TNF-α/Stearate Induced H3K9/18 Histone Acetylation Amplifies IL-6 Expression in 3T3-L1 Mouse Adipocytes
Abstract
1. Introduction
2. Results
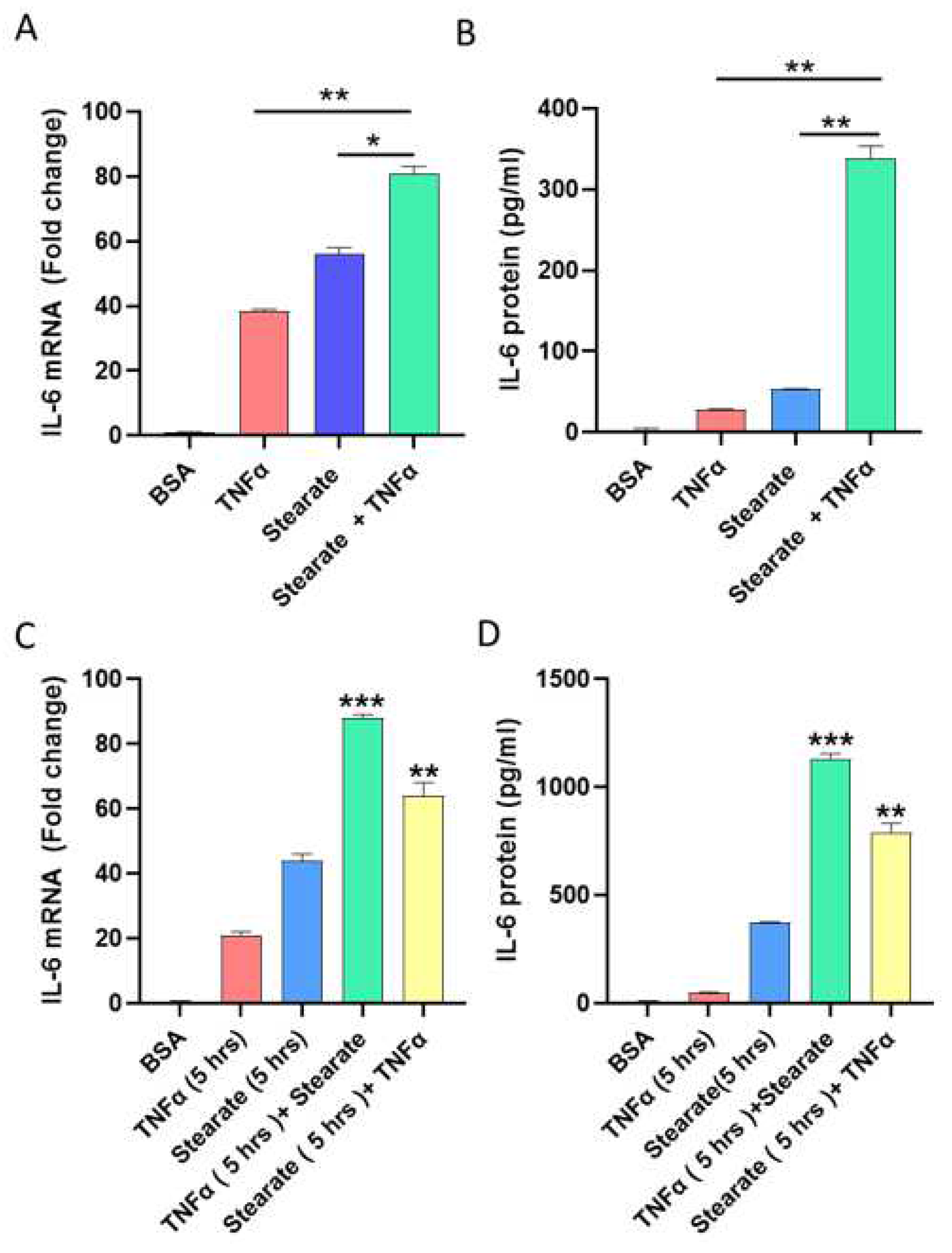
2.1. TNF-α and Stearate Act Synergistically to Enhance the IL-6 Gene Expression and Protein Production by Adipocytes
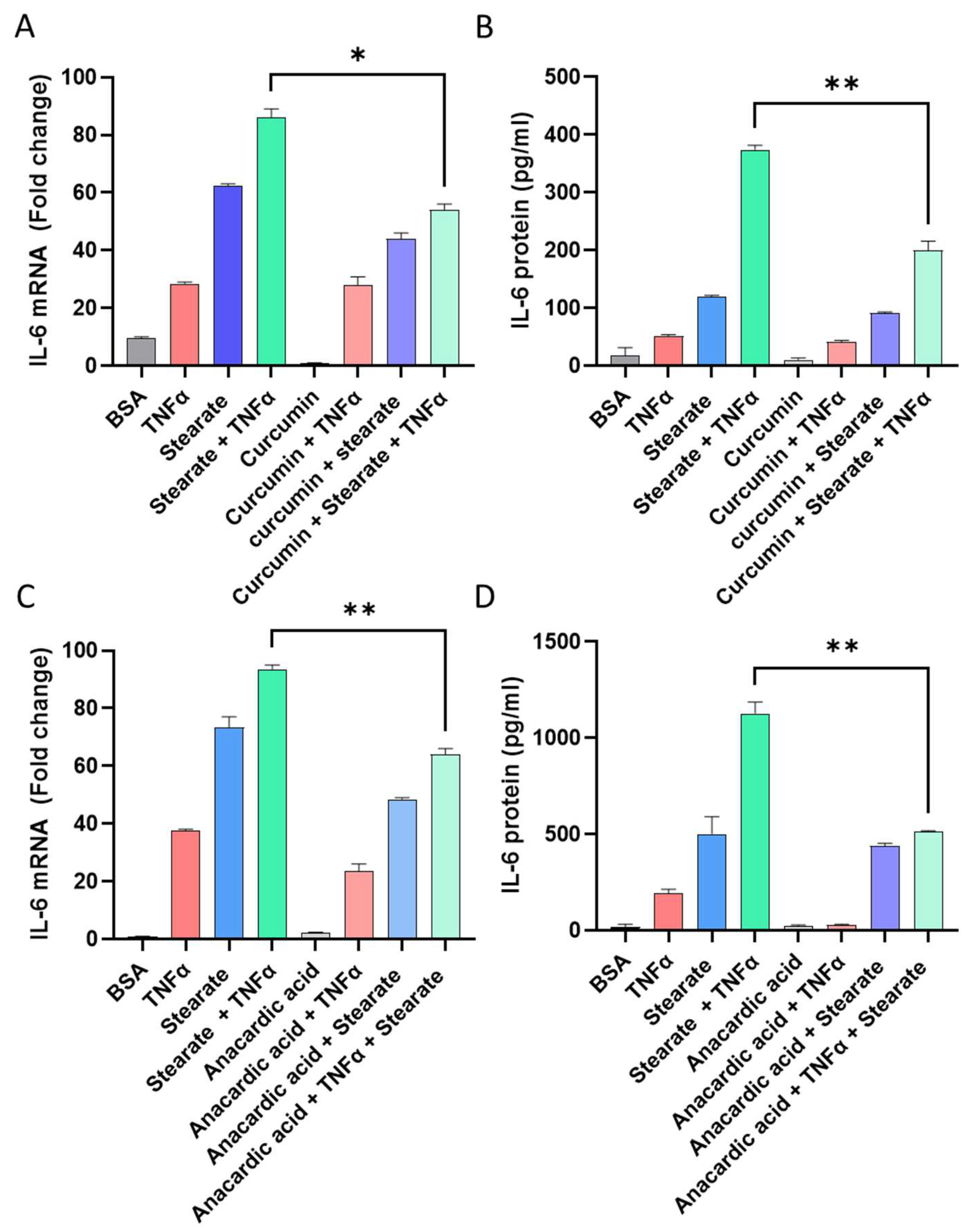
2.2. Inhibiting Acetylation Reduces the Synergistic Impact of TNF-α/Stearate Co-Stimulation on IL-6 Gene Expression and Protein Production
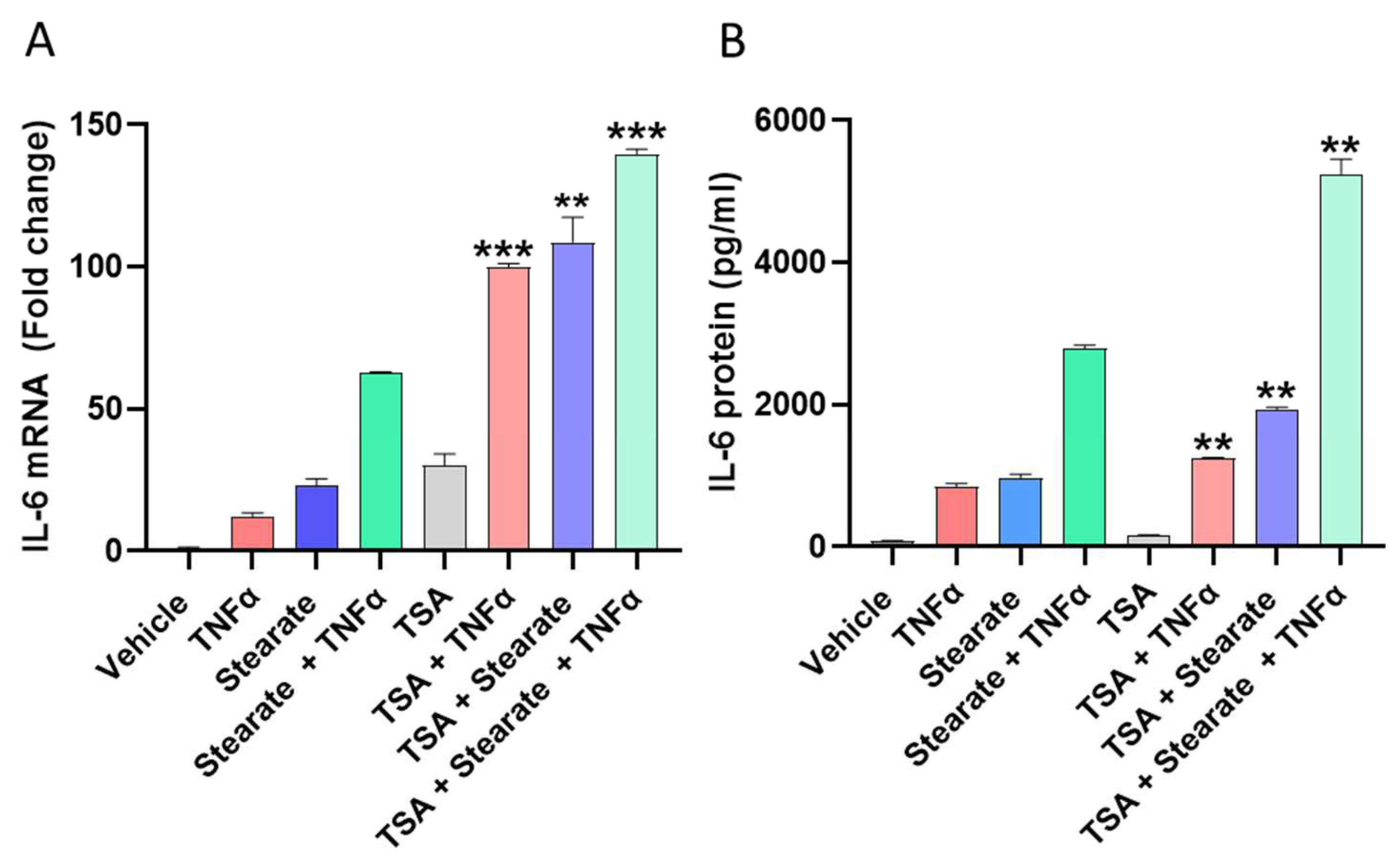
2.3. Pharmacological Induced Acetylation Substitutes the Effect of TNF-α or Stearate in the Synergistic Interaction between TNF-α and Stearate for Promoting IL-6 Gene Expression and Protein Production
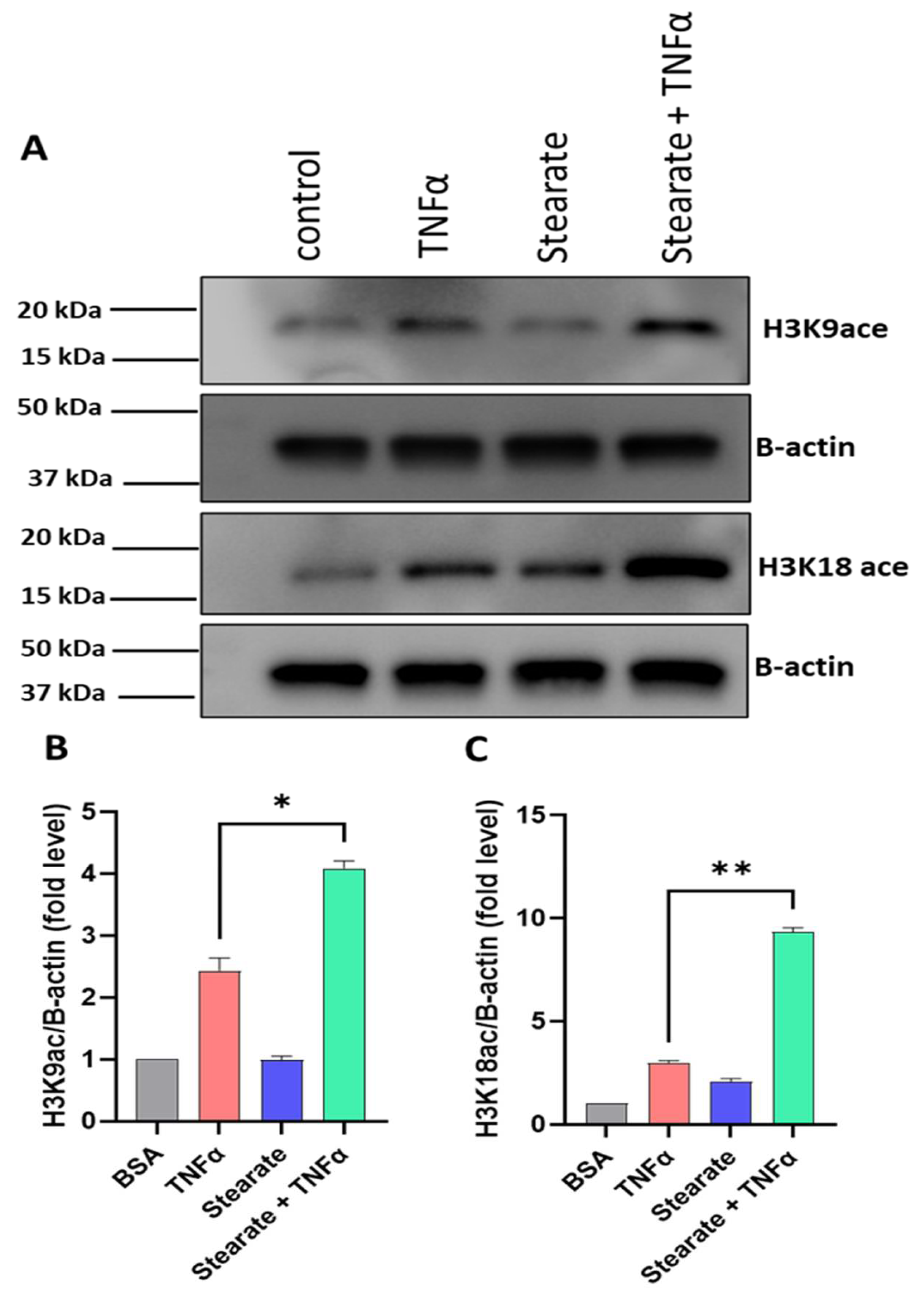
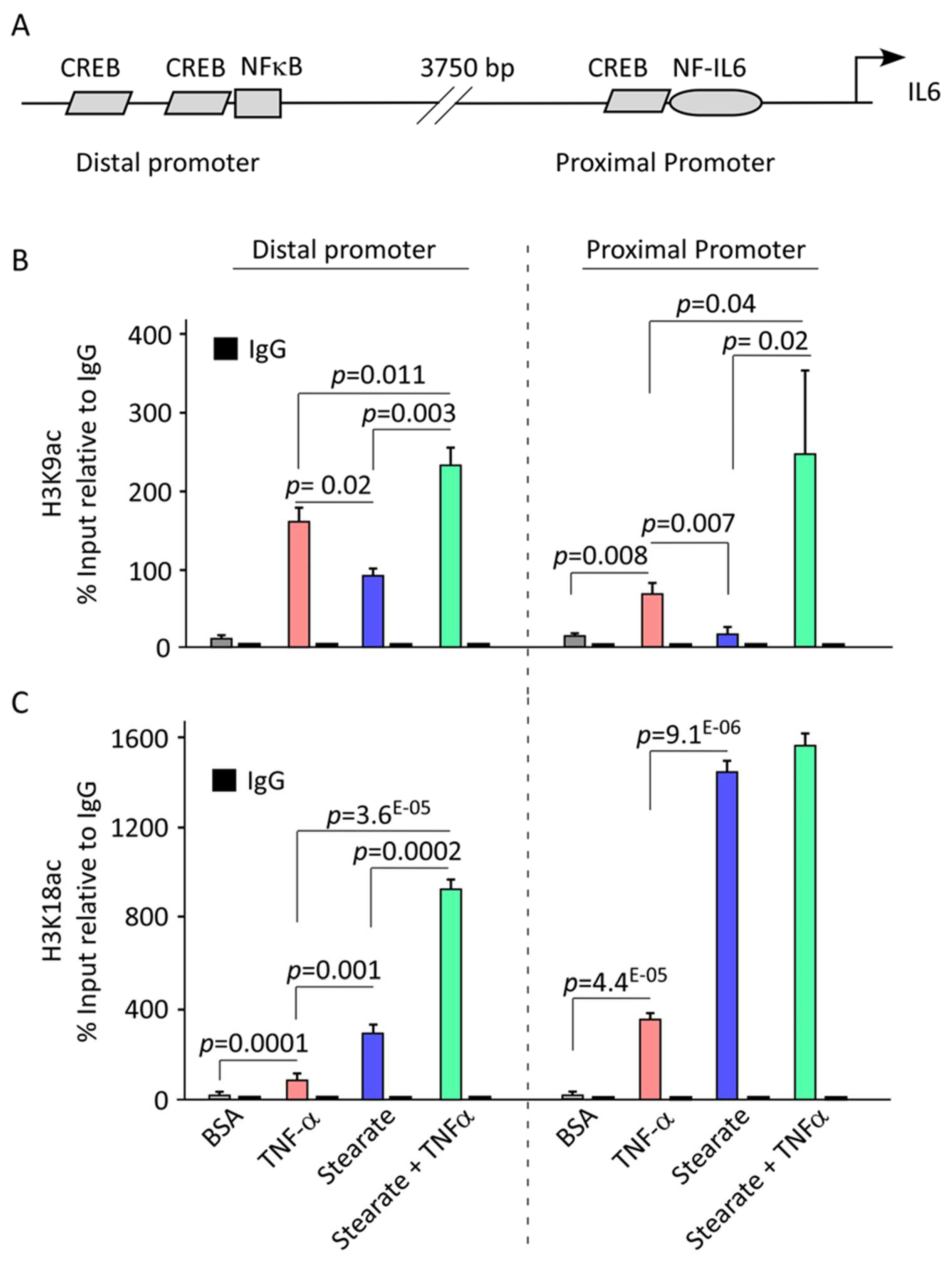
2.4. TNF-α/Stearate Co-Stimulation Increases Il6 Promoter H3K9 and H3K18 Acetylation in 3T3-L1 Adipocytes
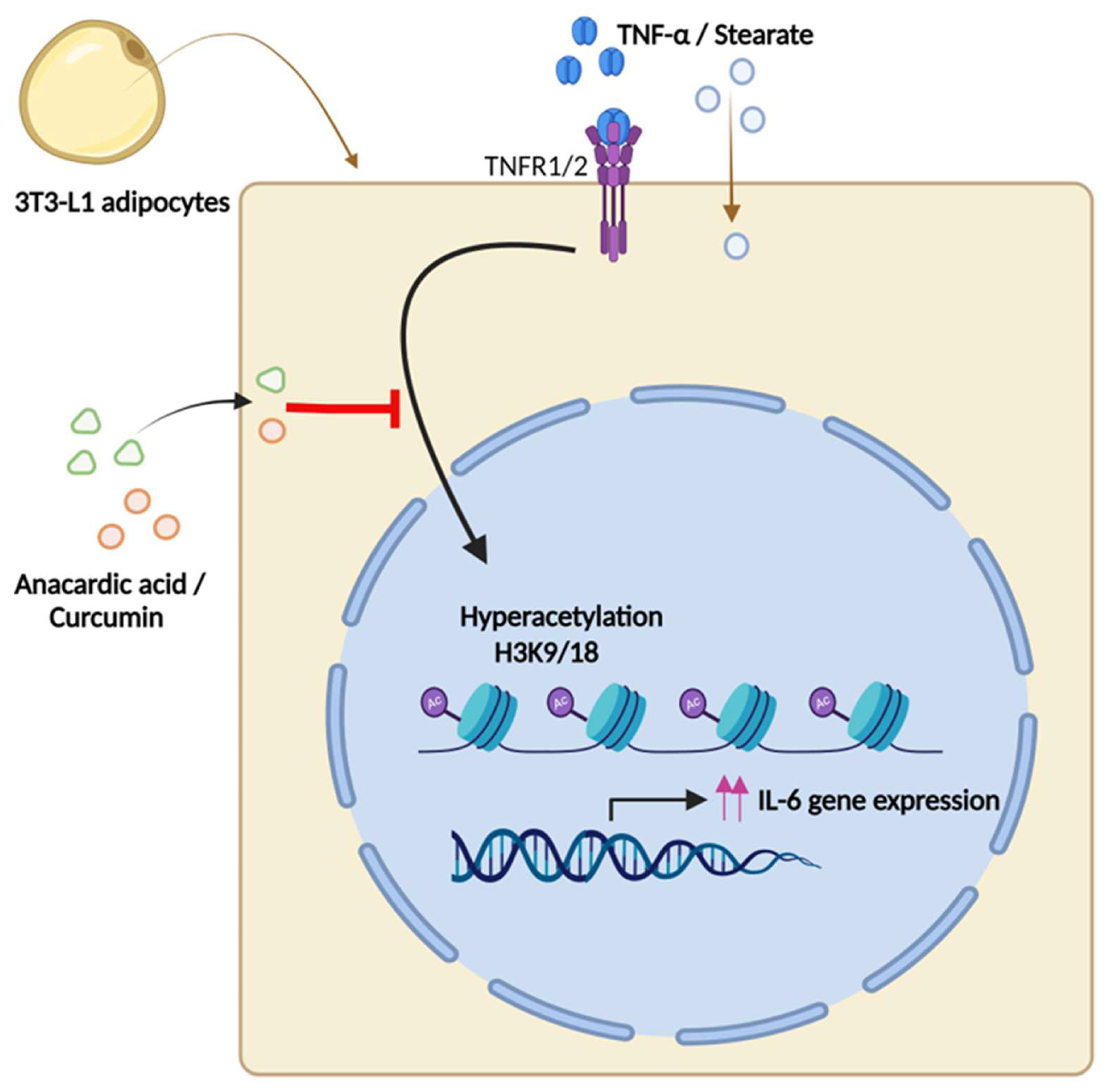
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Real-Time Quantitative RT-PCR (qPCR)
4.3. ELISA
4.4. Chromatin Immunoprecipitation (ChIP) Assay
4.5. Western Blotting
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jin, X.; Qiu, T.; Li, L.; Yu, R.; Chen, X.; Li, C.; Proud, C.G.; Jiang, T. Pathophysiology of obesity and its associated diseases. Acta Pharm. Sin. B 2023, 13, 2403–2424. [Google Scholar] [CrossRef]
- Donath, M.Y.; Shoelson, S.E. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 2011, 11, 98–107. [Google Scholar] [CrossRef]
- Guzik, T.J.; Skiba, D.S.; Touyz, R.M.; Harrison, D.G. The role of infiltrating immune cells in dysfunctional adipose tissue. Cardiovasc. Res. 2017, 113, 1009–1023. [Google Scholar] [CrossRef]
- Sindhu, S.; Thomas, R.; Shihab, P.; Sriraman, D.; Behbehani, K.; Ahmad, R. Obesity is a positive modulator of Il-6r and Il-6 expression in the subcutaneous adipose tissue: Significance for metabolic inflammation. PLoS ONE 2015, 10, e0133494. [Google Scholar] [CrossRef]
