Development of Bioactive Hybrid Poly(lactic acid)/Poly(methyl methacrylate) (PLA/PMMA) Electrospun Fibers Functionalized with Bioglass Nanoparticles for Bone Tissue Engineering Applications
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of Bioglass Nanoparticle (n-BG)
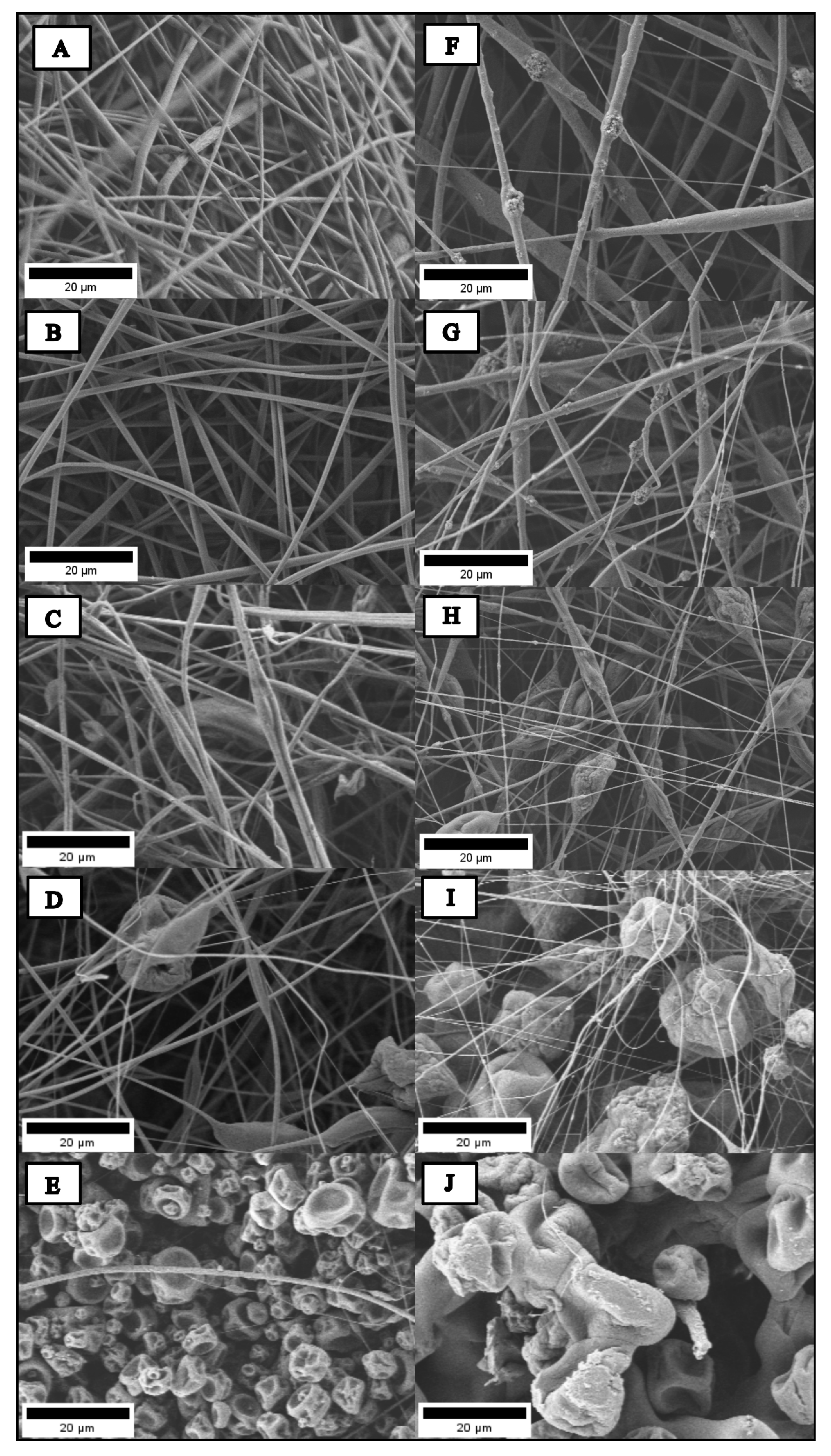
2.2. Morphology and Size of Hybrid Electrospun Fibers
2.3. Wettability of Hybrid Electrospun Fibers
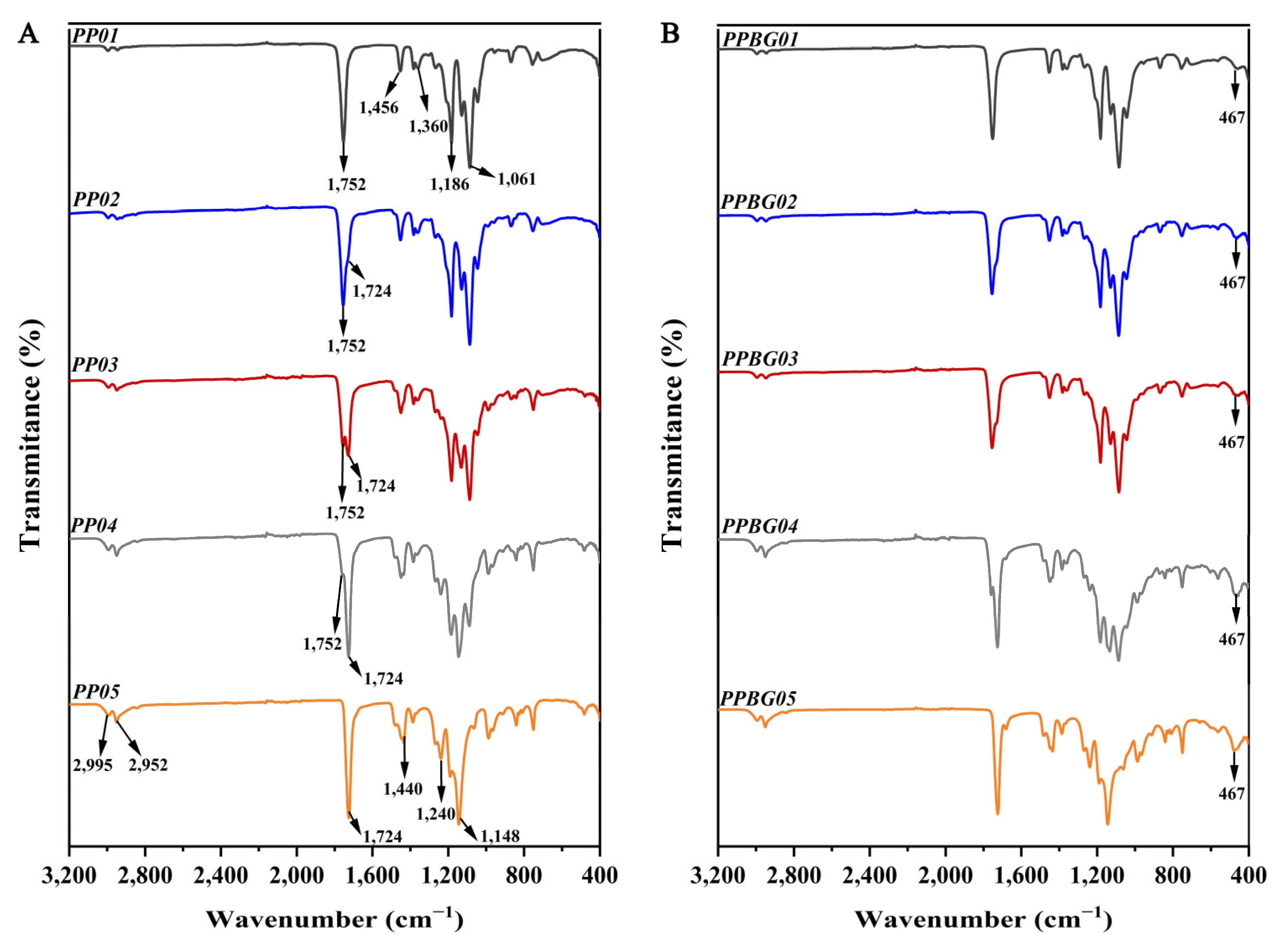
2.4. Infrared Spectroscopy Analysis of the Hybrid Electrospun Fibers
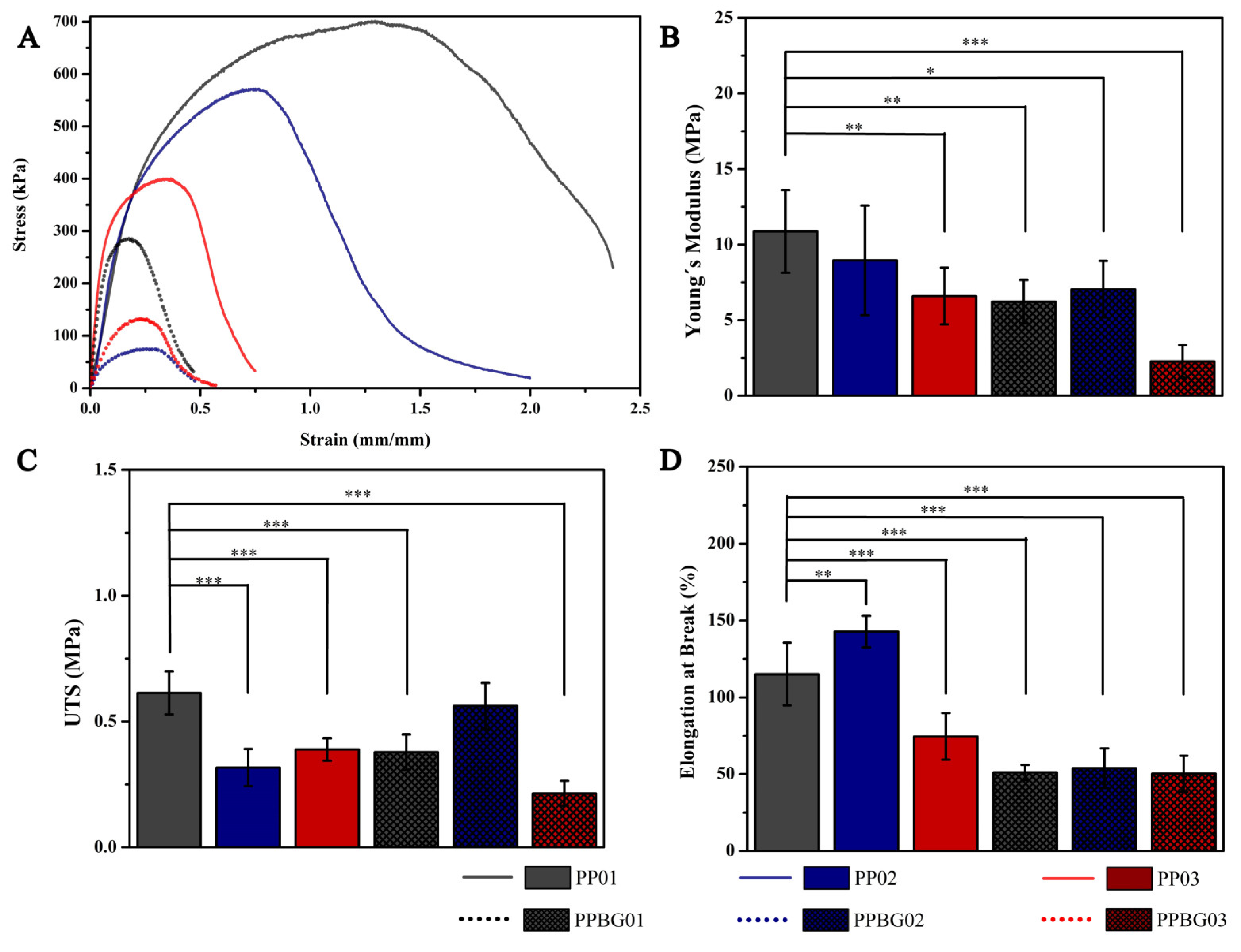
2.5. Mechanical Characterization of Hybrid Fibers
2.6. In Vitro Bioactivity
2.7. In Vitro Hydrolytic Degradation
2.8. In Vitro Cell Viability
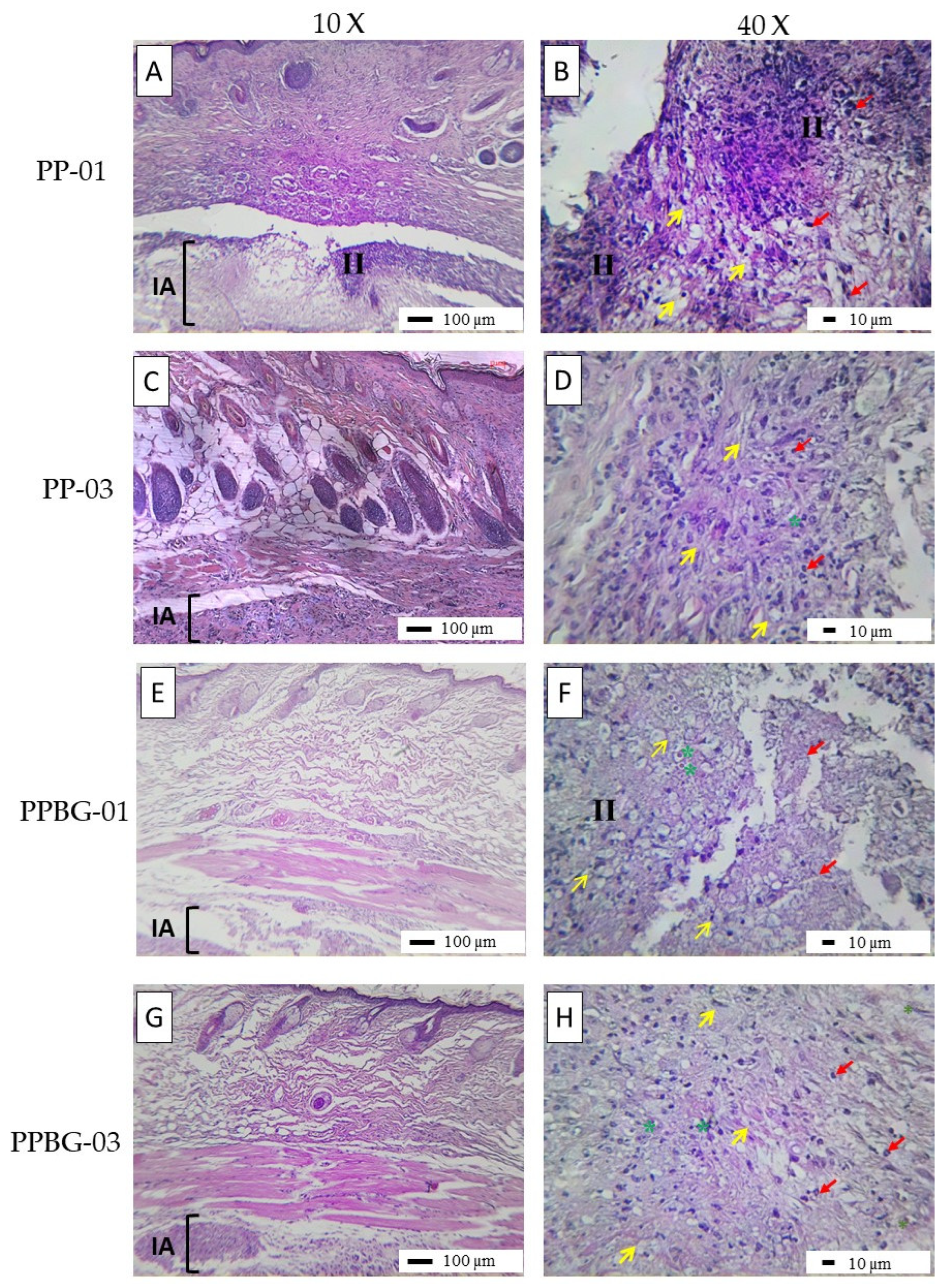
2.9. In Vivo Biocompatibility
3. Materials and Methods
3.1. Materials
3.2. Preparation of Hybrid PLA/PMMA and PLA/PMMA/n-BG Fibers via Electrospinning Technique
3.3. Characterization of n-BG Nanoparticles, Hybrid PLA/PMMA, and PLA/PMMA/n-BG Fibers
3.3.1. Nanoparticle Characterization
3.3.2. Morphology and Size of Hybrid Electrospun Fibers
3.3.3. Wettability of the Hybrid Electrospun Fibers
3.3.4. Infrared Spectroscopy Analysis of the Hybrid Electrospun Fibers
3.3.5. Mechanical Characterization of Hybrid Fibers
3.3.6. In Vitro Bioactivity in Simulated Body Fluid (SBF)
3.3.7. In Vitro Hydrolytic Degradation in Phosphate-Buffered Saline Solution (PBS)
3.3.8. In Vitro Cell Viability
3.3.9. In Vivo Biocompatibility
3.3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jang, J.W.; Min, K.E.; Kim, C.; Shin, J.; Lee, J.; Yi, S. Correction: Review: Scaffold Characteristics, Fabrication Methods, and Biomaterials for the Bone Tissue Engineering. Int. J. Precis. Eng. Manuf. 2023, 24, 887. [Google Scholar] [CrossRef]
- Ansari, M. Bone tissue regeneration: Biology, strategies and interface studies. Prog. Biomater. 2019, 8, 223–237. [Google Scholar] [CrossRef]
- Battafarano, G.; Rossi, M.; De Martino, V.; Marampon, F.; Borro, L.; Secinaro, A.; Del Fattore, A. Strategies for bone regeneration: From graft to tissue engineering. Int. J. Mol. Sci. 2021, 22, 1128. [Google Scholar] [CrossRef] [PubMed]
- Chocholata, P.; Kulda, V.; Babuska, V. Fabrication of scaffolds for bone-tissue regeneration. Materials 2019, 12, 568. [Google Scholar] [CrossRef] [PubMed]
- Alaribe, F.N.; Manoto, S.L.; Motaung, S.C.K.M. Scaffolds from biomaterials: Advantages and limitations in bone and tissue engineering. Biologia 2016, 71, 353–366. [Google Scholar] [CrossRef]
