New Insights into Aptamers: An Alternative to Antibodies in the Detection of Molecular Biomarkers
Abstract
1. Introduction
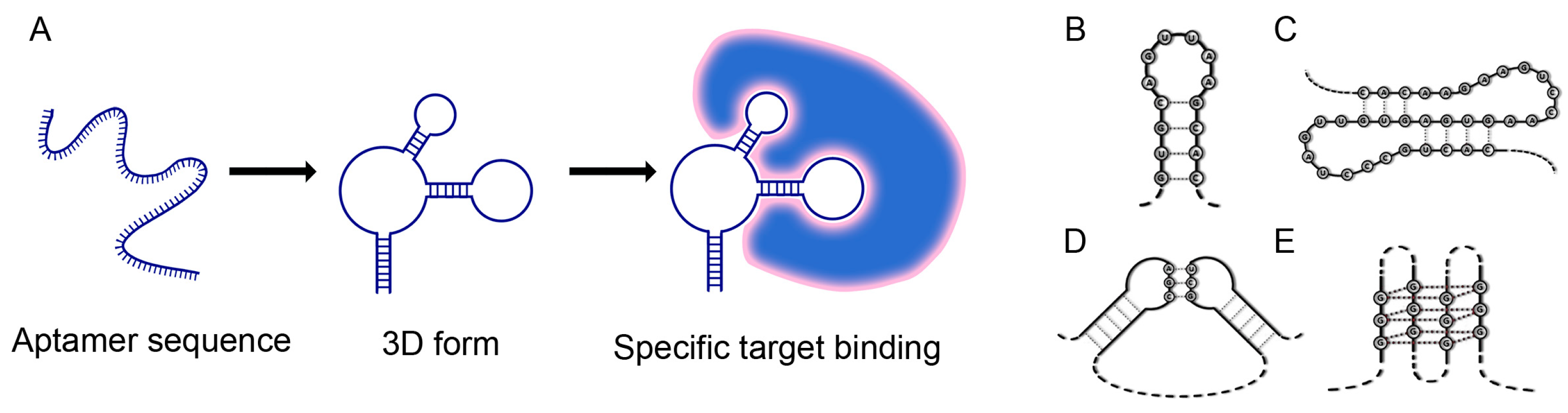
2. Aptamers
2.1. Aptamer Structure
2.2. Advantages of Aptamers
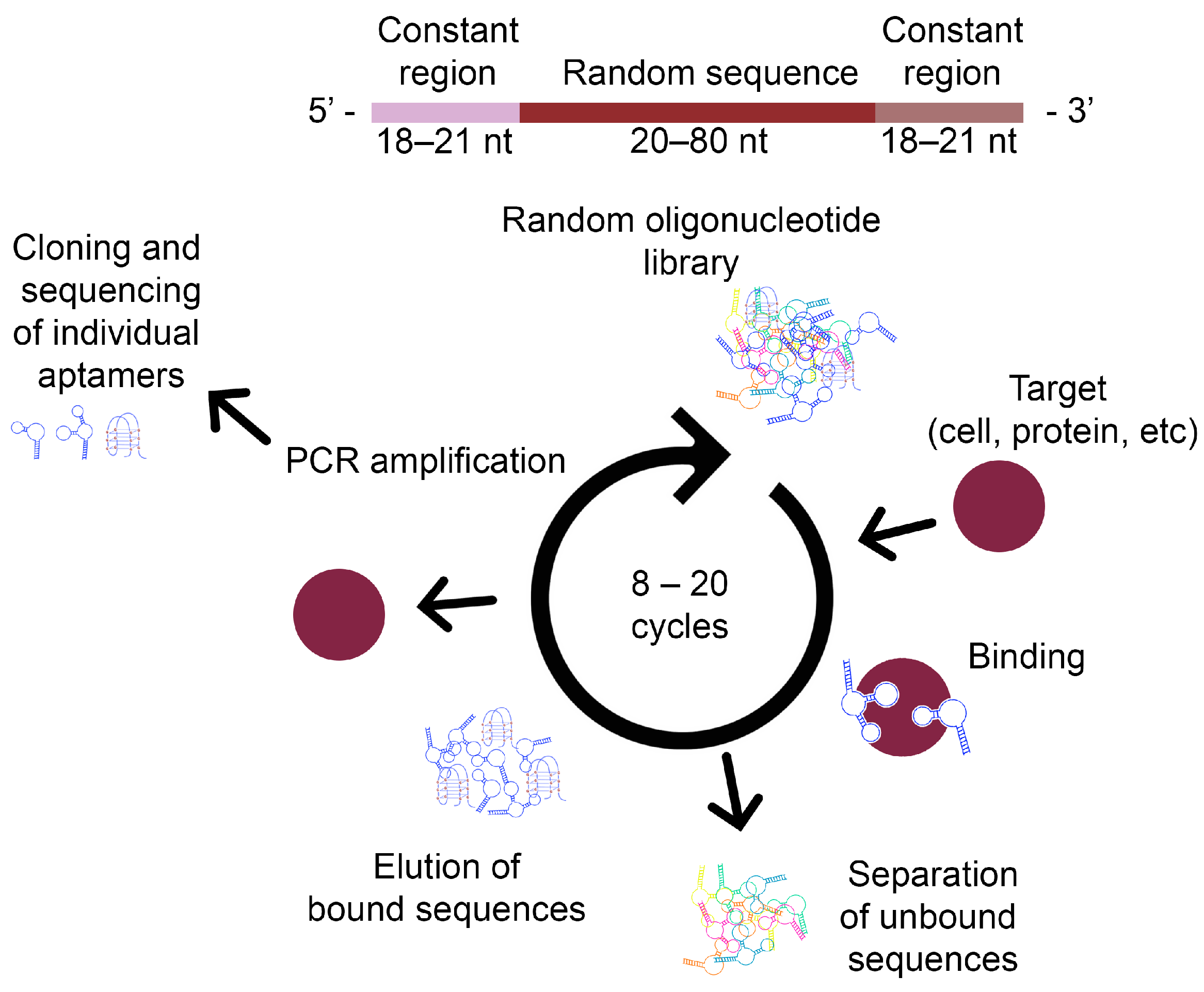
3. Generating Aptamers—SELEX System
3.1. Steps of the SELEX Process
3.1.1. DNA Library Design
3.1.2. Characterisation of Aptamer–Target Ligand Binding
3.1.3. Selection of Suitable Aptamers
3.1.4. Amplification of Selected Aptamers
3.1.5. Separation of Amplified Aptamers
3.2. Cell-SELEX, New Biomarker Discovery
3.3. SELEX Based on Cellular Uptake
3.4. Tissue-SELEX
3.5. In Vivo Whole Organism SELEX
4. Implementation of Aptamers
Mechanisms of Analysis and Detection Strategies
- Single-binding mode: Labelled aptamers are used to detect surface-bound target molecules [93].
- Sandwich mode: Two aptamers, each sensitive to different epitopes of target molecules, are used. Typically, one aptamer is immobilised on the surface of the sensor and binds a ligand to which a second labelled aptamer is then bound. This method involves the use of two aptamers, one for capturing the target ligand and the other for its detection [94].
- Competitive displacement: One component (aptamer or analyte) is immobilised on the sensor surface. In the case of immobilised aptamers, the labelled target ligand binds to the immobilised aptamer. This standard is competitively displaced from binding by the unlabelled ligand originating from the analyte which reduces the signal intensity of the labelled standard [95].
- Target-induced structure switching (TISS): This is based on the mechanism of induced binding adaptation. Conformational changes during the binding of a target molecule are used to generate a signal [96].
- Target-induced dissociation (TID): Because aptamers are oligonucleotides, it is possible to design complementary oligonucleotides that hybridise to the aptamer in the absence of the target molecule. In its presence, the complementary sequence dissociates from the aptamer and is replaced by the target molecule [97].
- Target-induced regeneration of aptamer fragments (TIR): The aptamer can be divided into two parts that do not interact with each other in the absence of the target molecule. In its presence, aptamer fragments re-join and form a trimolecular complex with the target molecule [98].
