Assessment of the Impact of Trace Essential Metals on Cancer Development
Abstract
1. Introduction
2. Methods and Search Criteria
2.1. Defining the Scope and Objectives
2.2. Search Strategy
2.3. Inclusion Criteria
2.4. Exclusion Criteria
3. The Influence of Specific Heavy Metals on the Carcinogenic Process
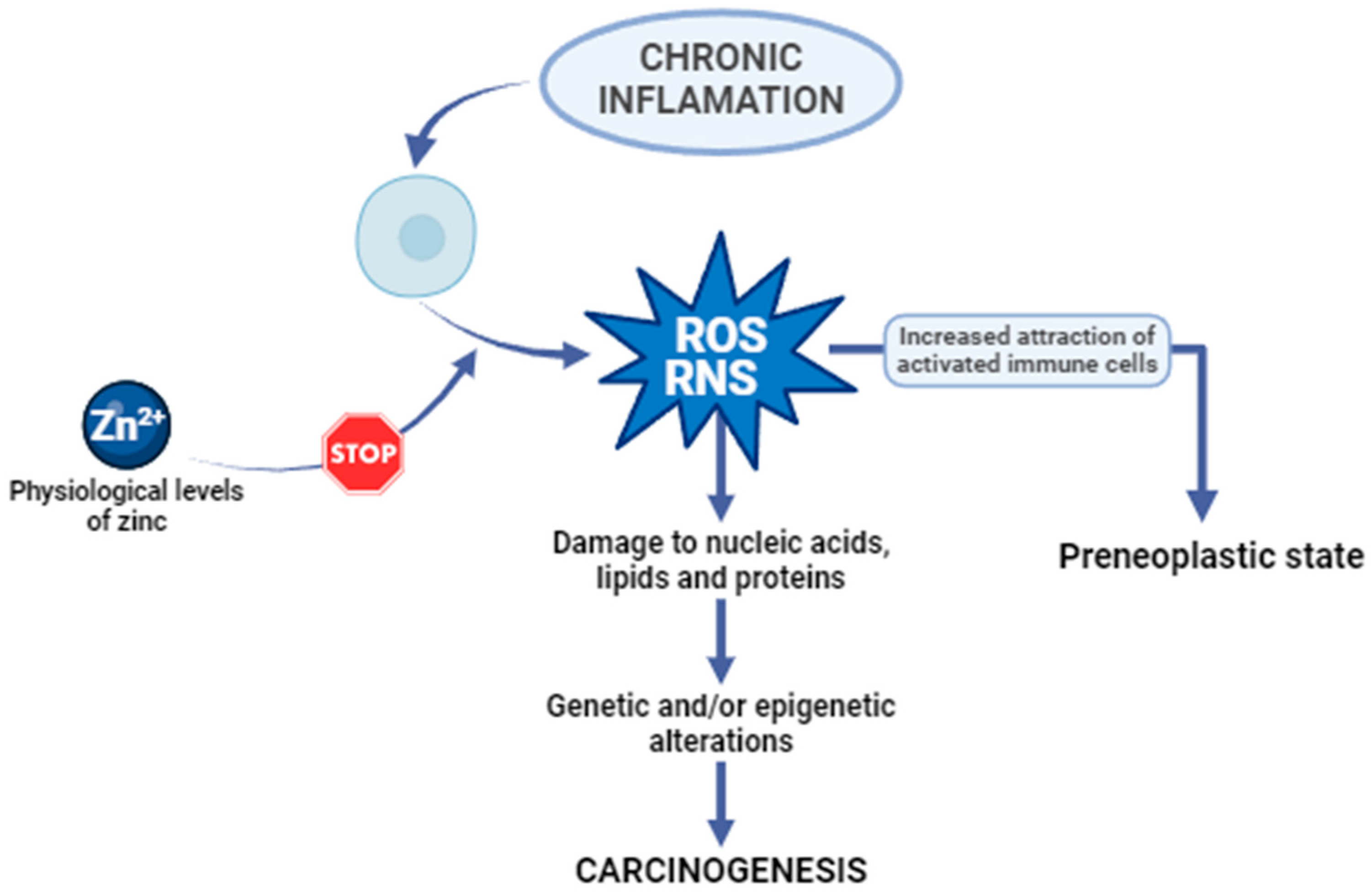
3.1. Zinc
| Type of Cancer | Characterization | Ref. |
|---|---|---|
| Breast cancer (BC) | Breast cancer, the most common cancer in women worldwide, has diverse types based on hormone and human epithelial growth factor receptor 2 (HER2) status: luminal A/B, HER2-positive, and triple-negative (TNBC). Zinc imbalance is linked to breast cancer, with low serum zinc but high zinc in cancer tissues. Zinc plays a crucial role in cancer progression, affecting cell transformation and tumor aggressiveness by influencing zinc transporters. | [16,18,19] |
| Prostate cancer (PCa) | Prostate cancer ranks as the second most common cancer in men globally, with high mortality rates, especially in cases with extracapsular disease. Unlike normal and benign prostate tissue, malignant prostate tissue shows decreased zinc levels, indicating a role for zinc alterations in cancer development. Zinc concentrations drop early in prostate cancer progression, inhibiting citrate oxidation, a key function of prostate cells. This loss of zinc may remove its inhibitory effects on cancer cells, potentially promoting prostate cancer initiation and progression. | [20,21] |
| Endometrial cancer (EC) | Endometrial cancer is a type of cancer that originates in the lining of the uterus known as the endometrium. Research has examined zinc metabolism in different cancers, including endometrial cancer. Although a direct link between serum zinc levels and endometrial cancer risk or progression hasn’t been established, studies suggest zinc may influence pathways related to cancer development and progression. Zinc is thought to possess anti-cancer properties by aiding in DNA repair, regulating cell growth, and impacting immune function. However, further research is required to comprehensively grasp the connection between zinc levels and endometrial cancer. | [22] |
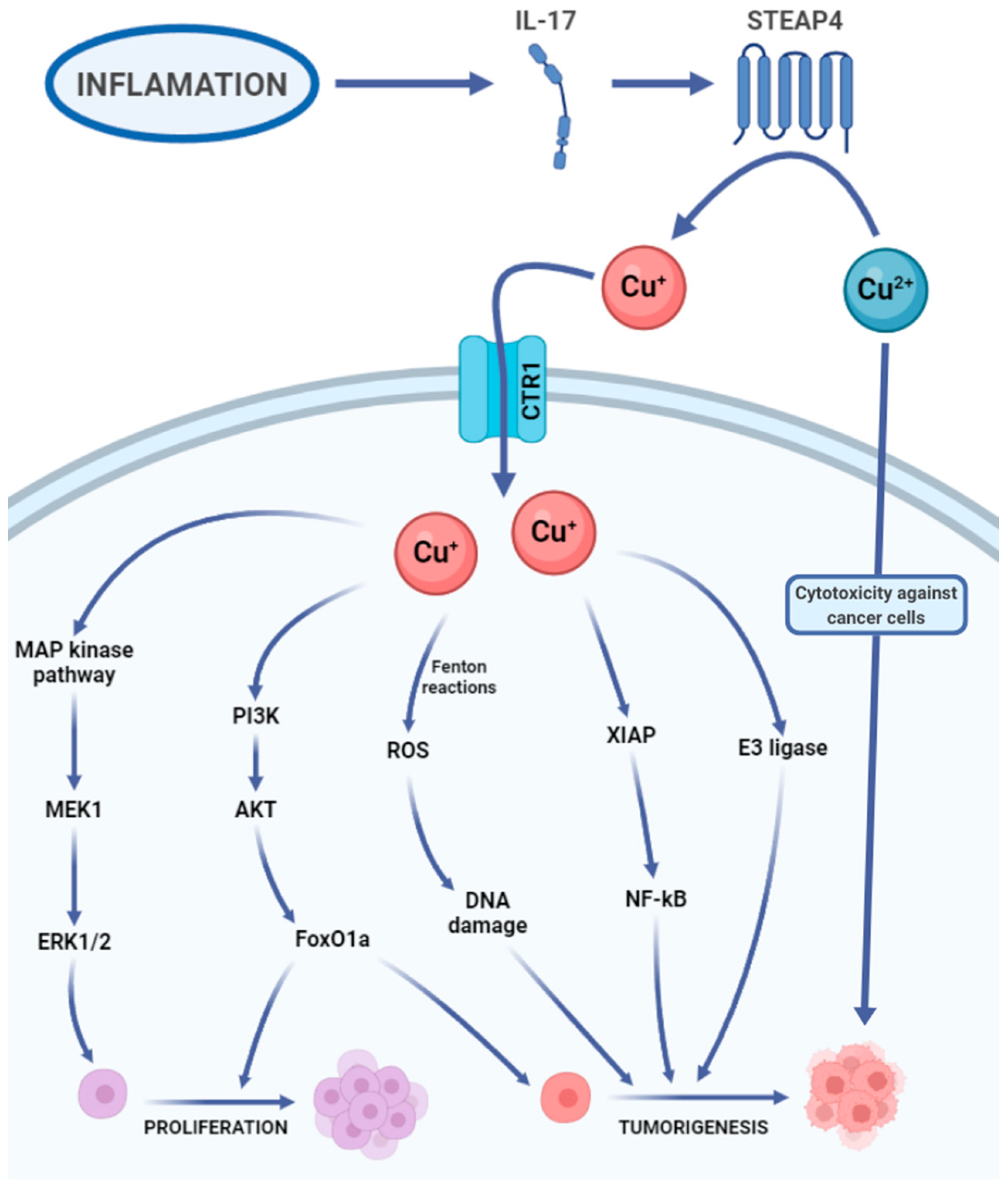
3.2. Copper
3.2.1. Copper’s Biological Role
3.2.2. Copper in Cancer
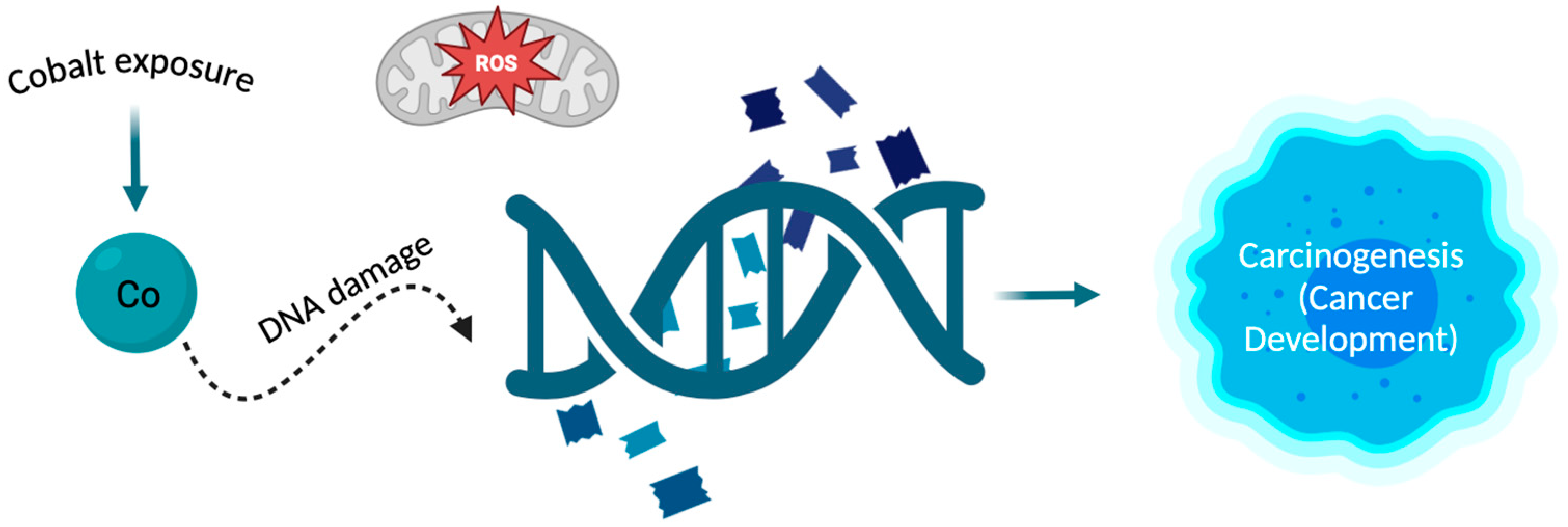
3.3. Cobalt
3.4. Iron
3.5. Manganese
3.6. Other Heavy Metals
4. Conclusions
- (1)
- Continuous monitoring of environment with increased levels of harmful substances for humans;
- (2)
- Standard use of protective equipment in accordance with procedures outlined by legal regulations and public health initiatives;
- (3)
- Ongoing monitoring of the health of individuals exposed to the harmful effects of various factors (harmful elements) present in the environment, conducting periodic (standard and additional) examinations;
- (4)
- Early implementation of medical procedures to prevent disease development, limiting the possibility of metastasis;
- (5)
- Establishing a procedural algorithm depending on the diagnosed disease and the impact of the harmful compound on the human body.
5. Study Limitations
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guo, Z.; Zhou, G.; Hu, W. Carcinogenesis induced by space radiation: A systematic review. Neoplasia 2022, 32, 100828. [Google Scholar] [CrossRef] [PubMed]
- Beyersmann, D.; Hartwig, A. Carcinogenic metal compounds: Recent insight into molecular and cellular mechanisms. Arch. Toxicol. 2008, 82, 493–512. [Google Scholar] [CrossRef] [PubMed]
