Identification of New Chemoresistance-Associated Genes in Triple-Negative Breast Cancer by Single-Cell Transcriptomic Analysis
Abstract
:1. Introduction
2. Results
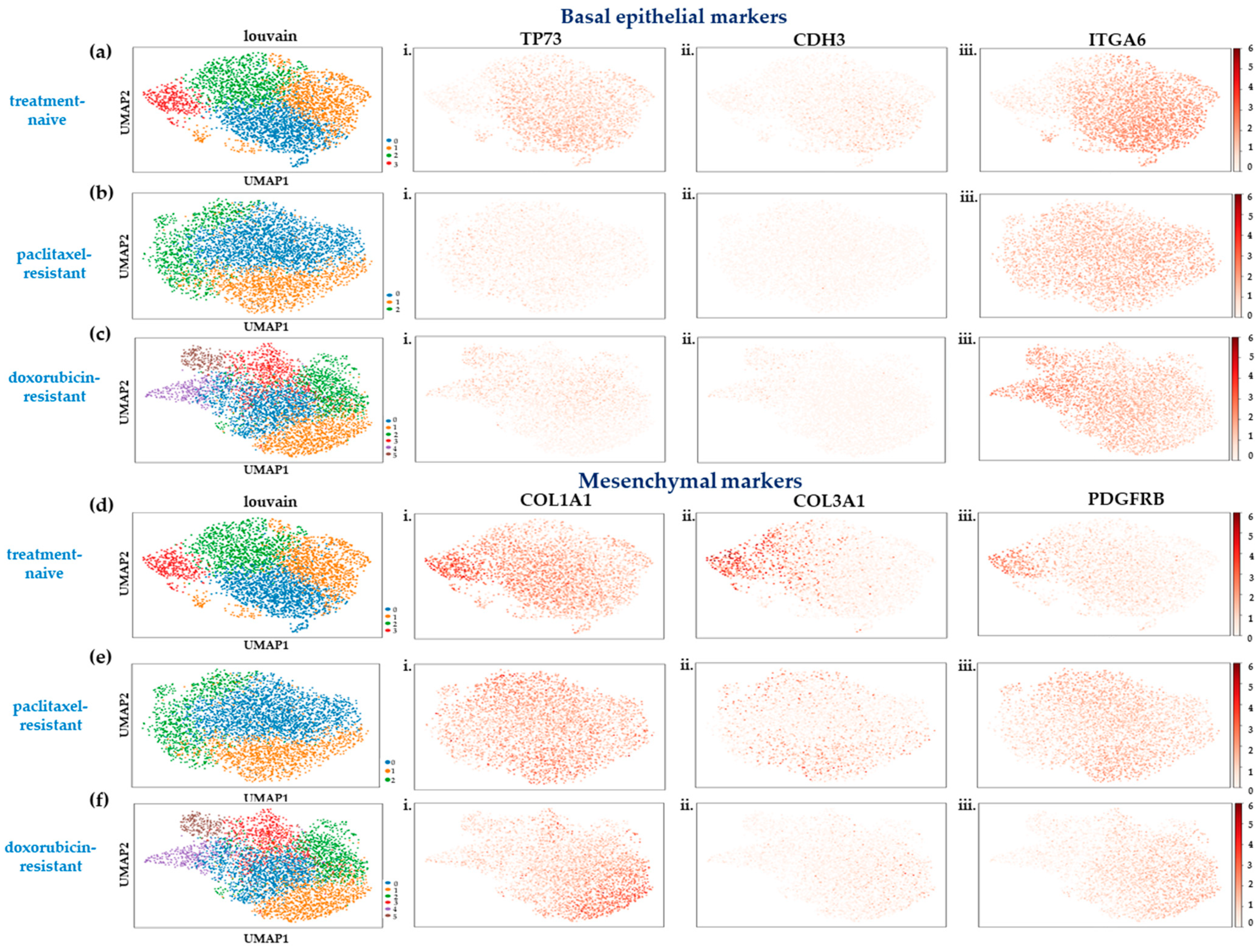
2.1. Single-Cell Transcriptome Analysis of SUM159 TNBC Cells Indicates a Partial Shift to a Highly