Behaviour Hallmarks in Alzheimer’s Disease 5xFAD Mouse Model
Abstract
1. Introduction
2. The 5xFAD Mouse Model
2.1. Rationale for the 5xFAD Mouse Model Development
2.2. Cognitive and Physiological Impairments Conditioning Behaviour
2.2.1. Motor Capacity
2.2.2. Sensory Capacity
2.2.3. Learning and Memory Capacity
2.2.4. Neuropsychiatric-like Behaviours
3. Final Considerations and Forthcoming Directions
Author Contributions
Funding
Conflicts of Interest
References
- Harman, D. The aging process. Proc. Natl. Acad. Sci. USA 1981, 78, 7124–7128. [Google Scholar] [CrossRef] [PubMed]
- Amarya, S.; Singh, K.; Sabharwal, M. Ageing process and physiological changes. In Gerontology; D’Onofrio, G., Greco, A., Sancarlo, D., Eds.; IntechOpen: London, UK, 2018; p. 276. [Google Scholar] [CrossRef]
- Kritsilis, M.V.; Rizou, S.; Koutsoudaki, P.N.; Evangelou, K.; Gorgoulis, V.G.; Papadopoulos, D. Ageing, cellular senescence and neurodegenerative disease. Int. J. Mol. Sci. 2018, 19, 2937. [Google Scholar] [CrossRef] [PubMed]
- Eurostat. Demography 2023 Edition. Available online: https://ec.europa.eu/eurostat/web/interactive-publications/demography-2023 (accessed on 10 November 2023).
- WHO (World Health Organization). Ageing and Health. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (accessed on 15 January 2024).
- Lane, C.A.; Hardy, J.; Schott, J.M. Alzheimer’s disease. Eur. J. Neurol. 2018, 25, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Long, S.; Benoist, C.; Weidner, W. World Alzheimer Report 2023: Reducing Dementia Risk: Never Too Early, Never Too Late; Alzheimer’s Disease International: London, UK, 2023. [Google Scholar]
- WHO (World Health Organization). Dementia. 2023. Available online: https://www.who.int/news-room/facts-in-pictures/detail/dementia (accessed on 15 January 2024).
- Nunes, B.; Silva, R.D.; Cruz, V.T.; Roriz, J.M.; Pais, J.; Silva, M.C. Prevalence and pattern of cognitive impairment in rural and urban populations from Northern Portugal. BMC Neurol. 2010, 10, 42. [Google Scholar] [CrossRef]
- Gonçalves-Pereira, M.; Cardoso, A.; Verdelho, A.; Alves da Silva, J.; Caldas de Almeida, M.; Fernandes, A.; Raminhos, C.; Ferri, C.P.; Prina, A.M.; Price, M.; et al. The prevalence of dementia in a Portuguese community sample: A 10/66 Dementia Research Group study. BMC Geriatr. 2017, 17, 261. [Google Scholar] [CrossRef]
- Ballard, C.; Gauthier, S.; Corbett, A.; Brayne, C.; Aarsland, D.; Jones, E. Alzheimer’s disease. Lancet 2011, 377, 1019–1031. [Google Scholar] [CrossRef]
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chételat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef]
- Murakami, S.; Lacayo, P. Biological and disease hallmarks of Alzheimer’s disease defined by Alzheimer’s disease genes. Front. Aging Neurosci. 2022, 14, 996030. [Google Scholar] [CrossRef]
- Cipriani, G.; Dolciotti, C.; Picchi, L.; Bonuccelli, U. Alzheimer and his disease: A brief history. Neurol. Sci. 2011, 32, 275–279. [Google Scholar] [CrossRef]
- Masters, C.L.; Bateman, R.; Blennow, K.; Rowe, C.C.; Sperling, R.A.; Cummings, J.L. Alzheimer’s disease. Nat. Rev. Dis. Primers 2015, 1, 15056. [Google Scholar] [CrossRef]
- Moloney, C.M.; Lowe, V.J.; Murray, M.E. Visualization of neurofibrillary tangle maturity in Alzheimer’s disease: A clinicopathologic perspective for biomarker research. Alzheimer’s Dement. 2021, 17, 1554–1574. [Google Scholar] [CrossRef] [PubMed]
- Ittner, L.M.; Götz, J. Amyloid-β and tau—A toxic pas de deux in Alzheimer’s disease. Nat. Rev. Neurosci. 2011, 12, 67–72. [Google Scholar] [CrossRef]
- Paroni, G.; Bisceglia, P.; Seripa, D. Understanding the amyloid hypothesis in Alzheimer’s disease. J. Alzheimer’s Dis. 2019, 68, 493–510. [Google Scholar] [CrossRef]
- Ranjan, V.D.; Qiu, L.; Tan, E.K.; Zeng, L.; Zhang, Y. Modelling Alzheimer’s disease: Insights from in vivo to in vitro three-dimensional culture platforms. J. Tissue Eng. Regen. Med. 2018, 12, 1944–1958. [Google Scholar] [CrossRef] [PubMed]
- Yiannopoulou, K.G.; Papageorgiou, S.G. Current and future treatments in Alzheimer disease: An update. J. Cent. Nerv. Syst. Dis. 2020, 12, 1179573520907397. [Google Scholar] [CrossRef]
- Elder, G.A.; Gama Sosa, M.A.; De Gasperi, R. Transgenic mouse models of Alzheimer’s disease. Mt. Sinai J. Med. 2010, 77, 69–81. [Google Scholar] [CrossRef]
- Tai, L.M.; Weng, J.M.; LaDu, M.J.; Brady, S.T. Relevance of transgenic mouse models for Alzheimer’s disease. Prog. Mol. Biol. Transl. Sci. 2021, 177, 1–48. [Google Scholar] [CrossRef]
- Saraceno, C.; Musardo, S.; Marcello, E.; Pelucchi, S.; Di Luca, M. Modeling Alzheimer’s disease: From past to future. Front. Pharmacol. 2013, 4, 77. [Google Scholar] [CrossRef] [PubMed]
- Drummond, E.; Wisniewski, T. Alzheimer’s disease: Experimental models and reality. Acta Neuropathol. 2017, 133, 155–175. [Google Scholar] [CrossRef]
- King, A. The search for better animal models of Alzheimer’s disease. Nature 2018, 559, S13–S15. [Google Scholar] [CrossRef]
- Vitek, M.P.; Araujo, J.A.; Fossel, M.; Greenberg, B.D.; Howell, G.R.; Rizzo, S.J.S.; Seyfried, N.T.; Tenner, A.J.; Territo, P.R.; Windisch, M.; et al. Translational animal models for Alzheimer’s disease: An Alzheimer’s Association Business Consortium think tank. Alzheimer’s Dement. Transl. Res. Clin. Int. 2020, 6, e12114. [Google Scholar] [CrossRef]
- Kitazawa, M.; Medeiros, R.; LaFerla, F.M. Transgenic mouse models of Alzheimer disease: Developing a better model as a tool for therapeutic interventions. Curr. Pharm. Des. 2012, 18, 1131–1147. [Google Scholar] [CrossRef]