- Kentsis, A. Why do young people get cancer? Pediatr. Blood Cancer 2020, 67, e28335. [Google Scholar] [CrossRef] [PubMed]
- Lewandowska, A.M.; Rudzki, M.; Rudzki, S.; Lewandowski, T.; Laskowska, B. Environmental risk factors for cancer—Review paper. Ann. Agric. Environ. Med. 2019, 26, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. Exp. Suppl. 2012, 101, 133–164. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Costa, M. Metals, and molecular carcinogenesis. Carcinogenesis 2020, 41, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Hussai, A.; Jiang, W.; Wang, X.; Shahid, S.; Saba, N.; Ahmad, M.; Dar, A.; Masood, S.U.; Imran, M.; Mustafa, A. Mechanic impact of zinc deficiency in human development. Front. Nutr. 2022, 9, 717064. [Google Scholar] [CrossRef]
- Guo, H.; Deng, H.; Liu, H.; Jian, Z.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; et al. Nickel carcinogenesis mechanism: Cell cycle dysregulation. Environ. Sci. Pollut. Res. 2021, 28, 4893–4901. [Google Scholar] [CrossRef]
- Choi, S.; Liu, X.; Pan, Z. Zinc deficiency and cellular oxidative stress: Prognostic implications in cardiovascular diseases. Acta Pharmacol. Sin. 2018, 39, 1120–1132. [Google Scholar] [CrossRef]
- Jomova, K.; Valko, M. Advances in metal-induced oxidative stress and human disease. Toxicology 2011, 283, 65–87. [Google Scholar] [CrossRef]
- Huang, L.; Drake, V.J.; Ho, E. Zinc. Adv. Nutr. 2015, 6, 224–226. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Makova, M.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Rhodes, C.J.; Valko, M. Essential metals in health and disease. Chem. Biol. Interact. 2022, 367, 110173. [Google Scholar] [CrossRef] [PubMed]
- Caliri, A.W.; Tommasi, S.; Besaratinia, A. Relationship among smoking, oxidative stress, inflammation, macromolecular damage and cancer. Mutat. Res./Rev. Mutat. Res. 2021, 787, 108365. [Google Scholar] [CrossRef] [PubMed]
- Jelic, M.D.; Mandic, A.D.; Maricic, S.M.; Srdjenovic, B.U. Oxidative stress and its role in cancer. J. Cancer Res. Ther. 2021, 17, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Olechnowicz, J.; Tinkov, A.; Skalny, A.; Suliburska, J. Zinc status is associated with inflammation, oxidative stress, lipid, and glucose metabolism. J. Physiol. Sci. 2018, 68, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Zannetti, A. Breast cancer: From pathophysiology to novel therapeutic approaches 2.0. Int. J. Mol. Sci. 2023, 24, 2542. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Pu, M.; Dong, X.; Ji, F.; Veeraraghavan, V.P.; Yang, H. Piperine loaded zinc oxide nanocomposite inhibits the PI3K/AKT/mTOR signalling pathway via attenuating the development of gastric carcinoma: In vitro and in vivo studies. Arab. J. Chem. 2020, 13, 5501–5516. [Google Scholar] [CrossRef]
- Qu, Z.; Liu, Q.; Kong, X.; Wang, X.; Wang, Z.; Wang, J.; Fang, Y. A Systematic study on zinc-related metabolism in breast cancer. Nutrients 2023, 15, 1703. [Google Scholar] [CrossRef] [PubMed]
- Dean-Colomb, W.; Esteva, F.J. Her-2 positive breast cancer: Herceptin and beyond. Eur. J. Cancer 2008, 44, 2806–2812. [Google Scholar] [CrossRef]
- Li, D.; Stovall, D.B.; Wang, W.; Sui, G. Advances of zinc signalling studies in prostate cancer. Int. J. Mol. Sci. 2021, 21, 667. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, H.; Xu, Z.; Cheng, X. Zinc dysregulation in cancers and its potential as a therapeutic target. Cancer Biol. Med. 2020, 17, 612–625. [Google Scholar] [CrossRef] [PubMed]