- Bastard, J.-P.; Maachi, M.; Van Nhieu, J.T.; Jardel, C.; Bruckert, E.; Grimaldi, A.; Robert, J.-J.; Capeau, J.; Hainque, B. Adipose tissue il-6 content correlates with resistance to insulin activation of glucose uptake both in vivo and in vitro. J. Clin. Endocrinol. Metab. 2002, 87, 2084–2089. [Google Scholar] [CrossRef]
- Kanemaki, T.; Kitade, H.; Kaibori, M.; Sakitani, K.; Hiramatsu, Y.; Kamiyama, Y.; Ito, S.; Okumura, T. Interleukin 1β and interleukin 6, but not tumor necrosis factor α, inhibit insulin-stimulated glycogen synthesis in rat hepatocytes. Hepatology 1998, 27, 1296–1303. [Google Scholar] [CrossRef]
- Ghanim, H.; Aljada, A.; Hofmeyer, D.; Syed, T.; Mohanty, P.; Dandona, P. Circulating mononuclear cells in the obese are in a proinflammatory state. Circulation 2004, 110, 1564–1571. [Google Scholar] [CrossRef]
- Tripathy, D.; Mohanty, P.; Dhindsa, S.; Syed, T.; Ghanim, H.; Aljada, A.; Dandona, P. Elevation of free fatty acids induces inflammation and impairs vascular reactivity in healthy subjects. Diabetes 2003, 52, 2882–2887. [Google Scholar] [CrossRef]
- Sampath, H.; Ntambi, J.M. The fate and intermediary metabolism of stearic acid. Lipids 2005, 40, 1187–1191. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.K.; Hill, A.A.; Hasty, A.H. Stearic acid accumulation in macrophages induces toll-like receptor 4/2-independent inflammation leading to endoplasmic reticulum stress–mediated apoptosis. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1687–1695. [Google Scholar] [CrossRef] [PubMed]
- Bunn, R.C.; Cockrell, G.E.; Ou, Y.; Thrailkill, K.M.; Lumpkin, C.K.; Fowlkes, J.L. Palmitate and insulin synergistically induce il-6 expression in human monocytes. Cardiovasc. Diabetol. 2010, 9, 73. [Google Scholar] [CrossRef]
