Highly Dispersive Gold Nanoclusters Confined within Micropores of Defective UiO-66 for Highly Efficient Aldehyde Oxidation at Mild Conditions
Abstract
:1. Introduction
2. Results
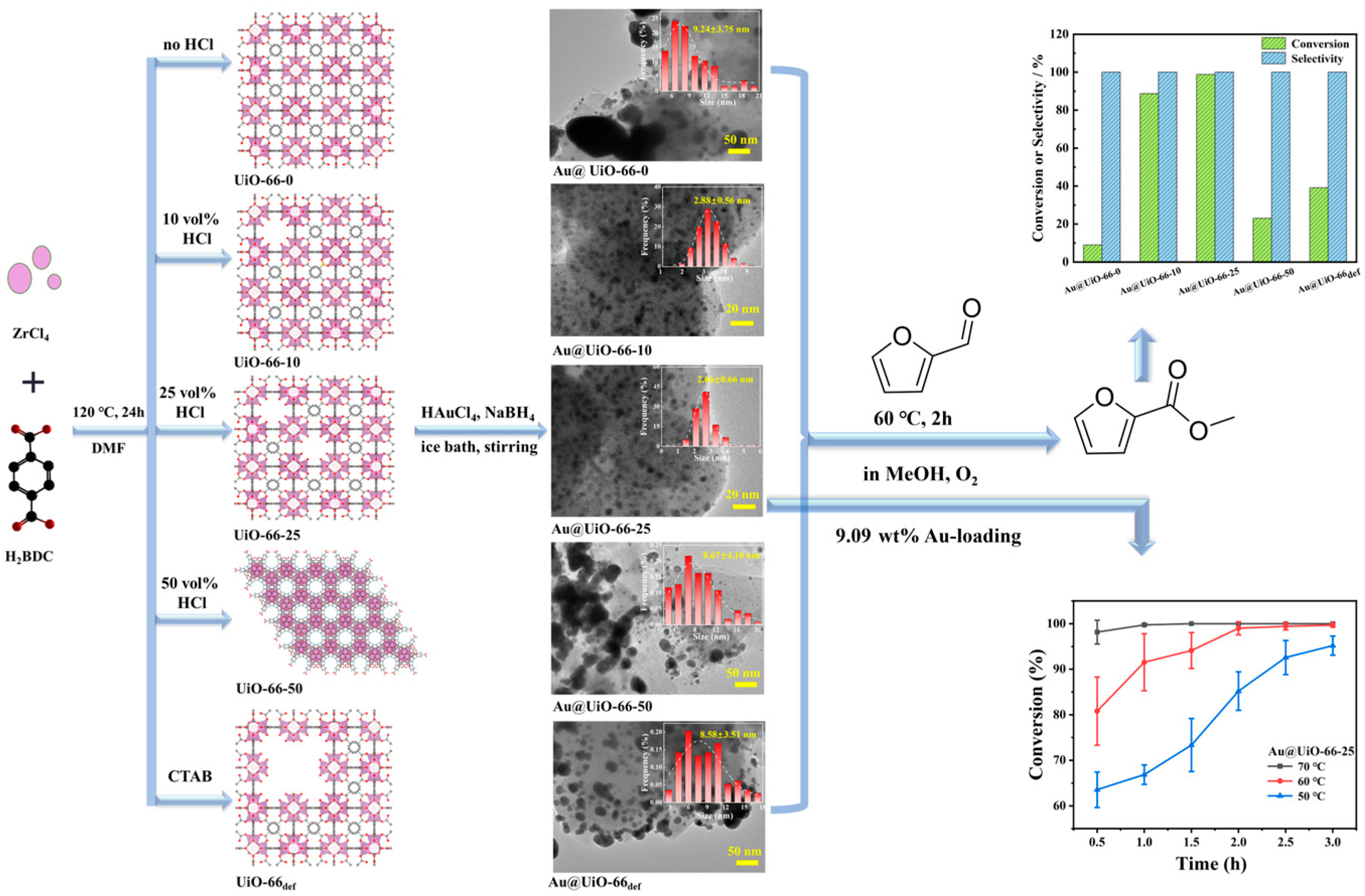
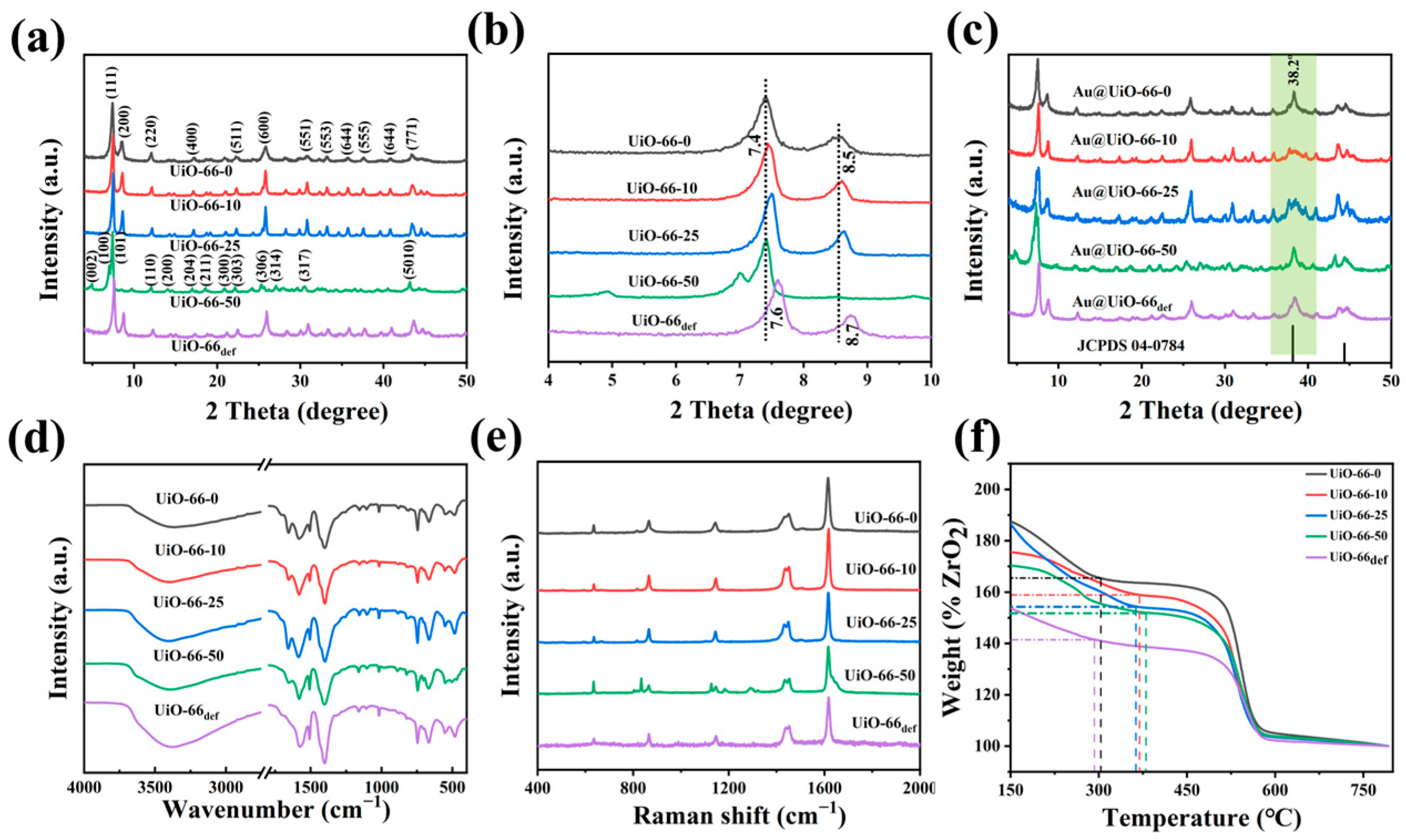
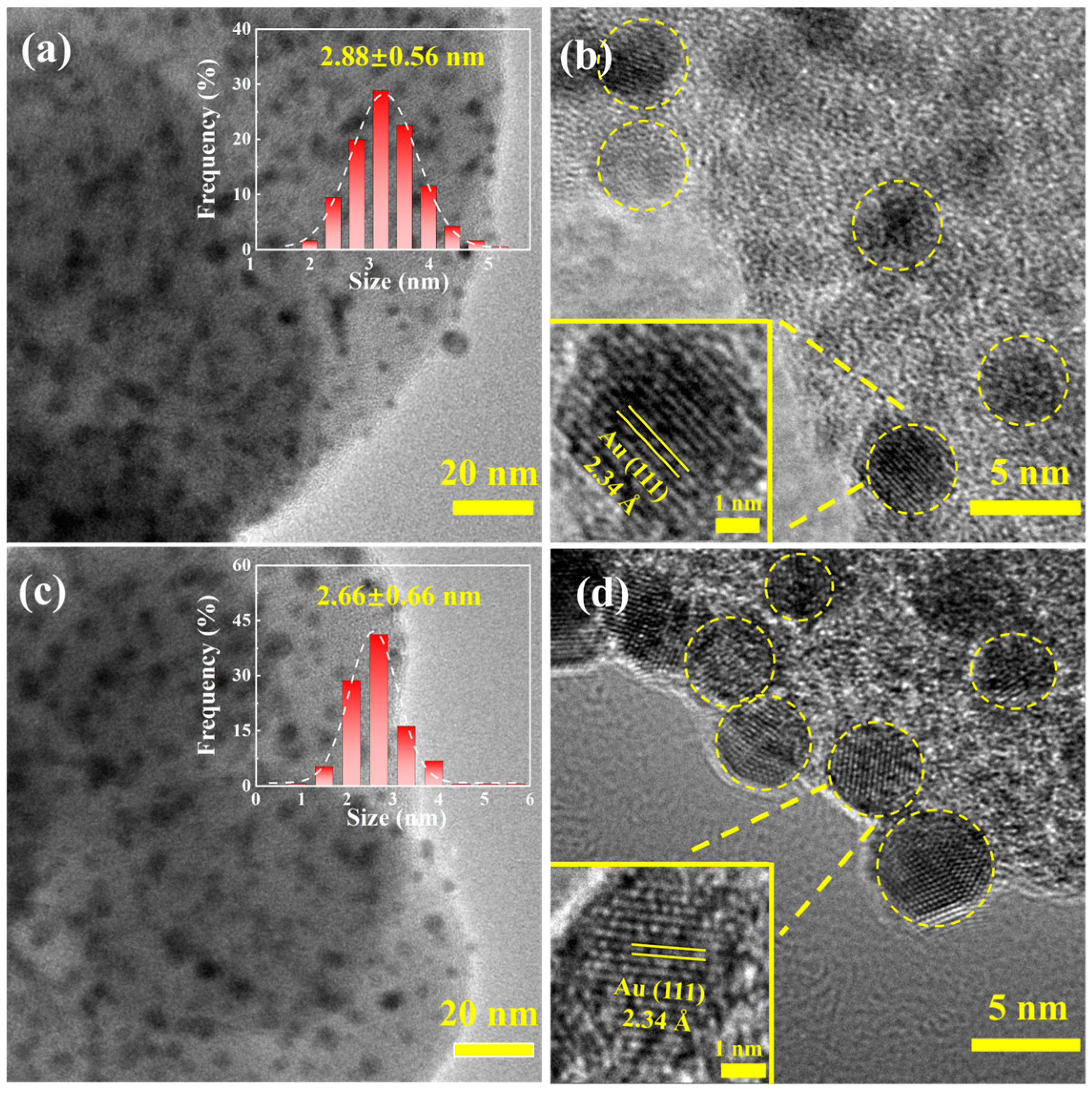
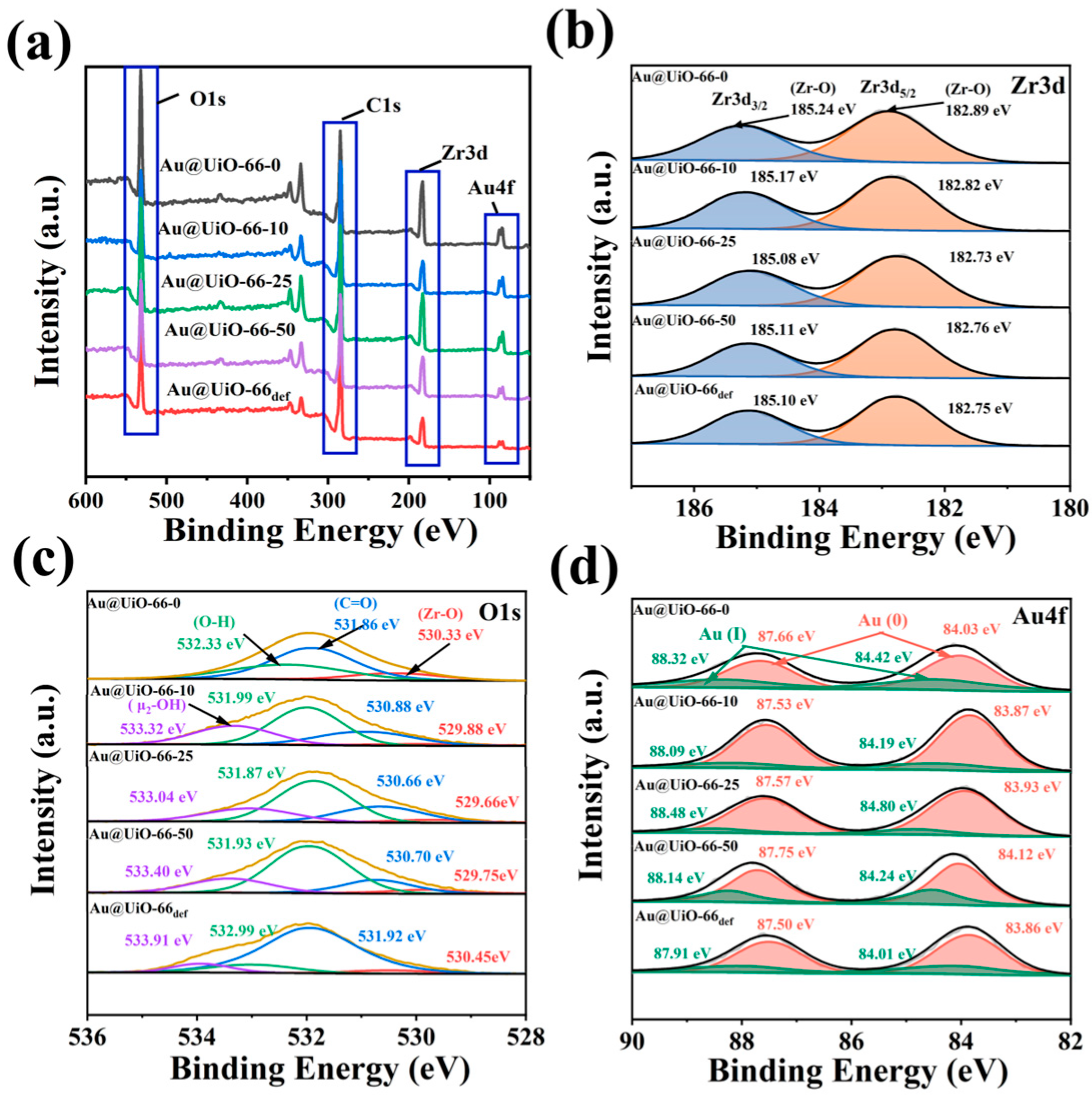
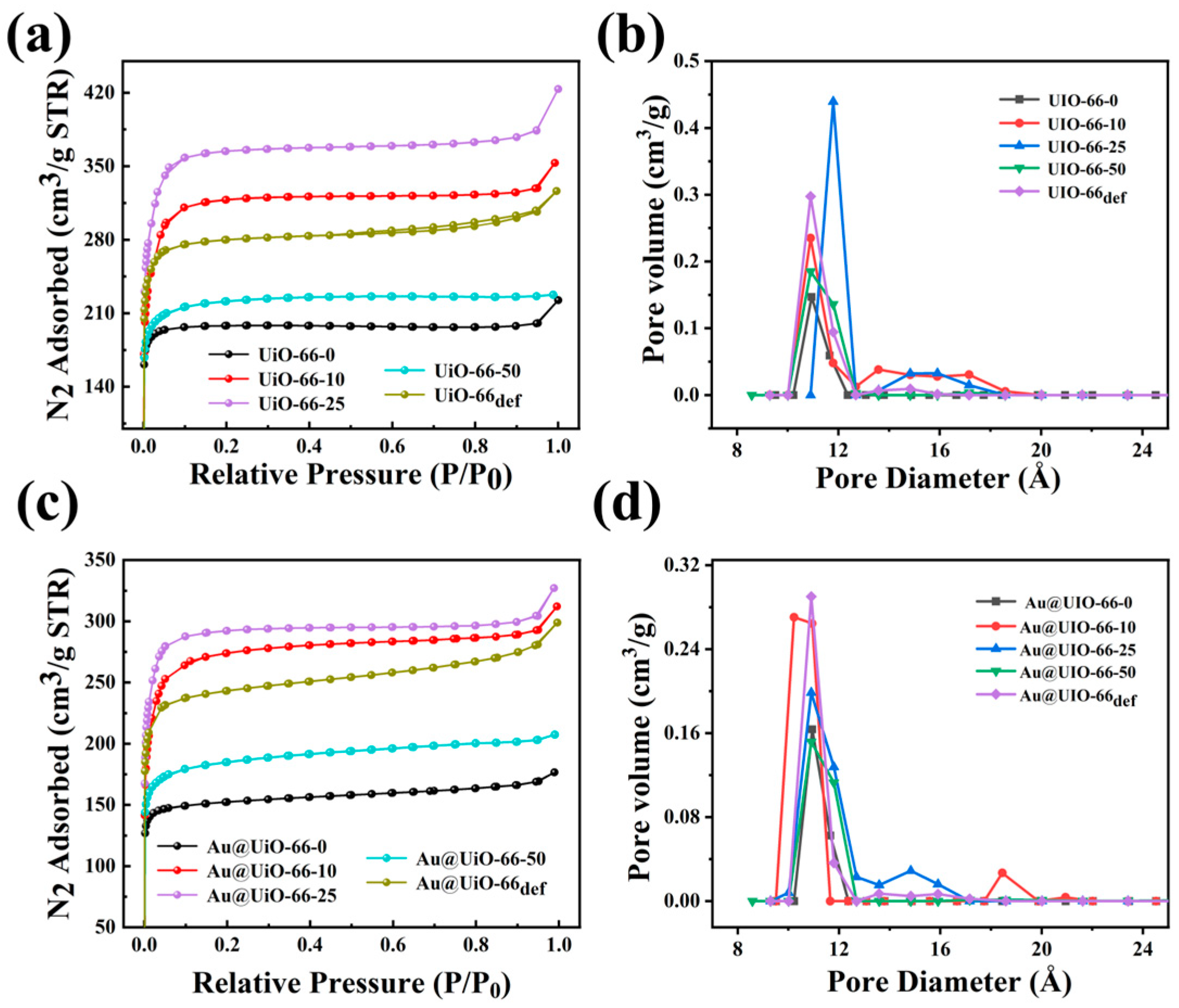
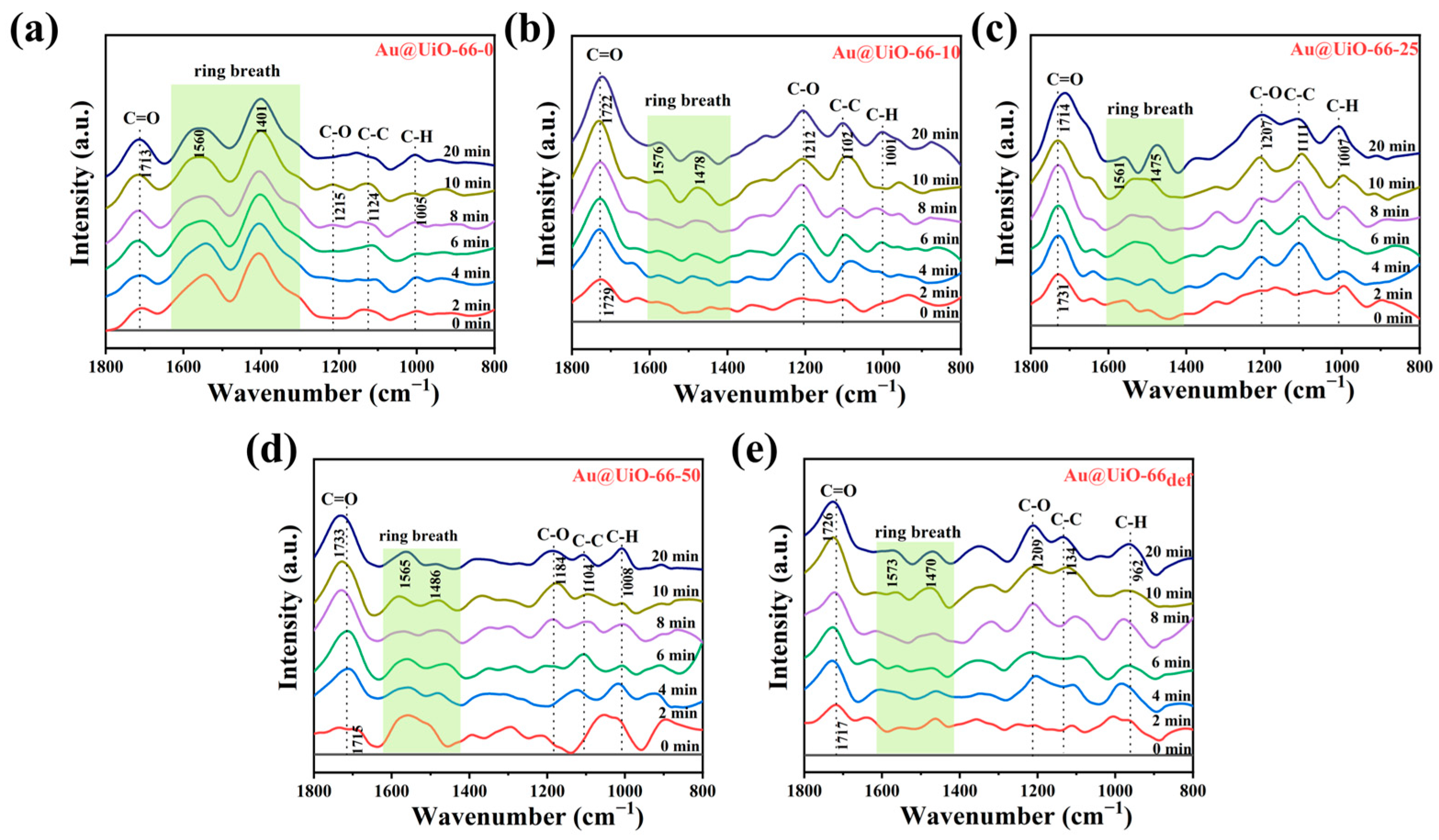
2.1. Preparation and Characterization of Catalysts
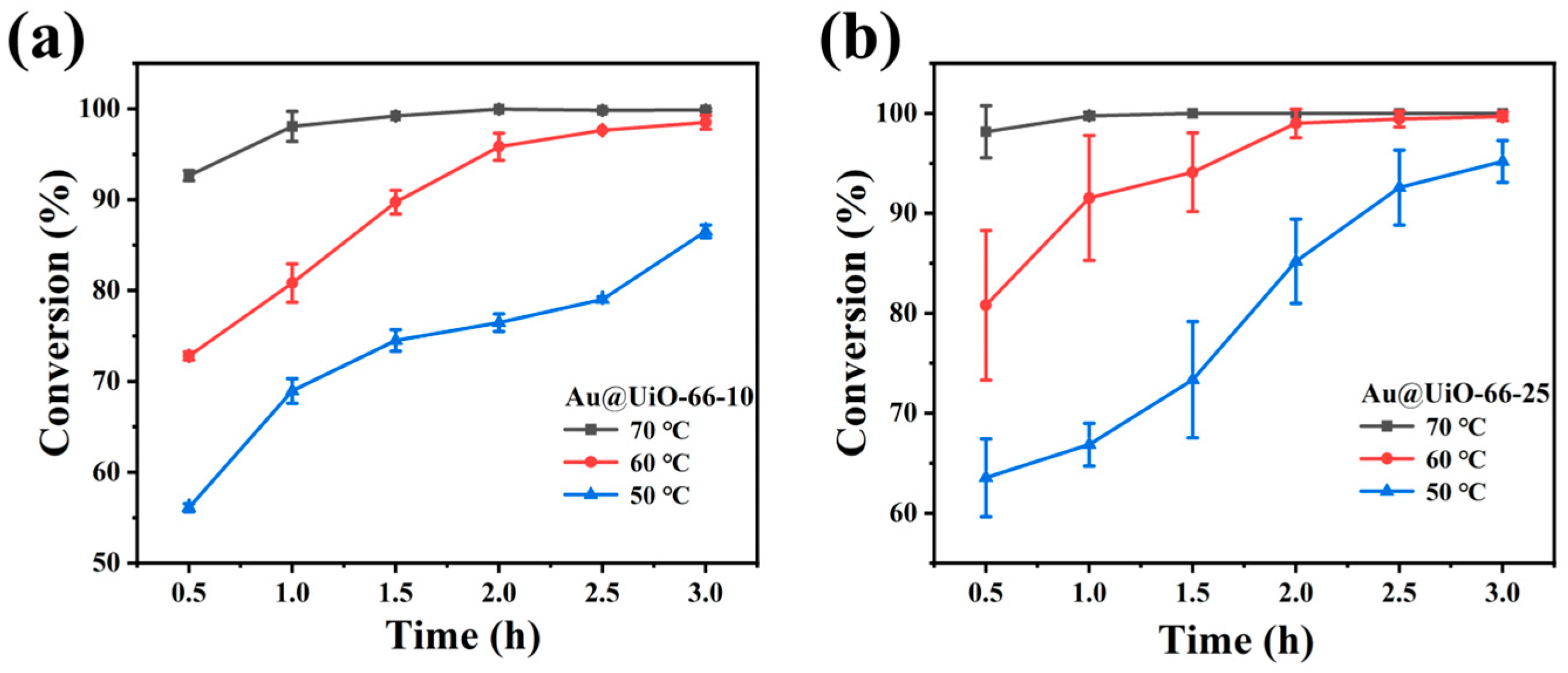
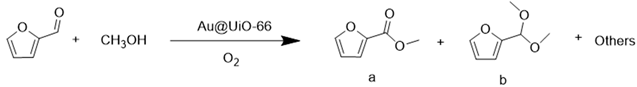
2.2. Catalytic Assay of Furfural Oxidation by Au@UiO-66-X
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis of Defective UiO-66
4.3. Synthesis of Au@UiO-66 Materials
4.4. Equipment for Characterizations
4.5. Oxidative Esterification Assay of Furfural in MeOH
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Signoretto, M.; Menegazzo, F.; Contessotto, L.; Pinna, F.; Manzoli, M.; Boccuzzi, F. Au/ZrO2: An efficient and reusable catalyst for the oxidative esterification of renewable furfural. Appl. Catal. B Environ. 2013, 129, 287–293. [Google Scholar] [CrossRef]
- Wang, H.; Liu, X.; Yang, W.; Mao, G.; Meng, Z.; Wu, Z.; Jiang, H.-L. Surface-Clean Au25 Nanoclusters in Modulated Microenvironment Enabled by Metal–Organic Frameworks for Enhanced Catalysis. J. Am. Chem. Soc. 2022, 144, 22008–22017. [Google Scholar] [CrossRef] [PubMed]
- Ning, L.; Liao, S.; Liu, X.; Guo, P.; Zhang, Z.; Zhang, H.; Tong, X. A regulatable oxidative valorization of furfural with aliphatic alcohols catalyzed by functionalized metal-organic frameworks-supported Au nanoparticles. J. Catal. 2018, 364, 1–13. [Google Scholar] [CrossRef]
- Nielsen, I.S.; Taarning, E.; Egeblad, K.; Madsen, R.; Christensen, C.H. Direct aerobic oxidation of primary alcohols to methyl esters catalyzed by a heterogeneous gold catalyst. Catal. Lett. 2007, 116, 35–40. [Google Scholar] [CrossRef]