Mesenchymal Phenotype upon Acquisition of Chemoresistance
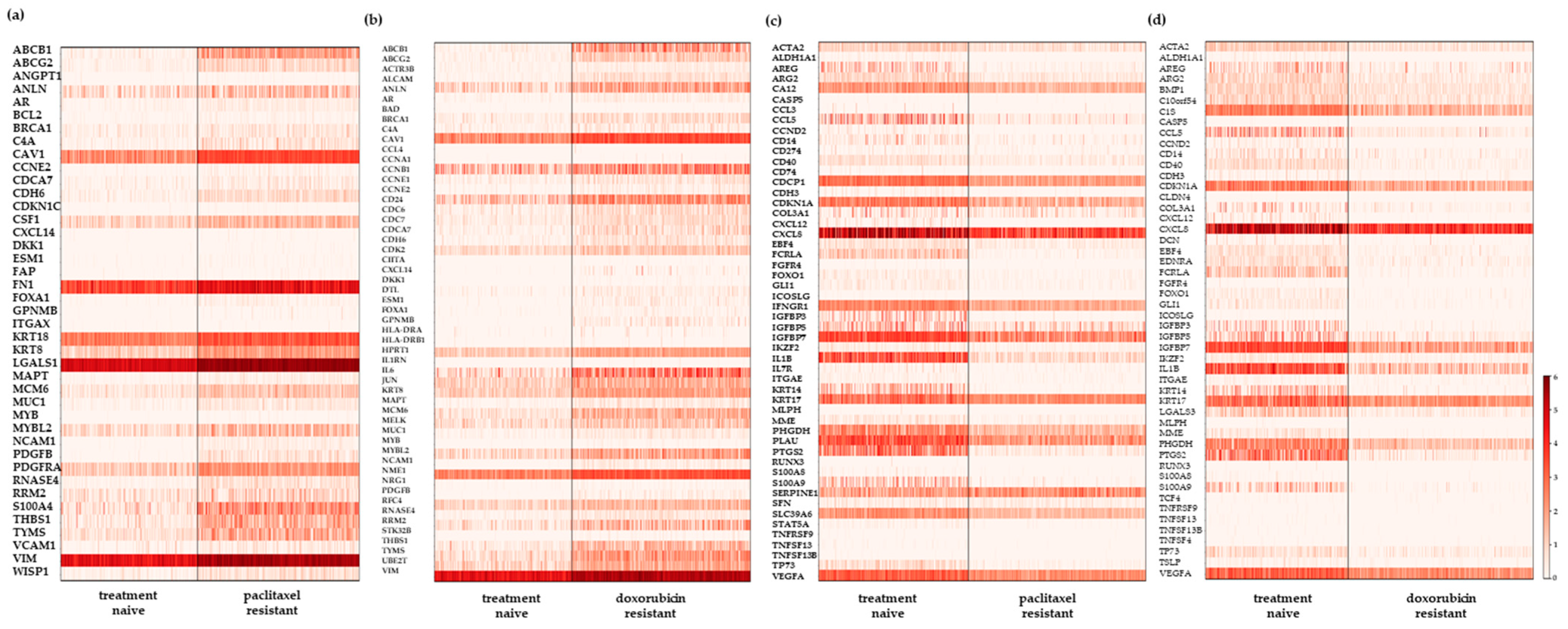
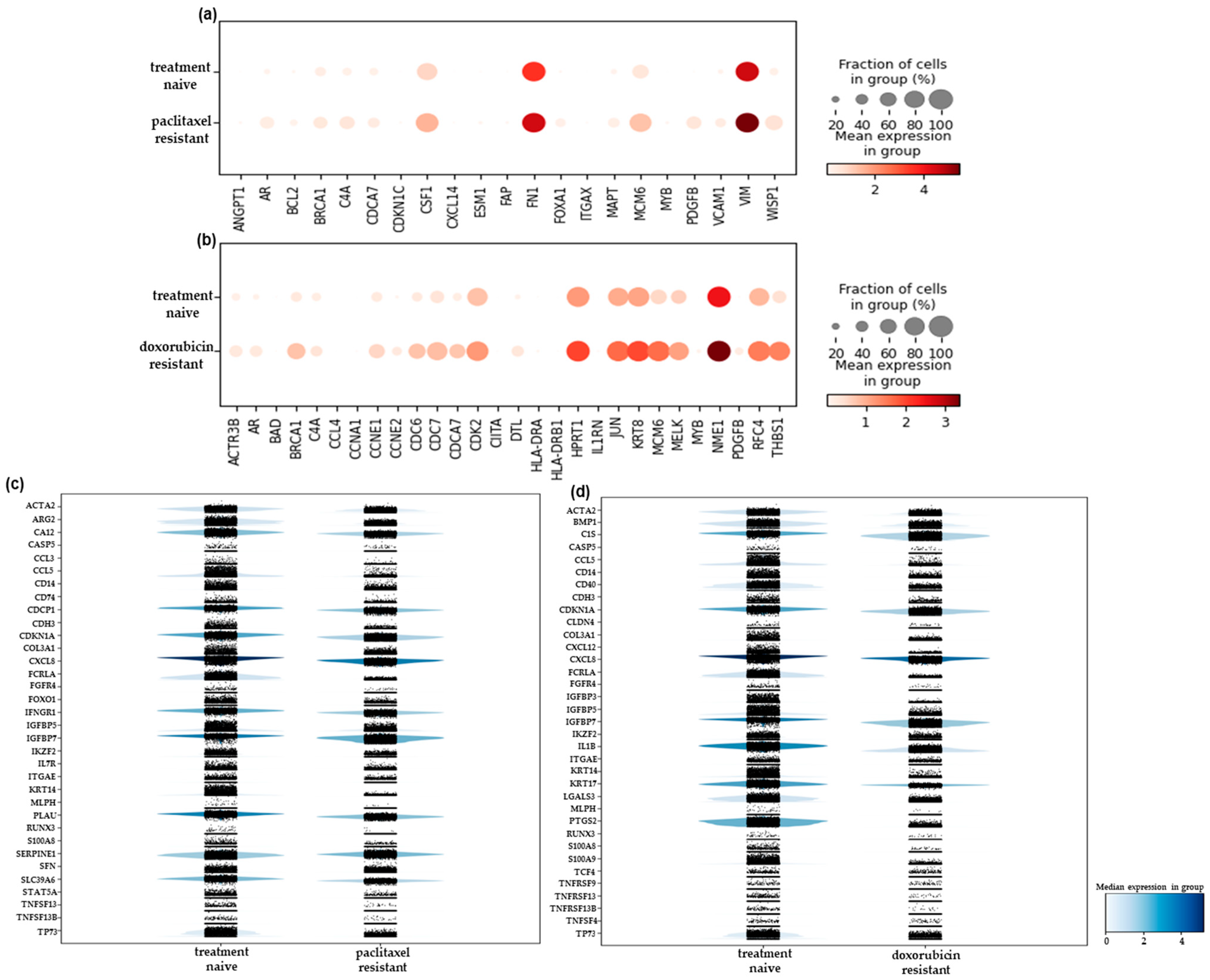