- Kochumon, S.; Arefanian, H.; Azim, R.; Shenouda, S.; Jacob, T.; Abu Khalaf, N.; Al-Rashed, F.; Hasan, A.; Sindhu, S.; Al-Mulla, F.; et al. Stearic Acid and TNF-α Co-Operatively Potentiate MIP-1α Production in Monocytic Cells via MyD88 Independent TLR4/TBK/IRF3 Signaling Pathway. Biomedicines 2020, 8, 403. [Google Scholar] [CrossRef] [PubMed]
- Qiu, T.; Yang, X.; Wang, J.; Pan, C.; Chu, X.; Xiong, J.; Xie, J.; Chang, Y.; Wang, C.; Zhang, J. Obesity-induced elevated palmitic acid promotes inflammation and glucose metabolism disorders through GPRs/NF-κB/KLF7 pathway. Nutr. Diabetes 2022, 12, 23. [Google Scholar] [CrossRef] [PubMed]
- Sindhu, S.; Al-Roub, A.; Koshy, M.; Thomas, R.; Ahmad, R. Palmitate-induced MMP-9 expression in the human monocytic cells is mediated through the TLR4-MyD88 dependent mechanism. Cell. Physiol. Biochem. 2016, 39, 889–900. [Google Scholar] [CrossRef]
- Korbecki, J.; Bajdak-Rusinek, K. The effect of palmitic acid on inflammatory response in macrophages: An overview of molecular mechanisms. Inflamm. Res. 2019, 68, 915–932. [Google Scholar] [CrossRef]
- Wang, Z.J.; Liang, C.L.; Li, G.M.; Yu, C.Y.; Yin, M. Stearic acid protects primary cultured cortical neurons against oxidative stress. Acta Pharmacol. Sin. 2007, 28, 315–326. [Google Scholar] [CrossRef]
- Pan, P.H.; Lin, S.Y.; Ou, Y.C.; Chen, W.Y.; Chuang, Y.H.; Yen, Y.J.; Liao, S.L.; Raung, S.L.; Chen, C.J. Stearic acid attenuates cholestasis-induced liver injury. Biochem. Biophys. Res. Commun. 2010, 391, 1537–1542. [Google Scholar] [CrossRef] [PubMed]
- Hung, H.-C.; Tsai, S.-F.; Chou, H.-W.; Tsai, M.-J.; Hsu, P.-L.; Kuo, Y.-M. Dietary fatty acids differentially affect secretion of pro-inflammatory cytokines in human THP-1 monocytes. Sci. Rep. 2023, 13, 5511. [Google Scholar] [CrossRef]