- Manzoli, M.; Menegazzo, F.; Signoretto, M.; Cruciani, G.; Pinna, F. Effects of synthetic parameters on the catalytic performance of Au/CeO2 for furfural oxidative esterification. J. Catal. 2015, 330, 465–473. [Google Scholar] [CrossRef]
- Tong, X.; Liu, Z.; Yu, L.; Li, Y. A tunable process: Catalytic transformation of renewable furfural with aliphatic alcohols in the presence of molecular oxygen. Chem. Commun. 2015, 51, 3674–3677. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, R.; Thiripuranthagan, S.; Devarajan, A.; Kumaravel, S.; Erusappan, E.; Kannan, K. Oxidative esterification of furfural by Au nanoparticles supported CMK-3 mesoporous catalysts. Appl. Catal. A-Gen. 2017, 545, 33–43. [Google Scholar] [CrossRef]
- Tian, J.; Cheng, X.; Liu, G.; Ren, Z.; Wang, Y.; Wei, T.; Zhang, D.; Guo, Y. Efficient aerobic oxidative esterification of furfural to methylfuroate over Au/Al2O3 catalysts in base-free medium. New J. Chem. 2024, 48, 7651–7659. [Google Scholar] [CrossRef]
- Dong, Y.; Chen, G.; Zuo, X.; Li, J.; Yu, J.; Zhang, G.; Kuang, J.; Akpinar, I.; Peng, L.; Tang, X.; et al. Nitrogen, Sulfur Co-doped Hollow Carbon-Encapsulated Cu/Co2P for Selective Oxidation Esterification of Furfurals. ACS Catal. 2024, 14, 6565–6576. [Google Scholar] [CrossRef]
- Shivhare, A.; Kumar, A.; Srivastava, R. The Size-Dependent Catalytic Performances of Supported Metal Nanoparticles and Single Atoms for the Upgrading of Biomass-Derived 5-Hydroxymethylfurfural, Furfural, and Levulinic acid. ChemCatChem 2022, 14, e202101423. [Google Scholar] [CrossRef]
- Sun, W.; Gao, T.; Zhu, G.; Cao, Q.; Fang, W. Influence of Support Properties and Particle Size on the Gold-Catalyzed Base-Free Aerobic Oxidation of 5-Hydroxymethylfurfural. ChemistrySelect 2020, 5, 1416–1423. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Asiri, A.M.; Garcia, H. Metal Organic Frameworks as Versatile Hosts of Au Nanoparticles in Heterogeneous Catalysis. ACS Catal. 2017, 7, 2896–2919. [Google Scholar] [CrossRef]