- Sanchez-Varo, R.; Mejias-Ortega, M.; Fernandez-Valenzuela, J.J.; Nuñez-Diaz, C.; Caceres-Palomo, L.; Vegas-Gomez, L.; Sanchez-Mejias, E.; Trujillo-Estrada, L.; Garcia-Leon, J.A.; Moreno-Gonzalez, I.; et al. Transgenic mouse models of Alzheimer’s disease: An integrative analysis. Int. J. Mol. Sci. 2022, 23, 5404. [Google Scholar] [CrossRef]
- Yokoyama, M.; Kobayashi, H.; Tatsumi, L.; Tomita, T. Mouse models of Alzheimer’s disease. Front. Mol. Neurosci. 2022, 15, 912995. [Google Scholar] [CrossRef]
- Myers, A.; McGonigle, P. Overview of transgenic mouse models for Alzheimer’s disease. Curr. Protoc. Neurosci. 2019, 89, e81. [Google Scholar] [CrossRef]
- Bohm, C.; Chen, F.; Sevalle, J.; Qamar, S.; Dodd, R.; Li, Y.; Schmitt-Ulms, G.; Fraser, P.E.; St George-Hyslop, P.H. Current and future implications of basic and translational research on amyloid-β peptide production and removal pathways. Mol. Cell. Neurosci. 2015, 66, 3–11. [Google Scholar] [CrossRef]
- Wang, C.; Holtzman, D.M. Bidirectional relationship between sleep and Alzheimer’s disease: Role of amyloid, tau, and other factors. Neuropsychopharmacology 2020, 45, 104–120. [Google Scholar] [CrossRef]
- Oakley, H.; Cole, S.L.; Logan, S.; Maus, E.; Shao, P.; Craft, J.; Guillozet-Bongaarts, A.; Ohno, M.; Disterhoft, J.; Van Eldik, L.; et al. Intraneuronal β-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: Potential factors in amyloid plaque formation. J. Neurosci. 2006, 26, 10129–10140. [Google Scholar] [CrossRef]
- Jankowsky, J.L.; Zheng, H. Practical considerations for choosing a mouse model of Alzheimer’s disease. Mol. Neurodegener. 2017, 12, 89. [Google Scholar] [CrossRef] [PubMed]
- Ayabe, T.; Ano, Y. Alzheimer model 5xfad mice and applications to dementia: Transgenic mouse models, a focus on neuroinflammation, microglia, and food-derived components. In Genetics, Neurology, Behavior, and Diet in Dementia; Academic Press: Cambridge, MA, USA, 2020; pp. 833–847. [Google Scholar]
- Ismeurt, C.; Giannoni, P.; Claeysen, S. The 5XFAD mouse model of Alzheimer’s disease. In Diagnosis and Management in Dementia; Academic Press: Cambridge, MA, USA, 2020; pp. 207–221. [Google Scholar]
- Eimer, W.A.; Vassar, R. Neuron loss in the 5XFAD mouse model of Alzheimer’s disease correlates with intraneuronal Aβ42 accumulation and caspase-3 activation. Mol. Neurodegener. 2013, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- Girard, S.D.; Baranger, K.; Gauthier, C.; Jacquet, M.; Bernard, A.; Escoffier, G.; Marchetti, E.; Khrestchatisky, M.; Rivera, S.; Roman, F.S. Evidence for early cognitive impairment related to frontal cortex in the 5XFAD mouse model of Alzheimer’s disease. J. Alzheimer’s Dis. 2013, 33, 781–796. [Google Scholar] [CrossRef]
- Boza-Serrano, A.; Yang, Y.; Paulus, A.; Deierborg, T. Innate immune alterations are elicited in microglial cells before plaque deposition in the Alzheimer’s disease mouse model 5xFAD. Sci. Rep. 2018, 8, 1550. [Google Scholar] [CrossRef]
- Forner, S.; Kawauchi, S.; Balderrama-Gutierrez, G.; Kramár, E.A.; Matheos, D.P.; Phan, J.; Javonillo, D.I.; Tran, K.M.; Hingco, E.; da Cunha, C.; et al. Systematic phenotyping and characterization of the 5xFAD mouse model of Alzheimer’s disease. Sci. Data 2021, 8, 270. [Google Scholar] [CrossRef]
- Jawhar, S.; Trawicka, A.; Jenneckens, C.; Bayer, T.A.; Wirths, O. Motor deficits, neuron loss, and reduced anxiety coinciding with axonal degeneration and intraneuronal Aβ aggregation in the 5XFAD mouse model of Alzheimer’s disease. Neurobiol. Aging 2012, 33, 196.e29–196.e40. [Google Scholar] [CrossRef]
- Wirths, O.; Bayer, T.A. Motor impairment in Alzheimer’s disease and transgenic Alzheimer’s disease mouse models. Genes Brain Behav. 2008, 7, 1–5. [Google Scholar] [CrossRef]
- Richard, B.C.; Kurdakova, A.; Baches, S.; Bayer, T.A.; Weggen, S.; Wirths, O. Gene dosage dependent aggravation of the neurological phenotype in the 5XFAD mouse model of Alzheimer’s disease. J. Alzheimer’s Dis. 2015, 45, 1223–1236. [Google Scholar] [CrossRef]
- Matchynski-Franks, J.J.; Pappas, C.; Rossignol, J.; Reinke, T.; Fink, K.; Crane, A.; Twite, A.; Lowrance, S.A.; Song, C.; Dunbar, G.L. Mesenchymal stem cells as treatment for behavioral deficits and neuropathology in the 5xFAD mouse model of Alzheimer’s disease. Cell Transplant. 2016, 25, 687–703. [Google Scholar] [CrossRef]
- Medina-Vera, D.; Zambrana-Infantes, E.N.; López-Gambero, A.J.; Verheul-Campos, J.; Santín, L.J.; Baixeras, E.; Suarez, J.; Pavon, F.J.; Rosell-Valle, C.; de Fonseca, F.R. Transcending the amyloid-beta dominance paradigm in Alzheimer’s disease: An exploration of behavioural, metabolic, and gut microbiota phenotypes in 5xFAD mice. Neurobiol. Dis. 2023, 187, 106295. [Google Scholar] [CrossRef]
- Shukla, V.; Zheng, Y.L.; Mishra, S.K.; Amin, N.D.; Steiner, J.; Grant, P.; Kesavapany, S.; Pant, H.C. A truncated peptide from p35, a Cdk5 activator, prevents Alzheimer’s disease phenotypes in model mice. FASEB J. 2013, 27, 174–186. [Google Scholar] [CrossRef]
- Schneider, F.; Baldauf, K.; Wetzel, W.; Reymann, K.G. Behavioral and EEG changes in male 5xFAD mice. Physiol. Behav. 2014, 135, 25–33. [Google Scholar] [CrossRef]
- Paesler, K.; Xie, K.; Hettich, M.M.; Siwek, M.E.; Ryan, D.P.; Schröder, S.; Papazoglou, A.; Broich, K.; Müller, R.; Trog, A.; et al. Limited effects of an eIF2α S51A allele on neurological impairments in the 5xFAD mouse model of Alzheimer’s disease. Neural Plast. 2015, 2015, 825157. [Google Scholar] [CrossRef]
- O’Leary, T.P.; Robertson, A.; Chipman, P.H.; Rafuse, V.F.; Brown, R.E. Motor function deficits in the 12 month-old female 5xFAD mouse model of Alzheimer’s disease. Behav. Brain Res. 2018, 337, 256–263. [Google Scholar] [CrossRef]