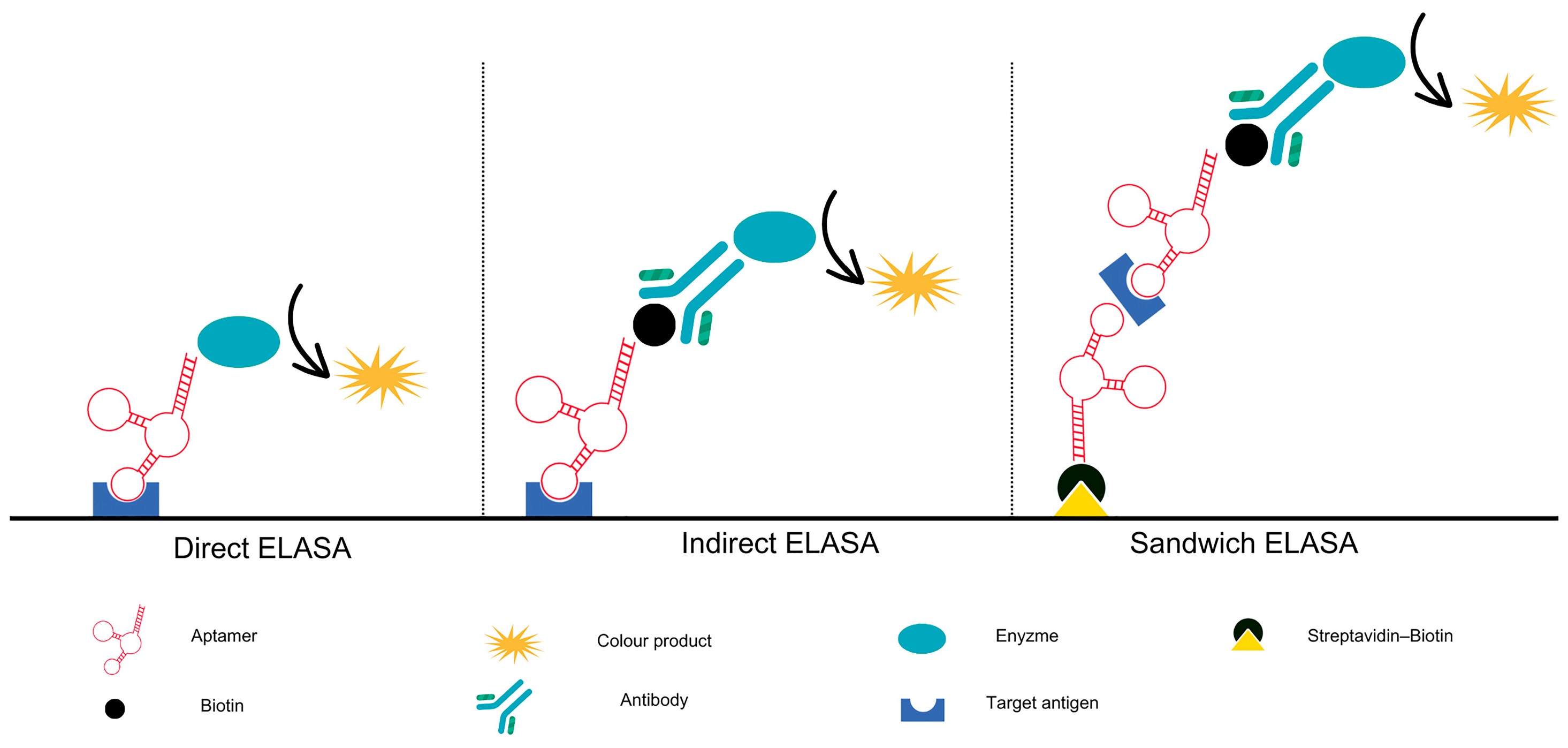
5. Immunodiagnostic Methods Based on Aptamers
5.1. Adoption of Enzyme-Linked Immunosorbent Assay (ELISA) Procedures for Aptamer-Based Analysis
5.2. Replacement of Immunophenotyping by Analysis Based on Aptamers
5.3. Histochemistry/Cytochemistry Based on Aptamers as an Alternative to Immunohisto-/Cyto-Chemistry
5.4. Lateral Flow Assays
6. Aptamer Nanoconjugates
- AuNPs can be used as labels to amplify the detection signal [141].
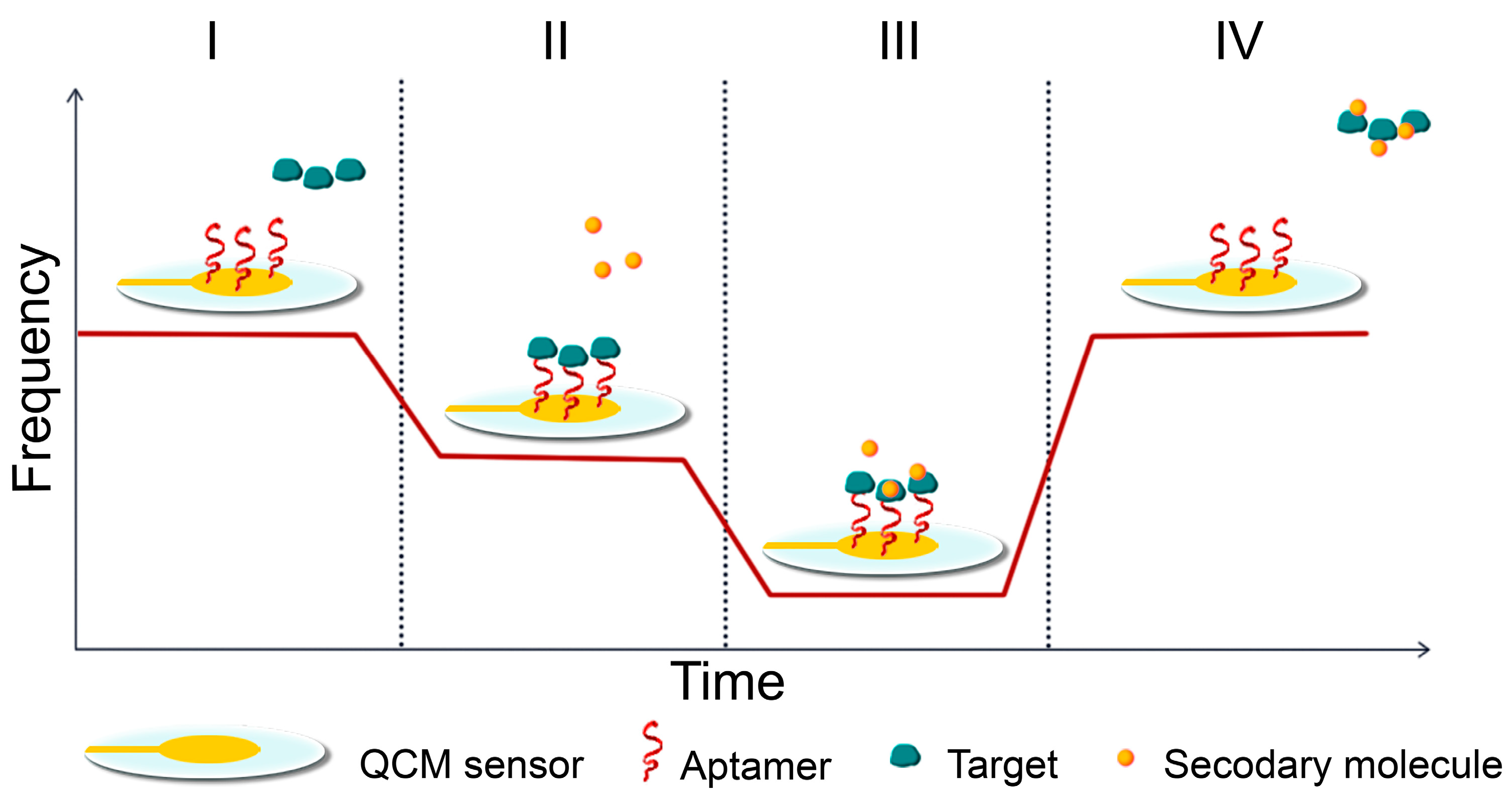
7. Aptasensors
Quartz Crystal Microbalance (QCM) Aptasensors
8. Conclusions and Future Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Thiviyanathan, V.; Gorenstein, D.G. Aptamers and the next generation of diagnostic reagents. Proteom.—Clin. Appl. 2012, 6, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Bruno, J.G.; Kumar, A.; Sharma, T.K. Aptamers in the Therapeutics and Diagnostics Pipelines. Theranostics 2018, 8, 4016–4032. [Google Scholar] [CrossRef] [PubMed]
- Ku, T.-H.; Zhang, T.; Luo, H.; Yen, T.M.; Chen, P.-W.; Han, Y.; Lo, Y.-H. Nucleic Acid Aptamers: An Emerging Tool for Biotechnology and Biomedical Sensing. Sensors 2015, 15, 16281–16313. [Google Scholar] [CrossRef]
- Patel, D.J.; Suri, A.K.; Jiang, F.; Jiang, L.; Fan, P.; Kumar, R.; Nonin, S. Structure, recognition and adaptive binding in RNA aptamer complexes. J. Mol. Biol. 1997, 272, 645–664. [Google Scholar] [CrossRef] [PubMed]
- Osborne, S.E.; Ellington, A.D. Nucleic Acid Selection and the Challenge of Combinatorial Chemistry. Chem. Rev. 1997, 97, 349–370. [Google Scholar] [CrossRef] [PubMed]
- Hermann, T.; Patel, D.J. Adaptive Recognition by Nucleic Acid Aptamers. Science 2000, 287, 820–825. [Google Scholar] [CrossRef] [PubMed]
- Song, K.-M.; Lee, S.; Ban, C. Aptamers and Their Biological Applications. Sensors 2012, 12, 612–631. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Rossi, J. Aptamers as targeted therapeutics: Current potential and challenges. Nat. Rev. Drug Discov. 2017, 16, 181–202, Erratum in Nat. Rev. Drug Discov. 2017, 16, 440. [Google Scholar] [CrossRef]
- Radi, A.-E. Electrochemical Aptamer-Based Biosensors: Recent Advances and Perspectives. Int. J. Electrochem. 2011, 2011, 863196. [Google Scholar] [CrossRef]
- Ștefan, G.; Hosu, O.; De Wael, K.; Lobo-Castañón, M.J.; Cristea, C. Aptamers in biomedicine: Selection strategies and recent advances. Electrochim. Acta 2021, 376, 137994. [Google Scholar] [CrossRef]
- Jenison, R.D.; Gill, S.C.; Pardi, A.; Polisky, B. High-Resolution Molecular Discrimination by RNA. Science 1994, 263, 1425–1429. [Google Scholar] [CrossRef] [PubMed]
- Geiger, A.; Burgstaller, P.; von der Eltz, H.; Roeder, A.; Famulok, M. RNA aptamers that bind L-arginine with sub-micromolar dissociation constants and high enantioselectivity. Nucleic Acids Res. 1996, 24, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Niazi, J.H.; Lee, S.J.; Kim, Y.S.; Gu, M.B. ssDNA aptamers that selectively bind oxytetracycline. Bioorganic Med. Chem. 2008, 16, 1254–1261. [Google Scholar] [CrossRef] [PubMed]
- Odeh, F.; Nsairat, H.; Alshaer, W.; Ismail, M.A.; Esawi, E.; Qaqish, B.; Al Bawab, A.; Ismail, S.I. Aptamers Chemistry: Chemical Modifications and Conjugation Strategies. Molecules 2019, 25, 3. [Google Scholar] [CrossRef] [PubMed]
- You, K.M.; Lee, S.H.; Im, A.; Lee, S.B. Aptamers as functional nucleic acids:In vitro selection and biotechnological applications. Biotechnol. Bioprocess Eng. 2003, 8, 64–75. [Google Scholar] [CrossRef]
- Lakhin, A.V.; Tarantul, V.Z.; Gening, L.V. Aptamers: Problems, solutions and prospects. Acta Nat. 2013, 5, 34–43. [Google Scholar] [CrossRef]
- Liu, S.; Xu, Y.; Jiang, X.; Tan, H.; Ying, B. Translation of aptamers toward clinical diagnosis and commercialization. Biosens. Bioelectron. 2022, 208, 114168. [Google Scholar] [CrossRef] [PubMed]
- Fischer, N.O.; Tarasow, T.M.; Tok, J.B.-H. Protein detection via direct enzymatic amplification of short DNA aptamers. Anal. Biochem. 2007, 373, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Gu, M.B. Advances in aptamer screening and small molecule aptasensors. Adv. Biochem. Eng.-Ing/Biotechnol. 2014, 140, 29–67. [Google Scholar] [CrossRef]
- Pfeiffer, F.; Mayer, G. Selection and Biosensor Application of Aptamers for Small Molecules. Front. Chem. 2016, 4, 25. [Google Scholar] [CrossRef]
- Hasegawa, H.; Savory, N.; Abe, K.; Ikebukuro, K. Methods for Improving Aptamer Binding Affinity. Molecules 2016, 21, 421. [Google Scholar] [CrossRef] [PubMed]
- Plach, M.; Schubert, T. Biophysical Characterization of Aptamer-Target Interactions. Adv. Biochem. Eng.-Ing/Biotechnol. 2020, 174, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ellington, A.D.; Szostak, J.W. In vitro selection of RNA molecules that bind specific ligands. Nature 1990, 346, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Robertson, D.L.; Joyce, G.F. Selection in vitro of an RNA enzyme that specifically cleaves single-stranded DNA. Nature 1990, 344, 467–468. [Google Scholar] [CrossRef] [PubMed]
- Tuerk, C.; Gold, L. Systematic Evolution of Ligands by Exponential Enrichment: RNA Ligands to Bacteriophage T4 DNA Polymerase. Science 1990, 249, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Komarova, N.; Kuznetsov, A. Inside the Black Box: What Makes SELEX Better? Molecules 2019, 24, 3598. [Google Scholar] [CrossRef]
- Ho, S. Potent antisense oligonucleotides to the human multidrug resistance-1 mRNA are rationally selected by mapping RNA-accessible sites with oligonucleotide libraries. Nucleic Acids Res. 1996, 24, 1901–1907. [Google Scholar] [CrossRef] [PubMed]
- Bock, L.C.; Griffin, L.C.; Latham, J.A.; Vermaas, E.H.; Toole, J.J. Selection of single-stranded DNA molecules that bind and inhibit human thrombin. Nature 1992, 355, 564–566. [Google Scholar] [CrossRef]
- Marshall, K.A.; Ellington, A.D. In vitro selection of RNA aptamers. Methods Enzymol. 2000, 318, 193–214. [Google Scholar] [CrossRef]
- Sampson, T. Aptamers and SELEX: The technology. World Pat. Inf. 2003, 25, 123–129. [Google Scholar] [CrossRef]
- Hamula, C.L.; Zhang, H.; Li, F.; Wang, Z.; Le, X.C.; Li, X.-F. Selection and analytical applications of aptamers binding microbial pathogens. TrAC Trends Anal. Chem. 2011, 30, 1587–1597. [Google Scholar] [CrossRef] [PubMed]