- Zadpoor, A.A. Bone tissue regeneration: The role of scaffold geometry. Biomater. Sci. 2014, 3, 231–245. [Google Scholar] [CrossRef]
- Zhang, K.; Fan, Y.; Dunne, N.; Li, X. Effect of microporosity on scaffolds for bone tissue engineering. Regen. Biomater. 2018, 5, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.-H.; Purevdorj, O.; Castano, O.; Planell, J.A.; Kim, H.-W. A short review: Recent advances in electrospinning for bone tissue regeneration. J. Tissue Eng. 2012, 3, 1–11. [Google Scholar] [CrossRef]
- Yang, X.; Wang, J.; Guo, H.; Liu, L.; Xu, W.; Duan, G. Structural design toward functional materials by electrospinning: A review. e-Polymers 2020, 20, 682–712. [Google Scholar] [CrossRef]
- Huang, C.; Thomas, N.L. Fabrication of porous fibers via electrospinning: Strategies and applications. Polym. Rev. 2020, 60, 595–647. [Google Scholar] [CrossRef]
- Jiang, T.; Carbone, E.J.; Lo, K.W.-H.; Laurencin, C.T. Electrospinning of polymer nanofibers for tissue regeneration. Prog. Polym. Sci. 2015, 46, 1–24. [Google Scholar] [CrossRef]
- Huang, C.; Thomas, N.L. Fabricating porous poly(lactic acid) fibres via electrospinning. Eur. Polym. J. 2018, 99, 464–476. [Google Scholar] [CrossRef]
- Peranidze, K.; Safronova, T.V.; Kildeeva, N.R. Fibrous polymer-based composites obtained by electrospinning for bone tissue engineering. Polymers 2022, 14, 96. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, F.; Dana, H.R. Poly lactic acid (PLA) polymers: From properties to biomedical applications. Int. J. Polym. Mater. Polym. Biomater. 2021, 71, 1117–1130. [Google Scholar] [CrossRef]
- Lasprilla, A.J.R.; Martinez, G.A.R.; Lunelli, B.H.; Jardini, A.L.; Filho, R.M. Poly-lactic acid synthesis for application in biomedical devices—A review. Biotechnol. Adv. 2012, 30, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Canales, D.A.; Reyes, F.; Saavedra, M.; Peponi, L.; Leonés, A.; Palza, H.; Boccaccini, A.R.; Grünewald, A.; Zapata, P.A. Electrospun fibers of poly (lactic acid) containing bioactive glass and magnesium oxide nanoparticles for bone tissue regeneration. Int. J. Biol. Macromol. 2022, 210, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Canales, D.; Moyano, D.; Alvarez, F.; Grande-Tovar, C.D.; Valencia-Llano, C.H.; Peponi, L.; Boccaccini, A.R.; Zapata, P.A. Preparation and characterization of novel poly (lactic acid)/calcium oxide nanocomposites by electrospinning as a potential bone tissue scaffold. Mater. Sci. Eng. C 2023, 153, 213578. [Google Scholar] [CrossRef] [PubMed]
