α-Chitosan and β-Oligochitosan Mixtures-Based Formula for In Vitro Assessment of Melanocyte Cells Response
Abstract
1. Introduction
2. Results
2.1. Physicochemical Characteristics of α-Chitosan and β-Chitosan
2.1.1. Scanning Electron Microscopy of the Powders
2.1.2. Biopolymer Solutions
2.1.3. Antioxidant Activity Assay
2.2. Biological Effects
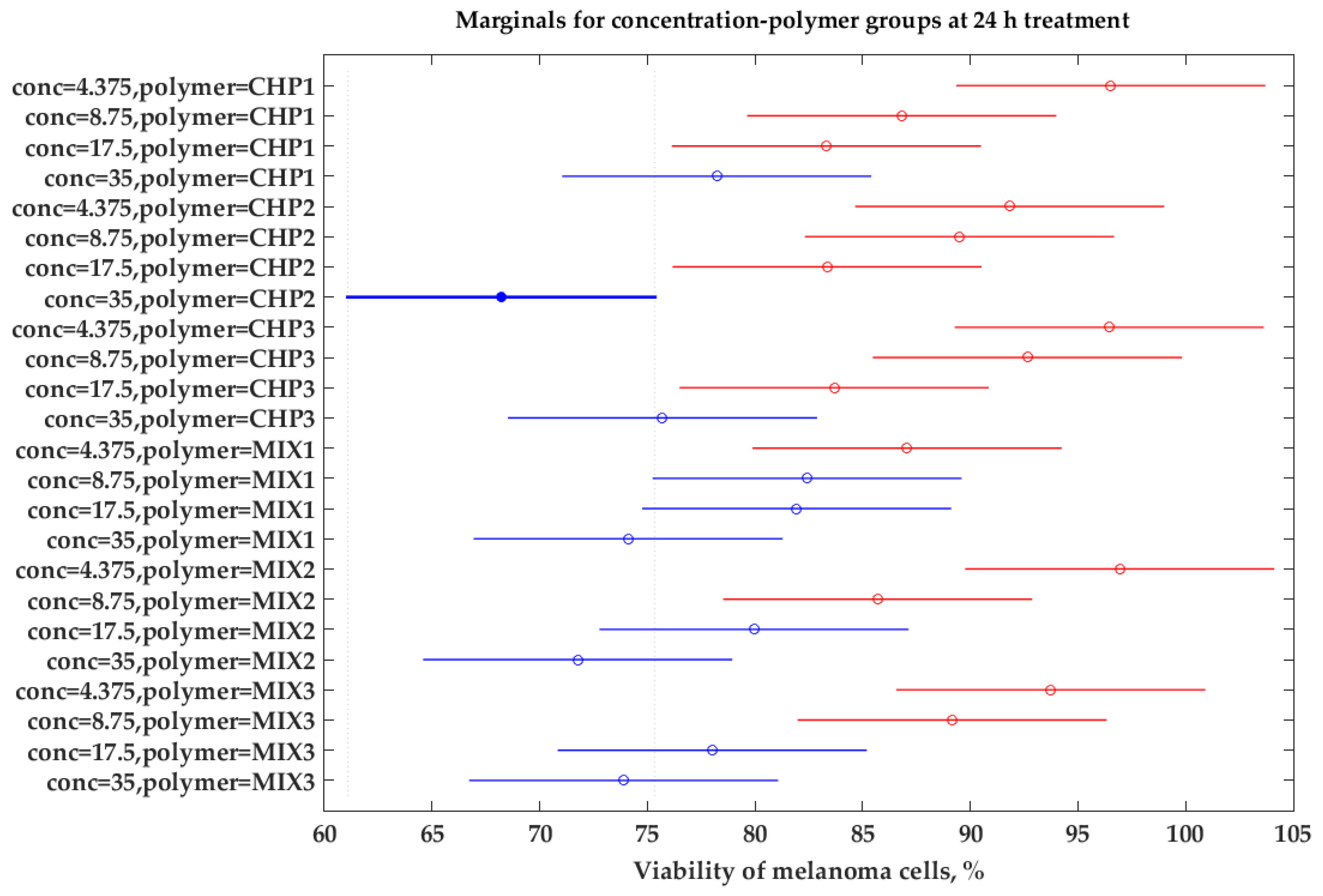
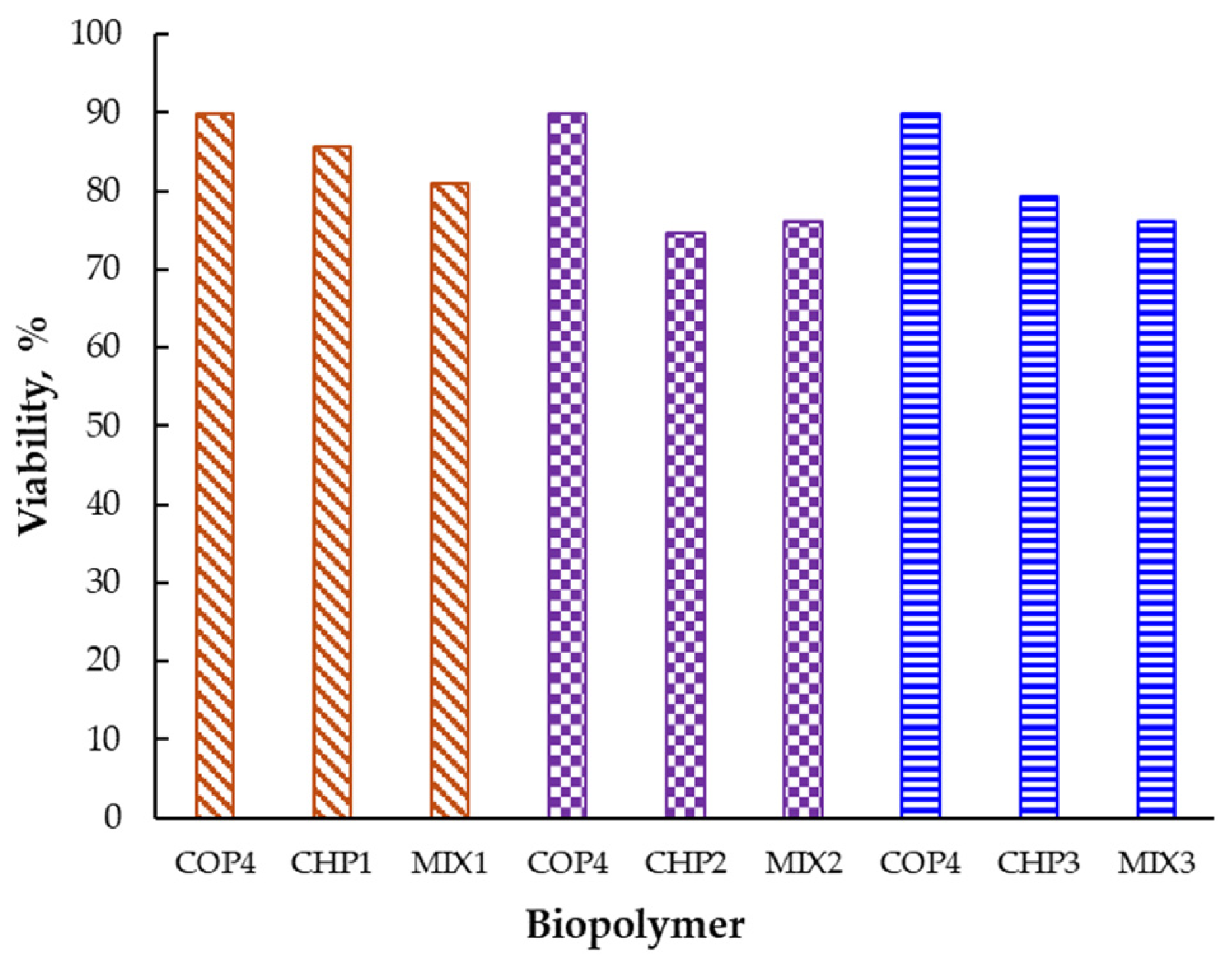
2.2.1. Evaluation of the Effects on Cell Viability by the MTT Method
2.2.2. The Cell Morphology Test
2.2.3. Determination of YKL-40 Protein Expression
2.3. Statistical Analysis of Experimental Data
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis and Characterization of Chitosan Samples
4.3. Antioxidant Activity
4.4. Cell Culturing Conditions
4.5. MTT Assay
4.6. Cell Morphology Test
4.7. Determination of YKL-40 Protein Expression
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, H.J.; Kim, Y.H. Molecular Frontiers in Melanoma: Pathogenesis, Diagnosis, and Therapeutic Advances. Int. J. Mol. Sci. 2024, 25, 2984. [Google Scholar] [CrossRef] [PubMed]
- Rambow, F.; Marine, J.-C.; Goding, C.R. Melanoma Plasticity and Phenotypic Diversity: Therapeutic Barriers and Opportunities. Genes Dev. 2019, 33, 1295–1318. [Google Scholar] [CrossRef] [PubMed]
- Hossain, S.M.; Eccles, M.R. Phenotype Switching and the Melanoma Microenvironment; Impact on Immunotherapy and Drug Resistance. Int. J. Mol. Sci. 2023, 24, 1601. [Google Scholar] [CrossRef]
- Xu, K.; Wang, Y.; Xie, Y.; Zhang, X.; Chen, W.; Li, Z.; Wang, T.; Yang, X.; Guo, B.; Wang, L.; et al. Anti-Melanoma Effect and Action Mechanism of a Novel Chitosan-Based Composite Hydrogel Containing Hydroxyapatite Nanoparticles. Regen. Biomater. 2022, 9, rbac050. [Google Scholar] [CrossRef] [PubMed]
- Adamus-Grabicka, A.A.; Hikisz, P.; Sikora, J. Nanotechnology as a Promising Method in the Treatment of Skin Cancer. Int. J. Mol. Sci. 2024, 25, 2165. [Google Scholar] [CrossRef] [PubMed]
- Lie, K.C.M.; Bonturi, C.R.; Salu, B.R.; de Oliveira, J.R.; Bonini Galo, M.; Paiva, P.M.G.; Correia, M.T.d.S.; Oliva, M.L.V. Impairment of SK-MEL-28 Development—A Human Melanoma Cell Line—By the Crataeva Tapia Bark Lectin and Its Sequence-Derived Peptides. Int. J. Mol. Sci. 2023, 24, 10617. [Google Scholar] [CrossRef] [PubMed]
- Czarnecka, A.M.; Bartnik, E.; Fiedorowicz, M.; Rutkowski, P. Targeted Therapy in Melanoma and Mechanisms of Resistance. Int. J. Mol. Sci. 2020, 21, 4576. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Wu, C.; Zhang, B.; Zhang, Y.; Li, J. Ferruginol Induced Apoptosis on SK-Mel-28 Human Malignant Melanoma Cells Mediated through P-P38 and NF-ΚB. Hum. Exp. Toxicol. 2019, 38, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Hou, K.; Yu, J.; Wang, L.; Chen, X. Nanoparticle-Based Combination Therapy for Melanoma. Front. Oncol. 2022, 12, 928797. [Google Scholar] [CrossRef] [PubMed]
- Piekarska, K.; Sikora, M.; Owczarek, M.; Jóźwik-Pruska, J.; Wiśniewska-Wrona, M. Chitin and Chitosan as Polymers of the Future—Obtaining, Modification, Life Cycle Assessment and Main Directions of Application. Polymers 2023, 15, 793. [Google Scholar] [CrossRef] [PubMed]
- Riccardo, A.A.; Gupta, N.S. (Eds.) Chitin. In Topics in Geobiology; Springer: Dordrecht, The Netherlands, 2011; Volume 34, ISBN 978-90-481-9683-8. [Google Scholar]