- Spill, F.; Weinstein, Z.B.; Shemirani, A.I.; Ho, N.; Desai, D.; Zaman, M.H. Controlling uncertainty in aptamer selection. Proc. Natl. Acad. Sci. USA 2016, 113, 12076–12081. [Google Scholar] [CrossRef] [PubMed]
- Sefah, K.; Shangguan, D.; Xiong, X.; O’Donoghue, M.B.; Tan, W. Development of DNA aptamers using Cell-SELEX. Nat. Protoc. 2010, 5, 1169–1185. [Google Scholar] [CrossRef] [PubMed]
- Fitzwater, T.; Polisky, B. A SELEX primer. Methods Enzymol. 1996, 267, 275–301. [Google Scholar] [CrossRef] [PubMed]
- Naimuddin, M.; Kitamura, K.; Kinoshita, Y.; Honda-Takahashi, Y.; Murakami, M.; Ito, M.; Yamamoto, K.; Hanada, K.; Husimi, Y.; Nishigaki, K. Selection-by-function: Efficient enrichment of cathepsin E inhibitors from a DNA library. J. Mol. Recognit. 2006, 20, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.; Avci-Adali, M.; Ziemer, G.; Wendel, H.P. Streptavidin-Coated Magnetic Beads for DNA Strand Separation Implicate a Multitude of Problems During Cell-SELEX. Oligonucleotides 2009, 19, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Reinemann, C.; Stoltenburg, R.; Strehlitz, B. Investigations on the Specificity of DNA Aptamers Binding to Ethanolamine. Anal. Chem. 2009, 81, 3973–3978. [Google Scholar] [CrossRef]
- White, R.; Rusconi, C.; Scardino, E.; Wolberg, A.; Lawson, J.; Hoffman, M.; Sullenger, B. Generation of Species Cross-reactive Aptamers Using “Toggle” SELEX. Mol. Ther. 2001, 4, 567–573. [Google Scholar] [CrossRef]
- Piotr, D.; Dariusz, M.; Krzysztof, J. Affinity Chromatography as a Tool for Quantification of Interactions Between Drug Molecules and Their Protein Targets. In Affinity Chromatography; Sameh, M., Ed.; IntechOpen: Rijeka, Croatia, 2012; Chapter 14. [Google Scholar]
- Bridonneau, P.; Chang, Y.-F.; Buvoli, A.V.-B.; O’Connell, D.; Parma, D. Site-Directed Selection of Oligonucleotide Antagonists by Competitive Elution. Antisense Nucleic Acid Drug Dev. 1999, 9, 1–11. [Google Scholar] [CrossRef]
- Yang, X.; Li, N.; Gorenstein, D.G. Strategies for the discovery of therapeutic aptamers. Expert Opin. Drug Discov. 2010, 6, 75–87. [Google Scholar] [CrossRef]
- Gopinath, S.C.B. Methods developed for SELEX. Anal. Bioanal. Chem. 2006, 387, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Fan, X.; NI, Z.; Lis, J.T. Evolutionary dynamics and population control during in vitro selection and amplification with multiple targets. RNA 2002, 8, 1461–1470. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ellington, A.D.; Szostak, J.W. Selection in vitro of single-stranded DNA molecules that fold into specific ligand-binding structures. Nature 1992, 355, 850–852. [Google Scholar] [CrossRef] [PubMed]
- Sola, M.; Menon, A.P.; Moreno, B.; Meraviglia-Crivelli, D.; Soldevilla, M.M.; Cartón-García, F.; Pastor, F. Aptamers Against Live Targets: Is In Vivo SELEX Finally Coming to the Edge? Mol. Ther.-Nucleic Acids 2020, 21, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lai, B.S.; Juhas, M. Recent Advances in Aptamer Discovery and Applications. Molecules 2019, 24, 941. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, M.; Yang, G.; Zhang, D.; Ding, H.; Wang, H.; Fan, M.; Shen, B.; Shao, N. Single-stranded DNA aptamers that bind differentiated but not parental cells: Subtractive systematic evolution of ligands by exponential enrichment. J. Biotechnol. 2003, 102, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Shangguan, D.; Li, Y.; Tang, Z.; Cao, Z.C.; Chen, H.W.; Mallikaratchy, P.; Sefah, K.; Yang, C.J.; Tan, W. Aptamers evolved from live cells as effective molecular probes for cancer study. Proc. Natl. Acad. Sci. USA 2006, 103, 11838–11843. [Google Scholar] [CrossRef] [PubMed]
- Conrad, R.C.; Baskerville, S.; Ellington, A.D. In vitro selection methodologies to probe RNA function and structure. Mol. Divers. 1995, 1, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Hoinka, J.; Zotenko, E.; Friedman, A.; Sauna, Z.E.; Przytycka, T.M. Identification of sequence–structure RNA binding motifs for SELEX-derived aptamers. Bioinformatics 2012, 28, i215–i223. [Google Scholar] [CrossRef]
- Cho, M.; Oh, S.S.; Nie, J.; Stewart, R.; Eisenstein, M.; Chambers, J.; Marth, J.D.; Walker, F.; Thomson, J.A.; Soh, H.T. Quantitative selection and parallel characterization of aptamers. Proc. Natl. Acad. Sci. USA 2013, 110, 18460–18465. [Google Scholar] [CrossRef]
- Elskens, J.P.; Elskens, J.M.; Madder, A. Chemical Modification of Aptamers for Increased Binding Affinity in Diagnostic Applications: Current Status and Future Prospects. Int. J. Mol. Sci. 2020, 21, 4522. [Google Scholar] [CrossRef]
- Takahashi, M.; Wu, X.; Ho, M.; Chomchan, P.; Rossi, J.J.; Burnett, J.C.; Zhou, J. High throughput sequencing analysis of RNA libraries reveals the influences of initial library and PCR methods on SELEX efficiency. Sci. Rep. 2016, 6, 33697. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, B.; Wu, Z.; Liu, G.; Li, W.; Tang, Y. In Silico discovery of aptamers with an enhanced library design strategy. Comput. Struct. Biotechnol. J. 2023, 21, 1005–1013. [Google Scholar] [CrossRef]
- Vant-Hull, B.; Payano-Baez, A.; Davis, R.H.; Gold, L. The mathematics of SELEX against complex targets. J. Mol. Biol. 1998, 278, 579–597. [Google Scholar] [CrossRef] [PubMed]
- Ohuchi, S.P.; Ohtsu, T.; Nakamura, Y. A novel method to generate aptamers against recombinant targets displayed on the cell surface. In Nucleic Acids Symposium Series; Oxford University Press: Oxford, UK, 2005; Volume 49, pp. 351–352. [Google Scholar] [CrossRef]
- Cerchia, L.; Ducongé, F.; Pestourie, C.; Boulay, J.; Aissouni, Y.; Gombert, K.; Tavitian, B.; de Franciscis, V.; Libri, D. Neutralizing Aptamers from Whole-Cell SELEX Inhibit the RET Receptor Tyrosine Kinase. PLoS Biol. 2005, 3, e123. [Google Scholar] [CrossRef]
- Rask-Andersen, M.; Almén, M.S.; Schiöth, H.B. Trends in the exploitation of novel drug targets. Nat. Rev. Drug Discov. 2011, 10, 579–590. [Google Scholar] [CrossRef]
- Quang, N.N.; Miodek, A.; Cibiel, A.; Ducongé, F. Selection of Aptamers Against Whole Living Cells: From Cell-SELEX to Identification of Biomarkers. Methods Mol. Biol. 2017, 1575, 253–272. [Google Scholar] [CrossRef]