- Miao, H.; Chen, L.; Hao, L.; Zhang, X.; Chen, Y.; Ruan, Z.; Liang, H. Stearic acid induces proinflammatory cytokine production partly through activation of lactate-HIF1α pathway in chondrocytes. Sci. Rep. 2015, 5, 13092. [Google Scholar] [CrossRef]
- Guria, S.; Hoory, A.; Das, S.; Chattopadhyay, D.; Mukherjee, S. Adipose tissue macrophages and their role in obesity-associated insulin resistance: An overview of the complex dynamics at play. Biosci. Rep. 2023, 43, BSR20220200. [Google Scholar] [CrossRef]
- Stephens, J.M.; Lee, J.; Pilch, P.F. Tumor Necrosis Factor-α-induced Insulin Resistance in 3T3-L1 Adipocytes Is Accompanied by a Loss of Insulin Receptor Substrate-1 and GLUT4 Expression without a Loss of Insulin Receptor-mediated Signal Transduction. J. Biol. Chem. 1997, 272, 971–976. [Google Scholar] [CrossRef] [PubMed]
- Putiri, E.L.; Robertson, K.D. Epigenetic mechanisms and genome stability. Clin. Epigenetics 2011, 2, 299–314. [Google Scholar] [CrossRef] [PubMed]
- Castellano-Castillo, D.; Ramos-Molina, B.; Cardona, F.; Queipo-Ortuño, M.I. Epigenetic regulation of white adipose tissue in the onset of obesity and metabolic diseases. Obes. Rev. 2020, 21, e13054. [Google Scholar] [CrossRef] [PubMed]
- Campión, J.; Milagro, F.; Martínez, J.A. Epigenetics and obesity. Prog. Mol. Biol. Transl. Sci. 2010, 94, 291–347. [Google Scholar] [PubMed]
- Suárez, R.; Chapela, S.P.; Álvarez-Córdova, L.; Bautista-Valarezo, E.; Sarmiento-Andrade, Y.; Verde, L.; Frias-Toral, E.; Sarno, G. Epigenetics in obesity and diabetes mellitus: New insights. Nutrients 2023, 15, 811. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Yeung, M.H.Y.; Yau, M.Y.C.; Lui, P.P.Y.; Wong, C.-M. Role of histone acetylation and methylation in obesity. Curr. Pharmacol. Rep. 2019, 5, 196–203. [Google Scholar] [CrossRef]
