Pivotal Role of mTOR in Non-Skin Manifestations of Psoriasis
Abstract
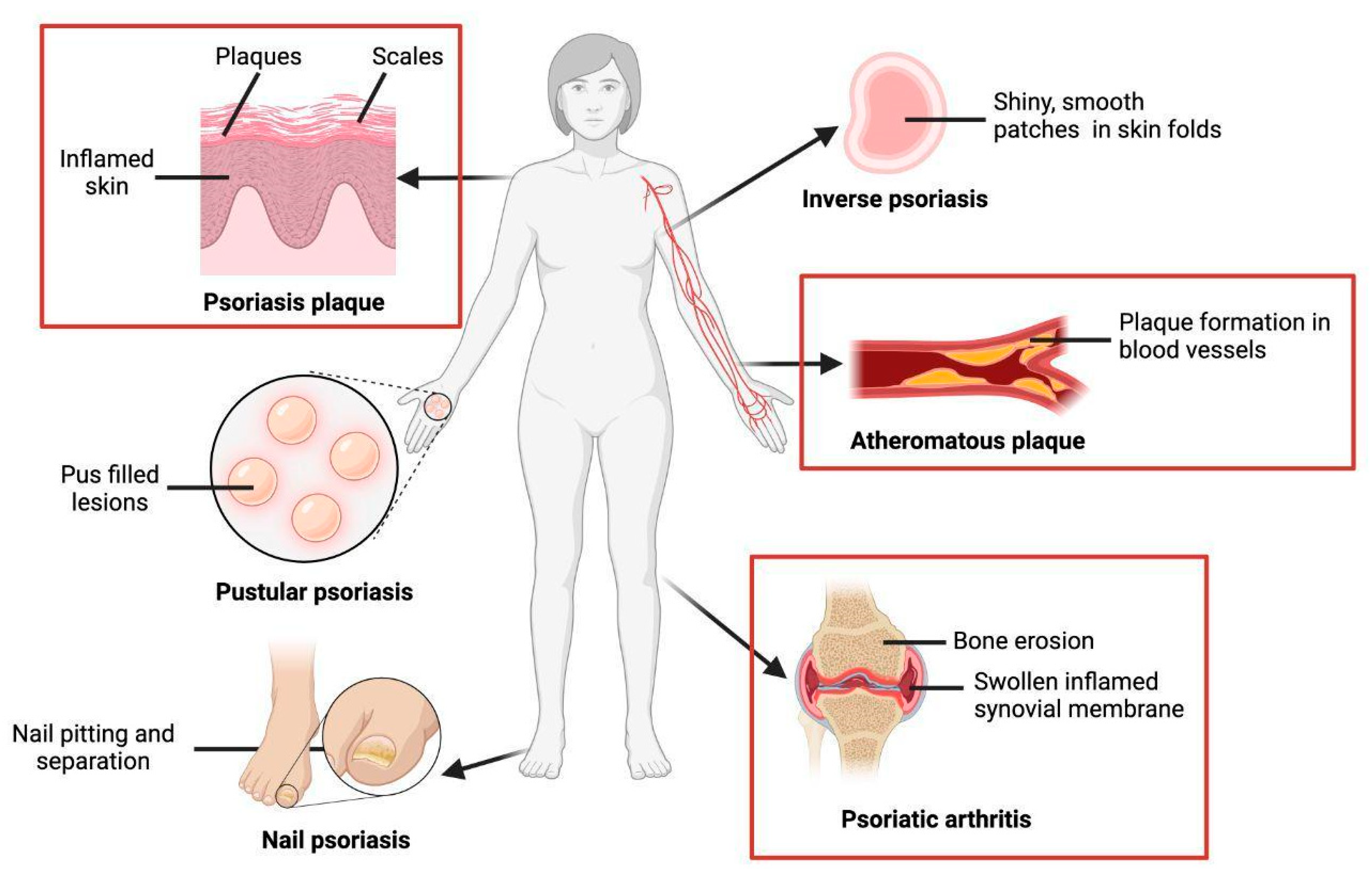
1. About Psoriasis
1.1. Clinical Manifestations
1.1.1. Skin Manifestations
1.1.2. Non-Skin Manifestations
2. Pathophysiology of the Skin and Non-Skin Manifestations in Psoriasis
2.1. Formation of Psoriatic Plaques
2.2. Increased Cardiovascular Risk
2.3. Psoriatic Arthritis
3. Key Evidence of the Pivotal Role of mTOR Non-Skin Manifestations
3.1. mTOR in the Transition from Psoriasis to Psoriatic Arthritis
3.2. mTOR in Atheromatous Plaque Formation
3.3. mTOR Inhibition Reduces Plaque Proliferation
3.4. mTOR Inhibition Reduces the Cardiovascular Risk
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Boehncke, W.H. Systemic Inflammation and Cardiovascular Comorbidity in Psoriasis Patients: Causes and Consequences. Front. Immunol. 2018, 9, 579. [Google Scholar] [CrossRef]
- World Health Organization. Global Report on Psoriasis; World Health Organization: Geneva, Switzerland, 2016; Available online: https://iris.who.int/handle/10665/204417 (accessed on 1 May 2024).