- Tang, Z.; Shangguan, D.; Wang, K.; Shi, H.; Sefah, K.; Mallikratchy, P.; Chen, H.W.; Li, Y.; Tan, W. Selection of Aptamers for Molecular Recognition and Characterization of Cancer Cells. Anal. Chem. 2007, 79, 4900–4907. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; You, M.; Pu, Y.; Liu, H.; Ye, M.; Tan, W. Recent developments in protein and cell-targeted aptamer selection and applications. Curr. Med. Chem. 2011, 18, 4117–4125. [Google Scholar] [CrossRef]
- Barman, J. Targeting cancer cells using aptamers: Cell-SELEX approach and recent advancements. RSC Adv. 2015, 5, 11724–11732. [Google Scholar] [CrossRef]
- Mallikaratchy, P.R.; Tang, Z.; Kwame, S.; Meng, L.; Shangguan, D.; Tan, W. Aptamer Directly Evolved from Live Cells Recognizes Membrane Bound Immunoglobin Heavy Mu Chain in Burkitt’s Lymphoma Cells. Mol. Cell. Proteom. 2007, 6, 2230–2238. [Google Scholar] [CrossRef] [PubMed]
- Blank, M.; Weinschenk, T.; Priemer, M.; Schluesener, H. Systematic Evolution of a DNA Aptamer Binding to Rat Brain Tumor Microvessels. J. Biol. Chem. 2001, 276, 16464–16468. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Yu, Y.; Jiang, F.; Zhou, J.; Li, Y.; Liang, C.; Dang, L.; Lu, A.; Zhang, G. Development of Cell-SELEX Technology and Its Application in Cancer Diagnosis and Therapy. Int. J. Mol. Sci. 2016, 17, 2079. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.A.; Lopez-Colon, D.; Zhu, Z.; Xu, Y.; Tan, W. Applications of aptamers in cancer cell biology. Anal. Chim. Acta 2008, 621, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Parekh, P.; Turner, P.; Moyer, R.W.; Tan, W. Generating Aptamers for Recognition of Virus-Infected Cells. Clin. Chem. 2009, 55, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Bakhtiari, H.; Palizban, A.A.; Khanahmad, H.; Mofid, M.R. Novel Approach to Overcome Defects of Cell-SELEX in Developing Aptamers against Aspartate β-Hydroxylase. ACS Omega 2021, 6, 11005–11014. [Google Scholar] [CrossRef] [PubMed]
- Kolm, C.; Cervenka, I.; Aschl, U.J.; Baumann, N.; Jakwerth, S.; Krska, R.; Mach, R.L.; Sommer, R.; DeRosa, M.C.; Kirschner, A.K.T.; et al. DNA aptamers against bacterial cells can be efficiently selected by a SELEX process using state-of-the art qPCR and ultra-deep sequencing. Sci. Rep. 2020, 10, 20917. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Wu, L.; Xu, X.; Wu, Q.; Wang, Y.; Shen, H.; Song, Y.; Wang, H.; Zhu, Z.; Kang, D.; et al. Aptamer Generated by Cell-SELEX for Specific Targeting of Human Glioma Cells. ACS Appl. Mater. Interfaces 2020, 13, 9306–9315. [Google Scholar] [CrossRef] [PubMed]
- Homann, M.; Göringer, H.U. Uptake and intracellular transport of RNA Aptamers in African trypanosomes suggest therapeutic “Piggy-Back” approach. Bioorganic Med. Chem. 2001, 9, 2571–2580. [Google Scholar] [CrossRef]
- Farokhzad, O.C.; Jon, S.; Khademhosseini, A.; Tran, T.N.; Lavan, D.A.; Langer, R. Nanoparticle-aptamer bioconjugates: A new approach for targeting prostate cancer cells. Cancer Res. 2004, 64, 7668–7672. [Google Scholar] [CrossRef]
- Wu, C.C.N.; Castro, J.E.; Motta, M.; Cottam, H.B.; Kyburz, D.; Kipps, T.J.; Corr, M.; Carson, D.A. Selection of Oligonucleotide Aptamers with Enhanced Uptake and Activation of Human Leukemia B Cells. Hum. Gene Ther. 2003, 14, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Kolesnikova, O.; Kazakova, H.; Comte, C.; Steinberg, S.; Kamenski, P.; Martin, R.P.; Tarassov, I.; Entelis, N. Selection of RNA aptamers imported into yeast and human mitochondria. RNA 2010, 16, 926–941. [Google Scholar] [CrossRef]
- Udofot, O.; Lin, L.-H.; Thiel, W.H.; Erwin, M.; Turner, E.; Miller, F.J.; Giangrande, P.H.; Yazdani, S.K. Delivery of Cell-Specific Aptamers to the Arterial Wall with an Occlusion Perfusion Catheter. Mol. Ther.-Nucleic Acids 2019, 16, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, X.; Volk, D.E.; Lokesh, G.L.-R.; Elizondo-Riojas, M.-A.; Li, L.; Nick, A.M.; Sood, A.K.; Rosenblatt, K.P.; Gorenstein, D.G. Morph-X-Select: Morphology-Based Tissue Aptamer Selection for Ovarian Cancer Biomarker Discovery. BioTechniques 2016, 61, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Chen, Y.H.; Lennox, K.A.; Behlke, M.A.; Davidson, B.L. In vivo SELEX for Identification of Brain-penetrating Aptamers. Mol. Ther. Nucleic Acids 2013, 2, e67. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; He, W.; Jiang, H.; Wu, L.; Xiong, W.; Li, B.; Zhou, Z.; Qian, Y. In vivo SELEX of bone targeting aptamer in prostate cancer bone metastasis model. Int. J. Nanomed. 2019, 14, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, Y.; Yang, H.; Qin, M.; Ding, X.; Liu, R.; Jiang, Y. In Vivo SELEX of an Inhibitory NSCLC-Specific RNA Aptamer from PEGylated RNA Library. Mol. Ther.-Nucleic Acids 2017, 10, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Mi, J.; Liu, Y.; Rabbani, Z.N.; Yang, Z.; Urban, J.H.; Sullenger, B.A.; Clary, B.M. In vivo selection of tumor-targeting RNA motifs. Nat. Chem. Biol. 2009, 6, 22–24. [Google Scholar] [CrossRef] [PubMed]
- Mi, J.; Ray, P.; Liu, J.; Kuan, C.-T.; Xu, J.; Hsu, D.; Sullenger, B.A.; White, R.R.; Clary, B.M. In Vivo Selection Against Human Colorectal Cancer Xenografts Identifies an Aptamer That Targets RNA Helicase Protein DHX9. Mol. Ther.-Nucleic Acids 2016, 5, e315. [Google Scholar] [CrossRef]
- Liu, Y.; Le, C.; Tyrrell, D.L.; Le, X.C.; Li, X.-F. Aptamer Binding Assay for the E Antigen of Hepatitis B Using Modified Aptamers with G-Quadruplex Structures. Anal. Chem. 2020, 92, 6495–6501. [Google Scholar] [CrossRef]
- Gao, F.; Yin, J.; Chen, Y.; Guo, C.; Hu, H.; Su, J. Recent advances in aptamer-based targeted drug delivery systems for cancer therapy. Front. Bioeng. Biotechnol. 2022, 10, 972933. [Google Scholar] [CrossRef]
- Kulabhusan, P.K.; Hussain, B.; Yüce, M. Current Perspectives on Aptamers as Diagnostic Tools and Therapeutic Agents. Pharmaceutics 2020, 12, 646. [Google Scholar] [CrossRef] [PubMed]
- Bohrmann, L.; Burghardt, T.; Haynes, C.; Saatchi, K.; Häfeli, U.O. Aptamers used for molecular imaging and theranostics—Recent developments. Theranostics 2022, 12, 4010–4050. [Google Scholar] [CrossRef] [PubMed]
- Perret, G.; Boschetti, E. Aptamer-Based Affinity Chromatography for Protein Extraction and Purification. Adv. Bio-Chem. Eng. /Biotechnol. 2020, 174, 93–139. [Google Scholar] [CrossRef] [PubMed]
- Ciancio, D.R.; Vargas, M.R.; Thiel, W.H.; Bruno, M.A.; Giangrande, P.H.; Mestre, M.B. Aptamers as Diagnostic Tools in Cancer. Pharmaceuticals 2018, 11, 86. [Google Scholar] [CrossRef] [PubMed]