- Jung, B.C.; Kang, S. Epigenetic regulation of inflammatory factors in adipose tissue. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2021, 1866, 159019. [Google Scholar] [CrossRef]
- Klimczak, S.; Śliwińska, A. Epigenetic regulation of inflammation in insulin resistance. Semin. Cell Dev. Biol. 2022, 154, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Mikula, M.; Majewska, A.; Ledwon, J.K.; Dzwonek, A.; Ostrowski, J. Obesity increases histone H3 lysine 9 and 18 acetylation at Tnfa and Ccl2 genes in mouse liver. Int. J. Mol. Med. 2014, 34, 1647–1654. [Google Scholar] [CrossRef]
- Małodobra-Mazur, M.; Cierzniak, A.; Myszczyszyn, A.; Kaliszewski, K.; Dobosz, T. Histone modifications influence the insulin-signaling genes and are related to insulin resistance in human adipocytes. Int. J. Biochem. Cell Biol. 2021, 137, 106031. [Google Scholar] [CrossRef]
- Castellano-Castillo, D.; Denechaud, P.-D.; Fajas, L.; Moreno-Indias, I.; Oliva-Olivera, W.; Tinahones, F.; Queipo-Ortuño, M.I.; Cardona, F. Human adipose tissue H3K4me3 histone mark in adipogenic, lipid metabolism and inflammatory genes is positively associated with BMI and HOMA-IR. PLoS ONE 2019, 14, e0215083. [Google Scholar] [CrossRef] [PubMed]
- Al-Roub, A.; Al Madhoun, A.; Akhter, N.; Thomas, R.; Miranda, L.; Jacob, T.; Al-Ozairi, E.; Al-Mulla, F.; Sindhu, S.; Ahmad, R. IL-1β and TNFα cooperativity in regulating IL-6 expression in adipocytes depends on CREB binding and H3K14 acetylationacetylation. Cells 2021, 10, 3228. [Google Scholar] [CrossRef] [PubMed]
- Kochumon, S.; Jacob, T.; Koshy, M.; Al-Rashed, F.; Sindhu, S.; Al-Ozairi, E.; Al-Mulla, F.; Rosen, E.D.; Ahmad, R. Palmitate potentiates lipopolysaccharide-induced il-6 production via coordinated acetylation of h3k9/h3k18, p300, and rna polymerase ii. J. Immunol. 