- Shahverdi, M.; Seifi, S.; Akbari, A.; Mohammadi, K.; Shamloo, A.; Movahhedy, M.R. Melt electrowriting of PLA, PCL, and composite PLA/PCL scaffolds for tissue engineering application. Sci. Rep. 2022, 12, 19935. [Google Scholar] [CrossRef]
- Magiera, A.; Markowski, J.; Menaszek, E.; Pilch, J.; Blazewicz, S. PLA-Based Hybrid and Composite Electrospun Fibrous Scaffolds as Potential Materials for Tissue Engineering. J. Nanomater. 2017, 2017, 9246802. [Google Scholar] [CrossRef]
- Lv, S.; Zhao, X.; Shi, L.; Zhang, G.; Wang, S.; Kang, W.; Zhuang, X. Preparation and properties of sc-PLA/PMMA transparent nanofiber air filter. Polymers 2018, 10, 996. [Google Scholar] [CrossRef]
- Ali, U.; Karim, K.J.B.A.; Buang, N.A. A Review of the Properties and Applications of Poly (Methyl Methacrylate) (PMMA). Polym. Rev. 2015, 55, 678–705. [Google Scholar] [CrossRef]
- Vedhanayagam, M.; Anandasadagopan, S.; Nair, B.U.; Sreeram, K.J. Polymethyl methacrylate (PMMA) grafted collagen scaffold reinforced by PdO–TiO2 nanocomposites. Mater. Sci. Eng. C 2019, 108, 110378. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, S.; Lin, Y.-C.; Thirumurugan, S.; Hu, C.-C.; Duann, Y.-F.; Chung, R.-J. Poly(methyl methacrylate) in Orthopedics: Strategies, Challenges, and Prospects in Bone Tissue Engineering. Polymers 2024, 16, 367. [Google Scholar] [CrossRef]
- Anakabe, J.; Huici, A.M.Z.; Eceiza, A.; Arbelaiz, A. Melt blending of polylactide and poly(methyl methacrylate): Thermal and mechanical properties and phase morphology characterization. J. Appl. Polym. Sci. 2015, 132, 24677. [Google Scholar] [CrossRef]
- Son, S.-R.; Linh, N.-T.B.; Yang, H.-M.; Lee, B.-T. In vitro and in vivo evaluation of electrospun PCL/PMMA fibrous scaffolds for bone regeneration. Sci. Technol. Adv. Mater. 2013, 14, 015009. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, S.; Yamaguchi, S.; Barbani, N.; Cazzola, M.; Cristallini, C.; Miola, M.; Vernè, E.; Spriano, S. Bioactive materials: In vitro investigation of different mechanisms of hydroxyapatite precipitation. Acta Biomater. 2020, 102, 468–480. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Hench, L.L. Bioactive materials. Ceram. Int. 1996, 22, 493–507. [Google Scholar] [CrossRef]
- Schätzlein, E.; Kicker, C.; Söhling, N.; Ritz, U.; Neijhoft, J.; Henrich, D.; Frank, J.; Marzi, I.; Blaeser, A. 3D-Printed PLA-Bioglass Scaffolds with Controllable Calcium Release and MSC Adhesion for Bone Tissue Engineering. Polymers 2022, 14, 2389. [Google Scholar] [CrossRef]