- Atakul, T.; Altinkaya, S.O.; Abas, B.I.; Yenisey, C. Serum copper and zinc levels in patients with endometrial cancer. Biol. Trace Elem. Res. 2020, 195, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Li, Y. Copper homeostasis: Emerging target for cancer treatment. IUBMB Life 2020, 72, 1900–1908. [Google Scholar] [CrossRef] [PubMed]
- Bost, M.; Houdart, S.; Oberli, M.; Kalonji, E.; Huneau, J.F.; Margaritis, I. Dietary copper, and human health: Current evidence and unresolved issues. J. Trace Elem. Med. Biol. 2016, 35, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Shanbhag, V.C.; Gudekar, N.; Jasmer, K.; Papageorgiou, C.; Singh, K.; Petris, M.J. Copper metabolism as a unique vulnerability in cancer. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 118893. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Yang, J.; Liu, T.; Jia, R.; Yang, L.; Sun, P.; Zhao, W. Copper metabolism and hepatocellular carcinoma: Current insights. Front. Oncol. 2023, 13, 1186659. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zheng, L.; Ma, S.; Lin, R.; Li, J.; Yang, S. Cuproptosis: Emerging biomarkers and potential therapeutics in cancers. Front. Oncol. 2023, 13, 1288504. [Google Scholar] [CrossRef] [PubMed]
- Martín Giménez, V.M.; Bergam, I.; Reiter, R.J.; Manucha, W. Metal ion homeostasis with emphasis on zinc and copper: Potential crucial link to explain the non-classical antioxidative properties of vitamin D and melatonin. Life Sci. 2021, 281, 119770. [Google Scholar] [CrossRef] [PubMed]
- Denoyer, D.; Clatworthy, S.A.S.; Cater, M.A. Copper complexes in cancer therapy. Met. Ions Life Sci. 2018, 18, 469–506. [Google Scholar] [CrossRef]
- Bian, C.; Zheng, Z.; Su, J.; Chang, S.; Yu, H.; Bao, J.; Xin, Y.; Jiang, X. Copper homeostasis and cuproptosis in tumour pathogenesis and therapeutic strategies. Front. Pharmacol. 2023, 14, 1271613. [Google Scholar] [CrossRef]
- Zhao, Q.; Qi, T. The implications and prospect of cuproptosis-related genes and copper transporters in cancer progression. Front. Oncol. 2023, 13, 1117164. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Yang, Y.; Gao, Y.; He, J. Cuproptosis: Mechanisms and links with cancers. Mol. Cancer 2023, 22, 46. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Singh, M.K.; Singh, T.B.; Bhartiya, S.K.; Singh, S.P.; Shukla, V.K. Heavy and trace metals in carcinoma of the gallbladder. World J. Surg. 2013, 37, 2641–2646. [Google Scholar] [CrossRef] [PubMed]
- Lopez, J.; Ramchandani, D.; Vahdat, L. Copper depletion as a therapeutic strategy in cancer. Met. Ions Life Sci. 2019, 19, 19. [Google Scholar] [CrossRef]
- He, F.; Chang, C.; Liu, B.; Li, Z.; Li, H.; Cai, N.; Wang, H.H. Copper (II) ions activate ligand-independent receptor tyrosine kinase (RTK) signalling pathway. Biomed. Res. Int. 2019, 2019, 4158415. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhou, M.; Liu, Y.; Si, Z. Cope with copper: From copper linked mechanisms to copper-based clinical cancer therapies. Cancer Lett. 2023, 561, 216157. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Zhao, J.; Bulek, K.; Tang, F.; Chen, X.; Cai, G.; Jia, S.; Fox, P.L.; Huang, E.; Pizarro, T.T.; et al. Inflammation mobilizes copper metabolism to promote colon tumorigenesis via an IL-17-STEAP4-XIAP axis. Nat. Commun. 2020, 11, 900. [Google Scholar] [CrossRef] [PubMed]