- Stie, M.B.; Thoke, H.S.; Issinger, O.-G.; Hochscherf, J.; Guerra, B.; Olsen, L.F. Delivery of Proteins Encapsulated in Chitosan-Tripolyphosphate Nanoparticles to Human Skin Melanoma Cells. Colloids Surf. B Biointerfaces 2019, 174, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Petroni, G.; Buqué, A.; Zitvogel, L.; Kroemer, G.; Galluzzi, L. Immunomodulation by Targeted Anticancer Agents. Cancer Cell 2021, 39, 310–345. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.-H.; Yuan, S.-S.; Huang, Y.-F.; Lin, P.-C.; Lu, C.-Y.; Chung, T.-W.; Tyan, Y.-C. A Proteomic View to Characterize the Effect of Chitosan Nanoparticle to Hepatic Cells: Is Chitosan Nanoparticle an Enhancer of PI3K/AKT1/MTOR Pathway? BioMed Res. Int. 2014, 2014, 789591. [Google Scholar] [CrossRef] [PubMed]
- Frank, L.A.; Onzi, G.R.; Morawski, A.S.; Pohlmann, A.R.; Guterres, S.S.; Contri, R.V. Chitosan as a Coating Material for Nanoparticles Intended for Biomedical Applications. React. Funct. Polym. 2020, 147, 104459. [Google Scholar] [CrossRef]
- Wu, J.; Liu, G.; Qin, Y.-X.; Meng, Y. Effect of Low-Intensity Pulsed Ultrasound on Biocompatibility and Cellular Uptake of Chitosan-Tripolyphosphate Nanoparticles. Biointerphases 2014, 9, 031016. [Google Scholar] [CrossRef] [PubMed]
- Apetroaei, M.R.; Zgârian, R.G.; Manea, A.-M.; Rau, I.; Tihan, G.T.; Schroder, V. New Source of Chitosan from Black Sea Marine Organisms Identification. Mol. Cryst. Liq. Cryst. 2016, 628, 102–109. [Google Scholar] [CrossRef]
- Schröder, V.; Rău, I.; Dobrin, N.; Stefanov, C.; Mihali, C.-V.; Pădureţu, C.-C.; Apetroaei, M.R. Micromorphological Details and Identification of Chitinous Wall Structures in Rapana Venosa (Gastropoda, Mollusca) Egg Capsules. Sci. Rep. 2020, 10, 14550. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, M.B.; Struszczyk-Swita, K.; Li, X.; Szczęsna-Antczak, M.; Daroch, M. Enzymatic Modifications of Chitin, Chitosan, and Chitooligosaccharides. Front. Bioeng. Biotechnol. 2019, 7, 243. [Google Scholar] [CrossRef] [PubMed]
- Morin-Crini, N.; Lichtfouse, E.; Torri, G.; Crini, G. Applications of Chitosan in Food, Pharmaceuticals, Medicine, Cosmetics, Agriculture, Textiles, Pulp and Paper, Biotechnology, and Environmental Chemistry. Environ. Chem. Lett. 2019, 17, 1667–1692. [Google Scholar] [CrossRef]
- Minh, N.C.; Nguyen, V.H.; Schwarz, S.; Stevens, W.F.; Trung, T.S. Preparation of Water Soluble Hydrochloric Chitosan from Low Molecular Weight Chitosan in the Solid State. Int. J. Biol. Macromol. 2019, 121, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Hosseinnejad, M.; Jafari, S.M. Evaluation of Different Factors Affecting Antimicrobial Properties of Chitosan. Int. J. Biol. Macromol. 2016, 85, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.F.; Aschi, A.; Nicolai, T. Formation and Characterization of Chitosan-Protein Particles with Fractal Whey Protein Aggregates. Colloids Surf. B Biointerfaces 2018, 169, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Sarabandi, K.; Jafari, S.M. Effect of Chitosan Coating on the Properties of Nanoliposomes Loaded with Flaxseed-Peptide Fractions: Stability during Spray-Drying. Food Chem. 2020, 310, 125951. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Qin, J.; Zhang, P.; Chen, X.; You, X.; Zhang, F.; Zuo, B.; Yao, M. Facile Preparation of a Strong Chitosan-Silk Biocomposite Film. Carbohydr. Polym. 2020, 229, 115515. [Google Scholar] [CrossRef] [PubMed]