- Liu, C.; Liu, Y.; Shi, Y.; Wang, Z.; Guo, W.; Bi, J.; Wu, L. Au nanoparticles-anchored defective metal–organic frameworks for photocatalytic transformation of amines to imines under visible light. J. Colloid Interface Sci. 2023, 631, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Cox, C.S.; Slavich, E.; Macreadie, L.K.; McKemmish, L.K.; Lessio, M. Understanding the Role of Synthetic Parameters in the Defect Engineering of UiO-66: A Review and Meta-analysis. Chem. Mater. 2023, 35, 3057–3072. [Google Scholar] [CrossRef]
- Feng, X.; Jena, H.S.; Krishnaraj, C.; Leus, K.; Wang, G.; Chen, H.; Jia, C.; Van Der Voort, P. Generating Catalytic Sites in UiO-66 through Defect Engineering. ACS Appl. Mater. Interfaces 2021, 13, 60715–60735. [Google Scholar] [CrossRef] [PubMed]
- Dissegna, S.; Epp, K.; Heinz, W.R.; Kieslich, G.; Fischer, R.A. Defective Metal-Organic Frameworks. Adv. Mater. 2018, 30, 1704501. [Google Scholar] [CrossRef] [PubMed]
- Clark, C.A.; Heck, K.N.; Powell, C.D.; Wong, M.S. Highly Defective UiO-66 Materials for the Adsorptive Removal of Perfluorooctanesulfonate. ACS Sustain. Chem. Eng. 2019, 7, 6619–6628. [Google Scholar] [CrossRef]
- Fu, J.; Lai, H.; Zhang, Z.; Li, G. UiO-66 metal-organic frameworks/gold nanoparticles based substrates for SERS analysis of food samples. Anal. Chim. Acta 2021, 1161, 338464. [Google Scholar] [CrossRef] [PubMed]
- Alnehia, A.; Al-Hammadi, A.H.; Al-Sharabi, A.; Alnahari, H. Optical, structural and morphological properties of ZnO and Fe3+ doped ZnO-NPs prepared by Foeniculum vulgare extract as capping agent for optoelectronic applications. Inorg. Chem. Commun. 2022, 143, 109699. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, H.; Ren, M.; Zhang, Y.; Li, Y.; Wang, L.; Chen, J. Low-temperature catalytic degradation of chlorinated aromatic hydrocarbons over bimetallic Ce-Zr/UiO-66 catalysts. Chem. Eng. J. 2021, 414, 128782. [Google Scholar] [CrossRef]
