The Application of Clinical and Molecular Diagnostic Techniques to Identify a Rare Haemoglobin Variant
Abstract
1. Introduction
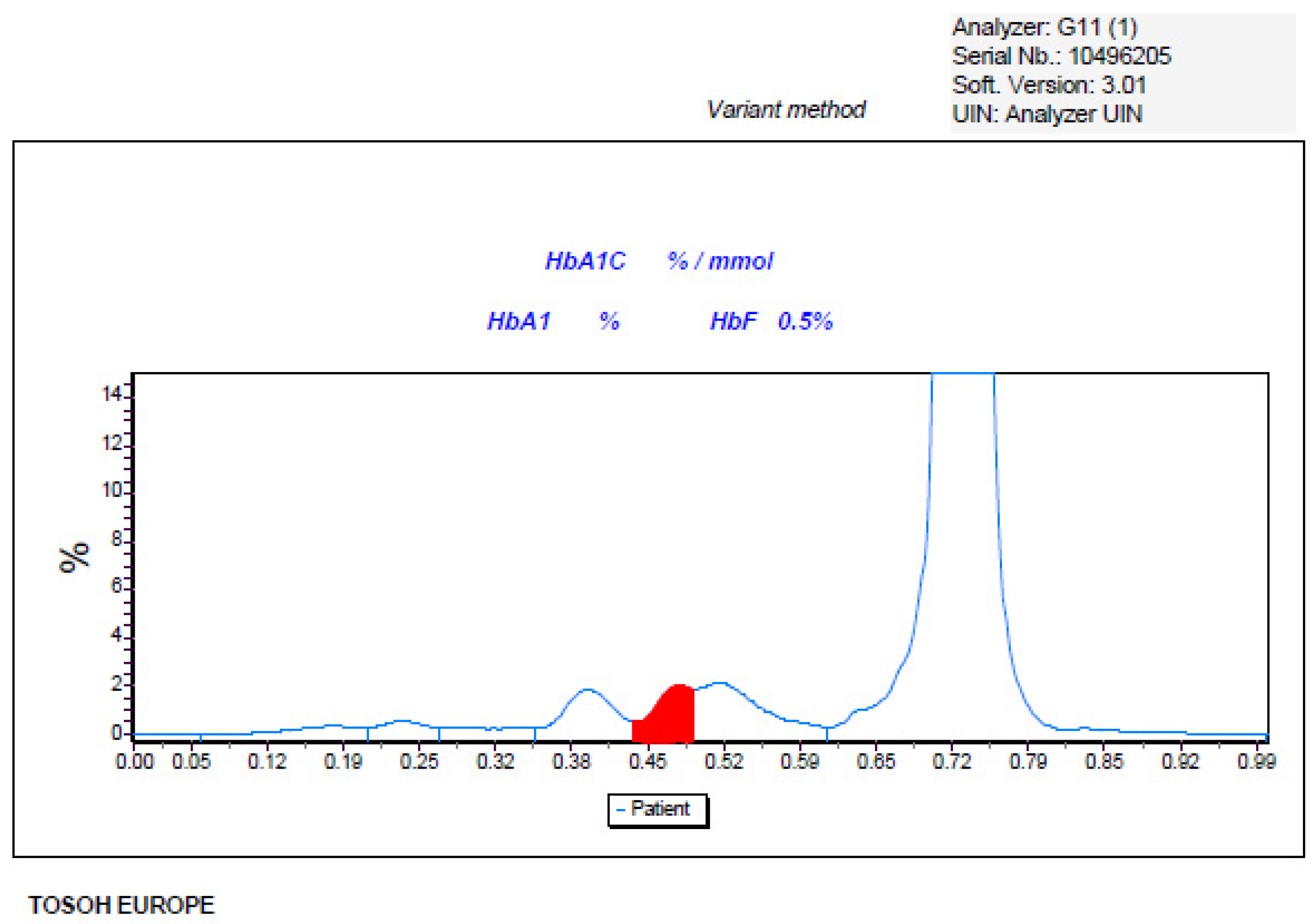
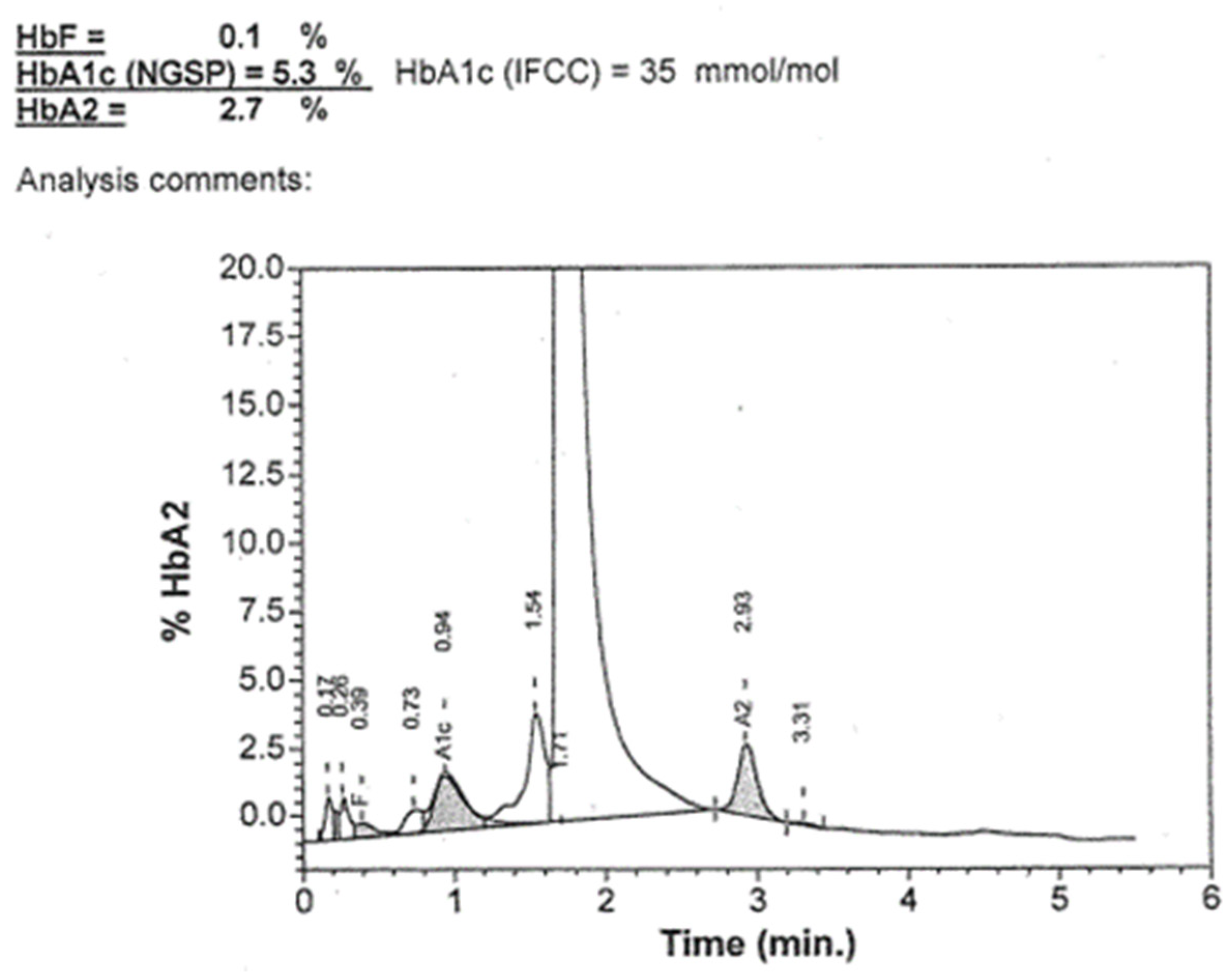
2. Presentation of an Adult Unusual Case of Haemoglobinopathy
3. Molecular Method Diagnosis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Old, J.; Harteveld, C.L.; Traeger-Synodinos, J.; Petrou, M.; Angastiniotis, M.; Galanello, R. Prevention of Thalassaemias and Other Haemoglobin Disorders, 2nd ed.; Volume 2: Laboratory Protocols; Thalassaemia International Federation: Nicosia, Cyprus, 2012. [Google Scholar]
- Ryan, K.; Bain, B.J.; Worthington, D.; James, J.; Plews, D.; Mason, A.; Roper, D.; Rees, D.C.; de la Salle, B.; Streetly, A. British Committee for Standards in Haematology. Significant haemoglobinopathies: Guidelines for screening and diagnosis. Br. J. Haematol. 2010, 149, 35–49. [Google Scholar] [CrossRef]
- Traeger-Synodinos, J.; Harteveld, C.L.; Old, J.M.; Petrou, M.; Galanello, R.; Giordano, P.; Angastioniotis, M.; De la Salle, B.; Henderson, S.; May, A. EMQN haemoglobinopathies best practice meeting. EMQN Best Practice Guidelines for molecular and haematology methods for carrier identification and prenatal diagnosis of the haemoglobinopathies. Eur. J. Hum. Genet. 2015, 23, 426–437. [Google Scholar] [CrossRef]
- Van Delft, P.; Lenters, E.; Bakker-Verweij, M.; de Korte, M.; Baylan, U.; Harteveld, C.L.; Giordano, P.C. Evaluating five dedicated automatic devices for haemoglobinopathy diagnostics in multi-ethnic populations. Int. J. Lab. Hematol. 2009, 31, 484–495. [Google Scholar] [CrossRef] [PubMed]
- Degandt, S.; Coens, R.; Cauwelier, B.; Devos, H.; Langlois, M.; Emmerechts, J. Evaluation of four hemoglobin separation analyzers for hemoglobinopathy diagnosis. J. Clin. Lab. Anal. 2018, 32, e22224. [Google Scholar] [CrossRef]
- Greene, D.N.; Vaughn, C.P.; Crews, B.O.; Agarwal, A.M. Advances in detection of hemoglobinopathies. Clin. Chim. Acta 2015, 439, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Joutovsky, A.; Hadzi-Nesic, J.; Nardi, M.A. HPLC retention time as a diagnostic tool for hemoglobin variants and hemoglobinopathies: A study of 60000 samples in a clinical diagnostic laboratory. Clin. Chem. 2004, 50, 1736–1747. [Google Scholar] [CrossRef]