- Tao, F.; Cheng, Y.; Shi, X.; Zheng, H.; Du, Y.; Xiang, W.; Deng, H. Applications of Chitin and Chitosan Nanofibers in Bone Regenerative Engineering. Carbohydr. Polym. 2020, 230, 115658. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Huang, Y.-H.; Cheng, K.-C.; Song, Y.-L. Investigations of the Influences of Processing Conditions on the Properties of Spray Dried Chitosan-Tripolyphosphate Particles Loaded with Theophylline. Sci. Rep. 2020, 10, 1155. [Google Scholar] [CrossRef] [PubMed]
- Mota, J.; Yu, N.; Caridade, S.G.; Luz, G.M.; Gomes, M.E.; Reis, R.L.; Jansen, J.A.; Walboomers, X.F.; Mano, J.F. Chitosan/Bioactive Glass Nanoparticle Composite Membranes for Periodontal Regeneration. Acta Biomater. 2012, 8, 4173–4180. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Elango, J.; Wu, W. Recent Advancement of Molecular Structure and Biomaterial Function of Chitosan from Marine Organisms for Pharmaceutical and Nutraceutical Application. Appl. Sci. 2020, 10, 4719. [Google Scholar] [CrossRef]
- Dinculescu, D.; Gîjiu, C.L.; Apetroaei, M.R.; Isopescu, R.; Rău, I.; Schröder, V. Optimization of Chitosan Extraction Process from Rapana Venosa Egg Capsules Waste Using Experimental Design. Materials 2023, 16, 525. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Lorenzo, C.; Blanco-Fernandez, B.; Puga, A.M.; Concheiro, A. Crosslinked Ionic Polysaccharides for Stimuli-Sensitive Drug Delivery. Adv. Drug Deliv. Rev. 2013, 65, 1148–1171. [Google Scholar] [CrossRef] [PubMed]
- Merz, C.R. Physicochemical and Colligative Investigation of α (Shrimp Shell)- and β (Squid Pen)-Chitosan Membranes: Concentration-Gradient-Driven Water Flux and Ion Transport for Salinity Gradient Power and Separation Process Operations. ACS Omega 2019, 4, 21027–21040. [Google Scholar] [CrossRef] [PubMed]
- Jampafuang, Y.; Tongta, A.; Waiprib, Y. Impact of Crystalline Structural Differences Between α- and β-Chitosan on Their Nanoparticle Formation Via Ionic Gelation and Superoxide Radical Scavenging Activities. Polymers 2019, 11, 2010. [Google Scholar] [CrossRef] [PubMed]
- Masselin, A.; Rousseau, A.; Pradeau, S.; Fort, L.; Gueret, R.; Buon, L.; Armand, S.; Cottaz, S.; Choisnard, L.; Fort, S. Optimizing Chitin Depolymerization by Lysozyme to Long-Chain Oligosaccharides. Mar. Drugs 2021, 19, 320. [Google Scholar] [CrossRef] [PubMed]
- Muzzarelli, R.A.A.; Boudrant, J.; Meyer, D.; Manno, N.; De Marchis, M.; Paoletti, M.G. Current Views on Fungal Chitin/Chitosan, Human Chitinases, Food Preservation, Glucans, Pectins and Inulin: A Tribute to Henri Braconnot, Precursor of the Carbohydrate Polymers Science, on the Chitin Bicentennial. Carbohydr. Polym. 2012, 87, 995–1012. [Google Scholar] [CrossRef]
- Chen, W.; Jiang, X.; Yang, Q. Glycoside Hydrolase Family 18 Chitinases: The Known and the Unknown. Biotechnol. Adv. 2020, 43, 107553. [Google Scholar] [CrossRef] [PubMed]
- Adrangi, S.; Faramarzi, M.A. From Bacteria to Human: A Journey into the World of Chitinases. Biotechnol. Adv. 2013, 31, 1786–1795. [Google Scholar] [CrossRef] [PubMed]