- Zhong, L.; Liao, X.; Cui, H.; Luo, H.; Lv, Y.; Liu, P. Hydrogenation of α,β-unsaturated aldehydes over defective UiO-66 with Frustrated Lewis Pairs: Modulation of densities of defect sites via tailoring ligand-vacancies. Appl. Catal. B Environ. 2024, 342, 123421. [Google Scholar] [CrossRef]
- Atzori, C.; Shearer, G.C.; Maschio, L.; Civalleri, B.; Bonino, F.; Lamberti, C.; Svelle, S.; Lillerud, K.P.; Bordiga, S. Effect of Benzoic Acid as a Modulator in the Structure of UiO-66: An Experimental and Computational Study. J. Phys. Chem. C 2017, 121, 9312–9324. [Google Scholar] [CrossRef]
- Han, Y.; Liu, M.; Li, K.; Zuo, Y.; Wei, Y.; Xu, S.; Zhang, G.; Song, C.; Zhang, Z.; Guo, X. Facile synthesis of morphology and size-controlled zirconium metal–organic framework UiO-66: The role of hydrofluoric acid in crystallization. CrystEngComm 2015, 17, 6434–6440. [Google Scholar] [CrossRef]
- Akita, T.; Tanaka, K.; Kohyama, M. TEM and HAADF-STEM study of the structure of Au nano-particles on CeO2. J. Mater. Sci. 2008, 43, 3917–3922. [Google Scholar] [CrossRef]
- Abou-Elyazed, A.S.; Shaban, E.A.; Sun, Y.; El-Nahas, A.M.; Kashar, T.I. Solvent-Free Synthesis and Characterization of Bimetallic UiO-66(Zr/Sn) Heterogeneous Catalyst for Biodiesel Production. Ind. Eng. Chem. Res. 2023, 62, 9211–9220. [Google Scholar] [CrossRef]
- Liu, L.; Qiao, Z.; Cui, X.; Pang, C.; Liang, H.; Xie, P.; Luo, X.; Huang, Z.; Zhang, Y.; Zhao, Z. Amino Acid Imprinted UiO-66s for Highly Recognized Adsorption of Small Angiotensin-Converting-Enzyme-Inhibitory Peptides. ACS Appl. Mater. Interfaces 2019, 11, 23039–23049. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, R.; Cui, Y.; Liang, X.; Lei, C.; Meng, S.; Ma, Y.; Lei, Z.; Yang, Z. PANI/FeUiO-66 nanohybrids with enhanced visible-light promoted photocatalytic activity for the selectively aerobic oxidation of aromatic alcohols. Appl. Catal. B Environ. 2017, 210, 484–494. [Google Scholar] [CrossRef]