- Mario, N.; Baudin, B.; Bruneel, A.; Janssens, J.; Vaubourdolle, M. Capillary zone electrophoresis for the diagnosis of congenital hemoglobinopathies. Clin. Chem. 1999, 45, 285–288. [Google Scholar] [CrossRef]
- Modell, B.; Darlison, M. Global epidemiology of haemoglobin disorders and derived service indicators. Bull. World Health Organ. 2008, 86, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Wada, Y.; Hayashi, A.; Fujita, T.; Matsuo, T.; Katakuse, I.; Matsuda, H. Structural analysis of human hemoglobin variants with field desorption mass spectrometry. Biochim. Biophys. Acta 1981, 667, 233–241. [Google Scholar] [CrossRef]
- Wild, B.J.; Green, B.N.; Stephens, A.D. The potential of electrospray ionization mass spectrometry for the diagnosis of hemoglobin variants found in newborn screening. Blood Cells Mol. Dis. 2004, 33, 308–317. [Google Scholar] [CrossRef]
- Daniel, Y.A.; Turner, C.; Haynes, R.M.; Hunt, B.J.; Dalton, R.N. Rapid and specific detection of clinically significant haemoglobinopathies using electrospray mass spectrometry—mass spectrometry. Br. J. Haematol. 2005, 130, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Old, J.M. Screening and genetic diagnosis of haemoglobinopathies. Scand. J. Clin. Lab. Investig. 2007, 67, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Arishi, W.A.; Alhadrami, H.A.; Zourob, M. Techniques for the Detection of Sickle Cell Disease: A Review. Micromachines 2021, 12, 519. [Google Scholar] [CrossRef] [PubMed]
- Harteveld, C.L.; Achour, A.; Arkesteijn, S.J.G.; Ter Huurne, J.; Verschuren, M.; Bhagwandien-Bisoen, S.; Schaap, R.; Vijfhuizen, L.; El Idrissi, H.; Koopmann, T.T. The hemoglobinopathies, molecular disease mechanisms and diagnostics. Int. J. Lab. Hematol. 2022, 44 (Suppl. S1), 28–36. [Google Scholar] [CrossRef] [PubMed]
- Achour, A.; Koopmann, T.T.; Baas, F.; Harteveld, C.L. The Evolving Role of Next-Generation Sequencing in Screening and Diagnosis of Hemoglobinopathies. Front. Physiol. 2021, 12, 686689. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, M.H.; Sebastiani, P. Genetic Modifiers of Sickle Cell Disease. Am. J. Hematol. 2012, 87, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Tukiainen, T.; Villani, A.C.; Yen, A.; Rivas, M.A.; Marshall, J.L.; Satija, R.; Aguirre, M.; Gauthier, L.; Fleharty, M.; Kirby, A.; et al. Landscape of X chromosome inactivation across human tissues. Nature 2017, 550, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Piel, F.B.; Steinberg, M.H.; Rees, D.C. Sickle cell disease. N. Engl. J. Med. 2017, 376, 1561–1573. [Google Scholar] [CrossRef] [PubMed]