- Boehncke, W.H.; Schön, M.P. Psoriasis. Lancet 2015, 386, 983–994. [Google Scholar] [CrossRef] [PubMed]
- Boehncke, W.H.; Brembilla, N.C. Unmet Needs in the Field of Psoriasis: Pathogenesis and Treatment. Clin. Rev. Allergy Immunol. 2018, 55, 295–311. [Google Scholar] [CrossRef] [PubMed]
- Lowes, M.A.; Russell, C.B.; Martin, D.A.; Towne, J.E.; Krueger, J.G. The IL-23/T17 pathogenic axis in psoriasis is amplified by keratinocyte responses. Trends Immunol. 2013, 34, 174–181. [Google Scholar] [CrossRef]
- Buerger, C. Epidermal mTORC1 Signaling Contributes to the Pathogenesis of Psoriasis and Could Serve as a Therapeutic Target. Front. Immunol. 2018, 9, 2786. [Google Scholar] [CrossRef]
- Conrad, C.; Gilliet, M. Psoriasis: From Pathogenesis to Targeted Therapies. Clin. Rev. Allergy Immunol. 2018, 54, 102–113. [Google Scholar] [CrossRef]
- Rendon, A.; Schäkel, K. Psoriasis Pathogenesis and Treatment. Int. J. Mol. Sci. 2019, 20, 1475. [Google Scholar] [CrossRef] [PubMed]
- Azuaga, A.B.; Ramírez, J.; Cañete, J.D. Psoriatic Arthritis: Pathogenesis and Targeted Therapies. Int. J. Mol. Sci. 2023, 24, 4901. [Google Scholar] [CrossRef]
- Zwain, A.; Aldiwani, M.; Taqi, H. The Association Between Psoriasis and Cardiovascular Diseases. Eur. Cardiol. 2021, 16, e19. [Google Scholar] [CrossRef]
- Stuart, P.E.; Nair, R.P.; Tsoi, L.C.; Tejasvi, T.; Das, S.; Kang, H.M.; Ellinghaus, E.; Chandran, V.; Callis-Duffin, K.; Ike, R.; et al. Genome-wide Association Analysis of Psoriatic Arthritis and Cutaneous Psoriasis Reveals Differences in Their Genetic Architecture. Am. J. Hum. Genet. 2015, 97, 816–836. [Google Scholar] [CrossRef]
- Thorarensen, S.M.; Lu, N.; Ogdie, A.; Gelfand, J.M.; Choi, H.K.; Love, T.J. Physical trauma recorded in primary care is associated with the onset of psoriatic arthritis among patients with psoriasis. Ann. Rheum. Dis. 2017, 76, 521–525. [Google Scholar] [CrossRef]
- Garshick, M.S.; Ward, N.L.; Krueger, J.G.; Berger, J.S. Cardiovascular Risk in Patients with Psoriasis: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2021, 77, 1670–1680. [Google Scholar] [CrossRef]
- E Adamopoulos, I.; Suzuki, E.; Chao, C.-C.; Gorman, D.; Adda, S.; Maverakis, E.; Zarbalis, K.; Geissler, R.; Asio, A.; Blumenschein, W.M.; et al. IL-17A gene transfer induces bone loss and epidermal hyperplasia associated with psoriatic arthritis. Ann. Rheum. Dis. 2015, 74, 1284–1292. [Google Scholar] [CrossRef] [PubMed]
- Manning, B.D.; Cantley, L.C. AKT/PKB signaling: Navigating downstream. Cell 2007, 129, 1261–1274. [Google Scholar] [CrossRef] [PubMed]
- Raychaudhuri, S.K.; Raychaudhuri, S.P. mTOR Signaling Cascade in Psoriatic Disease: Double Kinase mTOR Inhibitor a Novel Therapeutic Target. Indian J. Dermatol. 2014, 59, 67–70. [Google Scholar] [CrossRef]