- De Vos, A.; Hendrickx, K.; Van Der Voort, P.; Van Speybroeck, V.; Lejaeghere, K. Missing Linkers: An Alternative Pathway to UiO-66 Electronic Structure Engineering. Chem. Mater. 2017, 29, 3006–3019. [Google Scholar] [CrossRef]
- Pakrieva, E.; Kolobova, E.; Kotolevich, Y.; Pascual, L.; Carabineiro, S.A.C.; Kharlanov, A.N.; Pichugina, D.; Nikitina, N.; German, D.; Partida, T.A.Z.; et al. Effect of Gold Electronic State on the Catalytic Performance of Nano Gold Catalysts in n-Octanol Oxidation. Nanomaterials 2020, 10, 880. [Google Scholar] [CrossRef] [PubMed]
- Kar, A.K.; Sarkar, R.; Manal, A.K.; Kumar, R.; Chakraborty, S.; Ahuja, R.; Srivastava, R. Unveiling and understanding the remarkable enhancement in the catalytic activity by the defect creation in UIO-66 during the catalytic transfer hydrodeoxygenation of vanillin with isopropanol. Appl. Catal. B Environ. 2023, 325, 122385. [Google Scholar] [CrossRef]
- Wang, Q.; Feng, J.; Zheng, L.; Wang, B.; Bi, R.; He, Y.; Liu, H.; Li, D. Interfacial Structure-Determined Reaction Pathway and Selectivity for 5-(Hydroxymethyl)furfural Hydrogenation over Cu-Based Catalysts. ACS Catal. 2020, 10, 1353–1365. [Google Scholar] [CrossRef]
- Meng, X.; Wang, L.; Chen, L.; Xu, M.; Liu, N.; Zhang, J.; Yang, Y.; Wei, M. Charge-separated metal-couple-site in NiZn alloy catalysts towards furfural hydrodeoxygenation reaction. J. Catal. 2020, 392, 69–79. [Google Scholar] [CrossRef]
- Meng, X.; Yang, Y.; Chen, L.; Xu, M.; Zhang, X.; Wei, M. A Control over Hydrogenation Selectivity of Furfural via Tuning Exposed Facet of Ni Catalysts. ACS Catal. 2019, 9, 4226–4235. [Google Scholar] [CrossRef]
- Ren, Z.; Yang, Y.; Wang, S.; Li, X.; Feng, H.; Wang, L.; Li, Y.; Zhang, X.; Wei, M. Pt atomic clusters catalysts with local charge transfer towards selective oxidation of furfural. Appl. Catal. B Environ. 2021, 295, 120290. [Google Scholar] [CrossRef]