- Dupuy, A.M.; Cristol, J.P.; Bargnoux, A.S.; Plawecki, M.; Lotierzo, M.; Aguilar-Martinez, P.; Badiou, S. The pivotal role of HbA1c assay to detect hemoglobinopathies: A 5-year observational retrospective study in the population of Southern France. Health Sci. Rep. 2023, 6, e1270. [Google Scholar] [CrossRef]
- Dessi, M.; Pieri, M.; Pignalosa, S.; Martino, F.G.; Zenobi, R. Performances of capillary electrophoresis and HPLC methods in HbA1c determination: Diagnostic accuracy in HbS and HbD-Iran variants’ presence. J. Clin. Lab. Anal. 2015, 29, 57–60. [Google Scholar] [CrossRef]
- Sacks, D.B.; Arnold, M.; Bakris, G.L.; Bruns, D.E.; Horvath, A.R.; Kirkman, M.S.; Lernmark, A.; Metzger, B.E.; Nathan, D.M. National Academy of Clinical Biochemistry; Evidence-Based Laboratory Medicine Committee of the American Association for Clinical Chemistry. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Diabetes Care 2011, 34, 61–99. [Google Scholar] [CrossRef] [PubMed]
- Arbiol-Roca, A.; Pérez-Hernández, E.A.; Aisa-Abdellaoui, N.; Valls-Guallar, T.; Gálvez-Carmona, F.; Mariano-Serrano, E.; Medina-Casanovas, M.; Ruiz-Morer, M.R. The utility HBA1c test as a screening biomarker for detecting gestational diabetes mellitus. Clin. Biochem. 2021, 90, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Weykamp, C. HbA1c: A review of analytical and clinical aspects. Ann. Lab. Med. 2013, 33, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Little, R.R.; La’ulu, S.L.; Hanson, S.E.; Rohlfing, C.L.; Schmidt, R.L. Effects of 49 Different Rare Hb Variants on HbA1c Measurement in Eight Methods. J. Diabetes Sci. Technol. 2015, 9, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.S.; Chen, C.H.; Yang, H.C. Haemoglobin A1C Levels Constantly Below Lower Reference Limit in a Diabetic Patient with Microcytic Anaemia. Eur. J. Case Rep. Intern. Med. 2019, 6, 001338. [Google Scholar] [PubMed]
- Xu, A.; Ji, L.; Chen, W.; Xia, Y.; Zhou, Y. Effects of α-Thalassemia on HbA1c Measurement. J. Clin. Lab. Anal. 2016, 30, 1078–1080. [Google Scholar] [CrossRef] [PubMed]