- Roy, T.; Boateng, S.T.; Uddin, M.B.; Banang-Mbeumi, S.; Yadav, R.K.; Bock, C.R.; Folahan, J.T.; Siwe-Noundou, X.; Walker, A.L.; King, J.A.; et al. The PI3K-Akt-mTOR and Associated Signaling Pathways as Molecular Drivers of Immune-Mediated Inflammatory Skin Diseases: Update on Therapeutic Strategy Using Natural and Synthetic Compounds. Cells 2023, 12, 1671. [Google Scholar] [CrossRef]
- Harper, E.G.; Guo, C.; Rizzo, H.; Lillis, J.V.; Kurtz, S.E.; Skorcheva, I.; Purdy, D.; Fitch, E.; Iordanov, M.; Blauvelt, A. Th17 cytokines stimulate CCL20 expression in keratinocytes in vitro and in vivo: Implications for psoriasis pathogenesis. J. Investig. Dermatol. 2009, 129, 2175–2183. [Google Scholar] [CrossRef]
- Teunissen, M.B.; Koomen, C.W.; de Waal Malefyt, R.; Wierenga, E.A.; Bos, J.D. Interleukin-17 and interferon-gamma synergize in the enhancement of proinflammatory cytokine production by human keratinocytes. J. Investig. Dermatol. 1998, 111, 645–649. [Google Scholar] [CrossRef]
- Adamopoulos, I.E.; Tessmer, M.; Chao, C.-C.; Adda, S.; Gorman, D.; Petro, M.; Chou, C.-C.; Pierce, R.H.; Yao, W.; Lane, N.E.; et al. IL-23 is critical for induction of arthritis, osteoclast formation, and maintenance of bone mass. J. Immunol. 2011, 187, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Sherlock, J.P.; Joyce-Shaikh, B.; Turner, S.P.; Chao, C.C.; Sathe, M.; Grein, J.; Gorman, D.M.; Bowman, E.P.; McClanahan, T.K.; Yearley, J.H.; et al. IL-23 induces spondyloarthropathy by acting on ROR-γt+ CD3+CD4−CD8− entheseal resident T cells. Nat. Med. 2012, 18, 1069–1076. [Google Scholar] [CrossRef]
- Malemud, C.J. The PI3K/Akt/PTEN/mTOR pathway: A fruitful target for inducing cell death in rheumatoid arthritis? Future Med. Chem. 2015, 7, 1137–1147. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhang, Y.; Zhang, Y.; Huang, Q.; Meng, W.; Gao, J.; Mo, X.; Tian, H.; Li, S. Chloroquine Alleviates Atherosclerosis by Modulating Regulatory T Cells Through the ATM/AMPK/mTOR Signaling Pathway in ApoE −/− Mice. Exp. Clin. Endocrinol. Diabetes 2023, 131, 676–685. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Fan, Y.; Zhang, Z.; Fang, Y.; Cheng, X.; Yang, Q.; Liu, J.; Xie, J. mTOR in the Mechanisms of Atherosclerosis and Cardiovascular Disease. Discov. Med. 2021, 31, 129–140. [Google Scholar] [PubMed]
- Roy, T.; Banang-Mbeumi, S.; Boateng, S.T.; Ruiz, E.M.; Chamcheu, R.C.N.; Kang, L.; King, J.A.; Walker, A.L.; Nagalo, B.M.; Kousoulas, K.G.; et al. Dual targeting of mTOR/IL-17A and autophagy by fisetin alleviates psoriasis-like skin inflammation. Front. Immunol. 2023, 13, 1075804. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Du, W.; Che, W.; Wang, L.; Zhao, L. Apigenin Inhibits the Progression of Osteoarthritis by Mediating Macrophage Polarization. Molecules 2023, 28, 2915. [Google Scholar] [CrossRef]
- Zhou, Z.-X.; Ma, X.-F.; Xiong, W.-H.; Ren, Z.; Jiang, M.; Deng, N.-H.; Zhou, B.-B.; Liu, H.-T.; Zhou, K.; Hu, H.-J.; et al. TRIM65 promotes vascular smooth muscle cell phenotypic transformation by activating PI3K/Akt/mTOR signaling during atherogenesis. Atherosclerosis 2024, 390, 117430. [Google Scholar] [CrossRef] [PubMed]