- Aljohani, M.M.; Cialla-May, D.; Popp, J.; Chinnappan, R.; Al-Kattan, K.; Zourob, M. Aptamers: Potential Diagnostic and Therapeutic Agents for Blood Diseases. Molecules 2022, 27, 383. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhang, D.; Zeng, Z.; Huang, L.; Lin, X.; Hong, S. Aptamer-Based Probes for Cancer Diagnostics and Treatment. Life 2022, 12, 1937. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Cho, J.; Lee, B.-H.; Hwang, D.; Park, J.-W. Design and Prediction of Aptamers Assisted by In Silico Methods. Biomedicines 2023, 11, 356. [Google Scholar] [CrossRef]
- Emami, N.; Pakchin, P.S.; Ferdousi, R. Computational predictive approaches for interaction and structure of aptamers. J. Theor. Biol. 2020, 497, 110268. [Google Scholar] [CrossRef]
- Nimjee, S.M.; Sullenger, B.A. Therapeutic Aptamers: Evolving to Find their Clinical Niche. Curr. Med. Chem. 2020, 27, 4181–4193. [Google Scholar] [CrossRef]
- Hianik, T. Aptamer-Based Biosensors. In Encyclopedia of Interfacial Chemistry; Wandelt, K., Ed.; Elsevier: Oxford, UK, 2018; pp. 11–19. [Google Scholar]
- Prante, M.; Segal, E.; Scheper, T.; Bahnemann, J.; Walter, J. Aptasensors for Point-of-Care Detection of Small Molecules. Biosensors 2020, 10, 108. [Google Scholar] [CrossRef]
- Wang, S.; Liu, J.; Yong, W.; Chen, Q.; Zhang, L.; Dong, Y.; Su, H.; Tan, T. A direct competitive assay-based aptasensor for sensitive determination of tetracycline residue in Honey. Talanta 2015, 131, 562–569. [Google Scholar] [CrossRef]
- Liu, C.; Chen, J.; Mao, G.; Su, C.; Ji, X.; He, Z. Target-induced structure switching of a hairpin aptamer for the fluorescence detection of zeatin. Anal. Methods 2016, 8, 5957–5961. [Google Scholar] [CrossRef]
- Han, K.; Chen, L.; Lin, Z.; Li, G. Target induced dissociation (TID) strategy for the development of electrochemical aptamer-based biosensor. Electrochem. Commun. 2008, 11, 157–160. [Google Scholar] [CrossRef]
- Liu, X.; Shi, L.; Hua, X.; Huang, Y.; Su, S.; Fan, Q.; Wang, L.; Huang, W. Target-Induced Conjunction of Split Aptamer Fragments and Assembly with a Water-Soluble Conjugated Polymer for Improved Protein Detection. ACS Appl. Mater. Interfaces 2014, 6, 3406–3412. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, A.R.; Plückthun, A. Getting to reproducible antibodies: The rationale for sequenced recombinant characterized reagents. Protein Eng. Des. Sel. 2015, 28, 303–305. [Google Scholar] [CrossRef] [PubMed]
- van der Strate, B.; Longdin, R.; Geerlings, M.; Bachmayer, N.; Cavallin, M.; Litwin, V.; Patel, M.; Passe-Coutrin, W.; Schoelch, C.; Companjen, A.; et al. Best practices in performing flow cytometry in a regulated environment: Feedback from experience within the European Bioanalysis Forum. Bioanalysis 2017, 9, 1253–1264. [Google Scholar] [CrossRef]
- Han, J.; Gao, L.; Wang, J.; Wang, J. Application and development of aptamer in cancer: From clinical diagnosis to cancer therapy. J. Cancer 2020, 11, 6902–6915. [Google Scholar] [CrossRef]
- Song, Y.; Zhu, Z.; An, Y.; Zhang, W.; Zhang, H.; Liu, D.; Yu, C.; Duan, W.; Yang, C.J. Selection of DNA Aptamers against Epithelial Cell Adhesion Molecule for Cancer Cell Imaging and Circulating Tumor Cell Capture. Anal. Chem. 2013, 85, 4141–4149. [Google Scholar] [CrossRef]
- Wang, D.-L.; Song, Y.-L.; Zhu, Z.; Li, X.-L.; Zou, Y.; Yang, H.-T.; Wang, J.-J.; Yao, P.-S.; Pan, R.-J.; Yang, C.J.; et al. Selection of DNA aptamers against epidermal growth factor receptor with high affinity and specificity. Biochem. Biophys. Res. Commun. 2014, 453, 681–685. [Google Scholar] [CrossRef]
- Ferreira, C.S.M.; Matthews, C.S.; Missailidis, S. DNA Aptamers That Bind to MUC1 Tumour Marker: Design and Characterization of MUC1-Binding Single-Stranded DNA Aptamers. Tumor Biol. 2006, 27, 289–301. [Google Scholar] [CrossRef]
- Ranganathan, V.; Srinivasan, S.; Singh, A.; DeRosa, M.C. An aptamer-based colorimetric lateral flow assay for the detection of human epidermal growth factor receptor 2 (HER2). Anal. Biochem. 2020, 588, 113471. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Anand, A.; Jain, N.; Goswami, S.; Anantharaj, A.; Patil, S.; Singh, R.; Kumar, A.; Shrivastava, T.; Bhatnagar, S.; et al. A novel G-quadruplex aptamer-based spike trimeric antigen test for the detection of SARS-CoV-2. Mol. Ther.-Nucleic Acids 2021, 26, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.; Ruppert, C.; Rentschler, S.; Laufer, S.; Deigner, H.-P. Combining aptamers and antibodies: Lateral flow quantification for thrombin and interleukin-6 with smartphone readout. Sens. Actuators B Chem. 2020, 333, 129246. [Google Scholar] [CrossRef]
- Chen, X.; Feng, Y.; Chen, H.; Zhang, Y.; Wang, X.; Zhou, N. Fluorescent Aptasensor for Highly Specific Detection of ATP Using a Newly Screened Aptamer. Sensors 2022, 22, 2425. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J. Aptamer-based strategies for recognizing adenine, adenosine, ATP and related compounds. Analyst 2020, 145, 6753–6768. [Google Scholar] [CrossRef] [PubMed]
- Kibar, G.; Şahinoğlu, O.B.; Kılınçlı, B.; Erdem, E.Y.; Çetin, B.; Özalp, V.C. Biosensor for ATP detection via aptamer-modified PDA@POSS nanoparticles synthesized in a microfluidic reactor. Microchim. Acta 2024, 191, 153. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, K.; Stobiecka, M. DNA Aptamer Beacon Probe (ABP) for Monitoring of Adenosine Triphosphate Level in SW480 Cancer Cells Treated with Glycolysis Inhibitor 2-Deoxyglucose. Int. J. Mol. Sci. 2023, 24, 9295. [Google Scholar] [CrossRef]
- Esawi, E.; Alshaer, W.; Mahmoud, I.S.; Alqudah, D.A.; Azab, B.; Awidi, A. Aptamer-Aptamer Chimera for Targeted Delivery and ATP-Responsive Release of Doxorubicin into Cancer Cells. Int. J. Mol. Sci. 2021, 22, 12940. [Google Scholar] [CrossRef]
- Brito, F.d.A.; Santos, S.M.E.; Ferreira, G.A.; Pedrosa, W.; Gradisse, J.; Costa, L.C.; Neves, S.P.F. Diagnostic Evaluation of ELISA and Chemiluminescent Assays as Alternative Screening Tests to Indirect Immunofluorescence for the Detection of Antibodies to Cellular Antigens. Am. J. Clin. Pathol. 2016, 145, 323–331. [Google Scholar] [CrossRef]