2.2. Differential Gene Expression Analysis of the scRNA-Seq Data Reveals New Resistance-Associated Genes in Chemoresistant TNBC
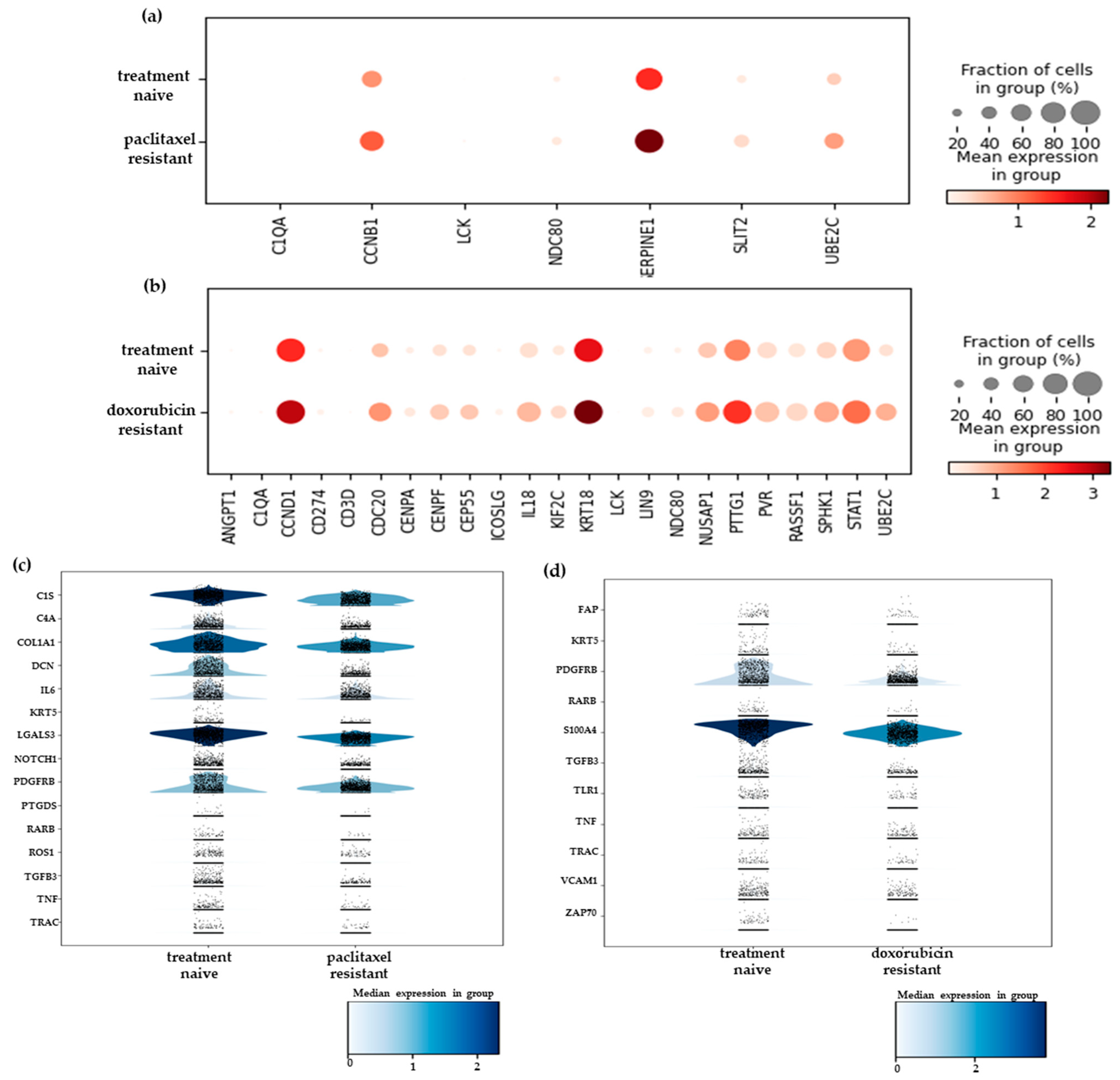