- Pham, A.N.; Xing, G.; Miller, C.J.; Waite, T.D. Fenton-like copper redox chemistry revisited: Hydrogen peroxide and superoxide mediation of copper-catalysed oxidant production. J. Catal. 2013, 301, 54–64. [Google Scholar] [CrossRef]
- Guan, D.; Zhao, L.; Shi, X.; Ma, X.; Chen, Z. Copper in cancer: From pathogenesis to therapy. Biomed. Pharmacother. 2023, 163, 114791. [Google Scholar] [CrossRef]
- Petruzzelli, R.; Polishchuk, R.S. Activity, and trafficking of copper-transporting ATPases in tumour development and defence against Platinum-Based Drugs. Cells 2019, 8, 1080. [Google Scholar] [CrossRef]
- Chen, L.; Min, J.; Wang, F. Copper homeostasis and cuproptosis in health and disease. Signal Transduct. Target Ther. 2022, 7, 378. [Google Scholar] [CrossRef] [PubMed]
- Tsvetkov, P.; Coy, S.; Petrova, B.; Dreishpoon, M.; Verma, A.; Abdusamad, M.; Rossen, J.; Joesch-Cohen, L.; Humeidi, R.; Spangler, R.D.; et al. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science 2022, 375, 1254–1261. [Google Scholar] [CrossRef] [PubMed]
- Mariani, D.; Ghasemishahrestani, Z.; Freitas, W.; Pezzuto, P.; Costa-da-Silva, A.C.; Tanuri, A.; Kanashiro, M.M.; Fernandes, C.; Horn, A., Jr.; Pereira, M.D. Antitumoral synergism between a copper (II) complex and cisplatin improves in vitro and in vivo anticancer activity against melanoma, lung and breast cancer cells. Biochim. Biophys. Acta Gen. Subj. 2021, 1865, 129963. [Google Scholar] [CrossRef] [PubMed]
- Maciel, L.L.F.; de Freitas, W.R.; Bull, E.S.; Fernandes, C.; Horn, A., Jr.; de Aquino Almeida, J.C.; Kanashiro, M.M. In vitro and in vivo anti-proliferative activity and ultrastructure investigations of a copper (II) complex toward human lung cancer cell NCI-H460. J. Inorg. Biochem. 2020, 210, 111166. [Google Scholar] [CrossRef] [PubMed]
- Romani, A.M.P. Cisplatin in cancer treatment. Biochem. Pharmacol. 2022, 206, 115323. [Google Scholar] [CrossRef] [PubMed]
- Czarnek, K.; Terpiłowska, S.; Siwicki, A.K. Selected aspects of the action of cobalt ions in the human body. Cent. Eur. J. Immunol. 2015, 40, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Ćwiertnia, A.; Kozłowski, M.; Cymbaluk-Płoska, A. The role of iron and cobalt in gynaecological diseases. Cells 2022, 12, 117. [Google Scholar] [CrossRef] [PubMed]
- Marques, H.M. The inorganic chemistry of the cobalt corrinoids—An update. J. Inorg. Biochem. 2023, 242, 112154. [Google Scholar] [CrossRef] [PubMed]
- Wahlqvist, F.; Bryngelsson, I.L.; Westberg, H.; Vihlborg, P.; Andersson, L. Dermal and inhalable cobalt exposure-uptake of cobalt for workers at Swedish hard metal plants. PLoS ONE 2020, 15, e0237100. [Google Scholar] [CrossRef]
- National Toxicology Program. Report on Carcinogens Monograph on Cobalt and Cobalt Compounds That Release Cobalt Ions In Vivo: RoC Monograph 06; National Toxicology Program: Research Triangle Park, NC, USA, 2016. [PubMed]
- Zhang, W.; Wang, C.; Zhu, W.; Liu, F.; Liu, Y. Ferrostatin-1 alleviates cytotoxicity of cobalt nanoparticles by inhibiting ferroptosis. Bioengineered 2022, 13, 6163–6172. [Google Scholar] [CrossRef]
- Liu, Y.K.; Ye, J.; Han, Q.L.; Tao, R.; Liu, F.; Wang, W. Toxicity, and bioactivity of cobalt nanoparticles on the monocytes. Orthop. Surg. 2015, 7, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Savi, M.; Bocchi, L.; Cacciani, F.; Vilella, R.; Buschini, A.; Perotti, A.; Galati, S.; Montalbano, S.; Pinelli, S.; Frati, C.; et al. Cobalt oxide nanoparticles induce oxidative stress and alter electromechanical function in rat ventricular myocytes. Part Fibre Toxicol. 2021, 18, 1. [Google Scholar] [CrossRef] [PubMed]