- Dong, H.; Locci, A.; Keszycki, R.M.; Orellana, H.D.; Rodriguez, G.; Fisher, D.W. Characterization and comparison of memory and affective behavior in common transgenic mouse models of Alzheimer’s disease: Molecular and cell biology/neurodegeneration and neuroprotection. Alzheimer’s Dement. 2020, 16, e045211. [Google Scholar] [CrossRef]
- Locci, A.; Orellana, H.; Rodriguez, G.; Gottliebson, M.; McClarty, B.; Dominguez, S.; Keszycki, R.; Dong, H. Comparison of memory, affective behavior, and neuropathology in APPNLGF knock-in mice to 5xFAD and APP/PS1 mice. Behav. Brain Res. 2021, 404, 113192. [Google Scholar] [CrossRef]
- Carter, R.J.; Morton, J.; Dunnett, S.B. Motor coordination and balance in rodents. Curr. Protoc. Neurosci. 2001, 15, 8–12. [Google Scholar] [CrossRef]
- Frydman-Marom, A.; Levin, A.; Farfara, D.; Benromano, T.; Scherzer-Attali, R.; Peled, S.; Vassar, R.; Segal, D.; Gazit, E.; Frenkel, D.; et al. Orally administrated cinnamon extract reduces β-amyloid oligomerization and corrects cognitive impairment in Alzheimer’s disease animal models. PLoS ONE 2011, 6, e16564. [Google Scholar] [CrossRef]
- Jawhar, S.; Wirths, O.; Schilling, S.; Graubner, S.; Demuth, H.U.; Bayer, T.A. Overexpression of glutaminyl cyclase, the enzyme responsible for pyroglutamate Aβ formation, induces behavioral deficits, and glutaminyl cyclase knock-out rescues the behavioral phenotype in 5XFAD mice. J. Biol. Chem. 2011, 286, 4454–4460. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Haertel, C.; Maelicke, A.; Montag, D. Galantamine slows down plaque formation and behavioral decline in the 5XFAD mouse model of Alzheimer’s disease. PLoS ONE 2014, 9, e89454. [Google Scholar] [CrossRef]
- O’Leary, T.P.; Mantolino, H.M.; Stover, K.R.; Brown, R.E. Age-related deterioration of motor function in male and female 5xFAD mice from 3 to 16 months of age. Genes Brain Behav. 2020, 19, e12538. [Google Scholar] [CrossRef]
- Gendron, W.H.; Fertan, E.; Pelletier, S.; Roddick, K.M.; O’Leary, T.P.; Anini, Y.; Brown, R.E. Age related weight loss in female 5xFAD mice from 3 to 12 months of age. Behav. Brain Res. 2021, 406, 113214. [Google Scholar] [CrossRef]
- Hüttenrauch, M.; Walter, S.; Kaufmann, M.; Weggen, S.; Wirths, O. Limited effects of prolonged environmental enrichment on the pathology of 5XFAD mice. Mol. Neurobiol. 2017, 54, 6542–6555. [Google Scholar] [CrossRef]
- Kameno, K.; Hasegawa, Y.; Hayashi, K.; Takemoto, Y.; Uchikawa, H.; Mukasa, A.; Kim-Mitsuyama, S. Loss of body weight in old 5xFAD mice and the alteration of gut microbiota composition. Exp. Gerontol. 2022, 166, 111885. [Google Scholar] [CrossRef]
- Sawmiller, D.; Li, S.; Mori, T.; Habib, A.; Rongo, D.; Delic, V.; Bradshaw, P.C.; Shytle, R.D.; Sanberg, C.; Bickford, P.; et al. Beneficial effects of a pyrroloquinolinequinone-containing dietary formulation on motor deficiency, cognitive decline and mitochondrial dysfunction in a mouse model of Alzheimer’s disease. Heliyon 2017, 3, e00279. [Google Scholar] [CrossRef]
- Oblak, A.L.; Lin, P.B.; Kotredes, K.P.; Pandey, R.S.; Garceau, D.; Williams, H.M.; Uyar, A.; O’Rourke, R.; O’Rourke, S.; Ingraham, C.; et al. Comprehensive evaluation of the 5xFAD mouse model for preclinical testing applications: A MODEL-AD study. Front. Aging Neurosci. 2021, 13, 713726. [Google Scholar] [CrossRef]
- Aartsma-Rus, A.; van Putten, M. Assessing functional performance in the mdx mouse model. J. Vis. Exp. 2014, 85, 51303. [Google Scholar] [CrossRef]
- Abe, Y.; Ikegawa, N.; Yoshida, K.; Muramatsu, K.; Hattori, S.; Kawai, K.; Murakami, M.; Tanaka, T.; Goda, W.; Goto, M.; et al. Behavioral and electrophysiological evidence for a neuroprotective role of aquaporin-4 in the 5xFAD transgenic mice model. Acta Neuropathol. Commun. 2020, 8, 67. [Google Scholar] [CrossRef]
- Romano III, R.R.; Carter, M.A.; Monroe, T.B. Narrative review of sensory changes as a biomarker for Alzheimer’s disease. Biol. Res. Nurs. 2021, 23, 223–230. [Google Scholar] [CrossRef]
- López-Gambero, A.J.; Rosell-Valle, C.; Medina-Vera, D.; Navarro, J.A.; Vargas, A.; Rivera, P.; Sanjuan, C.; de Fonseca, R.R.; Suárez, J. A negative energy balance is associated with metabolic dysfunctions in the hypothalamus of a humanized preclinical model of Alzheimer’s disease, the 5XFAD mouse. Int. J. Mol. Sci. 2021, 22, 5365. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, D.; Chiu, K.; Xu, Y. Brain and retinal abnormalities in the 5xFAD mouse model of Alzheimer’s disease at early stages. Front. Neurosci. 2021, 15, 681831. [Google Scholar] [CrossRef]
- Na, D.; Zhang, J.; Beaulac, H.J.; Kiernan, A.E.; White, P.M. Increased central auditory gain in 5xFAD Alzheimer’s disease mice as an early biomarker candidate for Alzheimer’s disease diagnosis. Front. Neurosci. 2023, 17, 1106570. [Google Scholar] [CrossRef]
- Kouzuki, M.; Ichikawa, J.; Shirasagi, D.; Katsube, F.; Kobashi, Y.; Matsumoto, H.; Chao, H.; Yoshida, S.; Urakami, K. Detection and recognition thresholds for five basic tastes in patients with mild cognitive impairment and Alzheimer’s disease dementia. BMC Neurol. 2020, 20, 110. [Google Scholar] [CrossRef]
- El Kadiri, W.; Perrignon-Sommet, M.; Delpont, B.; Graber, M.; Mohr, S.; Mouillot, T.; Devilliers, H.; Grall, S.; Lienard, F.; Georges, M.; et al. Changes in Taste Perception in patients with minor and major cognitive impairment linked to Alzheimer’s Disease recorded by gustatory evoked potentials. J. Alzheimer’s Dis. 2023, 96, 1593–1607. [Google Scholar] [CrossRef]