- Toh, S.Y.; Citartan, M.; Gopinath, S.C.; Tang, T.-H. Aptamers as a replacement for antibodies in enzyme-linked immunosorbent assay. Biosens. Bioelectron. 2015, 64, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhu, X.; Lu, P.Y.; Rosato, R.R.; Tan, W.; Zu, Y. Oligonucleotide Aptamers: New Tools for Targeted Cancer Therapy. Mol. Ther. Nucleic Acids 2014, 3, e182. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, S.C.; Kumar, P.K. Aptamers that bind to the hemagglutinin of the recent pandemic influenza virus H1N1 and efficiently inhibit agglutination. Acta Biomater. 2013, 9, 8932–8941. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.J.; Lee, J.-W.; Ellington, A.D. Applications of Aptamers as Sensors. Annu. Rev. Anal. Chem. 2009, 2, 241–264. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Z.; Li, J.; Huang, X.; Wei, J.; Yang, J.; Guan, L.; Wen, X.; Wang, S.; Qin, Q. A Novel Sandwich ELASA Based on Aptamer for Detection of Largemouth Bass Virus (LMBV). Viruses 2022, 14, 945. [Google Scholar] [CrossRef] [PubMed]
- Moshref, Z.S.; Jalali, T.; Adriani, R.R.; Soltati, E.; Gargari, S.L.M. Aptamer-based diagnosis of various SARS-CoV2 strains isolated from clinical specimens. Heliyon 2023, 9, e16458. [Google Scholar] [CrossRef] [PubMed]
- Thevendran, R.; Rogini, S.; Leighton, G.; Mutombwera, A.; Shigdar, S.; Tang, T.-H.; Citartan, M. The Diagnostic Potential of RNA Aptamers against the NS1 Protein of Dengue Virus Serotype 2. Biology 2023, 12, 722. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Fernández, A.; Ferapontova, E.E. Electrochemical ELASA: Improving early cancer detection and monitoring. Anal. Bioanal. Chem. 2023, 415, 3831–3846. [Google Scholar] [CrossRef]
- Wu, Y.; Sefah, K.; Liu, H.; Wang, R.; Tan, W. DNA aptamer–micelle as an efficient detection/delivery vehicle toward cancer cells. Proc. Natl. Acad. Sci. USA 2009, 107, 5–10. [Google Scholar] [CrossRef]
- Bauer, M.; Strom, M.; Hammond, D.S.; Shigdar, S. Anything You Can Do, I Can Do Better: Can Aptamers Replace Antibodies in Clinical Diagnostic Applications? Molecules 2019, 24, 4377. [Google Scholar] [CrossRef]
- Zeng, Z.; Zhang, P.; Zhao, N.; Sheehan, A.M.; Tung, C.-H.; Chang, C.-C.; Zu, Y. Using oligonucleotide aptamer probes for immunostaining of formalin-fixed and paraffin-embedded tissues. Mod. Pathol. 2010, 23, 1553–1558. [Google Scholar] [CrossRef] [PubMed]
- Koji, T. In Situ Localization of Gene-Specific Transcription Regulatory Factors by Southwestern Histochemistry. Acta Histochem. Cytochem. 1999, 32, 255–260. [Google Scholar] [CrossRef]
- Shin, M.; Hishikawa, Y.; Izumi, S.-I.; Koji, T. Southwestern histochemistry as a molecular histochemical tool for analysis of expression of transcription factors: Application to paraffin-embedded tissue sections. Med Mol. Morphol. 2002, 35, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xiong, H.; Chen, R.; Wan, L.; Kong, Y.; Rao, J.; Xie, Y.; Huang, C.; Zhang, X.-L. Aptamer Detection of Mycobaterium tuberculosis Mannose-Capped Lipoarabinomannan in Lesion Tissues for Tuberculosis Diagnosis. Front. Cell. Infect. Microbiol. 2021, 11, 34915. [Google Scholar] [CrossRef] [PubMed]
- Reid, R.; Chatterjee, B.; Das, S.J.; Ghosh, S.; Sharma, T.K. Application of aptamers as molecular recognition elements in lateral flow assays. Anal. Biochem. 2020, 593, 113574. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Yuan, J.; Zhang, Y.; Khan, I.M.; Ma, P.; Wang, Z. Lateral flow assays based on aptamers for food safety applications. Food Control. 2024, 155, 110051. [Google Scholar] [CrossRef]
- Bahadır, E.B.; Sezgintürk, M.K. Lateral flow assays: Principles, designs and labels. TrAC Trends Anal. Chem. 2016, 82, 286–306. [Google Scholar] [CrossRef]
- Le, T.T.; Chang, P.; Benton, D.J.; McCauley, J.W.; Iqbal, M.; Cass, A.E.G. Dual Recognition Element Lateral Flow Assay Toward Multiplex Strain Specific Influenza Virus Detection. Anal. Chem. 2017, 89, 6781–6786. [Google Scholar] [CrossRef] [PubMed]
- Jaisankar, A.; Krishnan, S.; Rangasamy, L. Recent developments of aptamer-based lateral flow assays for point-of-care (POC) diagnostics. Anal. Biochem. 2022, 655, 114874. [Google Scholar] [CrossRef]
- Urmann, K.; Modrejewski, J.; Scheper, P.T.; Walter, J.-G. Aptamer-modified nanomaterials: Principles and applications. BioNanoMaterials 2016, 18, 20160012. [Google Scholar] [CrossRef]
- Ajnai, G.; Chiu, A.; Kan, T.; Cheng, C.-C.; Tsai, T.-H.; Chang, J. Trends of Gold Nanoparticle-based Drug Delivery System in Cancer Therapy. J. Exp. Clin. Med. 2014, 6, 172–178. [Google Scholar] [CrossRef]
- Singh, R.; Lillard, J.W., Jr. Nanoparticle-based targeted drug delivery. Exp. Mol. Pathol. 2009, 86, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Doria, G.; Conde, J.; Veigas, B.; Giestas, L.; Almeida, C.; Assunção, M.; Rosa, J.; Baptista, P.V. Noble Metal Nanoparticles for Biosensing Applications. Sensors 2012, 12, 1657–1687. [Google Scholar] [CrossRef] [PubMed]
- Alivisatos, A.P.; Johnsson, K.P.; Peng, X.; Wilson, T.E.; Loweth, C.J.; Bruchez, M.P., Jr.; Schultz, P.G. Organization of ‘nanocrystal molecules’ using DNA. Nature 1996, 382, 609–611. [Google Scholar] [CrossRef] [PubMed]
- Mirkin, C.A.; Letsinger, R.L.; Mucic, R.C.; Storhoff, J.J. A DNA-based method for rationally assembling nanoparticles into macroscopic materials. Nature 1996, 382, 607–609. [Google Scholar] [CrossRef] [PubMed]
- Pavlov, V.; Xiao, Y.; Shlyahovsky, B.; Willner, I. Aptamer-Functionalized Au Nanoparticles for the Amplified Optical Detection of Thrombin. J. Am. Chem. Soc. 2004, 126, 11768–11769. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-C.; Huang, Y.-F.; Cao, Z.; Tan, W.; Chang, H.-T. Aptamer-Modified Gold Nanoparticles for Colorimetric Determination of Platelet-Derived Growth Factors and Their Receptors. Anal. Chem. 2005, 77, 5735–5741. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Li, B.; Wang, E. Analytical potential of gold nanoparticles in functional aptamer-based biosensors. Bioanal. Rev. 2010, 1, 187–208. [Google Scholar] [CrossRef]
- Kannan, P.; John, S.A. Synthesis of mercaptothiadiazole-functionalized gold nanoparticles and their self-assembly on Au substrates. Nanotechnology 2008, 19, 085602. [Google Scholar] [CrossRef]
- Zahra, Q.U.A.; Luo, Z.; Ali, R.; Khan, M.I.; Li, F.; Qiu, B. Advances in Gold Nanoparticles-Based Colorimetric Aptasensors for the Detection of Antibiotics: An Overview of the Past Decade. Nanomaterials 2021, 11, 840. [Google Scholar] [CrossRef]