2022, 209, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Eliseeva, E.D.; Valkov, V.; Jung, M.; Jung, M.O. Characterization of novel inhibitors of histone acetyltransferases. Mol. Cancer Ther. 2007, 6, 2391–2398. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; Gupta, S.C.; Park, B.; Goel, A.; Aggarwal, B.B. Epigenetic changes induced by curcumin and other natural compounds. Genes Nutr. 2011, 6, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xie, H.; Tang, W.; Zeng, X.; Lin, Y.; Xu, L.; Xiao, L.; Xu, J.; Wu, Z.; Yuan, D. Trichostatin a, a histone deacetylase inhibitor, alleviates eosinophilic meningitis induced by angiostrongylus cantonensis infection in mice. Front. Microbiol. 2019, 10, 2280. [Google Scholar] [CrossRef]
- Večeřa, J.; Bártová, E.; Krejčí, J.; Legartová, S.; Komůrková, D.; Rudá-Kučerová, J.; Štark, T.; Dražanová, E.; Kašpárek, T.; Šulcová, A.; et al. HDAC1 and HDAC3 underlie dynamic H3K9 acetylation during embryonic neurogenesis and in schizophrenia-like animals. J. Cell. Physiol. 2018, 233, 530–548. [Google Scholar] [CrossRef]
- Peng, B.; Peng, C.; Luo, X.; Wu, S.; Mao, Q.; Zhang, H.; Han, X. JNK signaling-dependent regulation of histone acetylation are involved in anacardic acid alleviates cardiomyocyte hypertrophy induced by phenylephrine. PLoS ONE 2021, 16, e0261388. [Google Scholar] [CrossRef]
- Caron-Jobin, M.; Mauvoisin, D.; Michaud, A.; Veilleux, A.; Noël, S.; Fortier, M.P.; Julien, P.; Tchernof, A.; Mounier, C. Stearic acid content of abdominal adipose tissues in obese women. Nutr. Diabetes 2012, 2, e23. [Google Scholar] [CrossRef]
- Bi, X.; Yeo, P.L.Q.; Loo, Y.T.; Henry, C.J. Associations between circulating fatty acid levels and metabolic risk factors. J. Nutr. Intermed. Metab. 2019, 15, 65–69. [Google Scholar] [CrossRef]