- Yunos, D.M.; Ahmad, Z.; Boccaccini, A.R. Fabrication and characterization of electrospun poly-DL-lactide (PDLLA) fibrous coatings on 45S5 Bioglass® substrates for bone tissue engineering applications. J. Chem. Technol. Biotechnol. 2010, 85, 768–774. [Google Scholar] [CrossRef]
- Canales, D.; Saavedra, M.; Flores, M.T.; Bejarano, J.; Ortiz, J.A.; Orihuela, P.; Alfaro, A.; Pabón, E.; Palza, H.; Zapata, P.A. Effect of bioglass nanoparticles on the properties and bioactivity of poly(lactic acid) films. J. Biomed. Mater. Res. A 2020, 108, 2032–2043. [Google Scholar] [CrossRef]
- Canales, D.A.; Piñones, N.; Saavedra, M.; Loyo, C.; Palza, H.; Peponi, L.; Leonés, A.; Baier, R.V.; Boccaccini, A.R.; Grünelwald, A.; et al. Fabrication and assessment of bifunctional electrospun poly(l-lactic acid) scaffolds with bioglass and zinc oxide nanoparticles for bone tissue engineering. Int. J. Biol. Macromol. 2023, 228, 78–88. [Google Scholar] [CrossRef]
- Tajbakhsh, S.; Hajiali, F. A comprehensive study on the fabrication and properties of biocomposites of poly(lactic acid)/ceramics for bone tissue engineering. Mater. Sci. Eng. C 2017, 70, 897–912. [Google Scholar] [CrossRef]
- Noh, K.-T.; Lee, H.-Y.; Shin, U.-S.; Kim, H.-W. Composite nanofiber of bioactive glass nanofiller incorporated poly(lactic acid) for bone regeneration. Mater. Lett. 2010, 64, 802–805. [Google Scholar] [CrossRef]
- Erol-Taygun, M.; Unalan, I.; Idris, M.I.B.; Mano, J.F.; Boccaccini, A.R. Bioactıve Glass-Polymer Nanocomposites for Bone Tıssue Regeneration Applicatıons: A Revıew. Adv. Eng. Mater. 2019, 21, 1900287. [Google Scholar] [CrossRef]
- Pajares-Chamorro, N.; Chatzistavrou, X. Bioactive Glass Nanoparticles for Tissue Regeneration. ACS Omega 2020, 5, 12716–12726. [Google Scholar] [CrossRef]
- Serio, F.; Miola, M.; Vernè, E.; Pisignano, D.; Boccaccini, A.R.; Liverani, L. Electrospun filaments embedding bioactive glass particles with ion release and enhanced mineralization. Nanomaterials 2019, 9, 182. [Google Scholar] [CrossRef]
- Liverani, L.; Lacina, J.; Roether, J.A.; Boccardi, E.; Killian, M.S.; Schmuki, P.; Schubert, D.W.; Boccaccini, A.R. Incorporation of bioactive glass nanoparticles in electrospun PCL/chitosan fibers by using benign solvents. Bioact. Mater. 2018, 3, 55–63. [Google Scholar] [CrossRef]
- Rong, Z.; Zeng, W.; Kuang, Y.; Zhang, J.; Liu, X.; Lu, Y.; Cheng, X. Enhanced bioactivity of osteoblast-like cells on poly(lactic acid)/poly(methyl methacrylate)/nano-hydroxyapatite scaffolds for bone tissue engineering. Fibers Polym. 2015, 16, 245–253. [Google Scholar] [CrossRef]