- Yu, J.; Yang, Y.; Chen, L.; Li, Z.; Liu, W.; Xu, E.; Zhang, Y.; Hong, S.; Zhang, X.; Wei, M. NiBi intermetallic compounds catalyst toward selective hydrogenation of unsaturated aldehydes. Appl. Catal. B Environ. 2020, 277, 119273. [Google Scholar] [CrossRef]
- Valekar, A.H.; Cho, K.-H.; Chitale, S.K.; Hong, D.-Y.; Cha, G.-Y.; Lee, U.-H.; Hwang, D.W.; Serre, C.; Chang, J.-S.; Hwang, Y.K. Catalytic transfer hydrogenation of ethyl levulinate to γ-valerolactone over zirconium-based metal–organic frameworks. Green Chem. 2016, 18, 4542–4552. [Google Scholar] [CrossRef]
- Song, J.; Wu, L.; Zhou, B.; Zhou, H.; Fan, H.; Yang, Y.; Meng, Q.; Han, B. A new porous Zr-containing catalyst with a phenate group: An efficient catalyst for the catalytic transfer hydrogenation of ethyl levulinate to γ-valerolactone. Green Chem. 2015, 17, 1626–1632. [Google Scholar] [CrossRef]
- Tang, X.; Chen, H.; Hu, L.; Hao, W.; Sun, Y.; Zeng, X.; Lin, L.; Liu, S. Conversion of biomass to γ-valerolactone by catalytic transfer hydrogenation of ethyl levulinate over metal hydroxides. Appl. Catal. B Environ. 2014, 147, 827–834. [Google Scholar] [CrossRef]
- Fan, C.; Wang, R.; Kong, P.; Wang, X.; Wang, J.; Zhang, X.; Zheng, Z. Modification of Au nanoparticles electronic state by MOFs defect engineering to realize highly active photocatalytic oxidative esterification of benzyl alcohol with methanol. Catal. Commun. 2020, 140, 106002. [Google Scholar] [CrossRef]
- Ma, M.; Hou, P.; Cao, J.; Liu, H.; Yan, X.; Xu, X.; Yue, H.; Tian, G.; Feng, S. Simple basic zirconium carbonate: Low temperature catalysis for hydrogen transfer of biomass-derived carboxides. Green Chem. 2019, 21, 5969–5979. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Y.; Song, L.; Chen, J.; Yang, Y.; Wang, Y. Enhanced adsorption performance of gaseous toluene on defective UiO-66 metal organic framework: Equilibrium and kinetic studies. J. Hazard. Mater. 2019, 365, 597–605. [Google Scholar] [CrossRef]