- De Cesaris, P.; Starace, D.; Riccioli, A.; Padula, F.; Filippini, A.; Ziparo, E. Tumor necrosis factor-alpha induces interleukin-6 production and integrin ligand expression by distinct transduction pathways. J. Biol. Chem. 1998, 273, 7566–7571. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, K.; Matsushima-Nishiwaki, R.; Yamaguchi, S.; Iida, H.; Dohi, S.; Kozawa, O. Mechanisms of tumor necrosis factor-alpha-induced interleukin-6 synthesis in glioma cells. J. Neuroinflammation 2010, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Confalone, E.; D’Alessio, G.; Furia, A. Il-6 induction by tnfα and il-1β in an osteoblast-like cell line. Int. J. Biomed. Sci. IJBS 2010, 6, 135–140. [Google Scholar] [PubMed]
- Sterner, D.E.; Berger, S.L. Acetylation of histones and transcription-related factors. Microbiol. Mol. Biol. Rev. 2000, 64, 435–459. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.P.; Zhao, Y.T.; Zhao, T.C. Histone deacetylases and mechanisms of regulation of gene expression. Crit. Rev. Oncog. 2015, 20, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Samuel, J.M.; Kelberman, D.; Smith, A.J.P.; Humphries, S.E.; Woo, P. Identification of a novel regulatory region in the inter-leukin-6 gene promoter. Cytokine 2008, 42, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.; Al-Rashed, F.; Akhter, N.; Al-Mulla, F.; Ahmad, R. Acsl1 regulates tnfα-induced gm-csf production by breast cancer mda-mb-231 cells. Biomolecules 2019, 9, 555. [Google Scholar] [CrossRef] [PubMed]
- Al Madhoun, A.; Haddad, D.; Al Tarrah, M.; Jacob, S.; Al-Ali, W.; Nizam, R.; Miranda, L.; Al-Rashed, F.; Sindhu, S.; Ahmad, R.; et al. Microarray analysis reveals onc201 mediated differential mechanisms of chop gene regulation in metastatic and nonmetastatic colorectal cancer cells. Sci. Rep. 2021, 11, 11893. [Google Scholar] [CrossRef]