- Luz, G.M.; Mano, J.F. Preparation and characterization of bioactive glass nanoparticles prepared by sol–gel for biomedical applications. Nanotechnology 2011, 22, 494014. [Google Scholar] [CrossRef]
- Duta, L.; Popescu, A.; Zgura, I.; Preda, N.; Mihailescu, I. Wettability of Nanostructured Surfaces. In Wetting and Wettability; InTech: London, UK, 2015. [Google Scholar] [CrossRef]
- Ehtesabi, H.; Massah, F. Improvement of hydrophilicity and cell attachment of polycaprolactone scaffolds using green synthesized carbon dots. Mater. Today Sustain. 2021, 13, 100075. [Google Scholar] [CrossRef]
- Raman, A.; Jayan, J.S.; Deeraj, B.; Saritha, A.; Joseph, K. Electrospun Nanofibers as Effective Superhydrophobic Surfaces: A Brief review. Surf. Interfaces 2021, 24, 101140. [Google Scholar] [CrossRef]
- Piperno, S.; Lozzi, L.; Rastelli, R.; Passacantando, M.; Santucci, S. PMMA nanofibers production by electrospinning. Appl. Surf. Sci. 2006, 252, 5583–5586. [Google Scholar] [CrossRef]
- Liao, G.Y.; Zhou, X.P.; Xie, X.L.; Mai, Y.W. Electrospun polymer scaffolds: Their biomedical and mechanical properties. In Springer Series in Biomaterials Science and Engineering; Springer Science and Business Media, LLC.: Berlin, Germany, 2016; pp. 237–270. [Google Scholar] [CrossRef]
- Rashid, T.U.; Gorga, R.E.; Krause, W.E. Mechanical Properties of Electrospun Fibers—A Critical Review. Adv. Eng. Mater. 2021, 23, 2100153. [Google Scholar] [CrossRef]
- Salimbeigi, G.; Cahill, P.; McGuinness, G. Solvent system effects on the physical and mechanical properties of electrospun Poly(ε-caprolactone) scaffolds for in vitro lung models. J. Mech. Behav. Biomed. Mater. 2022, 136, 105493. [Google Scholar] [CrossRef]
- O’Connor, R.; Cahill, P.; McGuinness, G. Effect of electrospinning parameters on the mechanical and morphological characteristics of small diameter PCL tissue engineered blood vessel scaffolds having distinct micro and nano fibre populations—A DOE approach. Polym. Test. 2021, 96, 107119. [Google Scholar] [CrossRef]
- Dolgin, J.; Hanumantharao, S.N.; Farias, S.; Simon, C.G.; Rao, S. Mechanical properties and morphological alterations in fiber-based scaffolds affecting tissue engineering outcomes. Fibers 2023, 11, 39. [Google Scholar] [CrossRef]