- Ton, T.T.; Kovi, R.C.; Peddada, T.N.; Chhabria, R.M.; Shockley, K.R.; Flagler, N.D.; Gerrish, K.E.; Herbert, R.A.; Behl, M.; Hoenerhoff, M.J.; et al. Cobalt-induced oxidative stress contributes to alveolar/bronchiolar carcinogenesis in B6C3F1/N mice. Arch. Toxicol. 2021, 95, 3171–3190. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Ke, Q.; Costa, M. Alterations of histone modifications by cobalt compounds. Carcinogenesis 2009, 30, 1243–1251. [Google Scholar] [CrossRef] [PubMed]
- Grochans, S.; Korbecki, J.; Simińska, D.; Żwierełło, W.; Rzeszotek, S.; Kolasa, A.; Kojder, K.; Tarnowski, M.; Chlubek, D.; Baranowska-Bosiacka, I. CCL18 expression is higher in a glioblastoma multiforme tumor than in the peritumoral area and causes the migration of tumour cells sensitized by hypoxia. Int. J. Mol. Sci. 2022, 23, 8536. [Google Scholar] [CrossRef] [PubMed]
- Korbecki, J.; Olbromski, M.; Dzięgiel, P. CCL18 in the progression of cancer. Int. J. Mol. Sci. 2020, 21, 7955. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Chen, Y.S.; Yao, Y.D.; Chen, J.Q.; Chen, J.N.; Huang, S.Y.; Zeng, Y.J.; Yao, H.R.; Zeng, S.H.; Fu, Y.S.; et al. CCL18 from tumor-associated macrophages promotes angiogenesis in breast cancer. Oncotarget 2015, 6, 34758–34773. [Google Scholar] [CrossRef] [PubMed]
- Pandrangi, S.L.; Chittineedi, P.; Chikati, R.; Lingareddy, J.R.; Nagoor, M.; Ponnada, S.K. Role of dietary iron revisited: In metabolism, ferroptosis and pathophysiology of cancer. Am. J. Cancer Res. 2022, 12, 974–985. [Google Scholar] [PubMed]
- Halcrow, P.W.; Lynch, M.L.; Geiger, J.D.; Ohm, J.E. Role of endolysosome function in iron metabolism and brain carcinogenesis. Semin. Cancer Biol. 2021, 76, 74–85. [Google Scholar] [CrossRef]
- Jomova, K.; Valko, M. Importance of iron chelation in free radical-induced oxidative stress and human disease. Curr. Pharm. Des. 2011, 17, 3460–3473. [Google Scholar] [CrossRef]
- Toyokuni, S.; Kong, Y.; Zheng, H.; Mi, D.; Katabuchi, M.; Motooka, Y.; Ito, F. Double-edged sword role of iron-loaded ferritin in extracellular vesicles. J. Cancer Prev. 2021, 26, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, L.; Ding, J.; Chen, Y. Iron metabolism in cancer. Int. J. Mol. Sci. 2018, 20, 95. [Google Scholar] [CrossRef] [PubMed]
- Toyokuni, S.; Kong, Y.; Cheng, Z.; Sato, K.; Hayashi, S.; Ito, F.; Jiang, L.; Yanatori, I.; Okazaki, Y.; Akatsuka, S. Carcinogenesis as side effects of iron and oxygen utilization: From the unveiled truth toward ultimate bioengineering. Cancers 2020, 12, 3320. [Google Scholar] [CrossRef] [PubMed]
- Torti, S.V.; Manz, D.H.; Paul, B.T.; Blanchette-Farra, N.; Torti, F.M. Iron and cancer. Annu. Rev. Nutr. 2018, 38, 97–125. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.; Mertens, C.; Tomat, E.; Brüne, B. Iron as a central player and promising target in cancer progression. Int. J. Mol. Sci. 2019, 20, 273. [Google Scholar] [CrossRef] [PubMed]
- Ying, J.F.; Lu, Z.B.; Fu, L.Q.; Tong, Y.; Wang, Z.; Li, W.F.; Mou, X.Z. The role of iron homeostasis and iron-mediated ROS in cancer. Am. J. Cancer Res. 2021, 11, 1895–1912. [Google Scholar] [PubMed Central]
- Ni, S.; Kuang, Y.; Yuan, Y.; Yu, B. Mitochondrion-mediated iron accumulation promotes carcinogenesis and Warburg effect through reactive oxygen species in osteosarcoma. Cancer Cell Int. 2020, 20, 399. [Google Scholar] [CrossRef] [PubMed]