- Swords, G.M.; Nguyen, L.T.; Mudar, R.A.; Llano, D.A. Auditory system dysfunction in Alzheimer disease and its prodromal states: A review. Ageing Res. Rev. 2018, 44, 49–59. [Google Scholar] [CrossRef]
- Heffner, H.E.; Heffner, R.S. Behavioral assessment of hearing in mice. In Handbook of Mouse Auditory Research: From Behavior to Molecular Biology; Willott, J.F., Ed.; CRC Press: Boca Raton, FL, USA, 2001; pp. 19–29. [Google Scholar] [CrossRef]
- Kim, Y.H.; Schrode, K.M.; Engel, J.; Vicencio-Jimenez, S.; Rodriguez, G.; Lee, H.K.; Lauer, A.M. Auditory behavior in adult-blinded mice. J. Assoc. Res. Otolaryngol. 2022, 23, 225–239. [Google Scholar] [CrossRef]
- O’Leary, T.P.; Shin, S.; Fertan, E.; Dingle, R.N.; Almuklass, A.; Gunn, R.K.; Yu, Z.; Wang, J.; Brown, R.E. Reduced acoustic startle response and peripheral hearing loss in the 5xFAD mouse model of Alzheimer’s disease. Genes Brain Behav. 2017, 16, 554–563. [Google Scholar] [CrossRef]
- Weible, A.P.; Stebritz, A.J.; Wehr, M. 5XFAD mice show early-onset gap encoding deficits in the auditory cortex. Neurobiol. Aging 2020, 94, 101–110. [Google Scholar] [CrossRef]
- Lian, T.-H.; Zhu, W.-L.; Li, S.-W.; Liu, Y.-O.; Guo, P.; Zuo, L.-J.; Hu, Y.; Yu, S.-Y.; Li, L.-X.; Jin, Z.; et al. Clinical, structural, and neuropathological features of olfactory dysfunction in patients with Alzheimer’s disease. J. Alzheimer’s Dis. 2019, 70, 413–423. [Google Scholar] [CrossRef]
- Papes, F.; Nakahara, T.S.; Camargo, A.P. Behavioral assays in the study of olfaction: A practical guide. Methods Mol. Biol. 2018, 1820, 289–388. [Google Scholar] [CrossRef]
- Xiao, N.A.; Zhang, J.; Zhou, M.; Wei, Z.; Wu, X.L.; Dai, X.M.; Zhu, Y.G.; Chen, X.C. Reduction of glucose metabolism in olfactory bulb is an earlier Alzheimer’s disease-related biomarker in 5xFAD mice. Chin. Med. J. 2015, 128, 2220–2227. [Google Scholar] [CrossRef]
- Baranger, K.; Giannoni, P.; Girard, S.D.; Girot, S.; Gaven, F.; Stephan, D.; Migliorati, M.; Khrestchatisky, M.; Bockaert, J.; Marchetti-Gauthier, E.; et al. Chronic treatments with a 5-HT4 receptor agonist decrease amyloid pathology in the entorhinal cortex and learning and memory deficits in the 5xFAD mouse model of Alzheimer’s disease. Neuropharmacology 2017, 126, 128–141. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Z.; Bai, Y.T.; Wu, G.Y.; Chen, G. Gad67 haploinsufficiency reduces amyloid pathology and rescues olfactory memory deficits in a mouse model of Alzheimer’s disease. Mol. Neurodegener. 2017, 12, 73. [Google Scholar] [CrossRef]
- Roddick, K.M.; Schellinck, H.M.; Brown, R.E. Olfactory delayed matching to sample performance in mice: Sex differences in the 5XFAD mouse model of Alzheimer’s disease. Behav. Brain Res. 2014, 270, 165–170. [Google Scholar] [CrossRef]
- Roddick, K.M.; Roberts, A.D.; Schellinck, H.M.; Brown, R.E. Sex and genotype differences in odor detection in the 3×Tg-AD and 5XFAD mouse models of Alzheimer’s disease at 6 months of age. Chem. Senses 2016, 41, 433–440. [Google Scholar] [CrossRef]
- Kosel, F.; Munoz, P.T.; Yang, J.R.; Wong, A.A.; Franklin, T.B. Age-related changes in social behaviours in the 5xFAD mouse model of Alzheimer’s disease. Behav. Brain Res. 2019, 362, 160–172. [Google Scholar] [CrossRef]
- O’Leary, T.P.; Stover, K.R.; Mantolino, H.M.; Darvesh, S.; Brown, R.E. Intact olfactory memory in the 5xFAD mouse model of Alzheimer’s disease from 3 to 15 months of age. Behav. Brain Res. 2020, 393, 112731. [Google Scholar] [CrossRef]
- Lenoir, H.; Siéroff, É. Les troubles de la perception visuelle dans la maladie d’Alzheimer. Geriatr. Psychol. Neuropsychiatr. Vieil. 2019, 17, 307–316. [Google Scholar]
- Pinto, L.H.; Enroth-Cugell, C. Tests of the mouse visual system. Mamm. Genome 2000, 11, 531–536. [Google Scholar] [CrossRef]
- Prusky, G.T.; West, P.W.; Douglas, R.M. Behavioral assessment of visual acuity in mice and rats. Vis. Res. 2000, 40, 2201–2209. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, D.; Ouyang, H.; Hu, S.; Li, A.; Luo, H.; Xu, Y. Neuroprotective effects of methyl 3, 4 dihydroxybenzoate in a mouse model of retinitis pigmentosa. Exp. Eye Res. 2017, 162, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Criscuolo, C.; Cerri, E.; Fabiani, C.; Capsoni, S.; Cattaneo, A.; Domenici, L. The retina as a window to early dysfunctions of Alzheimer’s disease following studies with a 5xFAD mouse model. Neurobiol. Aging 2018, 67, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.T.; Overman, A.A. The Memory Deficit in Alzheimers Disease; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2002; pp. 569–589. [Google Scholar]
- Berron, D.; van Westen, D.; Ossenkoppele, R.; Strandberg, O.; Hansson, O. Medial temporal lobe connectivity and its associations with cognition in early Alzheimer’s disease. Brain 2020, 143, 1233–1248. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, S.; Khamaj, A.; Asthana, M.K. Cognitive neuroscience perspective on memory: Overview and summary. Front. Hum. Neurosci. 2023, 17, 1217093. [Google Scholar] [CrossRef] [PubMed]
- Grayson, B.; Leger, M.; Piercy, C.; Adamson, L.; Harte, M.; Neill, J.C. Assessment of disease-related cognitive impairments using the novel object recognition (NOR) task in rodents. Behav. Brain Res. 2015, 285, 176–193. [Google Scholar] [CrossRef]