- Young, E.; Chatterton, C.; Vellodi, A.; Winchester, B. Plasma Chitotriosidase Activity in Gaucher Disease Patients Who Have Been Treated Either by Bone Marrow Transplantation or by Enzyme Replacement Therapy with Alglucerase. J. Inherit. Metab. Dis. 1997, 20, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Boot, R.G.; Blommaart, E.F.C.; Swart, E.; Ghauharali-van der Vlugt, K.; Bijl, N.; Moe, C.; Place, A.; Aerts, J.M.F.G. Identification of a Novel Acidic Mammalian Chitinase Distinct from Chitotriosidase. J. Biol. Chem. 2001, 276, 6770–6778. [Google Scholar] [CrossRef] [PubMed]
- Mazur, M.; Zielińska, A.; Grzybowski, M.M.; Olczak, J.; Fichna, J. Chitinases and Chitinase-Like Proteins as Therapeutic Targets in Inflammatory Diseases, with a Special Focus on Inflammatory Bowel Diseases. Int. J. Mol. Sci. 2021, 22, 6966. [Google Scholar] [CrossRef] [PubMed]
- Ziatabar, S.; Zepf, J.; Rich, S.; Danielson, B.T.; Bollyky, P.I.; Stern, R. Chitin, Chitinases, and Chitin Lectins: Emerging Roles in Human Pathophysiology. Pathophysiology 2018, 25, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Ismail, H.; Helby, J.; Hölmich, L.R.; Chakera, A.H.; Bastholt, L.; Klyver, H.; Sjøgren, P.; Schmidt, H.; Schöllhammer, L.; Johansen, J.S.; et al. Measured and Genetically Predicted Plasma YKL-40 Levels and Melanoma Mortality. Eur. J. Cancer 2019, 121, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.-Q.; Hassanein, T.; Qun-Fang, L.; Liu, W.; Zheng, Y.-H.; Chen, J. YKL-40 Expression in Human Hepatocellular Carcinoma: A Potential Biomarker? Hepatobiliary Pancreat. Dis. Int. 2011, 10, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Richard, B.; Price, P. YKL-40 MONOCLONAL AUTHORITY 2011. U.S. Patent US8053563B2, 8 November 2011. Available online: https://patents.google.com/patent/US8053563B2/en (accessed on 16 June 2024).
- Kognole, A.A.; Payne, C.M. Inhibition of Mammalian Glycoprotein YKL-40. J. Biol. Chem. 2017, 292, 2624–2636. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, E.; Çiçek, T. S71 THE ASSOCIATION OF SERUM YKL-40 LEVEL WITH TUMOR BURDEN AND METASTATIC STAGE OF PROSTATE CANCER. Eur. Urol. Suppl. 2011, 10, 593. [Google Scholar] [CrossRef]
- Saleh, A.A.; Alhanafy, A.M.; Elbahr, O.; El-Hefnawy, S.M. Chitinase 3-like 1 Gene (T/C) Polymorphism and Serum YKL-40 Levels in Patients with Hepatocellular Carcinoma. Meta Gene 2020, 24, 100686. [Google Scholar] [CrossRef]
- Bi, J.; Lau, S.-H.; Lv, Z.-L.; Xie, D.; Li, W.; Lai, Y.-R.; Zhong, J.-M.; Wu, H.; Su, Q.; He, Y.; et al. Overexpression of YKL-40 Is an Independent Prognostic Marker in Gastric Cancer. Hum. Pathol. 2009, 40, 1790–1797. [Google Scholar] [CrossRef] [PubMed]
- Pouyafar, A.; Heydarabad, M.Z.; Mahboob, S.; Mokhtarzadeh, A.; Rahbarghazi, R. Angiogenic Potential of YKL-40 in the Dynamics of Tumor Niche. Biomed. Pharmacother. 2018, 100, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.-C.; Chen, C.-T.; Chiang, P.-F.; Chiang, Y.-C. The Role of Chitinase-3-like Protein-1 (YKL40) in the Therapy of Cancer and Other Chronic-Inflammation-Related Diseases. Pharmaceuticals 2024, 17, 307. [Google Scholar] [CrossRef] [PubMed]
- Böckelmann, L.C.; Felix, T.; Calabrò, S.; Schumacher, U. YKL-40 Protein Expression in Human Tumor Samples and Human Tumor Cell Line Xenografts: Implications for Its Use in Tumor Models. Cell. Oncol. 2021, 44, 1183–1195. [Google Scholar] [CrossRef] [PubMed]