- Alharbi, N.; Daraei, A.; Lee, H.; Guthold, M. The effect of molecular weight and fiber diameter on the mechanical properties of single, electrospun PCL nanofibers. Mater. Today Commun. 2023, 35, 105773. [Google Scholar] [CrossRef]
- Boakye, M.A.D.; Rijal, N.P.; Adhikari, U.; Bhattarai, N. Fabrication and characterization of electrospun PCL-MgO-keratin-based composite nanofibers for biomedical applications. Materials 2015, 8, 4080–4095. [Google Scholar] [CrossRef]
- Luginina, M.; Schuhladen, K.; Orrú, R.; Cao, G.; Boccaccini, A.R.; Liverani, L. Electrospun PCL/PGS composite fibers incorporating bioactive glass particles for soft tissue engineering applications. Nanomaterials 2020, 10, 978. [Google Scholar] [CrossRef] [PubMed]
- Bretcanu, O.; Misra, S.K.; Yunos, D.M.; Boccaccini, A.R.; Roy, I.; Kowalczyk, T.; Blonski, S.; Kowalewski, T.A. Electrospun nanofibrous biodegradable polyester coatings on Bioglass®-based glass-ceramics for tissue engineering. Mater. Chem. Phys. 2009, 118, 420–426. [Google Scholar] [CrossRef]
- Filip, D.G.; Surdu, V.-A.; Paduraru, A.V.; Andronescu, E. Current Development in Biomaterials—Hydroxyapatite and Bioglass for Applications in Biomedical Field: A Review. J. Funct. Biomater. 2022, 13, 248. [Google Scholar] [CrossRef]
- Bi, H.; Feng, T.; Li, B.; Han, Y. In vitro and in vivo comparison study of electrospun pla and pla/pva/sa fiber membranes for wound healing. Polymers 2020, 12, 839. [Google Scholar] [CrossRef]
- Cole, K.A.; Funk, G.A.; Rahaman, M.N.; McIff, T.E. Mechanical and degradation properties of poly(methyl methacrylate) cement/borate bioactive glass composites. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 2765–2775. [Google Scholar] [CrossRef]
- Chelu, M.; Musuc, A.M. Advanced Biomedical Applications of Multifunctional Natural and Synthetic Biomaterials. Processes 2023, 11, 2696. [Google Scholar] [CrossRef]
- Bruinink, A.; Luginbuehl, R. Evaluation of biocompatibility using in vitro methods: Interpretation and limitations. Adv. Biochem. Eng. Biotechnol. 2012, 126, 117–152. [Google Scholar] [CrossRef]
- Vega-González, A.; Subra-Paternault, P.; López-Periago, A.M.; García-González, C.A.; Domingo, C. Supercritical CO2 antisolvent precipitation of polymer networks of l-PLA, PMMA and PMMA/PCL blends for biomedical applications. Eur. Polym. J. 2008, 44, 1081–1094. [Google Scholar] [CrossRef]
- Xing, Z.-C.; Han, S.-J.; Shin, Y.-S.; Koo, T.-H.; Moon, S.; Jeong, Y.; Kang, I.-K. Enhanced osteoblast responses to poly(methyl methacrylate)/hydroxyapatite electrospun nanocomposites for bone tissue engineering. J. Biomater. Sci. Polym. Ed. 2013, 24, 61–76. [Google Scholar] [CrossRef]