- Joyashiki, E.; Matsuya, Y.; Tohda, C. Sominone improves memory impairments and increases axonal density in Alzheimer’s disease model mice, 5XFAD. Int. J. Neurosci. 2011, 121, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Tohda, C.; Nakada, R.; Urano, T.; Okonogi, A.; Kuboyama, T. Kamikihi-to (KKT) rescues axonal and synaptic degeneration associated with memory impairment in a mouse model of Alzheimer’s disease, 5XFAD. Int. J. Neurosci. 2011, 121, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Tohda, C.; Urano, T.; Umezaki, M.; Nemere, I.; Kuboyama, T. Diosgenin is an exogenous activator of 1, 25D3-MARRS/Pdia3/ERp57 and improves Alzheimer’s disease pathologies in 5XFAD mice. Sci. Rep. 2012, 2, 535. [Google Scholar] [CrossRef] [PubMed]
- Giannoni, P.; Gaven, F.; De Bundel, D.; Baranger, K.; Marchetti-Gauthier, E.; Roman, F.S.; Valjent, E.; Marin, P.; Bockaert, J.; Rivera, S.; et al. Early administration of RS 67333, a specific 5-HT4 receptor agonist, prevents amyloidogenesis and behavioral deficits in the 5XFAD mouse model of Alzheimer’s disease. Front. Aging Neurosci. 2013, 5, 96. [Google Scholar] [CrossRef] [PubMed]
- Fragkouli, A.; Tsilibary, E.C.; Tzinia, A.K. Neuroprotective role of MMP-9 overexpression in the brain of Alzheimer’s 5xFAD mice. Neurobiol. Dis. 2014, 70, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Creighton, S.D.; Mendell, A.L.; Palmer, D.; Kalisch, B.E.; MacLusky, N.J.; Prado, V.F.; Prado, M.A.M.; Winters, B.D. Dissociable cognitive impairments in two strains of transgenic Alzheimer’s disease mice revealed by a battery of object-based tests. Sci. Rep. 2019, 9, 57. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.; Huang, Z.; Goo, N.; Bae, H.J.; Jeong, Y.; Park, H.J.; Cai, M.; Cho, K.; Jung, S.Y.; et al. Theracurmin ameliorates cognitive dysfunctions in 5XFAD mice by improving synaptic function and mitigating oxidative stress. Biomol. Ther. 2019, 27, 327–335. [Google Scholar] [CrossRef]
- Medina-Vera, D.; Rosell-Valle, C.; López-Gambero, A.J.; Navarro, J.A.; Zambrana-Infantes, E.N.; Rivera, P.; Santín, L.J.; Suarez, J.; de Fonseca, F.R. Imbalance of endocannabinoid/lysophosphatidylinositol receptors marks the severity of alzheimer’s disease in a preclinical model: A therapeutic opportunity. Biology 2020, 9, 377. [Google Scholar] [CrossRef] [PubMed]
- Sil, A.; Erfani, A.; Lamb, N.; Copland, R.; Riedel, G.; Platt, B. Sex differences in behavior and molecular pathology in the 5XFAD model. J. Alzheimer’s Dis. 2022, 85, 755–778. [Google Scholar] [CrossRef] [PubMed]
- Viola, K.L.; Bicca, M.A.; Bebenek, A.M.; Kranz, D.L.; Nandwana, V.; Waters, E.A.; Haney, C.R.; Lee, M.; Gupta, A.; Brahmbhatt, Z.; et al. The therapeutic and diagnostic potential of amyloid β oligomers selective antibodies to treat Alzheimer’s disease. Front. Neurosci. 2022, 15, 768646. [Google Scholar] [CrossRef] [PubMed]
- Faisal, M.; Aid, J.; Nodirov, B.; Lee, B.; Hickey, M.A. Preclinical trials in Alzheimer’s disease: Sample size and effect size for behavioural and neuropathological outcomes in 5xFAD mice. PLoS ONE 2023, 18, e0281003. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Silva, A.; Sharma, J.; Nguyen, J.; Pizzo, D.P.; Hinz, D.; Sahoo, D.; Cherqui, S. Rescue of Alzheimer’s disease phenotype in a mouse model by transplantation of wild-type hematopoietic stem and progenitor cells. Cell Rep. 2023, 42, 112956. [Google Scholar] [CrossRef] [PubMed]
- Fanselow, M.S.; Poulos, A.M. The neuroscience of mammalian associative learning. Annu. Rev. Psychol. 2005, 56, 207–234. [Google Scholar] [CrossRef]
- Shoji, H.; Takao, K.; Hattori, S.; Miyakawa, T. Contextual and cued fear conditioning test using a video analyzing system in mice. J. Vis. Exp. 2014, 85, 50871. [Google Scholar] [CrossRef] [PubMed]
- Ohno, M.; Chang, L.; Tseng, W.; Oakley, H.; Citron, M.; Klein, W.L.; Vassar, R.; Disterhoft, J.F. Temporal memory deficits in Alzheimer’s mouse models: Rescue by genetic deletion of BACE1. Eur. J. Neurosci. 2006, 23, 251–260. [Google Scholar] [CrossRef]
- Kimura, R.; Ohno, M. Impairments in remote memory stabilization precede hippocampal synaptic and cognitive failures in 5XFAD Alzheimer mouse model. Neurobiol. Dis. 2009, 33, 229–235. [Google Scholar] [CrossRef]
- Ohno, M. Failures to reconsolidate memory in a mouse model of Alzheimer’s disease. Neurobiol. Learn. Mem. 2009, 92, 455–459. [Google Scholar] [CrossRef]
- Devi, L.; Ohno, M. Genetic reductions of β-site amyloid precursor protein-cleaving enzyme 1 and amyloid-β ameliorate impairment of conditioned taste aversion memory in 5XFAD Alzheimer’s disease model mice. Eur. J. Neurosci. 2010, 31, 110–118. [Google Scholar] [CrossRef]
- Kaczorowski, C.C.; Sametsky, E.; Shah, S.; Vassar, R.; Disterhoft, J.F. Mechanisms underlying basal and learning-related intrinsic excitability in a mouse model of Alzheimer’s disease. Neurobiol. Aging 2011, 32, 1452–1465. [Google Scholar] [CrossRef]
- Bouter, Y.; Kacprowski, T.; Weissmann, R.; Dietrich, K.; Borgers, H.; Brauß, A.; Sperling, C.; Wirths, O.; Albrecht, M.; Jensen, L.R.; et al. Deciphering the molecular profile of plaques, memory decline and neuron loss in two mouse models for Alzheimer’s disease by deep sequencing. Front. Aging Neurosci. 2014, 6, 75. [Google Scholar] [CrossRef] [PubMed]
- Dinkins, M.B.; Enasko, J.; Hernandez, C.; Wang, G.; Kong, J.; Helwa, I.; Liu, Y.; Terry, A.V., Jr.; Bieberich, E. Neutral sphingomyelinase-2 deficiency ameliorates Alzheimer’s disease pathology and improves cognition in the 5XFAD mouse. J. Neurosci. 2016, 36, 8653–8667. [Google Scholar] [CrossRef] [PubMed]
- Lebois, E.P.; Schroeder, J.P.; Esparza, T.J.; Bridges, T.M.; Lindsley, C.W.; Conn, P.J.; Brody, D.L.; Daniels, J.S.; Levey, A.I. Disease-modifying effects of M1 muscarinic acetylcholine receptor activation in an Alzheimer’s disease mouse model. ACS Chem. Neurosci. 2017, 8, 1177–1187. [Google Scholar] [CrossRef]