- Pădurețu, C.-C.; Isopescu, R.; Rău, I.; Apetroaei, M.R.; Schröder, V. Influence of the Parameters of Chitin Deacetylation Process on the Chitosan Obtained from Crab Shell Waste. Korean J. Chem. Eng. 2019, 36, 1890–1899. [Google Scholar] [CrossRef]
- Khanmohammadi, M.; Elmizadeh, H.; Ghasemi, K. Investigation of Size and Morphology of Chitosan Nanoparticles Used in Drug Delivery System Employing Chemometric Technique. Iran. J. Pharm. Res. IJPR 2015, 14, 665–675. [Google Scholar] [PubMed]
- Duarte, A.R.C.; Gordillo, M.D.; Cardoso, M.M.; Simplício, A.L.; Duarte, C.M.M. Preparation of Ethyl Cellulose/Methyl Cellulose Blends by Supercritical Antisolvent Precipitation. Int. J. Pharm. 2006, 311, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Apetroaei, M.R.; Rau, I.; Schroder, V. Chitosan: Biopolymer Identified in Rapana Venosa (Gastropoda, Mollusca) [Available in Romanian]; Editura Pim: Iasi, Romania, 2020; ISBN 978-606-13-5543-3. [Google Scholar]
- Joseph, S.M.; Krishnamoorthy, S.; Paranthaman, R.; Moses, J.A.; Anandharamakrishnan, C. A Review on Source-Specific Chemistry, Functionality, and Applications of Chitin and Chitosan. Carbohydr. Polym. Technol. Appl. 2021, 2, 100036. [Google Scholar] [CrossRef]
- Negm, N.A.; Hefni, H.H.H.; Abd-Elaal, A.A.A.; Badr, E.A.; Abou Kana, M.T.H. Advancement on Modification of Chitosan Biopolymer and Its Potential Applications. Int. J. Biol. Macromol. 2020, 152, 681–702. [Google Scholar] [CrossRef]
- Gutteridge, J.M.C.; Halliwell, B. Free Radicals and Antioxidants in the Year 2000: A Historical Look to the Future. Ann. N. Y. Acad. Sci. 2000, 899, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Zhou, D.; Xie, J.; Mao, F. Preparation of Chitosan Oligomers and Their Antioxidant Activity. Eur. Food Res. Technol. 2007, 225, 451–456. [Google Scholar] [CrossRef]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Heras Caballero, A.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef]
- Si Trung, T.; Bao, H.N.D. Physicochemical Properties and Antioxidant Activity of Chitin and Chitosan Prepared from Pacific White Shrimp Waste. Int. J. Carbohydr. Chem. 2015, 2015, 706259. [Google Scholar] [CrossRef]
- Xing, R.; Liu, S.; Guo, Z.; Yu, H.; Wang, P.; Li, C.; Li, Z.; Li, P. Relevance of Molecular Weight of Chitosan and Its Derivatives and Their Antioxidant Activities in Vitro. Bioorg. Med. Chem. 2005, 13, 1573–1577. [Google Scholar] [CrossRef] [PubMed]
- Boudier, A.; Aubert-Pouëssel, A.; Gérardin, C.; Devoisselle, J.-M.; Bégu, S. PH-Sensitive Double-Hydrophilic Block Copolymer Micelles for Biological Applications. Int. J. Pharm. 2009, 379, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Zou, P.; Yang, X.; Zhang, Y.; Du, P.; Yuan, S.; Yang, D.; Wang, J. Antitumor Effects of Orally and Intraperitoneally Administered Chitosan Oligosaccharides (COSs) on S180-Bearing/Residual Mouse. J. Food Sci. 2016, 81, H3035–H3042. [Google Scholar] [CrossRef]
- Farhadihosseinabadi, B.; Zarebkohan, A.; Eftekhary, M.; Heiat, M.; Moosazadeh Moghaddam, M.; Gholipourmalekabadi, M. Crosstalk between Chitosan and Cell Signaling Pathways. Cell. Mol. Life Sci. 2019, 76, 2697–2718. [Google Scholar] [CrossRef] [PubMed]