- Zhao, Y.; Trewyn, B.G.; Slowing, I.I.; Lin, V.S.-Y. Mesoporous Silica Nanoparticle-Based Double Drug Delivery System for Glucose-Responsive Controlled Release of Insulin and Cyclic AMP. J. Am. Chem. Soc. 2009, 131, 8398–8400. [Google Scholar] [CrossRef]
- Subudhi, S.; Mansingh, S.; Tripathy, S.P.; Mohanty, A.; Mohapatra, P.; Rath, D.; Parida, K. The fabrication of Au/Pd plasmonic alloys on UiO-66-NH2: An efficient visible light-induced photocatalyst towards the Suzuki Miyaura coupling reaction under ambient conditions. Catal. Sci. Technol. 2019, 9, 6585–6597. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y.; Fu, Q.; Qin, H.; Lv, J.; Yang, K.; Zhang, Q.; Zhang, H.; Wang, M. An efficient modulated synthesis of zirconium metal–organic framework UiO-66. RSC Adv. 2022, 12, 6083–6092. [Google Scholar] [CrossRef] [PubMed]
- Geravand, E.; Farzaneh, F.; Ghiasi, M. Metalation and DFT studies of metal organic frameworks UiO-66(Zr) with vanadium chloride as allyl alcohol epoxidation catalyst. J. Mol. Struct. 2019, 1198, 126940. [Google Scholar] [CrossRef]
- Fayyazi, M.; Nazar, A.R.S.; Farhadian, M.; Tangestaninejad, S. Adsorptive removal of ibuprofen to binary and amine-functionalized UiO-66 in the aquatic environment: Synergistic/antagonistic evaluation. Environ. Sci. Pollut. Res. 2022, 29, 69502–69516. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, X.; Bi, F.; Zheng, Z.; Sheng, L.; Xu, J.; Wang, Z.; Yang, Y. Effective toluene adsorption over defective UiO-66-NH2: An experimental and computational exploration. J. Mol. Liq. 2020, 316, 113812. [Google Scholar] [CrossRef]
- Andrade, P.H.M.; Henry, N.; Volkringer, C.; Loiseau, T.; Vezin, H.; Hureau, M.; Moissette, A. Iodine Uptake by Zr-/Hf-Based UiO-66 Materials: The Influence of Metal Substitution on Iodine Evolution. ACS Appl. Mater. Interfaces 2022, 14, 29916–29933. [Google Scholar] [CrossRef]