- Kraeuter, A.-K.; Guest, P.C.; Sarnyai, Z. The Y-maze for assessment of spatial working and reference memory in mice. Methods Mol. Biol. 2019, 1916, 105–111. [Google Scholar] [CrossRef]
- d’Isa, R.; Comi, G.; Leocani, L. Apparatus design and behavioural testing protocol for the evaluation of spatial working memory in mice through the spontaneous alternation T-maze. Sci. Rep. 2021, 11, 21177. [Google Scholar] [CrossRef]
- Ohno, M.; Cole, S.L.; Yasvoina, M.; Zhao, J.; Citron, M.; Berry, R.; Disterhoft, J.F.; Vassar, R. BACE1 gene deletion prevents neuron loss and memory deficits in 5XFAD APP/PS1 transgenic mice. Neurobiol. Dis. 2007, 26, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Devi, L.; Ohno, M. Phospho-eIF2α level is important for determining abilities of BACE1 reduction to rescue cholinergic neurodegeneration and memory defects in 5XFAD mice. PLoS ONE 2010, 5, e12974. [Google Scholar] [CrossRef]
- Hüttenrauch, M.; Baches, S.; Gerth, J.; Bayer, T.A.; Weggen, S.; Wirths, O. Neprilysin deficiency alters the neuropathological and behavioral phenotype in the 5XFAD mouse model of Alzheimer’s disease. J. Alzheimer’s Dis. 2015, 44, 1291–1302. [Google Scholar] [CrossRef]
- Wei, Z.; Chen, X.-C.; Song, Y.; Pan, X.-D.; Dai, X.-M.; Zhang, J.; Cui, X.-L.; Wu, X.-L.; Zhu, Y.-G. Amyloid β protein aggravates neuronal senescence and cognitive deficits in 5XFAD mouse model of Alzheimer’s disease. Chin. Med. J. 2016, 129, 1835–1844. [Google Scholar] [CrossRef] [PubMed]
- Datta, M.; Staszewski, O.; Raschi, E.; Frosch, M.; Hagemeyer, N.; Tay, T.L.; Blank, T.; Kreutzfeldt, M.; Merkler, D.; Ziegler-Waldkirch, S.; et al. Histone deacetylases 1 and 2 regulate microglia function during development, homeostasis, and neurodegeneration in a context-dependent manner. Immunity 2018, 48, 514–529.e6. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Peng, Q.; Lv, N.; Yuan, J.; Deng, Z.; Liang, X.; Chen, S.; Wang, L. Paeoniflorin exerts neuroprotective effects in a transgenic mouse model of Alzheimer’s disease via activation of adenosine A1 receptor. Neurosci. Lett. 2020, 730, 135016. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Xi, Y.; Wang, Q.; Liu, J.; Li, P.; Meng, X.; Liu, K.; Chen, W.; Liu, X.; Liu, Z. Mannan oligosaccharide attenuates cognitive and behavioral disorders in the 5xFAD Alzheimer’s disease mouse model via regulating the gut microbiota-brain axis. Brain Behav. Immun. 2021, 95, 330–343. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.; Kang, J.-H.; Jo, K.W.; Shin, W.-S.; Jeong, Y.-H.; Kang, B.; Rho, T.-T.; Jeon, S.Y.; Lee, J.; Song, I.-S.; et al. Synthetic PPAR agonist DTMB alleviates Alzheimer’s Disease pathology by inhibition of chronic microglial inflammation in 5xFAD mice. Neurotherapeutics 2022, 19, 1546–1565. [Google Scholar] [CrossRef] [PubMed]
- Keszycki, R.; Rodriguez, G.; Dunn, J.T.; Locci, A.; Orellana, H.; Haupfear, I.; Dominguez, S.; Fisher, D.W.; Dong, H. Characterization of apathy-like behaviors in the 5xFAD mouse model of Alzheimer’s disease. Neurobiol. Aging 2023, 126, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Poon, C.H.; Wong, S.T.N.; Roy, J.; Wang, Y.; Chan, H.W.H.; Steinbusch, H.; Blokland, A.; Temel, Y.; Aquili, L.; Lim, L.W. Sex differences between neuronal loss and the early onset of amyloid deposits and behavioral consequences in 5xFAD transgenic mouse as a model for Alzheimer’s disease. Cells 2023, 12, 780. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, T.P.; Brown, R.E. Age-related changes in species-typical behaviours in the 5xFAD mouse model of Alzheimer’s disease from 4–16 months of age. Behav. Brain Res. 2024, 465, 114970. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Ryoo, J.E.; Hong, S.; Kim, H.Y.; Kim, Y. Carprofen alleviates Alzheimer-like phenotypes of 5XFAD transgenic mice by targeting the pathological hallmarks induced by amyloid-β aggregation. Sci. Rep. 2023, 13, 10889. [Google Scholar] [CrossRef]
- Bromley-Brits, K.; Deng, Y.; Song, W. Morris water maze test for learning and memory deficits in Alzheimer’s disease model mice. J. Vis. Exp. 2011, 53, 2920. [Google Scholar] [CrossRef]
- Tang, X.; Wu, D.; Gu, L.-H.; Nie, B.-B.; Qi, X.-Y.; Wang, Y.-J.; Wu, F.-F.; Li, X.-L.; Bai, F.; Chen, X.-C.; et al. Spatial learning and memory impairments are associated with increased neuronal activity in 5xFAD mouse as measured by manganese-enhanced magnetic resonance imaging. Oncotarget 2016, 7, 57556–57570. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Wu, D.; Tang, X.; Qi, X.; Li, X.; Bai, F.; Chen, X.; Ren, Q.; Zhang, Z. Myelin changes at the early stage of 5xFAD mice. Brain Res. Bull. 2018, 137, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Tang, X.; Gu, L.-H.; Li, X.-L.; Qi, X.-Y.; Bai, F.; Chen, X.-C.; Wang, J.-Z.; Ren, Q.-G.; Zhang, Z.J. LINGO-1 antibody ameliorates myelin impairment and spatial memory deficits in the early stage of 5XFAD mice. CNS Neurosci. Ther. 2018, 24, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Hongpaisan, J.; Sun, M.K.; Alkon, D.L. PKC ε activation prevents synaptic loss, Aβ elevation, and cognitive deficits in Alzheimer’s disease transgenic mice. J. Neurosci. 2011, 31, 630–643. [Google Scholar] [CrossRef] [PubMed]
- Kanno, T.; Tsuchiya, A.; Nishizaki, T. Hyperphosphorylation of Tau at Ser396 occurs in the much earlier stage than appearance of learning and memory disorders in 5XFAD mice. Behav. Brain Res. 2014, 274, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, X.; Schroeder, J.P.; Chan, C.-B.; Song, M.; Yu, S.P.; Weinshenker, D.; Ye, K. 7, 8-dihydroxyflavone prevents synaptic loss and memory deficits in a mouse model of Alzheimer’s disease. Neuropsychopharmacology 2014, 39, 638–650. [Google Scholar] [CrossRef] [PubMed]