- Shemesh, A.; Pickering, H.; Roybal, K.T.; Lanier, L.L. Differential IL-12 signaling induces human natural killer cell activating receptor-mediated ligand-specific expansion. J. Exp. Med. 2022, 219, e20212434. [Google Scholar] [CrossRef] [PubMed]
- Loyola, K.; Karsulovic, C.; Cabrera, R.; Perez, C.; Hojman, L. New Markers for Cardiovascular Disease in Psoriatic Patients: Preliminary Study on Monocyte Phenotype, ADAMTS7, and mTOR Activity. Metabolites 2023, 13, 116. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; He, Y.; Chen, Y. Role of Mammalian Target of Rapamycin in Atherosclerosis. Curr. Mol. Med. 2018, 18, 216–232. [Google Scholar] [CrossRef] [PubMed]
- Aramwit, P.; Fongsodsri, K.; Tuentam, K.; Reamtong, O.; Thiangtrongjit, T.; Kanjanapruthipong, T.; Yadavalli, V.K.; Ampawong, S. Sericin coated thin polymeric films reduce keratinocyte proliferation via the mTOR pathway and epidermal inflammation through IL17 signaling in psoriasis rat model. Sci. Rep. 2023, 13, 12133. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, F.; Ma, W.; Sun, Q. Metformin inhibits proliferation and proinflammatory cytokines of human keratinocytes in vitro via mTOR-signaling pathway. Pharm. Biol. 2016, 54, 1173–1178. [Google Scholar] [CrossRef] [PubMed]
- Dorjsembe, B.; Joo, H.; Nho, C.; Ham, J.; Kim, J.C. Aruncus dioicus var. kamtschaticus Extract Ameliorates Psoriasis-like Skin Inflammation via Akt/mTOR and JAK2/STAT3 Signaling Pathways in a Murine Model. Nutrients 2022, 14, 5094. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-Y. DNA Damage Protection and Anti-inflammatory Activity of Different Solvent Fractions from Aruncus dioicus var. kamtschaticus. Korean J. Plant Resour. 2014, 27, 714–719. [Google Scholar] [CrossRef]
- Ahn, H.; Kim, J.; Kim, J.; Auh, J.; Choe, E. In vitro α-glucosidase and pancreatic lipase inhibitory activities and antioxidants of Samnamul (Aruncus dioicus) during rehydration and cooking. Food Sci. Biotechnol. 2014, 23, 1287–1293. [Google Scholar] [CrossRef]
- Shen, J.W.; Wu, P.Y.; Kuo, Y.H.; Chang, Q.X.; Wen, K.C.; Chiang, H.M. Fermented Taiwanofungus camphoratus Extract Ameliorates Psoriasis-Associated Response in HaCaT Cells via Modulating NF-κB and mTOR Pathways. Int. J. Mol. Sci. 2022, 23, 14623. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.J.; Chao, C.H.; Lu, M.K. Large-scale preparation of sulfated polysaccharides with anti-angionenic and anti-inflammatory properties from Antrodia cinnamomia. Int. J. Biol. Macromol. 2018, 113, 1198–1205. [Google Scholar] [CrossRef] [PubMed]