- Wei, R.; Gaggioli, C.A.; Li, G.; Islamoglu, T.; Zhang, Z.; Yu, P.; Farha, O.K.; Cramer, C.J.; Gagliardi, L.; Yang, D.; et al. Tuning the Properties of Zr6O8 Nodes in the Metal Organic Framework UiO-66 by Selection of Node-Bound Ligands and Linkers. Chem. Mater. 2019, 31, 1655–1663. [Google Scholar] [CrossRef]
- Menegazzo, F.; Signoretto, M.; Pinna, F.; Manzoli, M.; Aina, V.; Cerrato, G.; Boccuzzi, F. Oxidative esterification of renewable furfural on gold-based catalysts: Which is the best support? J. Catal. 2014, 309, 241–247. [Google Scholar] [CrossRef]

 | ||||
|---|---|---|---|---|
| No. | Catalysts | Con. (%) | Sel. (%, a) | Sel. (%, b) |
| 1 | Au@UiO-66-0 | 9.04 | 100.0 | N.D. |
| 2 | Au@UiO-66-10 | 88.71 | 100.0 | N.D. |
| 3 | Au@UiO-66-25 | 98.79 | 100.0 | N.D. |
| 4 | Au@UiO-66-50 | 23.07 | 100.0 | N.D. |
| 5 | Au@UiO-66def | 39.14 | 100.0 | N.D. |
| Samples | UiO-66-0 | UiO-66-10 | UiO-66-25 | UiO-66-50 | UiO-66def |
|---|---|---|---|---|---|
| Acid quantity (mmol/g) | 1.039 | 1.353 | 1.468 | 1.014 | 1.247 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, M.-Q.; Chang, X.-Y.; Li, H.-W.; Wu, Y. Highly Dispersive Gold Nanoclusters Confined within Micropores of Defective UiO-66 for Highly Efficient Aldehyde Oxidation at Mild Conditions. Int. J. Mol. Sci. 2024, 25, 6779. https://doi.org/10.3390/ijms25126779
He M-Q, Chang X-Y, Li H-W, Wu Y. Highly Dispersive Gold Nanoclusters Confined within Micropores of Defective UiO-66 for Highly Efficient Aldehyde Oxidation at Mild Conditions. International Journal of Molecular Sciences. 2024; 25(12):6779. https://doi.org/10.3390/ijms25126779
Chicago/Turabian StyleHe, Ming-Qin, Xin-Yu Chang, Hong-Wei Li, and Yuqing Wu. 2024. "Highly Dispersive Gold Nanoclusters Confined within Micropores of Defective UiO-66 for Highly Efficient Aldehyde Oxidation at Mild Conditions" International Journal of Molecular Sciences 25, no. 12: 6779. https://doi.org/10.3390/ijms25126779