- Urano, T.; Tohda, C. Icariin improves memory impairment in Alzheimer’s disease model mice (5xFAD) and attenuates amyloid β-induced neurite atrophy. Phytother. Res. 2010, 24, 1658–1663. [Google Scholar] [CrossRef] [PubMed]
- Flanigan, T.J.; Xue, Y.; Kishan Rao, S.; Dhanushkodi, A.; McDonald, M.P. Abnormal vibrissa-related behavior and loss of barrel field inhibitory neurons in 5xFAD transgenics. Genes Brain Behav. 2014, 13, 488–500. [Google Scholar] [CrossRef] [PubMed]
- Gee, M.S.; Son, S.H.; Jeon, S.H.; Do, J.; Kim, N.; Ju, Y.-J.; Lee, S.J.; Chung, E.K.; Inn, K.-S.; Kim, N.-J.; et al. A selective p38α/β MAPK inhibitor alleviates neuropathology and cognitive impairment, and modulates microglia function in 5XFAD mouse. Alzheimer’s Res. Ther. 2020, 12, 45. [Google Scholar] [CrossRef]
- O’Leary, T.P.; Brown, R.E. Visuo-spatial learning and memory impairments in the 5xFAD mouse model of Alzheimer’s disease: Effects of age, sex, albinism, and motor impairments. Genes Brain Behav. 2022, 21, e12794. [Google Scholar] [CrossRef]
- Hart, D.J.; Craig, D.; Compton, S.A.; Critchlow, S.; Kerrigan, B.M.; McIlroy, S.P.; Passmore, A.P. A retrospective study of the behavioural and psychological symptoms of mid and late phase Alzheimer’s disease. Int. J. Geriatr. Psychiatry 2003, 18, 1037–1042. [Google Scholar] [CrossRef] [PubMed]
- Desmarais, P.; Lanctôt, K.L.; Masellis, M.; Black, S.E.; Herrmann, N. Social inappropriateness in neurodegenerative disorders. Int. Psychogeriatr. 2017, 30, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Kosel, F.; Pelley, J.M.; Franklin, T.B. Behavioural and psychological symptoms of dementia in mouse models of Alzheimer’s disease-related pathology. Neurosci. Biobehav. Rev. 2020, 112, 634–647. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhao, J.; Lai, L.; Xia, Y.; Wan, C.; Wei, S.; Liang, J.; Chen, Y.; Xu, N. Loss of SST and PV positive interneurons in the ventral hippocampus results in anxiety-like behavior in 5xFAD mice. Neurobiol. Aging 2022, 117, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Jirkof, P. Burrowing and nest building behavior as indicators of well-being in mice. J. Neurosci. Methods 2014, 234, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Cathomas, F.; Hartmann, M.N.; Seifritz, E.; Pryce, C.R.; Kaiser, S. The translational study of apathy—An ecological approach. Front. Behav. Neurosci. 2015, 9, 241. [Google Scholar] [CrossRef] [PubMed]
- Devi, L.; Ohno, M. TrkB reduction exacerbates Alzheimer’s disease-like signaling aberrations and memory deficits without affecting β-amyloidosis in 5XFAD mice. Transl. Psychiatry 2015, 5, e562. [Google Scholar] [CrossRef]
- Kalueff, A.V.; Tuohimaa, P. Experimental modeling of anxiety and depression. Acta Neurobiol. Exp. 2004, 64, 439–448. [Google Scholar] [CrossRef]
- Belovicova, K.; Bogi, E.; Csatlosova, K.; Dubovicky, M. Animal tests for anxiety-like and depression-like behavior in rats. Interdiscip. Toxicol. 2017, 10, 40–43. [Google Scholar] [CrossRef]
- Wirths, O.; Erck, C.; Martens, H.; Harmeier, A.; Geumann, C.; Jawhar, S.; Kumar, S.; Multhaup, G.; Walter, J.; Ingelsson, M.; et al. Identification of low molecular weight pyroglutamate Aβ oligomers in Alzheimer disease: A novel tool for therapy and diagnosis. J. Biol. Chem. 2010, 285, 41517–41524. [Google Scholar] [CrossRef]
- Smith, S.; Hopp, S.C. The 5XFAD mouse model of Alzheimer’s disease displays age-dependent deficits in habituation to a novel environment. Aging Brain 2023, 3, 100078. [Google Scholar] [CrossRef] [PubMed]
- Belzung, C. Chapter 4.11 Measuring rodent exploratory behavior. Tech. Behav. Neural Sci. 1999, 13, 738–749. [Google Scholar] [CrossRef]
- Crusio, W.E. Genetic dissection of mouse exploratory behaviour. Behav. Brain Res. 2001, 125, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Gould, T.D.; Dao, D.T.; Kovacsics, C.E. The open field test. In Mood and Anxiety Related Phenotypes in Mice: Characterization Using Behavioral Tests; Springer Protocols; Humana Press: Totowa, NJ, USA, 2009; pp. 1–20. [Google Scholar] [CrossRef]
- Uslu, Z.S.A. Recent advancements in behavioral testing in rodents. MethodsX 2021, 8, 101536. [Google Scholar] [CrossRef] [PubMed]
- Voikar, V. Reproducibility of behavioral phenotypes in mouse models—A short history with critical and practical notes. J Rep. Neurosci. 2020, 1, 1375. [Google Scholar] [CrossRef]
- Saré, R.M.; Lemons, A.; Smith, C.B. Behavior testing in rodents: Highlighting potential confounds affecting variability and reproducibility. Brain Sci. 2021, 11, 522. [Google Scholar] [CrossRef] [PubMed]
- Schellinck, H.M.; Cyr, D.P.; Brown, R.E. How many ways can mouse behavioral experiments go wrong? Confounding variables in mouse models of neurodegenerative diseases and how to control them. Adv. Stud. Behav. 2010, 41, 255–366. [Google Scholar] [CrossRef]
- Nigri, M.; Åhlgren, J.; Wolfer, D.P.; Voikar, V. Role of environment and experimenter in reproducibility of behavioral studies with laboratory mice. Front. Behav. Neurosci. 2022, 16, 835444. [Google Scholar] [CrossRef] [PubMed]
- Rosso, M.; Wirz, R.; Loretan, A.V.; Sutter, N.A.; da Cunha, C.T.P.; Jaric, I.; Würbel, H.; Voelkl, B. Reliability of common mouse behavioural tests of anxiety: A systematic review and meta-analysis on the effects of anxiolytics. Neurosci. Biobehav. Rev. 2022, 143, 104928. [Google Scholar] [CrossRef]