- Gibot, L.; Chabaud, S.; Bouhout, S.; Bolduc, S.; Auger, F.A.; Moulin, V.J. Anticancer Properties of Chitosan on Human Melanoma Are Cell Line Dependent. Int. J. Biol. Macromol. 2015, 72, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Amirani, E.; Hallajzadeh, J.; Asemi, Z.; Mansournia, M.A.; Yousefi, B. Effects of Chitosan and Oligochitosans on the Phosphatidylinositol 3-Kinase-AKT Pathway in Cancer Therapy. Int. J. Biol. Macromol. 2020, 164, 456–467. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Mendis, E.; Rajapakse, N.; Kim, S.-K. Strong Electronic Charge as an Important Factor for Anticancer Activity of Chitooligosaccharides (COS). Life Sci. 2006, 78, 2399–2408. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.W.; Thomas, R.L. Antioxidative Activity of Chitosans with Varying Molecular Weights. Food Chem. 2007, 101, 308–313. [Google Scholar] [CrossRef]
- Kong, C.-S.; Kim, J.-A.; Ahn, B.; Byun, H.-G.; Kim, S.-K. Carboxymethylations of Chitosan and Chitin Inhibit MMP Expression and ROS Scavenging in Human Fibrosarcoma Cells. Process Biochem. 2010, 45, 179–186. [Google Scholar] [CrossRef]
- Wimardhani, Y.S.; Suniarti, D.F.; Freisleben, H.J.; Wanandi, S.I.; Siregar, N.C.; Ikeda, M.-A. Chitosan Exerts Anticancer Activity through Induction of Apoptosis and Cell Cycle Arrest in Oral Cancer Cells. J. Oral Sci. 2014, 56, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, H.; Kanayairam, V.; Ravichandran, R. Chitin and Chitosan Preparation from Shrimp Shells Penaeus Monodon and Its Human Ovarian Cancer Cell Line, PA-1. Int. J. Biol. Macromol. 2018, 107, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Chokradjaroen, C.; Rujiravanit, R.; Watthanaphanit, A.; Theeramunkong, S.; Saito, N.; Yamashita, K.; Arakawa, R. Enhanced Degradation of Chitosan by Applying Plasma Treatment in Combination with Oxidizing Agents for Potential Use as an Anticancer Agent. Carbohydr. Polym. 2017, 167, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.-H.; Sekine, K.; Chao, H.-M.; Hsu, S.; Chern, E. Chitosan Promotes Cancer Progression and Stem Cell Properties in Association with Wnt Signaling in Colon and Hepatocellular Carcinoma Cells. Sci. Rep. 2017, 7, 45751. [Google Scholar] [CrossRef] [PubMed]
- Yeo, I.J.; Lee, C.-K.; Han, S.-B.; Yun, J.; Hong, J.T. Roles of Chitinase 3-like 1 in the Development of Cancer, Neurodegenerative Diseases, and Inflammatory Diseases. Pharmacol. Ther. 2019, 203, 107394. [Google Scholar] [CrossRef] [PubMed]
- He, C.H.; Lee, C.G.; Dela Cruz, C.S.; Lee, C.-M.; Zhou, Y.; Ahangari, F.; Ma, B.; Herzog, E.L.; Rosenberg, S.A.; Li, Y.; et al. Chitinase 3-like 1 Regulates Cellular and Tissue Responses via IL-13 Receptor A2. Cell Rep. 2013, 5, 1156. [Google Scholar] [CrossRef]
- Zhao, T.; Su, Z.; Li, Y.; Zhang, X.; You, Q. Chitinase-3 like-Protein-1 Function and Its Role in Diseases. Signal Transduct. Target. Ther. 2020, 5, 201. [Google Scholar] [CrossRef] [PubMed]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Tissue-Based Map of the Human Proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, R.B.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination Therapy in Combating Cancer. Oncotarget 2017, 8, 38022–38043. [Google Scholar] [CrossRef] [PubMed]
- Wande, D.P.; Qiu, Y.; Chen, S.; Yao, L.; Xu, Y.; Yao, J.; Xiong, H. Modified Chitosan Nanogel-Polymersomes for Oral Co-Delivery of Oxaliplatin and Rapamycin for Synergistic Chemotherapy. J. Drug Deliv. Sci. Technol. 2022, 77, 103852. [Google Scholar] [CrossRef]