- Al Madhoun, A.S.; Voronova, A.; Ryan, T.; Zakariyah, A.; McIntire, C.; Gibson, L.; Shelton, M.; Ruel, M.; Skerjanc, I.S. Testosterone enhances cardiomyogenesis in stem cells and recruits the androgen receptor to the mef2c and hcn4 genes. J. Mol. Cell. Cardiol. 2013, 60, 164–171. [Google Scholar] [CrossRef]
| No | Primer | Sequence |
|---|---|---|
| 1 | IL6-distal-F | AACTGATAAAAAGGAAGGGAGGT |
| 2 | IL6-distal-R | CCTCTCTCTGTGGGGTTGAT |
| 3 | IL6-proximal-F | TAGGGCTAGCCTCAAGGATG |
| 4 | IL6-proximal-R | AGGAAGGGGAAAGTGTGCTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bahman, F.; Al-Roub, A.; Akhter, N.; Al Madhoun, A.; Wilson, A.; Almansour, N.; Al-Rashed, F.; Sindhu, S.; Al-Mulla, F.; Ahmad, R. TNF-α/Stearate Induced H3K9/18 Histone Acetylation Amplifies IL-6 Expression in 3T3-L1 Mouse Adipocytes. Int. J. Mol. Sci. 2024, 25, 6776. https://doi.org/10.3390/ijms25126776
Bahman F, Al-Roub A, Akhter N, Al Madhoun A, Wilson A, Almansour N, Al-Rashed F, Sindhu S, Al-Mulla F, Ahmad R. TNF-α/Stearate Induced H3K9/18 Histone Acetylation Amplifies IL-6 Expression in 3T3-L1 Mouse Adipocytes. International Journal of Molecular Sciences. 2024; 25(12):6776. https://doi.org/10.3390/ijms25126776
Chicago/Turabian StyleBahman, Fatemah, Areej Al-Roub, Nadeem Akhter, Ashraf Al Madhoun, Ajit Wilson, Nourah Almansour, Fatema Al-Rashed, Sardar Sindhu, Fahd Al-Mulla, and Rasheed Ahmad. 2024. "TNF-α/Stearate Induced H3K9/18 Histone Acetylation Amplifies IL-6 Expression in 3T3-L1 Mouse Adipocytes" International Journal of Molecular Sciences 25, no. 12: 6776. https://doi.org/10.3390/ijms25126776
APA StyleBahman, F., Al-Roub, A., Akhter, N., Al Madhoun, A., Wilson, A., Almansour, N., Al-Rashed, F., Sindhu, S., Al-Mulla, F., & Ahmad, R. (2024). TNF-α/Stearate Induced H3K9/18 Histone Acetylation Amplifies IL-6 Expression in 3T3-L1 Mouse Adipocytes. International Journal of Molecular Sciences, 25(12), 6776. https://doi.org/10.3390/ijms25126776











