metal chelation, nutrient homeostasis, cell cycle regulation and the DNA damage response in cadmium-exposed Arabidopsis thaliana. Plant Physiol. Biochem. 2020, 154, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Wang, Y.; Lü, Q.; Wen, H.; Han, B.; Chen, S.; Zheng, X.; Lin, R. Responses of glutathione and phytochelatins biosysthesis in a cadmium accumulator of Perilla frutescens (L.) Britt. under cadmium contaminated conditions. Ecotoxicol. Environ. Saf. 2020, 201, 110805. [Google Scholar] [CrossRef] [PubMed]
- Bellini, E.; Varotto, C.; Borsò, M.; Rugnini, L.; Bruno, L.; Sanità di Toppi, L. Eukaryotic and Prokaryotic Phytochelatin Synthases Differ Less in Functional Terms Than Previously Thought: A Comparative Analysis of Marchantia polymorpha and Geitlerinema sp. PCC 7407. Plants 2020, 9, 914. [Google Scholar] [CrossRef] [PubMed]
- Klapheck, S.; Fliegner, W.; Zimmer, I. Hydroxymethyl-phytochelatins [(gamma-glutamylcysteine)n-serine] are metal-induced peptides of the Poaceae. Plant Physiol. 1994, 104, 1325–1332. [Google Scholar] [CrossRef]
- Vögeli-Lange, R.; Wagner, G.J. Relationship between cadmium, glutathione and cadmium-binding peptides (phytochelatins) in leaves of intact tobacco seedlings. Plant Sci. 1996, 114, 11–18. [Google Scholar] [CrossRef]
- Jemal, F.; Didierjean, L.; Ghrir, R.; Ghorbal, M.H.; Burkard, G. Characterization of cadmium binding peptides from pepper (Capsicum annuum). Plant Sci. 1998, 137, 143–154. [Google Scholar] [CrossRef]
- Navarrete, A.; González, A.; Gómez, M.; Contreras, R.A.; Díaz, P.; Lobos, G.; Brown, M.T.; Sáez, C.A.; Moenne, A. Copper excess detoxification is mediated by a coordinated and complementary induction of glutathione, phytochelatins and metallothioneins in the green seaweed Ulva compressa. Plant Physiol. Biochem. 2019, 135, 423–431. [Google Scholar] [CrossRef]
- Clemens, S.; Kim, E.J.; Neumann, D.; Schroeder, J.I. Tolerance to toxic metals by a gene family of phytochelatin synthases from plants and yeast. EMBO J. 1999, 18, 3325–3333. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.B.; Smith, A.P.; Howden, R.; Dietrich, W.M.; Bugg, S.; O’Connell, M.J.; Goldsbrough, P.B.; Cobbett, C.S. Phytochelatin synthase genes from Arabidopsis and the yeast Schizosaccharomyces pombe. Plant Cell 1999, 11, 1153–1164. [Google Scholar] [CrossRef] [PubMed]
- Grill, E.; Löffler, S.; Winnacker, E.L.; Zenk, M.H. Phytochelatins, the heavy-metal-binding peptides of plants, are synthesized from glutathione by a specific gamma-glutamylcysteine dipeptidyl transpeptidase (phytochelatin synthase). Proc. Natl. Acad. Sci. USA 1989, 86, 6838–6842. [Google Scholar] [CrossRef]
- Howden, R.; Goldsbrough, P.B.; Andersen, C.R.; Cobbett, C.S. Cadmium-Sensitive, cad1 Mutants of Arabidopsis thaliana Are Phytochelatin Deficient. Plant Physiol. 1995, 107, 1059–1066. [Google Scholar] [CrossRef]
- Klapheck, S.; Schlunz, S.; Bergmann, L. Synthesis of Phytochelatins and Homo-Phytochelatins in Pisum sativum L. Plant Physiol. 1995, 107, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhou, J.; Goldsbrough, P.B. Characterization of phytochelatin synthase from tomato. Physiol. Plant. 1997, 101, 165–172. [Google Scholar] [CrossRef]
- Ranieri, A.; Castagna, A.; Scebba, F.; Careri, M.; Zagnoni, I.; Predieri, G.; Pagliari, M.; di Toppi, L.S. Oxidative stress and phytochelatin characterisation in bread wheat exposed to cadmium excess. Plant Physiol. Biochem. 2005, 43, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Srivastava, S.; Tripathi, R.D.; Govindarajan, R.; Kuriakose, S.V.; Prasad, M.N.V. Phytochelatin synthesis and response of antioxidants during cadmium stress in Bacopa monnieri L◊. Plant Physiol. Biochem. 2006, 44, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Goldsbrough, P.B. Phytochelatin accumulation and cadmium tolerance in selected tomato cell lines. Plant Physiol. 1991, 97, 306–312. [Google Scholar] [CrossRef]
- Gupta, M.; Tripathi, R.D.; Rai, U.N.; Chandra, P. Role of glutathione and phytochelatin in Hydrilla verticillata (I.f.) royle and Valusneria spiraus L. under mercury stress. Chemosphere 1998, 37, 785–800. [Google Scholar] [CrossRef]