- Sangnim, T.; Dheer, D.; Jangra, N.; Huanbutta, K.; Puri, V.; Sharma, A. Chitosan in Oral Drug Delivery Formulations: A Review. Pharmaceutics 2023, 15, 2361. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-L.; Chen, H.-H.; Zheng, L.-L.; Sun, L.-P.; Shi, L. Angiogenic Signaling Pathways and Anti-Angiogenic Therapy for Cancer. Signal Transduct. Target. Ther. 2023, 8, 198. [Google Scholar] [CrossRef]
- Dinculescu, D.D.; Apetroaei, M.R.; Gîjiu, C.L.; Anton, M.; Enache, L.; Schröder, V.; Isopescu, R.; Rău, I. Simultaneous Optimization of Deacetylation Degree and Molar Mass of Chitosan from Shrimp Waste. Polymers 2024, 16, 170. [Google Scholar] [CrossRef] [PubMed]
- Pădurețu, C.-C.; Apetroaei, M.R.; Rau, I.; Schröder, V. Characterization of Chitosan Extracted from Different Romanian Black Sea Crustaceans. UPB Sci. Bull. B Chem. Mater. Sci. 2018, 80, 13–24. [Google Scholar]
- Apetroaei, M.R.; Manea, A.M.; Tihan, G.; Zgârian, R.; Schröder, V.; Rău, I. Improved Method of Chitosan Extraction from Different Crustacean Species of Romanian Black Sea Coast. UPB Sci. Bull. B Chem. Mater. Sci. 2017, 79, 13–24. [Google Scholar]
- Al Sagheer, F.A.; Al-Sughayer, M.A.; Muslim, S.; Elsabee, M.Z. Extraction and Characterization of Chitin and Chitosan from Marine Sources in Arabian Gulf. Carbohydr. Polym. 2009, 77, 410–419. [Google Scholar] [CrossRef]
- Vunjak-Novakovic, G.; Freshney, R.I. Culture of Cells for Tissue Engineering; Wiley: New York, NY, USA, 2006; ISBN 9780471741800. [Google Scholar]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Laville, N.; Aït-Aïssa, S.; Gomez, E.; Casellas, C.; Porcher, J. Effects of Human Pharmaceuticals on Cytotoxicity, EROD Activity and ROS Production in Fish Hepatocytes. Toxicology 2004, 196, 41–55. [Google Scholar] [CrossRef] [PubMed]
- van Meerloo, J.; Kaspers, G.J.L.; Cloos, J. Cell Sensitivity Assays: The MTT Assay. Methods Mol. Biol. 2011, 731, 237–245. [Google Scholar] [PubMed]
- Stockert, J.C.; Blázquez-Castro, A.; Cañete, M.; Horobin, R.W.; Villanueva, Á. MTT Assay for Cell Viability: Intracellular Localization of the Formazan Product Is in Lipid Droplets. Acta Histochem. 2012, 114, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Cann, A.J. Maths from Scratch for Biologists; Wiley: New York, NY, USA, 2002; ISBN 978-0-471-49835-3. [Google Scholar]

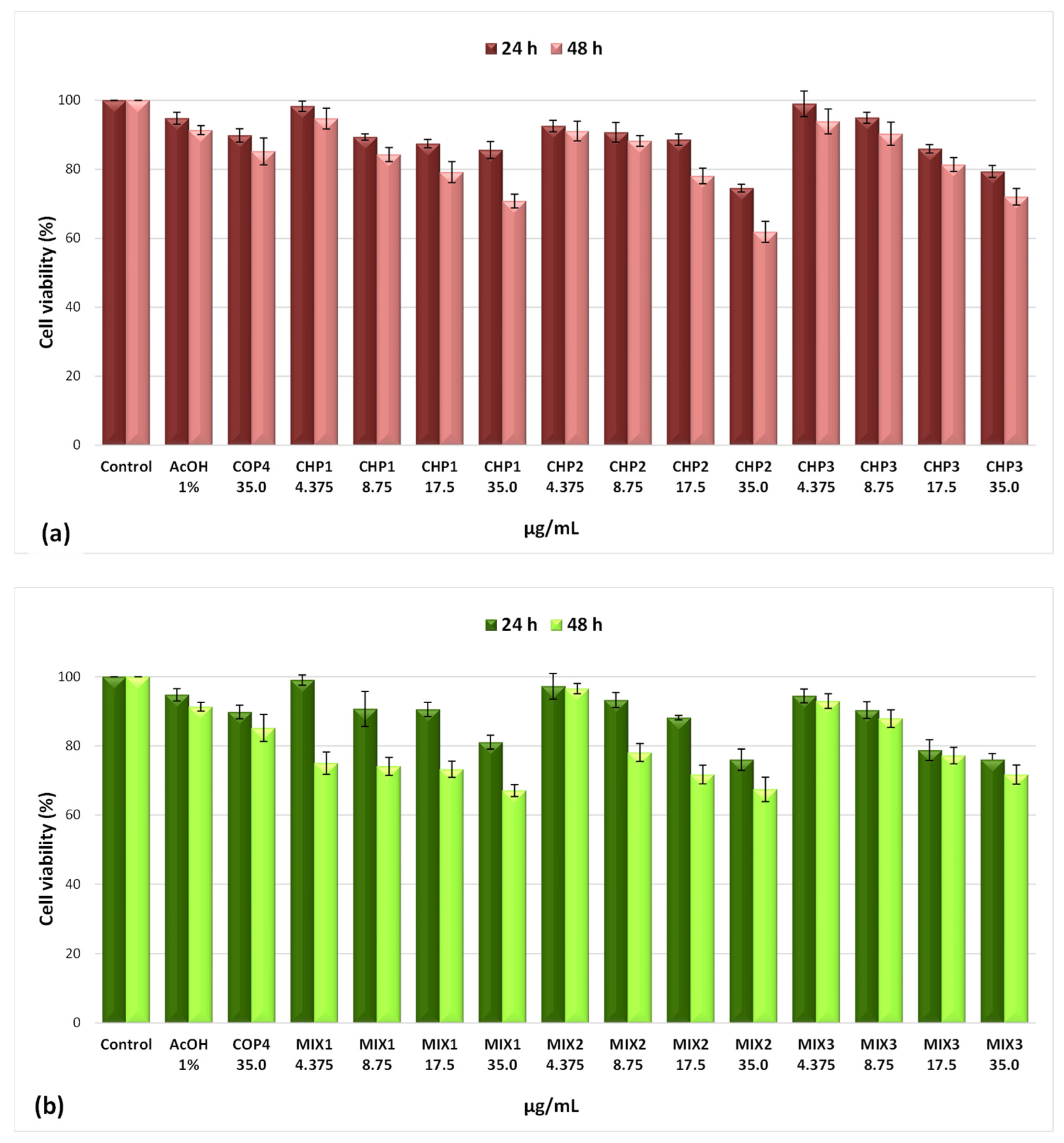
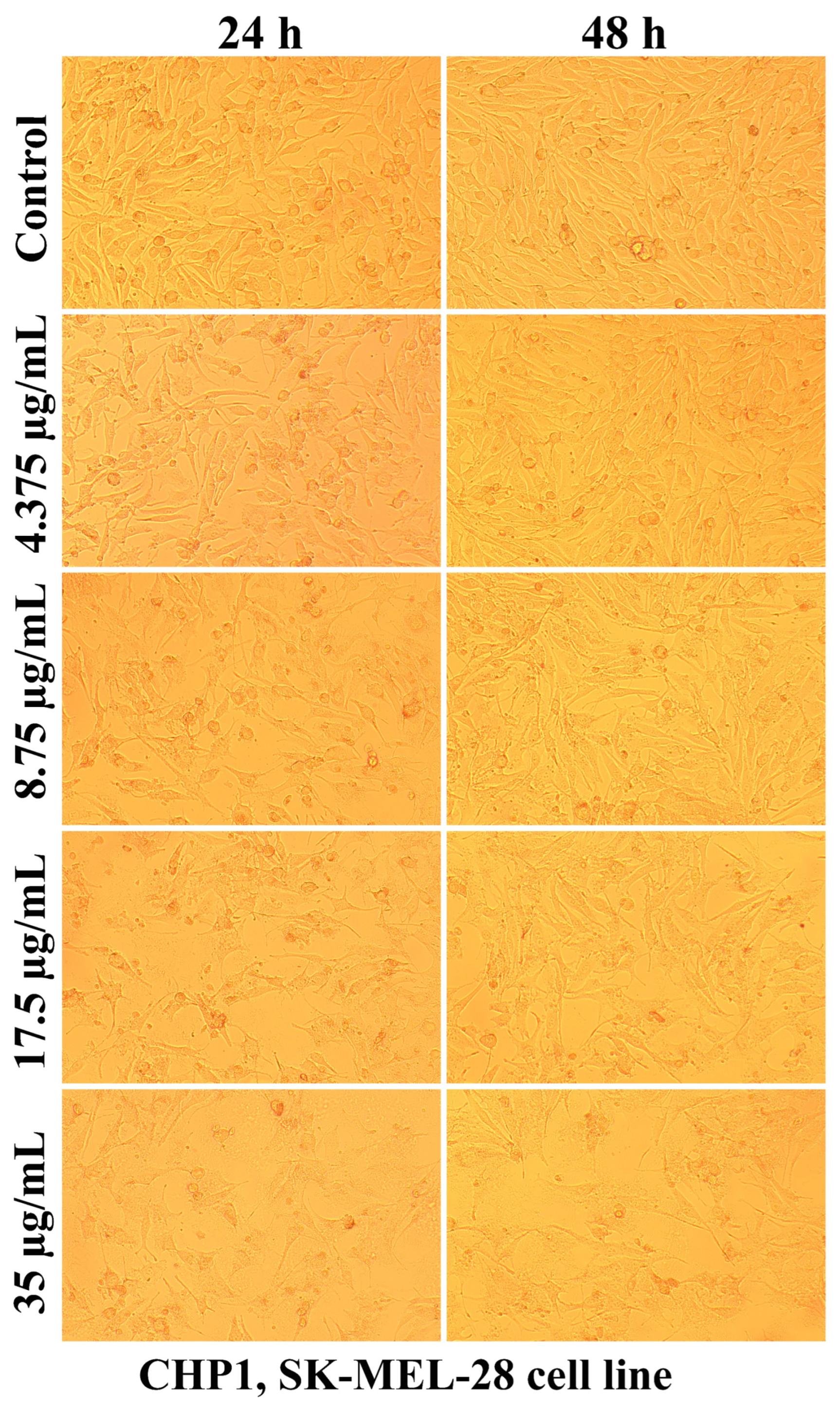
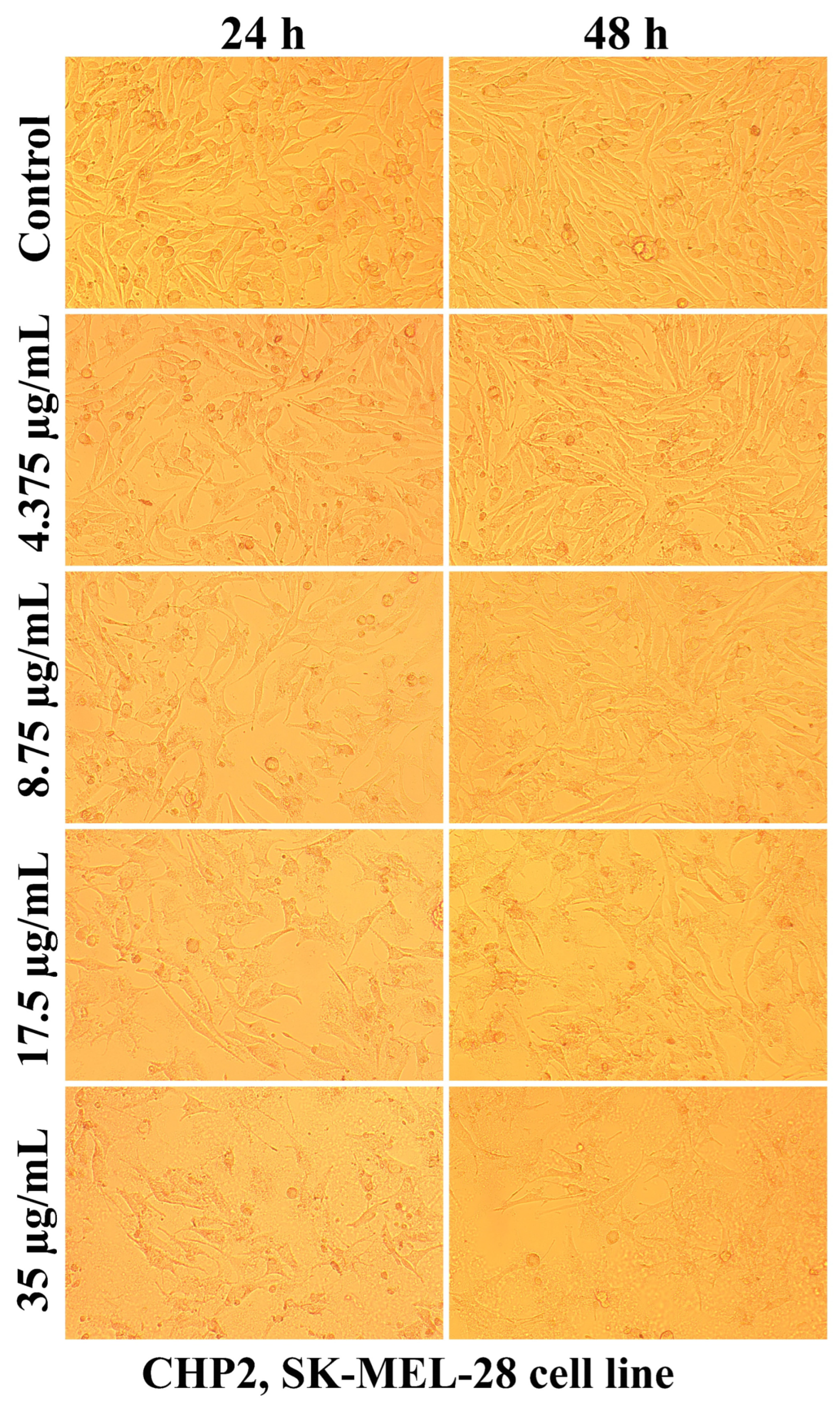

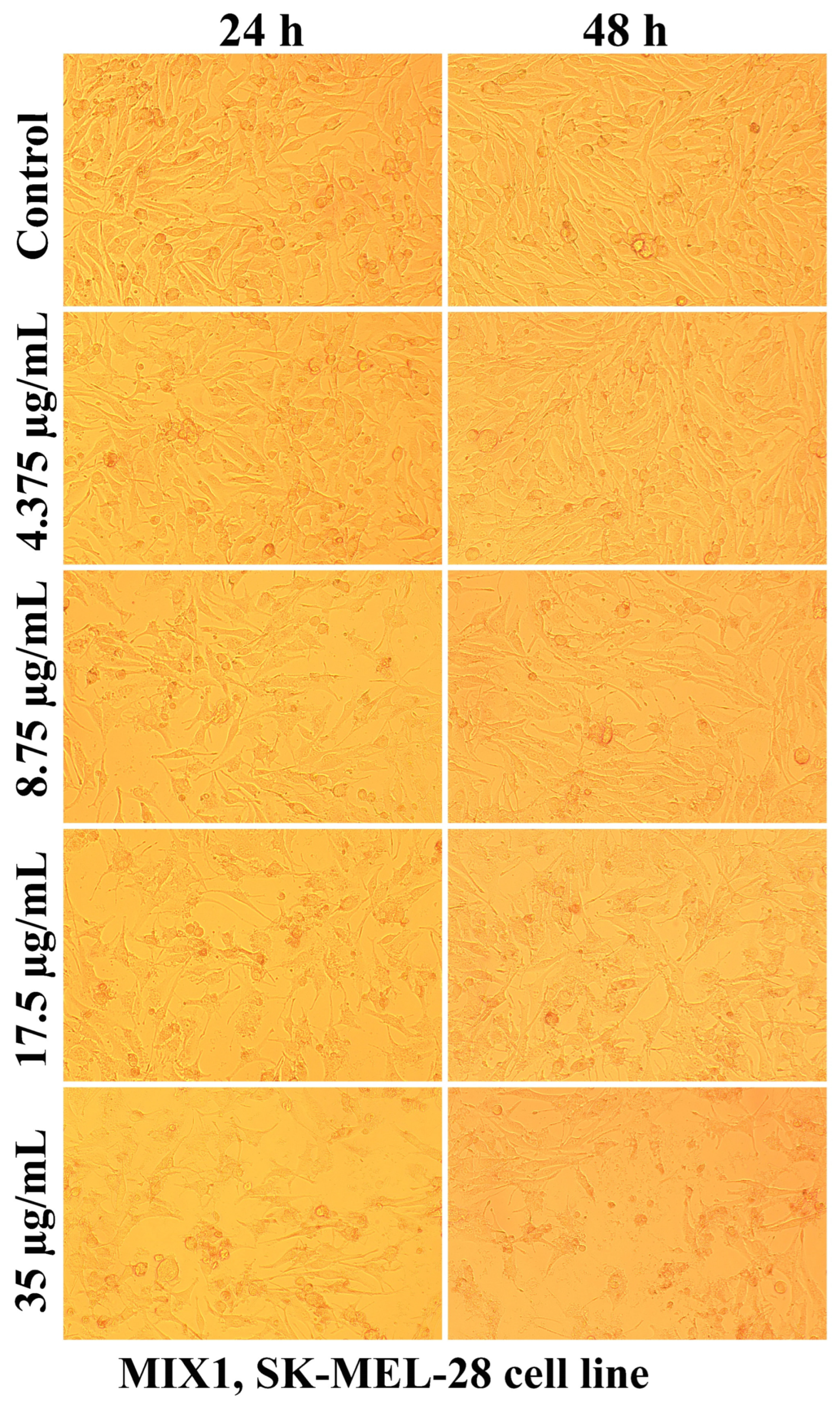
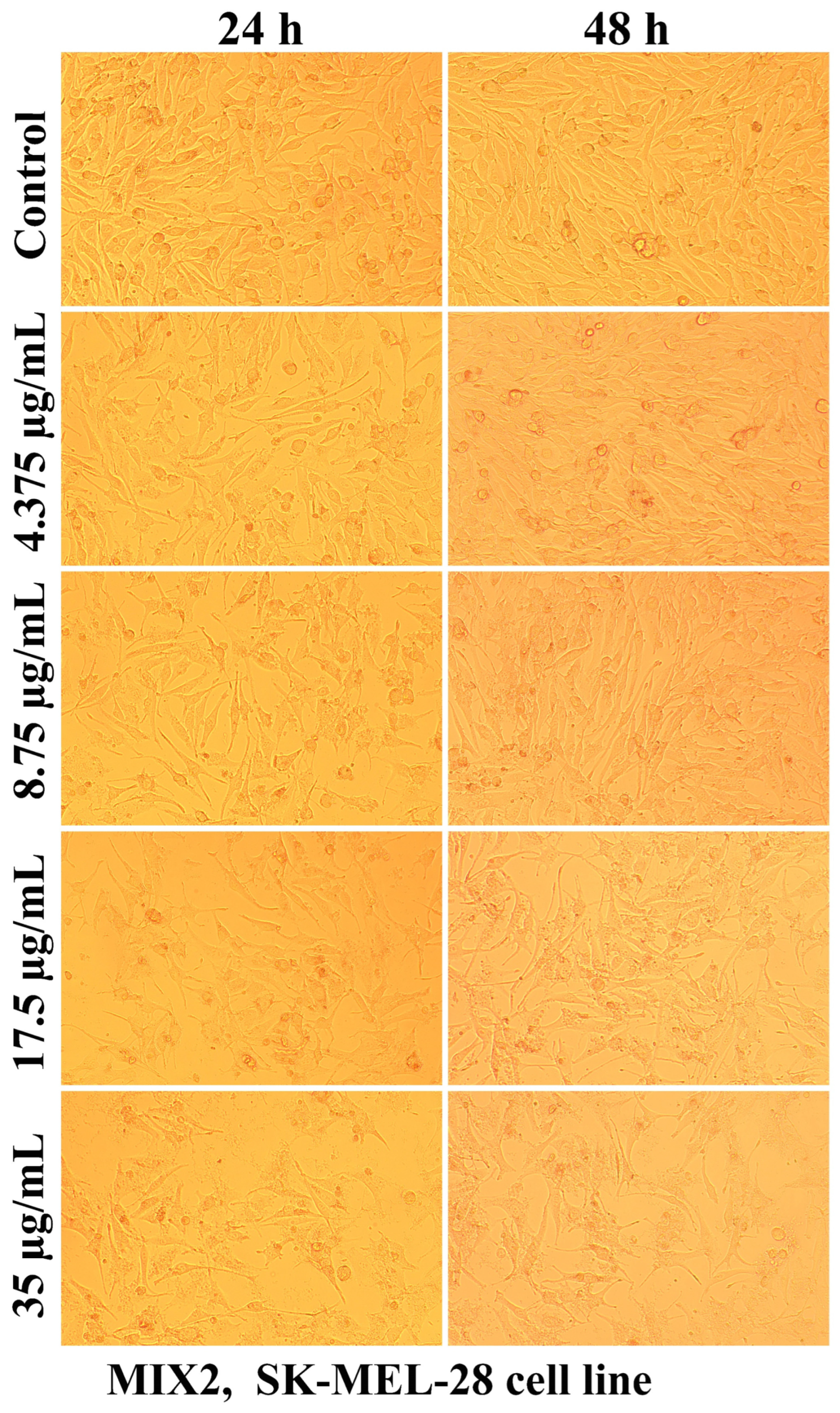
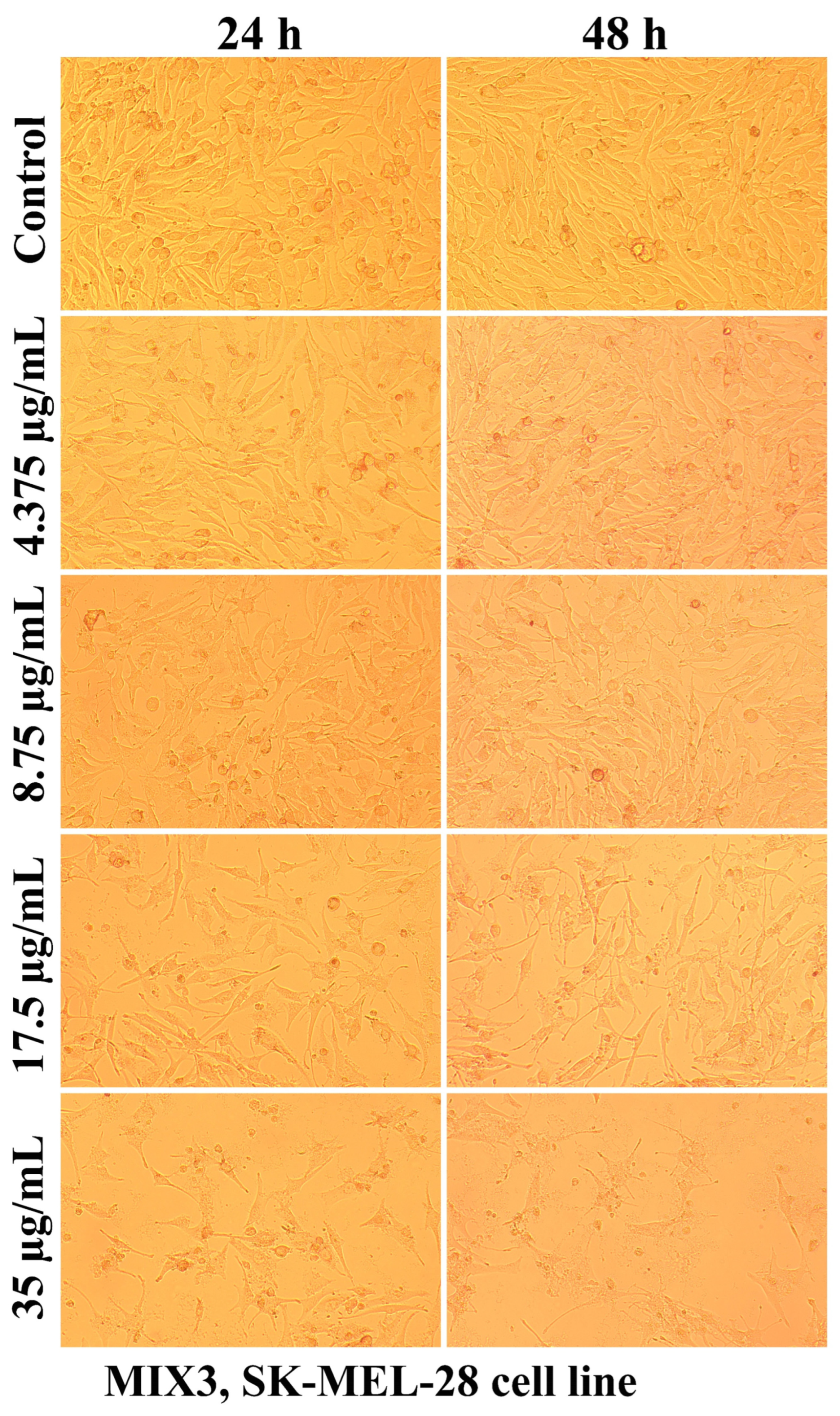
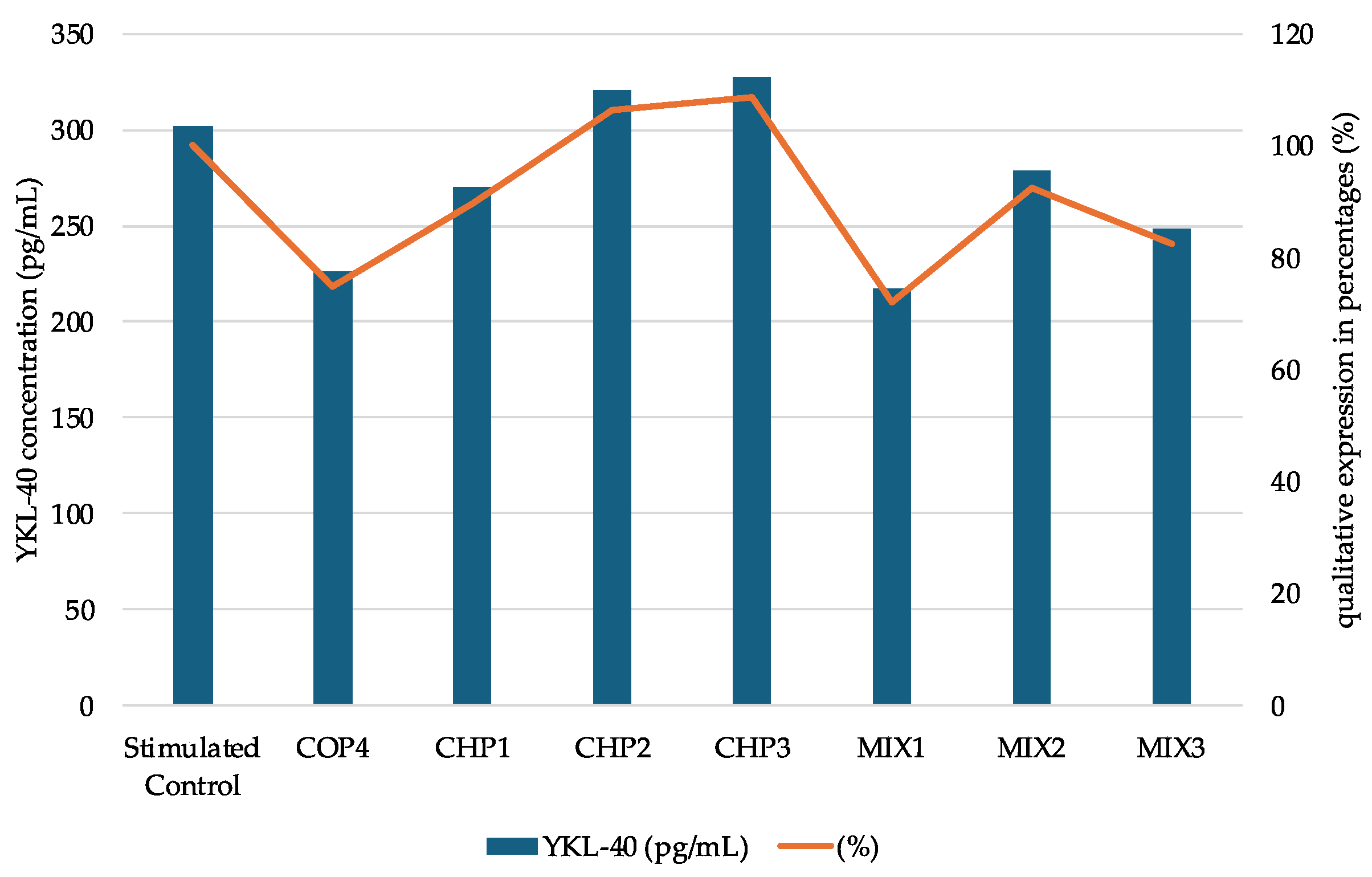

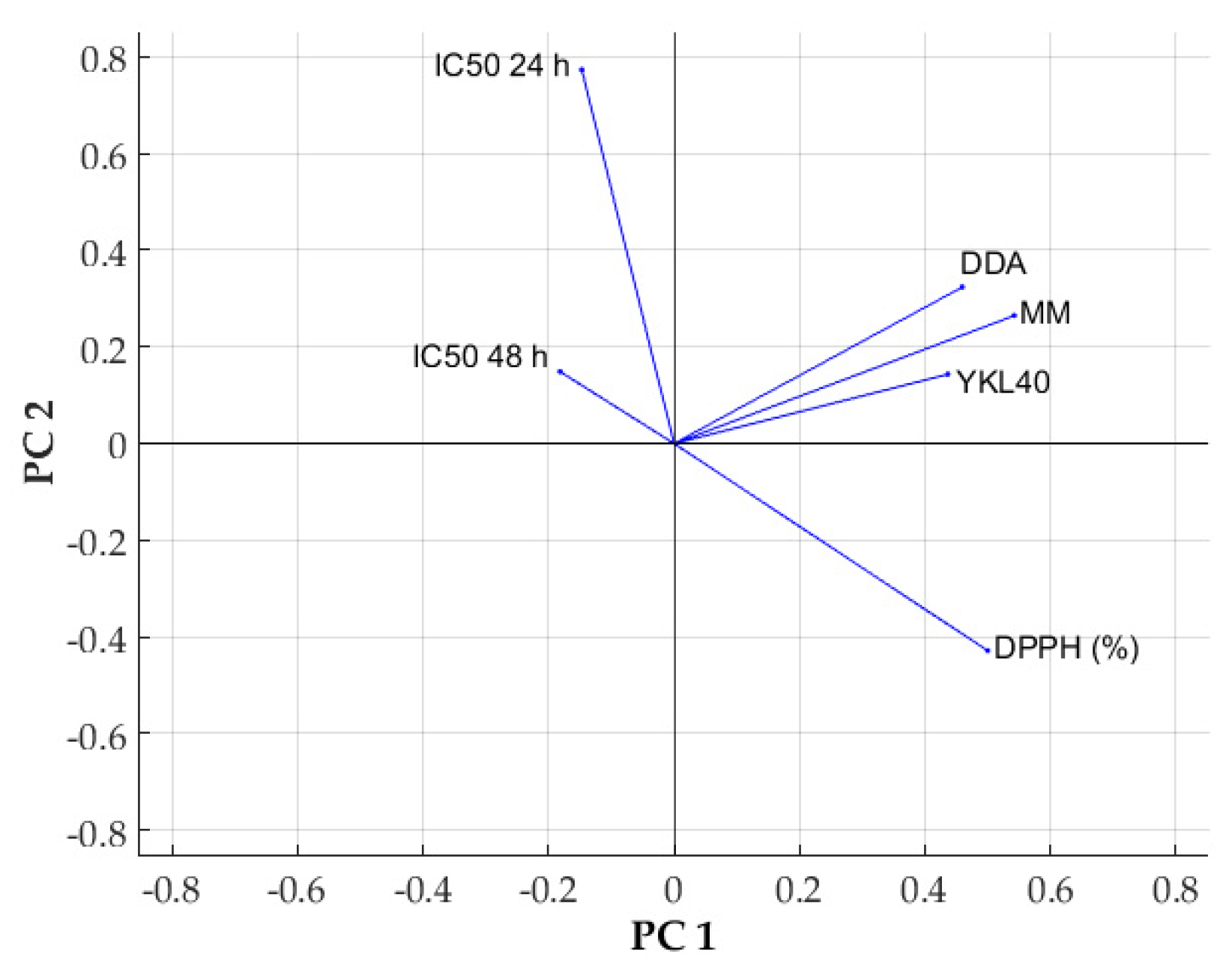
| Sample Code | DDA (%) | MM (kDa) | Chitosan Conformation |
|---|---|---|---|
| CHP1 | 96.27 | 819.99 | α-chitosan |
| CHP2 | 86.65 | 804.33 | α-chitosan |
| CHP3 | 88.50 | 475.43 | α-chitosan |
| COP4 | 70.00 | 1.50 | β-oligochitosan |
| MIX1(mixture CHP1 + COP4) * | 78.76 | 2.25 | α-chitosan and β-oligochitosan (1:2) |
| MIX2 (mixture CHP2 + COP4) * | 75.55 | 2.25 | |
| MIX3 (mixture CHP3 + COP4) * | 76.17 | 2.25 |
| Samples | Antioxidant Activity (%), ± SD |
|---|---|
| CHP1 | 13.28 ± 1.59 |
| CHP2 | 18.79 ± 1.04 |
| CHP3 | 15.15 ± 0.05 |
| COP4 | 11.57 ± 0.11 |
| MIX1 | 14.27 ± 1.81 |
| MIX2 | 10.52 ± 0.16 |
| MIX3 | 11.63 ± 1.37 |