- Liberti, M.V.; Locasale, J.W. The Warburg effect: How does it benefit cancer cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Ni, S.; Zhuge, A.; Li, B.; Li, L. Iron regulates the Warburg effect and ferroptosis in colorectal cancer. Front. Oncol. 2021, 11, 614778. [Google Scholar] [CrossRef]
- Muto, Y.; Moroishi, T.; Ichihara, K.; Nishiyama, M.; Shimizu, H.; Eguchi, H.; Moriya, K.; Koike, K.; Mimori, K.; Mori, M.; et al. Disruption of FBXL5-mediated cellular iron homeostasis promotes liver carcinogenesis. J. Exp. Med. 2019, 216, 950–965. [Google Scholar] [CrossRef]
- Islam, S.; Hoque, N.; Nasrin, N.; Hossain, M.; Rizwan, F.; Biswas, K.; Asaduzzaman, M.; Rahman, S.; Hoskin, D.W.; Sultana, S.; et al. Iron overload and breast cancer: Iron chelation as a potential therapeutic approach. Life 2022, 12, 963. [Google Scholar] [CrossRef] [PubMed]
- Ploug, M.; Kroijer, R.; Qvist, N.; Lindahl, C.H.; Knudsen, T. Iron deficiency in colorectal cancer patients: A cohort study on prevalence and associations. Color. Dis. 2020, 23, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Y.; Guo, W.; Ling, J.; Xu, D.; Liao, Y.; Zhao, H.; Du, X.; Wang, H.; Xu, M.; Song, H.; et al. Iron-dependent CDK1 activity promotes lung carcinogenesis via activation of the GP130/STAT3 signalling pathway. Cell Death Dis. 2019, 10, 297. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.; Mertens, C.; Brüne, B. Macrophage iron homeostasis and polarization in the context of cancer. Immunobiology 2015, 220, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.; Mertens, C.; Bauer, R.; Rehwald, C.; Brüne, B. Lipocalin-2 and iron trafficking in the tumour microenvironment. Pharmacol Res. 2017, 120, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Crescenzi, E.; Leonardi, A.; Pacifico, F. Iron metabolism in cancer and senescence: A cellular perspective. Biology 2023, 12, 989. [Google Scholar] [CrossRef]
- Özdemir, A.; Şimay Demir, Y.D.; Yeşilyurt, Z.E.; Ark, M. Senescent cells and SASP in cancer microenvironment: New approaches in cancer therapy. Adv. Protein Chem. Struct. Biol. 2023, 133, 115–158. [Google Scholar] [CrossRef] [PubMed]
- Basak, T.; Kanwar, R.K. Iron imbalance in cancer: Intersection of deficiency and overload. Cancer Med. 2022, 11, 3837–3853. [Google Scholar] [CrossRef] [PubMed]
- Murata, M. Inflammation, and cancer. Environ. Health Prev. Med. 2018, 23, 50. [Google Scholar] [CrossRef]
- Hassannia, B.; Vandenabeele, P.; Vanden Berghe, T. Targeting ferroptosis to iron out cancer. Cancer Cell 2019, 35, 830–849. [Google Scholar] [CrossRef]
- Liang, D.; Minikes, A.M.; Jiang, X. Ferroptosis at the intersection of lipid metabolism and cellular signalling. Mol. Cell 2022, 82, 2215–2227. [Google Scholar] [CrossRef] [PubMed]
- Candelaria, P.V.; Leoh, L.S.; Penichet, M.L.; Daniels-Wells, T.R. Antibodies targeting the transferrin receptor 1 (TfR1) as direct anti-cancer agents. Front. Immunol. 2021, 12, 607692. [Google Scholar] [CrossRef] [PubMed]
- El Hout, M.; Dos Santos, L.; Hamaï, A.; Mehrpour, M. A promising new approach to cancer therapy: Targeting iron metabolism in cancer stem cells. Semin. Cancer Biol. 2018, 53, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Mertens, C.; Akam, E.A.; Rehwald, C.; Brüne, B.; Tomat, E.; Jung, M. Intracellular iron chelation modulates the macrophage iron phenotype with consequences on tumour progression. PLoS ONE 2016, 11, e0166164. [Google Scholar] [CrossRef] [PubMed]
- Evans, G.R.; Masullo, L.N. Manganese toxicity. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar] [PubMed]