- Zaszczyńska, A.; Kołbuk, D.; Gradys, A.; Sajkiewicz, P. Development of Poly (methyl methacrylate)/nano-hydroxyapatite (PMMA/nHA) Nanofibers for Tissue Engineering Regeneration Using an Electrospinning Technique. Polymers 2024, 16, 531. [Google Scholar] [CrossRef]
- Chandorkar, Y.; Ravikumar, K.; Basu, B. The Foreign Body Response Demystified. ACS Biomater. Sci. Eng. 2019, 5, 19–44. [Google Scholar] [CrossRef]
- Cui, X.; Huang, C.; Zhang, M.; Ruan, C.; Peng, S.; Li, L.; Liu, W.; Wang, T.; Li, B.; Huang, W.; et al. Enhanced osteointegration of poly(methylmethacrylate) bone cements by incorporating strontium-containing borate bioactive glass. J. R. Soc. Interface 2017, 14, 20161057. [Google Scholar] [CrossRef]
- Sharifi, E.; Sadati, S.A.; Yousefiasl, S.; Sartorius, R.; Zafari, M.; Rezakhani, L.; Alizadeh, M.; Zare, E.N.; Omidghaemi, S.; Ghanavatinejad, F.; et al. Cell loaded hydrogel containing Ag-doped bioactive glass–ceramic nanoparticles as skin substitute: Antibacterial properties, immune response, and scarless cutaneous wound regeneration. Bioeng. Transl. Med. 2022, 7, e10386. [Google Scholar] [CrossRef] [PubMed]
- Barbeck, M.; Alkildani, S.; Mandlule, A.; Radenković, M.; Najman, S.; Stojanović, S.; Jung, O.; Ren, Y.; Cai, B.; Görke, O.; et al. In Vivo Analysis of the Immune Response to Strontium- and Copper-doped Bioglass. In Vivo 2022, 36, 2149–2165. [Google Scholar] [CrossRef] [PubMed]
- D882-12; American Society for Testing and Materials. Standard Test Method for Tensile Properties of Thin Plastic Sheeting. ASTM International: West Conshohocken, PA, USA, 2018. [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhong, T.; Huang, R.; Wan, A. Preparation of hydrophilic poly(lactic acid) tissue engineering scaffold via (PLA)-(PLA-b-PEG)-(PEG) solution casting and thermal-induced surface structural transformation. J. Biomater. Sci. Polym. Ed. 2015, 26, 1286–1296. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-6:2016; Biological Evaluation of Medical Devices—Part 6: Tests for Local Effects after Implantation. International Organization for Standardization: Geneva, Switzerland, 2016. [CrossRef]


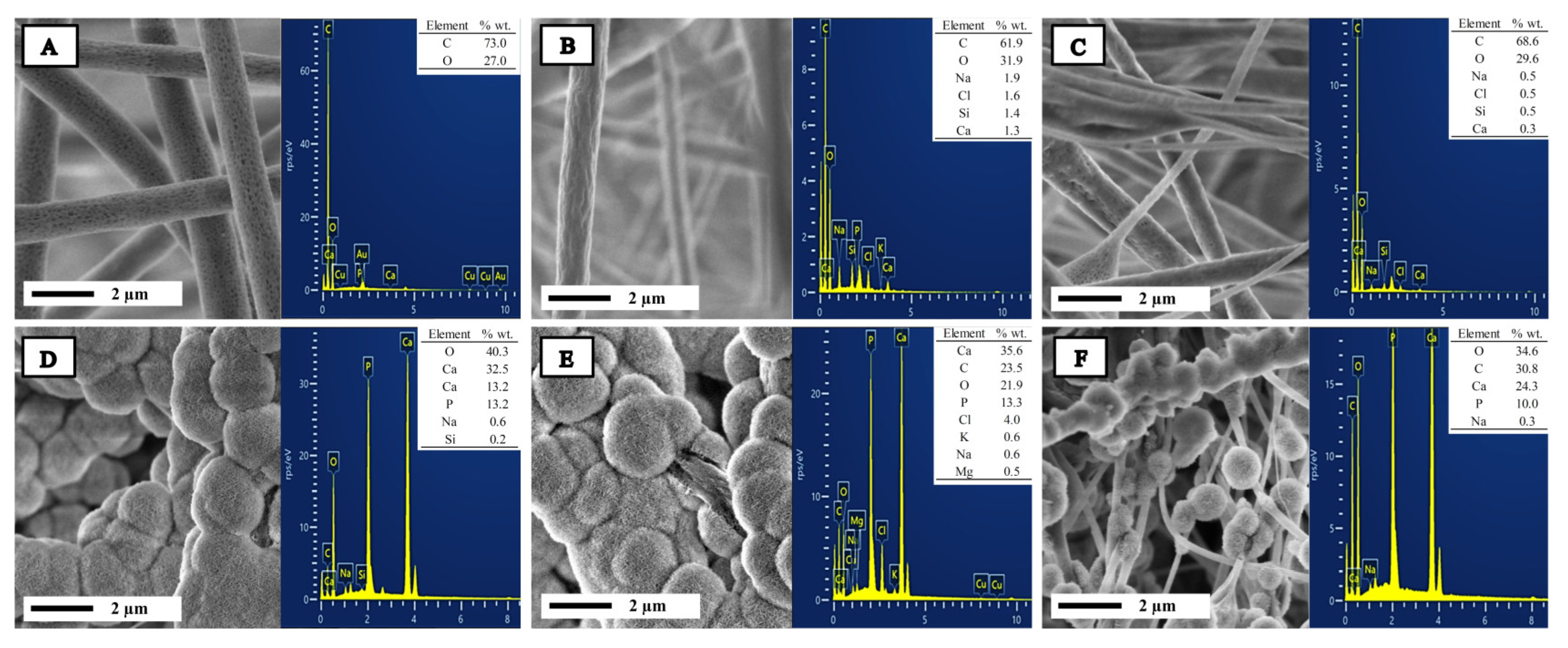


| Sample | Ca (% wt./wt.) | P (% wt./wt.) | Ca/P Relation |
|---|---|---|---|
| PP01 | 0 | 0 | 0 |
| PP02 | 0 | 0 | 0 |
| PP03 | 0 | 0 | 0 |
| PPBG01 | 32.5 | 13.3 | 1.90 |
| PPBG02 | 35.5 | 13.2 | 2.06 |
| PPBG03 | 24.3 | 10.0 | 1.87 |