| Samples | IC50 24 h (µg/mL) | IC50 48 h (µg/mL) |
|---|---|---|
| CHP1 | 88.74 | 62.91 |
| CHP2 | 78.45 | 47.01 |
| CHP3 | 73.98 | 64.58 |
| MIX1 | 75.01 | 59.21 |
| MIX2 | 73.40 | 53.06 |
| MIX3 | 75.01 | 64.22 |
| Source of Variation | SS | df | MS | F | p-Value |
|---|---|---|---|---|---|
| Time | 685.58 | 1 | 685.58 | 51.58 | 0 |
| Concentration | 2927.71 | 3 | 975.90 | 73.43 | 0 |
| Polymer | 238.21 | 5 | 47.64 | 3.58 | 0.0248 |
| Time × Concentration | 28.18 | 3 | 9.39 | 0.71 | 0.5628 |
| Time × Polymer | 305.56 | 5 | 61.11 | 4.60 | 0.0096 |
| Concentration × Polymer | 299.70 | 15 | 19.98 | 1.50 | 0.2195 |
| Within | 199.37 | 15 | 13.29 | ||
| Total | 4684.31 | 47 |
| Source of Variation | SS | df | MS | F | p-Value |
|---|---|---|---|---|---|
| Polymers | 284.46 | 5 | 56.89 | 3.69 | 0.0066 |
| Concentration | 3065.01 | 3 | 1021.67 | 66.31 | 0.0000 |
| Interaction | 389.28 | 15 | 25.95 | 1.68 | 0.0867 |
| Within | 739.55 | 48 | 15.41 | ||
| Total | 4478.30 | 71 |
| (a) | Source of Variation | SS | df | MS | F | p-Value |
| Between Groups | 936.24 | 5 | 187.2 | 7.91 | 0.00167 | |
| Within Groups | 284.03 | 12 | 23.67 | |||
| Total | 1220.26 | 17 | ||||
| (b) | Source of Variation | SS | df | MS | F | p-Value |
| Between Groups | 616.52 | 5 | 123.3 | 6.52 | 0.00375 | |
| Within Groups | 226.89 | 12 | 18.91 | |||
| Total | 843.41 | 17 | ||||
| (c) | Source of Variation | SS | df | MS | F | p-Value |
| Between Groups | 198.82 | 5 | 39.76 | 2.15 | 0.12908 | |
| Within Groups | 222.20 | 12 | 18.52 | |||
| Total | 421.03 | 17 | ||||
| (d) | Source of Variation | SS | df | MS | F | p-Value |
| Between Groups | 226.83 | 5 | 45.37 | 2.17 | 0.12598 | |
| Within Groups | 250.80 | 12 | 20.9 | |||
| Total | 477.63 | 17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schröder, V.; Gherghel, D.; Apetroaei, M.R.; Gîjiu, C.L.; Isopescu, R.; Dinculescu, D.; Apetroaei, M.-M.; Enache, L.E.; Mihai, C.-T.; Rău, I.; et al. α-Chitosan and β-Oligochitosan Mixtures-Based Formula for In Vitro Assessment of Melanocyte Cells Response. Int. J. Mol. Sci. 2024, 25, 6768. https://doi.org/10.3390/ijms25126768
Schröder V, Gherghel D, Apetroaei MR, Gîjiu CL, Isopescu R, Dinculescu D, Apetroaei M-M, Enache LE, Mihai C-T, Rău I, et al. α-Chitosan and β-Oligochitosan Mixtures-Based Formula for In Vitro Assessment of Melanocyte Cells Response. International Journal of Molecular Sciences. 2024; 25(12):6768. https://doi.org/10.3390/ijms25126768
Chicago/Turabian StyleSchröder, Verginica, Daniela Gherghel, Manuela Rossemary Apetroaei, Cristiana Luminița Gîjiu, Raluca Isopescu, Daniel Dinculescu, Miruna-Maria Apetroaei, Laura Elena Enache, Cosmin-Teodor Mihai, Ileana Rău, and et al. 2024. "α-Chitosan and β-Oligochitosan Mixtures-Based Formula for In Vitro Assessment of Melanocyte Cells Response" International Journal of Molecular Sciences 25, no. 12: 6768. https://doi.org/10.3390/ijms25126768
APA StyleSchröder, V., Gherghel, D., Apetroaei, M. R., Gîjiu, C. L., Isopescu, R., Dinculescu, D., Apetroaei, M.-M., Enache, L. E., Mihai, C.-T., Rău, I., & Vochița, G. (2024). α-Chitosan and β-Oligochitosan Mixtures-Based Formula for In Vitro Assessment of Melanocyte Cells Response. International Journal of Molecular Sciences, 25(12), 6768. https://doi.org/10.3390/ijms25126768