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Bissé, E.; Schauber, C.; Zorn, N.; Epting, T.; Eigel, A.; Van Dorsselaer, A.; Wieland, H.; Kister, J.; Kiger, L. Hemoglobin Görwihl [α2β25(A2)Pro→Ala], an electrophoretically silent variant with impaired glycation. Clin. Chem. 2003, 49, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Vinciguerra, M.; Passarello, C.; Leto, F.; Cassarà, F.; Cannata, M.; Maggio, A.; Giambona, A. Co-inheritance of the rare β hemoglobin variants Hb Yaounde, Hb Görwihl and Hb City of Hope with other alterations in globin genes: Impact in genetic counselling. Eur. J. Haematol. 2015, 94, 322–329. [Google Scholar] [CrossRef]
- Bellia, C.; Zaninotto, M.; Cosma, C.; Agnello, L.; Bivona, G.; Marinova, M.; Lo Sasso, B.; Plebani, M.; Ciaccio, M. Clinical usefulness of Glycated Albumin in the diagnosis of diabetes: Results from an Italian study. Clin. Biochem. 2018, 54, 68–72. [Google Scholar] [CrossRef]
- Farmakis, D.; Aessopos, A. Pulmonary Hypertension Associated with Hemoglobinopathies. Circulation 2011, 123, 1227–1232. [Google Scholar] [CrossRef] [PubMed]

| Characteristics of Routine Laboratory Methods | |||
|---|---|---|---|
| Complete Blood Count (CBC) | High-Performance Liquid Chromatography (HPLC) | Haemoglobin Electrophoresis | Molecular Genetic Testing |
| This routine test provides valuable information about the cellular components of blood, including red blood cell (RBC) count, haemoglobin concentration, haematocrit, mean corpuscular volume (MCV), mean corpuscular haemoglobin (MCH), and mean corpuscular haemoglobin concentration (MCHC). Anomalies in these parameters, such as microcytosis, hypochromia, or anisocytosis, may indicate the presence of haemoglobinopathies. | This provides high sensitivity, accuracy, and ability to quantify different haemoglobin variants. HPLC separates haemoglobin variants based on their interaction with a chromatographic column and detection system. This method allows for the identification and quantification of both common and rare haemoglobin variants, making it a valuable tool for diagnosing haemoglobinopathies. | This technique relies on the principle that haemoglobin variants migrate at different rates under an electric field due to variations in their charge and size. Haemoglobin variants commonly assessed include haemoglobin A (HbA), haemoglobin A2 (HbA2), haemoglobin F (HbF), and various abnormal haemoglobins associated with haemoglobinopathies. | Molecular genetic