| Sample | Code | Amount PLA [g] | Amount PMMA [g] | Amount n-BG [g] |
|---|---|---|---|---|
| Neat PLA | PP-01 | 1.250 | 0 | 0 |
| PLA/PMMA (75/25) | PP-02 | 0.9375 | 0.3125 | 0 |
| PLA/PMMA (50/50) | PP-03 | 0.6250 | 0.6250 | 0 |
| PLA/PMMA (25/75) | PP-04 | 0.3125 | 0.9375 | 0 |
| Neat PMMA | PP-05 | 0 | 1.250 | 0 |
| PLA + 10 wt.% n-BG | PPBG-01 | 1.125 | 0 | 0.1250 |
| PLA/PMMA (75/25) + 10 wt.% n-BG | PPBG-02 | 0.8437 | 0.2813 | 0.1250 |
| PLA/PMMA (50/50) + 10 wt.% n-BG | PPBG-03 | 0.5625 | 0.5625 | 0.1250 |
| PLA/PMMA (25/75) + 10 wt.% n-BG | PPBG-04 | 0.2813 | 0.8437 | 0.1250 |
| PMMA + 10 wt.% n-BG | PPBG-05 | 0 | 1.125 | 0.1250 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Álvarez-Carrasco, F.; Varela, P.; Sarabia-Vallejos, M.A.; García-Herrera, C.; Saavedra, M.; Zapata, P.A.; Zárate-Triviño, D.; Martínez, J.J.; Canales, D.A. Development of Bioactive Hybrid Poly(lactic acid)/Poly(methyl methacrylate) (PLA/PMMA) Electrospun Fibers Functionalized with Bioglass Nanoparticles for Bone Tissue Engineering Applications. Int. J. Mol. Sci. 2024, 25, 6843. https://doi.org/10.3390/ijms25136843
Álvarez-Carrasco F, Varela P, Sarabia-Vallejos MA, García-Herrera C, Saavedra M, Zapata PA, Zárate-Triviño D, Martínez JJ, Canales DA. Development of Bioactive Hybrid Poly(lactic acid)/Poly(methyl methacrylate) (PLA/PMMA) Electrospun Fibers Functionalized with Bioglass Nanoparticles for Bone Tissue Engineering Applications. International Journal of Molecular Sciences. 2024; 25(13):6843. https://doi.org/10.3390/ijms25136843
Chicago/Turabian StyleÁlvarez-Carrasco, Fabián, Pablo Varela, Mauricio A. Sarabia-Vallejos, Claudio García-Herrera, Marcela Saavedra, Paula A. Zapata, Diana Zárate-Triviño, Juan José Martínez, and Daniel A. Canales. 2024. "Development of Bioactive Hybrid Poly(lactic acid)/Poly(methyl methacrylate) (PLA/PMMA) Electrospun Fibers Functionalized with Bioglass Nanoparticles for Bone Tissue Engineering Applications" International Journal of Molecular Sciences 25, no. 13: 6843. https://doi.org/10.3390/ijms25136843
APA StyleÁlvarez-Carrasco, F., Varela, P., Sarabia-Vallejos, M. A., García-Herrera, C., Saavedra, M., Zapata, P. A., Zárate-Triviño, D., Martínez, J. J., & Canales, D. A. (2024). Development of Bioactive Hybrid Poly(lactic acid)/Poly(methyl methacrylate) (PLA/PMMA) Electrospun Fibers Functionalized with Bioglass Nanoparticles for Bone Tissue Engineering Applications. International Journal of Molecular Sciences, 25(13), 6843. https://doi.org/10.3390/ijms25136843