- Gulinello, M.; Mitchell, H.A.; Chang, Q.; O’Brien, W.T.; Zhou, Z.; Abel, T.; Wang, L.; Corbin, J.G.; Veeraragavan, S.; Samaco, R.C.; et al. Rigor and reproducibility in rodent behavioral research. Neurobiol. Learn. Mem. 2019, 165, 106780. [Google Scholar] [CrossRef]
- Harach, T.; Marungruang, N.; Duthilleul, N.; Cheatham, V.; Mc Coy, K.D.; Frisoni, G.; Neher, J.J.; Fåk, F.; Jucker, M.; Lasser, T.; et al. Reduction of Abeta amyloid pathology in APPPS1 transgenic mice in the absence of gut microbiota. Sci. Rep. 2017, 7, 41802. [Google Scholar] [CrossRef] [PubMed]
- Kulesskaya, N.; Rauvala, H.; Voikar, V. Evaluation of social and physical enrichment in modulation of behavioural phenotype in C57BL/6J female mice. PLoS ONE 2011, 6, e24755. [Google Scholar] [CrossRef]
- Sorge, R.E.; Martin, L.J.; Isbester, K.A.; Sotocinal, S.G.; Rosen, S.; Tuttle, A.H.; Wieskopf, J.S.; Acland, E.L.; Dokova, A.; Kadoura, B.; et al. Olfactory exposure to males, including men, causes stress and related analgesia in rodents. Nat. Methods 2014, 11, 629–632. [Google Scholar] [CrossRef]
- van Driel, K.S.; Talling, J.C. Familiarity increases consistency in animal tests. Behav. Brain Res. 2005, 159, 243–245. [Google Scholar] [CrossRef] [PubMed]
- Chesler, E.J.; Wilson, S.G.; Lariviere, W.R.; Rodriguez-Zas, S.L.; Mogil, J.S. Identification and ranking of genetic and laboratory environment factors influencing a behavioral trait, thermal nociception, via computational analysis of a large data archive. Neurosci. Biobehav. Rev. 2002, 26, 907–923. [Google Scholar] [CrossRef] [PubMed]
- Aksoy, A.; Schulz, D.; Yilmaz, A.; Canbeyli, R. Seasonal variability in behavioral despair in female rats. Int. J. Neurosci. 2004, 114, 1513–1520. [Google Scholar] [CrossRef] [PubMed]
- Richetto, J.; Polesel, M.; Weber-Stadlbauer, U. Effects of light and dark phase testing on the investigation of behavioural paradigms in mice: Relevance for behavioural neuroscience. Pharmacol. Biochem. Behav. 2019, 178, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Gerdin, A.-K.; Igosheva, N.; Roberson, L.-A.; Ismail, O.; Karp, N.; Sanderson, M.; Cambridge, E.; Shannon, C.; Sunter, D.; Ramirez-Solis, R.; et al. Experimental and husbandry procedures as potential modifiers of the results of phenotyping tests. Physiol. Behav. 2012, 106, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Valentinuzzi, V.S.; Buxton, O.M.; Chang, A.M.; Scarbrough, K.; Ferrari, E.A.; Takahashi, J.S.; Turek, F.W. Locomotor response to an open field during C57BL/6J active and inactive phases: Differences dependent on conditions of illumination. Physiol. Behav. 2000, 69, 269–275. [Google Scholar] [CrossRef]
- Brown, R.E.; Wong, A.A. The influence of visual ability on learning and memory performance in 13 strains of mice. Learn. Mem. 2007, 14, 134–144. [Google Scholar] [CrossRef]
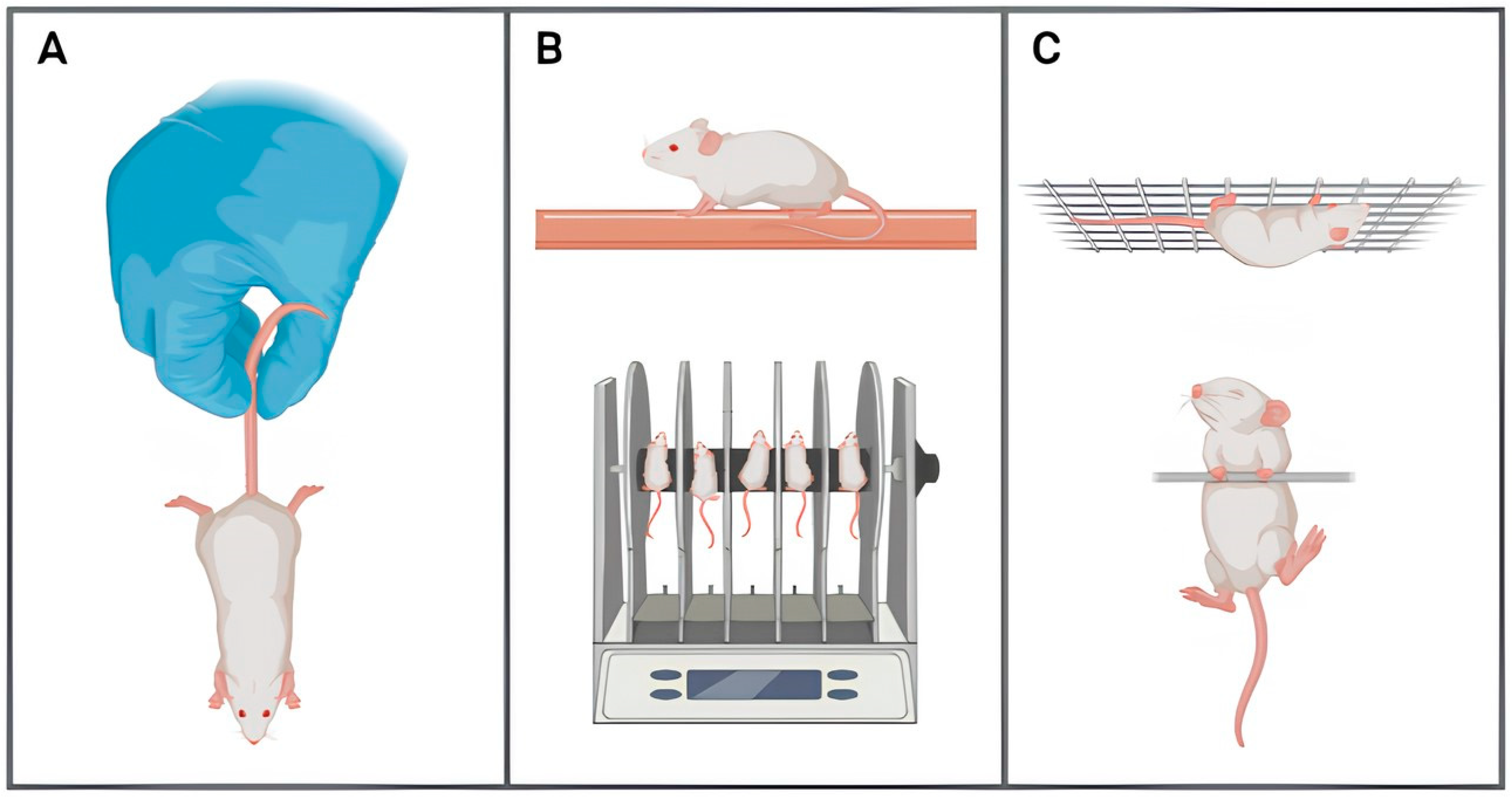
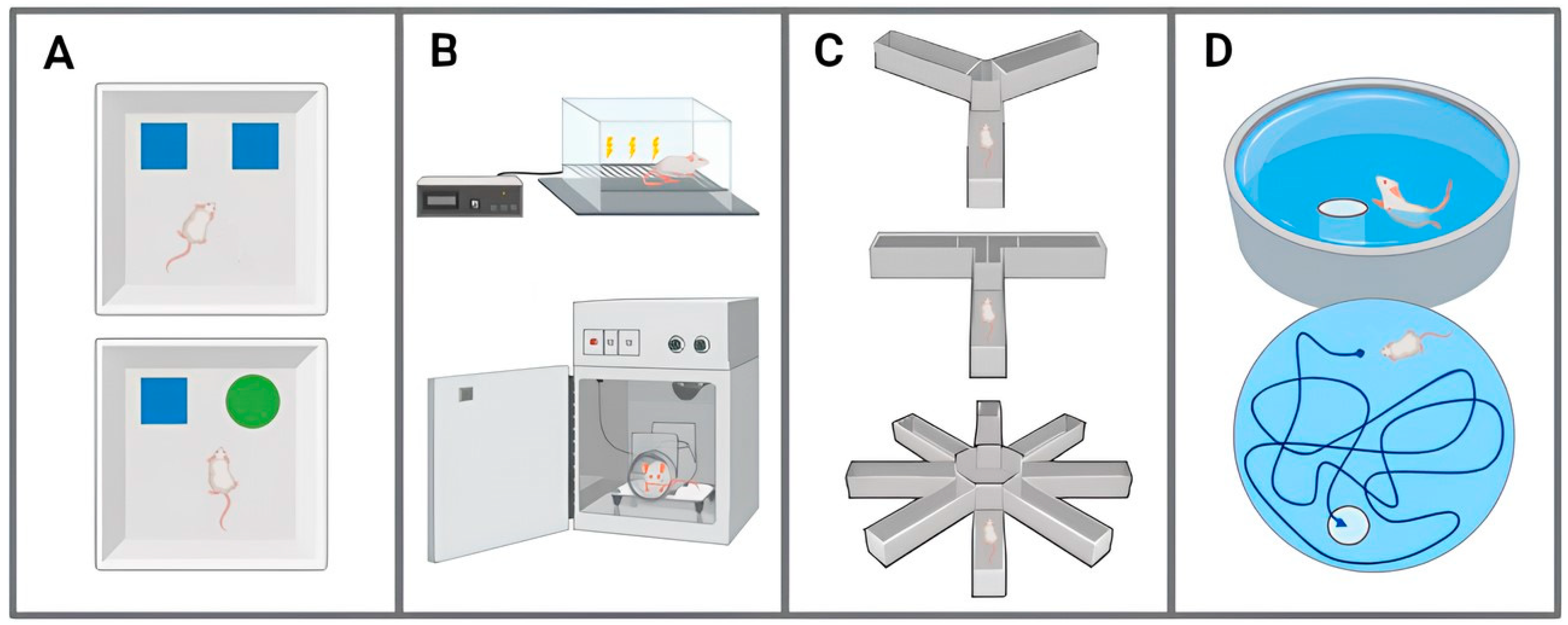
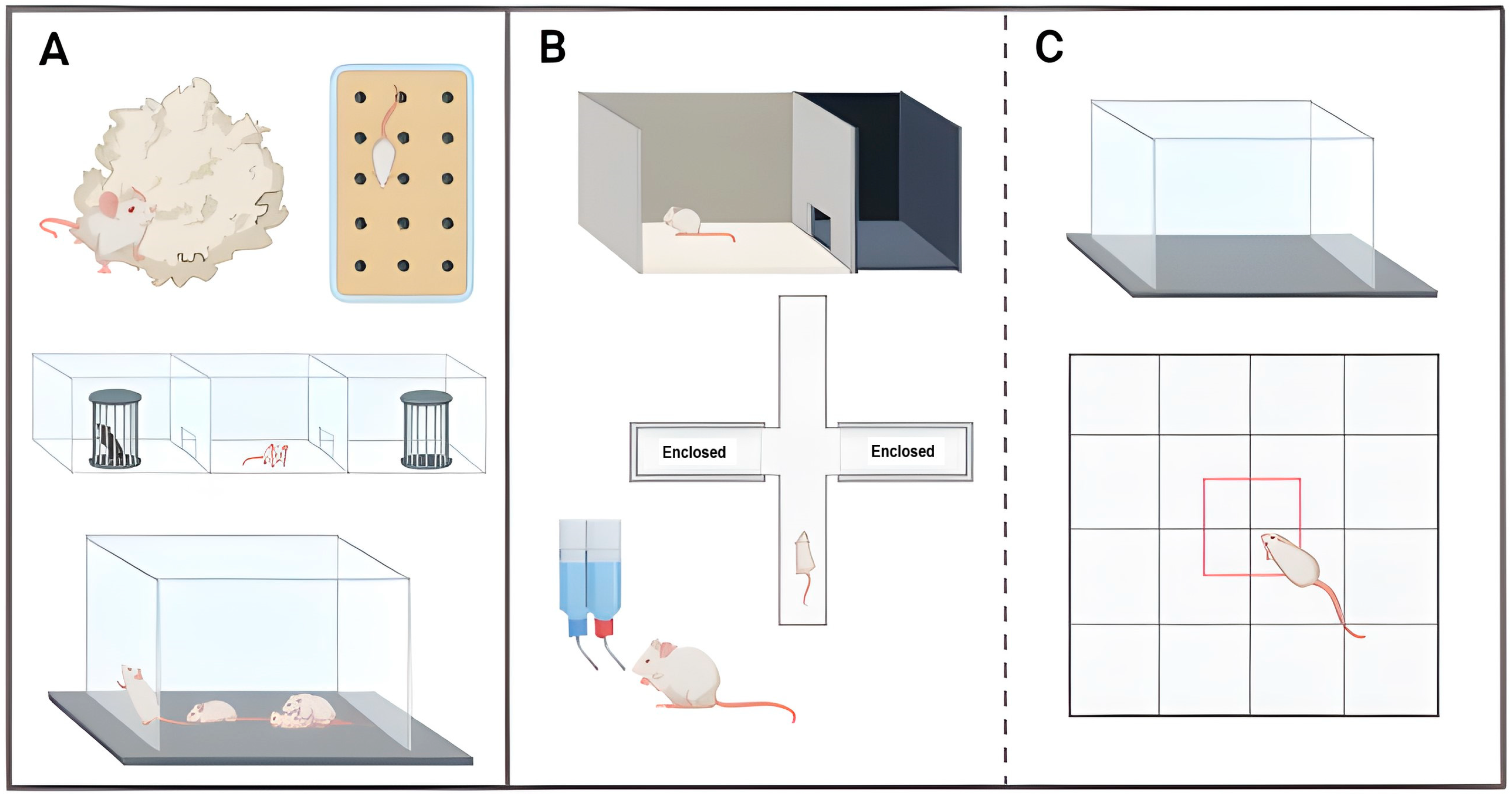
| Early Onset (1–3 Months) | Mid-Onset (4–8 Months) | Late-Onset (9–15 Months) | Absence | |
|---|---|---|---|---|
| Abnormal agility and reflex movements | - | [43] | [41,46,47,48,49,50,51] | [45] |
| Loss of balance and coordination | - | [60] | [41,46,49,56,57,58,59] | [61] |
| Deficits in skeletal muscle function | - | - | [41,43,48,49,54,55,56,58,59,63] | - |
| Early Onset (1–3 Months) | Mid-Onset (4–8 Months) | Late-Onset (9–15 Months) | Absence | |
|---|---|---|---|---|
| Altered taste profile | - | [45] | - | - |
| Auditory dysfunction | [49,73,74] | - | - | [45] |
| Olfactory dysfunction | - | [38,77,78,79] | - | [45,82,83] |
| Impaired visual acuity | [88] | [66] | - | - |
| Early Onset (1–3 Months) | Mid-Onset (4–8 Months) | Late-Onset (9–18 Months) | Absence | |
|---|---|---|---|---|
| Impaired visual recognition memory | - | [53,93,94,95,96,97,99,101,102,103,104] | [51,98,100] | [58] |
| Impaired associative learning and memory | - | [60,107,108,109,110,111,113,114] | [48,112] | [40] |
| Impaired spatial working memory | [128] | [33,41,44,46,54,63,118,119,120,122,123,124,126] | [51,58,59,117,121,125] | [45,61,103] |
| Impaired spatial reference learning and memory | [120,130,131,132] | [60,66,77,97,99,103,107,114,122,123,124,126,133,134,135,136] | [47,48,49,50,58,59,112,121,137,138,139] | [45] |
| Early Onset (1–3 Months) | Mid-Onset (4–8 Months) | Late-Onset (9–18 Months) | Absence | |
|---|---|---|---|---|
| Apathy-like behaviour | - | [50,101,123,127,146] | [51,82,125,137] | - |
| Anxiety-like behaviour | - | - | ↑ [49,50,51] | [56,137] |
| ↓ [40,41,43,44,55,104,149,150] | ↓ [47,58,59,127] | |||
| Depressive-like behaviour | - | [45] | - | - |
| Abnormal exploratory behaviour | - | ↑ [48,61,101,104] | ↑ [103,137] | - |
| - | ↓ [60,126] | ↓ [40,49,56,57,127] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pádua, M.S.; Guil-Guerrero, J.L.; Lopes, P.A. Behaviour Hallmarks in Alzheimer’s Disease 5xFAD Mouse Model. Int. J. Mol. Sci. 2024, 25, 6766. https://doi.org/10.3390/ijms25126766
Pádua MS, Guil-Guerrero JL, Lopes PA. Behaviour Hallmarks in Alzheimer’s Disease 5xFAD Mouse Model. International Journal of Molecular Sciences. 2024; 25(12):6766. https://doi.org/10.3390/ijms25126766
Chicago/Turabian StylePádua, Mafalda Soares, José L. Guil-Guerrero, and Paula Alexandra Lopes. 2024. "Behaviour Hallmarks in Alzheimer’s Disease 5xFAD Mouse Model" International Journal of Molecular Sciences 25, no. 12: 6766. https://doi.org/10.3390/ijms25126766
APA StylePádua, M. S., Guil-Guerrero, J. L., & Lopes, P. A. (2024). Behaviour Hallmarks in Alzheimer’s Disease 5xFAD Mouse Model. International Journal of Molecular Sciences, 25(12), 6766. https://doi.org/10.3390/ijms25126766