- Seregin, I.V.; Kozhevnikova, A.D. Phytochelatins: Sulfur-Containing Metal(loid)-Chelating Ligands in Plants. Int. J. Mol. Sci. 2023, 24, 2430. [Google Scholar] [CrossRef]
- Mathis, C.L.; Barrios, A.M. Histidine phosphorylation in metalloprotein binding sites. J. Inorg. Biochem. 2021, 225, 111606. [Google Scholar] [CrossRef] [PubMed]
- Feyer, V.; Plekan, O.; Tsud, N.; Cháb, V.; Matolín, V.; Prince, K.C. Adsorption of histidine and histidine-containing peptides on Au(111). Langmuir ACS J. Surf. Colloids 2010, 26, 8606–8613. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.H.; Dong, C.D.; Chen, C.W.; Sheu, Y.T.; Kao, C.M. Using poly-glutamic acid as soil-washing agent to remediate heavy metal-contaminated soils. Environ. Sci. Pollut. Res. Int. 2018, 25, 5231–5242. [Google Scholar] [CrossRef] [PubMed]
- Bukietyńska, K.; Podsiadly, H.; Karwecka, Z. Complexes of vanadium(III) with L-alanine and L-aspartic acid. J. Inorg. Biochem. 2003, 94, 317–325. [Google Scholar] [CrossRef]
- Ye, X.; Li, H.; Wang, Q.; Chai, R.; Ma, C.; Gao, H.; Mao, J. Influence of aspartic acid and lysine on the uptake of gold nanoparticles in rice. Ecotoxicol. Environ. Saf. 2018, 148, 418–425. [Google Scholar] [CrossRef]
| Bioremediation Methods | Advantage | Disadvantages |
|---|---|---|
| Microbial remediation | Efficient, environmentally friendly, low cost, sustainable, and widely applicable | Longer restoration process, unstable restoration effect, susceptible to environmental conditions, suitable for small-scale restoration, and higher restoration costs |
| Phytoremediation | Green, low-cost, sustainable, pollution-free, widely applicable, and biodiversity conservation | Inefficient, species-restricted, resource-intensive, difficult to control, and risk-transferring restoration effects |
| Animal restoration | Efficient, widely applicable, promotes biodiversity conservation, recyclable and controllable heavy metals, does not damage soil structure, and enhances soil productivity | Inability to degrade high concentrations of heavy metals, longer remediation times, and cumulative food chain effects |
| Repair Method | Advantage | Disadvantages |
|---|---|---|
| Physical rehabilitation | Simple operation, short time-consumption, high efficiency, reusable | High cost, small application range, incomplete restoration, easy to cause secondary pollution |
| Chemical remediation | Low cost, suitable for large area remediation, no damage to soil structure | Long remediation time, remediation effect is affected by catalysts, easy to pollute the environment and destroy ecosystems |
| Bioremediation | Green, low cost, small damage to the environment, not easy to cause secondary pollution. | Time-consuming, limited applicability, high requirements for plant vigor, growth habit, and species |
| Combined restoration | Beneficial, low cost, no damage to soil environment, no secondary pollution, not time-consuming | Limited to laboratory simulations, less research on field experiments, immature technology, less consideration of restoration risks |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Y.; Zhang, Y.; Xiong, Z.; Chen, X.; Sha, A.; Xiao, W.; Peng, L.; Zou, L.; Han, J.; Li, Q. Peptides Used for Heavy Metal Remediation: A Promising Approach. Int. J. Mol. Sci. 2024, 25, 6717. https://doi.org/10.3390/ijms25126717
Luo Y, Zhang Y, Xiong Z, Chen X, Sha A, Xiao W, Peng L, Zou L, Han J, Li Q. Peptides Used for Heavy Metal Remediation: A Promising Approach. International Journal of Molecular Sciences. 2024; 25(12):6717. https://doi.org/10.3390/ijms25126717
Chicago/Turabian StyleLuo, Yingyong, Yunfeng Zhang, Zhuang Xiong, Xiaodie Chen, Ajia Sha, Wenqi Xiao, Lianxin Peng, Liang Zou, Jialiang Han, and Qiang Li. 2024. "Peptides Used for Heavy Metal Remediation: A Promising Approach" International Journal of Molecular Sciences 25, no. 12: 6717. https://doi.org/10.3390/ijms25126717
APA StyleLuo, Y., Zhang, Y., Xiong, Z., Chen, X., Sha, A., Xiao, W., Peng, L., Zou, L., Han, J., & Li, Q. (2024). Peptides Used for Heavy Metal Remediation: A Promising Approach. International Journal of Molecular Sciences, 25(12), 6717. https://doi.org/10.3390/ijms25126717