testing plays a crucial role in confirming the diagnosis of haemoglobinopathies and identifying specific genetic mutations responsible for the disorder. Techniques such as polymerase chain reaction (PCR), DNA sequencing, and gene-specific mutation analysis are used to detect point mutations, deletions, or insertions in the globin genes. Molecular genetic testing provides valuable information about the genotype of the patient, which is essential for genetic counselling and family planning. |
| Parameter | Results | Reference Intervals |
|---|---|---|
| Haematology results | ||
| RBC (×1012/L) | 4.4 | 4.5–6.00 |
| Haemoglobin (g/L) | 146 | 140–180 |
| MCV (fL) | 93 | 80–95 |
| MCH (pg) | 32 | 26–32 |
| MCHC (g/L) | 357 | 320–360 |
| Biochemistry results | ||
| ALT | 24 | ≤55 |
| AST | 19 | ≤34 |
| Total Cholesterol | 215 * | ≤190 |
| Triglycerides | 185 * | ≤150 |
| HDL-cholesterol | 40 | ≥40 |
| OGTT | ||
| Glucose (0 h) mg/dL | 112 * | 70–100 |
| Glucose (1 h) mg/dL | 211 * | <155 |
| Glucose (2 h) mg/dL | 108 | <140 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salvatici, M.; Caslini, C.; Alesci, S.; Arosio, G.; Meroni, G.; Ceriotti, F.; Ammirabile, M.; Drago, L. The Application of Clinical and Molecular Diagnostic Techniques to Identify a Rare Haemoglobin Variant. Int. J. Mol. Sci. 2024, 25, 6781. https://doi.org/10.3390/ijms25126781
Salvatici M, Caslini C, Alesci S, Arosio G, Meroni G, Ceriotti F, Ammirabile M, Drago L. The Application of Clinical and Molecular Diagnostic Techniques to Identify a Rare Haemoglobin Variant. International Journal of Molecular Sciences. 2024; 25(12):6781. https://doi.org/10.3390/ijms25126781
Chicago/Turabian StyleSalvatici, Michela, Cecilia Caslini, Simona Alesci, Grazia Arosio, Giuliana Meroni, Ferruccio Ceriotti, Massimiliano Ammirabile, and Lorenzo Drago. 2024. "The Application of Clinical and Molecular Diagnostic Techniques to Identify a Rare Haemoglobin Variant" International Journal of Molecular Sciences 25, no. 12: 6781. https://doi.org/10.3390/ijms25126781
APA StyleSalvatici, M., Caslini, C., Alesci, S., Arosio, G., Meroni, G., Ceriotti, F., Ammirabile, M., & Drago, L. (2024). The Application of Clinical and Molecular Diagnostic Techniques to Identify a Rare Haemoglobin Variant. International Journal of Molecular Sciences, 25(12), 6781. https://doi.org/10.3390/ijms25126781