- Dulkeith, E.; Morteani, A.C.; Niedereichholz, T.; Klar, T.A.; Feldmann, J.; Levi, S.A.; van Veggel, F.C.J.M.; Reinhoudt, D.N.; Möller, M.; Gittins, D.I. Fluorescence Quenching of Dye Molecules near Gold Nanoparticles: Radiative and Nonradiative Effects. Phys. Rev. Lett. 2002, 89, 203002. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Qi, H.; Shen, L.; Gao, Q.; Zhang, C. Electrochemical Aptasensor for the Determination of Cocaine Incorporating Gold Nanoparticles Modification. Electroanalysis 2008, 20, 1475–1482. [Google Scholar] [CrossRef]
- Wang, J.; Meng, W.; Zheng, X.; Liu, S.; Li, G. Combination of aptamer with gold nanoparticles for electrochemical signal amplification: Application to sensitive detection of platelet-derived growth factor. Biosens. Bioelectron. 2009, 24, 1598–1602. [Google Scholar] [CrossRef] [PubMed]
- Walter, J.-G.; Heilkenbrinker, A.; Austerjost, J.; Timur, S.; Stahl, F.; Schepe, T. Aptasensors for Small Molecule Detection. Z. Fur Naturforschung Sect. B-A J. Chem. Sci. 2012, 67, 976–986. [Google Scholar] [CrossRef]
- Garzón, V.; Pinacho, D.G.; Bustos, R.-H.; Garzón, G.; Bustamante, S. Optical Biosensors for Therapeutic Drug Monitoring. Biosensors 2019, 9, 132. [Google Scholar] [CrossRef] [PubMed]
- McConnell, E.M.; Nguyen, J.; Li, Y. Aptamer-Based Biosensors for Environmental Monitoring. Front. Chem. 2020, 8, 434. [Google Scholar] [CrossRef] [PubMed]
- Hara, T.O.; Singh, B. Electrochemical Biosensors for Detection of Pesticides and Heavy Metal Toxicants in Water: Recent Trends and Progress. ACS ES T Water 2021, 1, 462–478. [Google Scholar] [CrossRef]
- Li, D.; Song, S.; Fan, C. Target-Responsive Structural Switching for Nucleic Acid-Based Sensors. Acc. Chem. Res. 2010, 43, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.K. Point of Care Diagnostic Devices for Rapid Detection of Novel Coronavirus (SARS-nCoV19) Pandemic: A Review. Front. Nanotechnol. 2021, 2, 593619. [Google Scholar] [CrossRef]
- Liu, L.; Han, Z.; An, F.; Gong, X.; Zhao, C.; Zheng, W.; Mei, L.; Zhou, Q. Aptamer-based biosensors for the diagnosis of sepsis. J. Nanobiotechnol. 2021, 19, 216. [Google Scholar] [CrossRef]
- Kashefi-Kheyrabadi, L.; Kim, J.; Chakravarty, S.; Park, S.; Gwak, H.; Kim, S.-I.; Mohammadniaei, M.; Lee, M.-H.; Hyun, K.-A.; Jung, H.-I. Detachable microfluidic device implemented with electrochemical aptasensor (DeMEA) for sequential analysis of cancerous exosomes. Biosens. Bioelectron. 2020, 169, 112622. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Liu, C.; Pan, W.; Shen, J.; Guo, J.; Luo, T.; Feng, J.; Situ, B.; An, T.; Zhang, Y.; et al. Facile fluorescent aptasensor using aggregation-induced emission luminogens for exosomal proteins profiling towards liquid biopsy. Biosens. Bioelectron. 2020, 168, 112520. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, T.; Sharma, T.K. Aptasensors for full body health checkup. Biosens. Bioelectron. X 2022, 11, 100199. [Google Scholar] [CrossRef]
- Dhiman, A.; Kumar, C.; Mishra, S.K.; Sikri, K.; Datta, I.; Sharma, P.; Singh, T.P.; Haldar, S.; Sharma, N.; Bansal, A.; et al. Theranostic Application of a Novel G-Quadruplex-Forming DNA Aptamer Targeting Malate Synthase of Mycobacterium tuberculosis. Mol. Ther.-Nucleic Acids 2019, 18, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, X.; Guo, S.; Cao, J.; Zhou, J.; Zuo, J.; Bai, L. A sandwich-type electrochemical aptasensor for Mycobacterium tuberculosis MPT64 antigen detection using C60NPs decorated N-CNTs/GO nanocomposite coupled with conductive PEI-functionalized metal-organic framework. Biomaterials 2019, 216, 119253. [Google Scholar] [CrossRef] [PubMed]
- Kashefi-Kheyrabadi, L.; Mehrgardi, M.A.; Wiechec, E.; Turner, A.; Tiwari, A. Ultrasensitive Detection of Human Liver Hepatocellular Carcinoma Cells Using a Label-Free Aptasensor. Anal. Chem. 2014, 86, 4956–4960. [Google Scholar] [CrossRef]
- Waiwinya, W.; Putnin, T.; Pimalai, D.; Chawjiraphan, W.; Sathirapongsasuti, N.; Japrung, D. Immobilization-Free Electrochemical Sensor Coupled with a Graphene-Oxide-Based Aptasensor for Glycated Albumin Detection. Biosensors 2021, 11, 85. [Google Scholar] [CrossRef] [PubMed]
- Grabowska, I.; Sharma, N.; Vasilescu, A.; Iancu, M.; Badea, G.; Boukherroub, R.; Ogale, S.; Szunerits, S. Electrochemical Aptamer-Based Biosensors for the Detection of Cardiac Biomarkers. ACS Omega 2018, 3, 12010–12018. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Li, Y. Hydrogel based QCM aptasensor for detection of avian influenzavirus. Biosens. Bioelectron. 2013, 42, 148–155. [Google Scholar] [CrossRef]
- Ferreira, G.N.; Da-Silva, A.-C.; Tomé, B. Acoustic wave biosensors: Physical models and biological applications of quartz crystal microbalance. Trends Biotechnol. 2009, 27, 689–697. [Google Scholar] [CrossRef]
- Huang, X.; Bai, Q.; Hu, J.; Hou, D. A Practical Model of Quartz Crystal Microbalance in Actual Applications. Sensors 2017, 17, 1785. [Google Scholar] [CrossRef] [PubMed]
- Zu, H.; Wu, H.; Wang, Q.-M. High-Temperature Piezoelectric Crystals for Acoustic Wave Sensor Applications. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2016, 63, 486–505. [Google Scholar] [CrossRef] [PubMed]
- Janshoff, A.; Galla, H.J.; Steinem, C. Piezoelectric Mass-Sensing Devices as Biosensors-An Alternative to Optical Biosensors? Angew. Chem. (Int. Ed. Engl.) 2000, 39, 4004–4032. [Google Scholar] [CrossRef]
- Pohanka, M. Overview of Piezoelectric Biosensors, Immunosensors and DNA Sensors and Their Applications. Materials 2018, 11, 448. [Google Scholar] [CrossRef] [PubMed]
- Perez-Alfaro, I.; Gil-Hernandez, D.; Muñoz-Navascues, O.; Casbas-Gimenez, J.; Sanchez-Catalan, J.C.; Murillo, N. Low-Cost Piezoelectric Sensors for Time Domain Load Monitoring of Metallic Structures During Operational and Maintenance Processes. Sensors 2020, 20, 1471. [Google Scholar] [CrossRef] [PubMed]
- Bragazzi, N.L.; Amicizia, D.; Panatto, D.; Tramalloni, D.; Valle, I.; Gasparini, R. Quartz-Crystal Microbalance (QCM) for Public Health: An Overview of Its Applications. Adv. Protein Chem. Struct. Biol. 2015, 101, 149–211. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, K.K.; Gordon, J.G. The oscillation frequency of a quartz resonator in contact with liquid. Anal. Chim. Acta 1985, 175, 99–105. [Google Scholar] [CrossRef]
- Martin, S.J.; Granstaff, V.E.; Frye, G.C. Characterization of a quartz crystal microbalance with simultaneous mass and liquid loading. Anal. Chem. 1991, 63, 2272–2281. [Google Scholar] [CrossRef]