- Aschner, M.; Erikson, K. Manganese. Adv. Nutr. 2017, 8, 520–521. [Google Scholar] [CrossRef] [PubMed]
- Brzóska, M.M.; Gałażyn-Sidorczuk, M.; Kozłowska, M.; Smereczański, N.M. The body status of manganese and activity of this element-dependent mitochondrial superoxide dismutase in a rat model of human exposure to cadmium and Co-administration of Aronia melanocarpa L. Extract. Nutr. 2022, 14, 4773. [Google Scholar] [CrossRef] [PubMed]
- Kim, A. Modulation of MnSOD in cancer: Epidemiological and experimental evidence. Toxicol. Res. 2010, 26, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Holley, A.K.; Dhar, S.K.; St Clair, D.K. Curbing cancer’s sweet tooth: Is there a role for MnSOD in regulation of the Warburg effect? Mitochondrion. 2013, 13, 170–188. [Google Scholar] [CrossRef] [PubMed]
- Bonetta Valentino, R. The structure-function relationships and physiological roles of MnSOD mutants. Biosci. Rep. 2022, 42, BSR20220202. [Google Scholar] [CrossRef]
- Funke, S.; Risch, A.; Nieters, A.; Hoffmeister, M.; Stegmaier, C.; Seiler, C.M.; Brenner, H.; Chang-Claude, J. Genetic polymorphisms in genes related to oxidative stress (GSTP1, GSTM1, GSTT1, CAT, MnSOD, MPO, eNOS) and survival of rectal cancer patients after radiotherapy. J. Cancer Epidemiol. 2009, 2009, 302047. [Google Scholar] [CrossRef]
- Liu, M.; Sun, X.; Chen, B.; Dai, R.; Xi, Z.; Xu, H. Insights into manganese superoxide dismutase and human diseases. Int. J. Mol. Sci. 2022, 23, 15893. [Google Scholar] [CrossRef]
- Weydert, C.J.; Waugh, T.A.; Ritchie, J.M.; Iyer, K.S.; Smith, J.L.; Li, L.; Spitz, D.R.; Oberley, L.W. Overexpression of manganese or copper-zinc superoxide dismutase inhibits breast cancer growth. Free Radic. Biol. Med. 2006, 41, 226–237. [Google Scholar] [CrossRef]
- Du, D.; Fu, H.J.; Ren, W.; Li, X.L.; Guo, L.H. PSA targeted dual-modality manganese oxide-mesoporus silica nanoparticles for prostate cancer imaging. Biomed. Pharmacother. 2020, 121, 109614. [Google Scholar] [CrossRef] [PubMed]
- Eybl, V.; Kotyzová, D. Protective effect of manganese in cadmium-induced hepatic oxidative damage, changes in cadmium distribution and trace elements level in mice. Interdiscip. Toxicol. 2010, 3, 68–72. [Google Scholar] [CrossRef]
- Zhang, W.; Li, H.; Tan, X.; Li, Z.; Zhong, C.; Xiao, W.; Xiong, Y.; Zhang, W.; Yang, L.; Wu, G. Fe-Mn plaque formation mechanism underlying the inhibition of cadmium absorption by rice under oxidation conditions. Environ. Eng. Sci. 2021, 38, 676–684. [Google Scholar] [CrossRef]
- Lener, M.R.; Reszka, E.; Marciniak, W.; Lesicka, M.; Baszuk, P.; Jabłońska, E.; Białkowska, K.; Muszyńska, M.; Pietrzak, S.; Derkacz, R.; et al. Blood cadmium levels as a marker for early lung cancer detection. J. Trace Elem. Med. Biol. 2021, 64, 126682. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Khalili, N.; Razi, S.; Keshavarz-Fatki, M.; Khalili, N.; Rezaei, N. Effects of lead and cadmium on the immune system and cancer progression. J. Environ. Health Sci. Eng. 2020, 18, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Zwolak, I. The role of selenium in arsenic and cadmium toxicity: An updated review of scientific literature. Biol. Trace Elem. Res. 2020, 193, 44–63. [Google Scholar] [CrossRef] [PubMed]
- Massányi, P.; Massányi, M.; Madeddu, R.; Stawarz, R.; Lukáč, N. Effects of cadmium, lead, and mercury on the structure and function of reproductive organs. Toxics 2020, 8, 94. [Google Scholar] [CrossRef]