- Samarasekera, E.J.; Neilson, J.M.; Warren, R.B.; Parnham, J.; Smith, C.H. Incidence of cardiovascular disease in individuals with psoriasis: A systematic review and meta-analysis. J. Investig. Dermatol. 2013, 133, 2340–2346. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Gao, L.; Sun, Q.; Jia, L.; Liu, D. Increased levels of IL-17 and autoantibodies following Bisphenol A exposure were associated with activation of PI3K/AKT/mTOR pathway and abnormal autophagy in MRL/lpr mice. Ecotoxicol. Environ. Saf. 2023, 255, 114788. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.; Zhang, W. Role of mTOR in Glucose and Lipid Metabolism. Int. J. Mol. Sci. 2018, 19, 2043. [Google Scholar] [CrossRef]
- Li, Y.; Pan, J.; Yu, J.J.J.; Wu, X.; Yang, G.; Pan, X.; Sui, G.; Wang, M.; Cheng, M.; Zhu, S.; et al. Huayu Qutan Recipe promotes lipophagy and cholesterol efflux through the mTORC1/TFEB/ABCA1-SCARB1 signal axis. J. Cell Mol. Med. 2024, 28, e18257. [Google Scholar] [CrossRef]
- Chen, X.; Cao, Y.; Guo, Y.; Liu, J.; Ye, X.; Li, H.; Zhang, L.; Feng, W.; Xian, S.; Yang, Z.; et al. microRNA-125b-1-3p mediates autophagy via the RRAGD/mTOR/ULK1 signaling pathway and mitigates atherosclerosis progression. Cell. Signal. 2024, 118, 111136. [Google Scholar] [CrossRef] [PubMed]

| Aspect | Implications |
|---|---|
| mTOR in the transition from psoriasis to psoriatic arthritis | Contributes to synovial inflammation and bone destruction by inducing IL-17A production and activating the abnormal proliferation of synovial fibroblasts. |
| mTOR in atheromatous plaque formation | Associated with the proliferation of macrophages and smooth muscle cells, contributing to atheroma formation and the production of pro-inflammatory cytokines. |
| mTOR inhibition reduces plaque proliferation | The inhibition of mTOR by certain substances has been shown to reduce skin inflammation and keratinocyte hyperproliferation. |
| mTOR inhibition reduces the cardiovascular risk | mTOR activation may increase the cardiovascular risk by promoting the production of pro-inflammatory cytokines and affecting lipid and glucose metabolism, suggesting a potential role in the pathogenesis of cardiovascular disease in psoriasis patients. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joo, K.; Karsulovic, C.; Sore, M.; Hojman, L. Pivotal Role of mTOR in Non-Skin Manifestations of Psoriasis. Int. J. Mol. Sci. 2024, 25, 6778. https://doi.org/10.3390/ijms25126778
Joo K, Karsulovic C, Sore M, Hojman L. Pivotal Role of mTOR in Non-Skin Manifestations of Psoriasis. International Journal of Molecular Sciences. 2024; 25(12):6778. https://doi.org/10.3390/ijms25126778
Chicago/Turabian StyleJoo, Ka, Claudio Karsulovic, Milisa Sore, and Lia Hojman. 2024. "Pivotal Role of mTOR in Non-Skin Manifestations of Psoriasis" International Journal of Molecular Sciences 25, no. 12: 6778. https://doi.org/10.3390/ijms25126778
APA StyleJoo, K., Karsulovic, C., Sore, M., & Hojman, L. (2024). Pivotal Role of mTOR in Non-Skin Manifestations of Psoriasis. International Journal of Molecular Sciences, 25(12), 6778. https://doi.org/10.3390/ijms25126778