- Ning, Y.; Hu, J.; Lu, F. Aptamers used for biosensors and targeted therapy. Biomed. Pharmacother. 2020, 132, 110902. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Xie, Z.; Qu, M.; Zhang, Q.; Fu, Y.; Xie, J. Virtual Sensor Array Based on Butterworth–Van Dyke Equivalent Model of QCM for Selective Detection of Volatile Organic Compounds. ACS Appl. Mater. Interfaces 2021, 13, 47043–47051. [Google Scholar] [CrossRef]
- Chang, Q.; Wu, D.; Huang, Y.; Liang, C.; Liu, L.; Liu, H.; He, Y.; Huang, Q.; Qiu, J.; Tang, X. A lead-free K2CuBr3 microwires-based humidity sensor realized via QCM for real-time breath monitoring. Sens. Actuators B Chem. 2022, 367, 132112. [Google Scholar] [CrossRef]
- Osypova, A.; Thakar, D.; Dejeu, J.; Bonnet, H.; Van der Heyden, A.; Dubacheva, G.V.; Richter, R.P.; Defrancq, E.; Spinelli, N.; Coche-Guérente, L.; et al. Sensor Based on Aptamer Folding to Detect Low-Molecular Weight Analytes. Anal. Chem. 2015, 87, 7566–7574. [Google Scholar] [CrossRef] [PubMed]
- Bayramoglu, G.; Ozalp, V.C.; Yilmaz, M.; Guler, U.; Salih, B.; Arica, M.Y. Lysozyme specific aptamer immobilized MCM-41 silicate for single-step purification and quartz crystal microbalance (QCM)-based determination of lysozyme from chicken egg white. Microporous Mesoporous Mater. 2015, 207, 95–104. [Google Scholar] [CrossRef]
- Xi, X.; Niyonshuti, I.I.; Yu, N.; Yao, L.; Fu, Y.; Chen, J.; Li, Y. Label-Free Quartz Crystal Microbalance Biosensor Based on Aptamer-Capped Gold Nanocages Loaded with Polyamidoamine for Thrombin Detection. ACS Appl. Nano Mater. 2021, 4, 10047–10054. [Google Scholar] [CrossRef]
- Iijima, M.; Yamada, Y.; Nakano, H.; Nakayama, T.; Kuroda, S. Bio-nanocapsules for oriented immobilization of DNA aptamers on aptasensors. Analyst 2022, 147, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Yue, X.; Hu, H.; Zhou, X. A new analytical experimental setup combining quartz crystal microbalance with surface enhancement Raman spectroscopy and its application in determination of thrombin. Microchem. J. 2017, 132, 385–390. [Google Scholar] [CrossRef]
- Yao, C.; Qi, Y.; Zhao, Y.; Xiang, Y.; Chen, Q.; Fu, W. Aptamer-based piezoelectric quartz crystal microbalance biosensor array for the quantification of IgE. Biosens. Bioelectron. 2009, 24, 2499–2503. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Chen, W.-Y.; Hwu, E.-T.; Hu, W.-P. In-Silico Selection of Aptamer Targeting SARS-CoV-2 Spike Protein. Int. J. Mol. Sci. 2022, 23, 5810. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, S.; Zhang, X.; Du, C.; Si, S.; Chen, J. A high-frequency QCM biosensing platform for label-free detection of the SARS-CoV-2 spike receptor-binding domain: An aptasensor and an immunosensor. Analyst 2023, 148, 719–723. [Google Scholar] [CrossRef] [PubMed]
- Bayramoglu, G.; Ozalp, V.C.; Oztekin, M.; Arica, M.Y. Rapid and label-free detection of Brucella melitensis in milk and milk products using an aptasensor. Talanta 2019, 200, 263–271. [Google Scholar] [CrossRef]
- Wang, R.; Wang, L.; Callaway, Z.T.; Lu, H.; Huang, T.J.; Li, Y. A nanowell-based QCM aptasensor for rapid and sensitive detection of avian influenza virus. Sens. Actuators B Chem. 2017, 240, 934–940. [Google Scholar] [CrossRef]
- Giamblanco, N.; Conoci, S.; Russo, D.; Marletta, G. Single-step label-free hepatitis B virus detection by a piezoelectric biosensor. RSC Adv. 2015, 5, 38152–38158. [Google Scholar] [CrossRef]
- Skládal, P.; Riccardi, C.d.S.; Yamanaka, H.; da Costa, P.I. Piezoelectric biosensors for real-time monitoring of hybridization and detection of hepatitis C virus. J. Virol. Methods 2004, 117, 145–151. [Google Scholar] [CrossRef]
- Yuan, M.; Song, Z.; Fei, J.; Wang, X.; Xu, F.; Cao, H.; Yu, J. Aptasensor for lead(II) based on the use of a quartz crystal microbalance modified with gold nanoparticles. Microchim. Acta 2017, 184, 1397–1403. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, L.; Ma, Y.; Wang, X.; Zhang, J.; Bai, B.; Yu, L.; Guo, C.; Zhang, F.; Qin, S. A novel metal–organic frameworks composite-based label-free point-of-care quartz crystal microbalance aptasensing platform for tetracycline detection. Food Chem. 2022, 392, 133302. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Huang, J.; Guo, Z.; Jia, P.; Sun, Z.; Guo, Y.; Sun, X. Unleashing the potential of QCM: A comprehensive review of aptamer-based QCM sensing analysis. Microchem. J. 2024, 200, 110344. [Google Scholar] [CrossRef]
- Nübel, C.; Appel, B.; Hospach, I.; Mai, M.; Krasteva, N.; Nelles, G.; Petruschka, L.; Müller, S. Challenges and Opportunities in the Development of Aptamers for TNFα. Appl. Biochem. Biotechnol. 2016, 179, 398–414. [Google Scholar] [CrossRef] [PubMed]
- Lino, C.; Barrias, S.; Chaves, R.; Adega, F.; Fernandes, J.R.; Martins-Lopes, P. Development of a QCM-based biosensor for the detection of non-small cell lung cancer biomarkers in liquid biopsies. Talanta 2023, 260, 124624. [Google Scholar] [CrossRef]
- Shan, W.; Pan, Y.; Fang, H.; Guo, M.; Nie, Z.; Huang, Y.; Yao, S. An aptamer-based quartz crystal microbalance biosensor for sensitive and selective detection of leukemia cells using silver-enhanced gold nanoparticle label. Talanta 2014, 126, 130–135. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domsicova, M.; Korcekova, J.; Poturnayova, A.; Breier, A. New Insights into Aptamers: An Alternative to Antibodies in the Detection of Molecular Biomarkers. Int. J. Mol. Sci. 2024, 25, 6833. https://doi.org/10.3390/ijms25136833
Domsicova M, Korcekova J, Poturnayova A, Breier A. New Insights into Aptamers: An Alternative to Antibodies in the Detection of Molecular Biomarkers. International Journal of Molecular Sciences. 2024; 25(13):6833. https://doi.org/10.3390/ijms25136833
Chicago/Turabian StyleDomsicova, Michaela, Jana Korcekova, Alexandra Poturnayova, and Albert Breier. 2024. "New Insights into Aptamers: An Alternative to Antibodies in the Detection of Molecular Biomarkers" International Journal of Molecular Sciences 25, no. 13: 6833. https://doi.org/10.3390/ijms25136833
APA StyleDomsicova, M., Korcekova, J., Poturnayova, A., & Breier, A. (2024). New Insights into Aptamers: An Alternative to Antibodies in the Detection of Molecular Biomarkers. International Journal of Molecular Sciences, 25(13), 6833. https://doi.org/10.3390/ijms25136833