- Saintilnord, W.N.; Fondufe-Mittendorf, Y. Arsenic-induced epigenetic changes in cancer development. Semin. Cancer Biol. 2021, 76, 195–205. [Google Scholar] [CrossRef]
- Son, Y.O. Molecular mechanisms of nickel-induced carcinogenesis. Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 1015–1023. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, M.; Metin, M.; Altay, V.; Bhat, R.A.; Ejaz, M.; Gul, A.; Unal, B.T.; Hasanuzzaman, M.; Nibir, L.; Nahar, K.; et al. Arsenic and human health: Genotoxicity, epigenomic effects, and cancer signalling. Biol. Trace Elem. Res. 2022, 200, 988–1001. [Google Scholar] [CrossRef]
- Strupp, C. Beryllium metal II. A review of the available toxicity data. Ann. Occup. Hyg. 2011, 55, 43–56. [Google Scholar] [CrossRef]
- Skalny, A.V.; Aschner, M.; Sekacheva, M.I.; Santamaria, A.; Barbosa, F.; Ferrer, B.; Aaseth, J.; Paoliello, M.M.; Rocha, J.B.; Tinkov, A.A. Mercury and cancer: Where are we now after two decades of research? Food Chem. Toxicol. 2022, 164, 113001. [Google Scholar] [CrossRef]
- Sánchez-Alarcón, J.; Milić, M.; Bustamante-Montes, L.P.; Isaac-Olivé, K.; Valencia-Quintana, R.; Ramírez-Durán, N. Genotoxicity of mercury and its derivatives demonstrated in vitro and in vivo in human populations studies. Systematic review. Toxics 2021, 9, 326. [Google Scholar] [CrossRef]
- Vincent, J.B. New evidence against chromium as an essential trace element. J. Nutr. 2017, 147, 2212–2219. [Google Scholar] [CrossRef]
- Levina, A.; Crans, D.C.; Lay, P.A. Speciation of metal drugs, supplements and toxins in media and bodily fluids controls in vitro activities. Coord. Chem. Rev. 2017, 352, 473–498. [Google Scholar] [CrossRef]
- Pontoni, L.; La Vecchia, C.; Boguta, P.; Sirakov, M.; D’Aniello, E.; Fabbricino, M.; Locascio, A. Natural organic matter controls metal speciation and toxicity for marine organisms: A review. Environ. Chem. Lett. 2022, 20, 797–812. [Google Scholar] [CrossRef]
- Medici, S.; Peana, M.; Pelucelli, A.; Zoroddu, M.A. An updated overview on metal nanoparticles toxicity. Semin. Cancer Biol. 2021, 76, 17–26. [Google Scholar] [CrossRef]
- Fouani, L.; Menezes, S.V.; Paulson, M.; Richardson, D.R.; Kovacevic, Z. Metals and metastasis: Exploiting the role of metals in cancer metastasis to develop novel anti-metastatic agents. Pharmacol. Res. 2017, 115, 275–287. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Górska, A.; Markiewicz-Gospodarek, A.; Trubalski, M.; Żerebiec, M.; Poleszak, J.; Markiewicz, R. Assessment of the Impact of Trace Essential Metals on Cancer Development. Int. J. Mol. Sci. 2024, 25, 6842. https://doi.org/10.3390/ijms25136842
Górska A, Markiewicz-Gospodarek A, Trubalski M, Żerebiec M, Poleszak J, Markiewicz R. Assessment of the Impact of Trace Essential Metals on Cancer Development. International Journal of Molecular Sciences. 2024; 25(13):6842. https://doi.org/10.3390/ijms25136842
Chicago/Turabian StyleGórska, Aleksandra, Agnieszka Markiewicz-Gospodarek, Mateusz Trubalski, Marta Żerebiec, Julia Poleszak, and Renata Markiewicz. 2024. "Assessment of the Impact of Trace Essential Metals on Cancer Development" International Journal of Molecular Sciences 25, no. 13: 6842. https://doi.org/10.3390/ijms25136842
APA StyleGórska, A., Markiewicz-Gospodarek, A., Trubalski, M., Żerebiec, M., Poleszak, J., & Markiewicz, R. (2024). Assessment of the Impact of Trace Essential Metals on Cancer Development. International Journal of Molecular Sciences, 25(13), 6842. https://doi.org/10.3390/ijms25136842