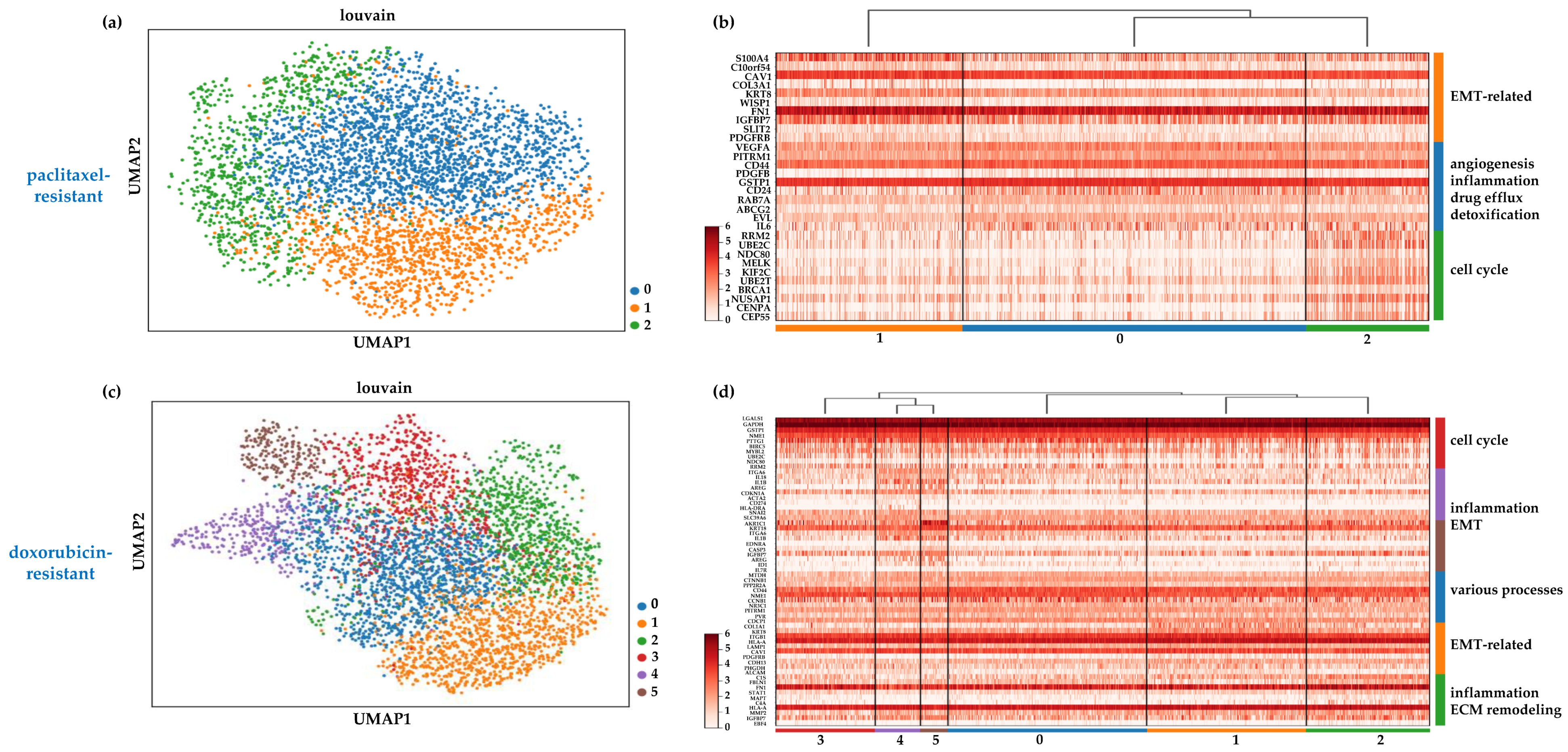
2.3. Heterogeneity in the Chemoresistant TNBC Populations Reflects Distinct Mechanisms of Resistance
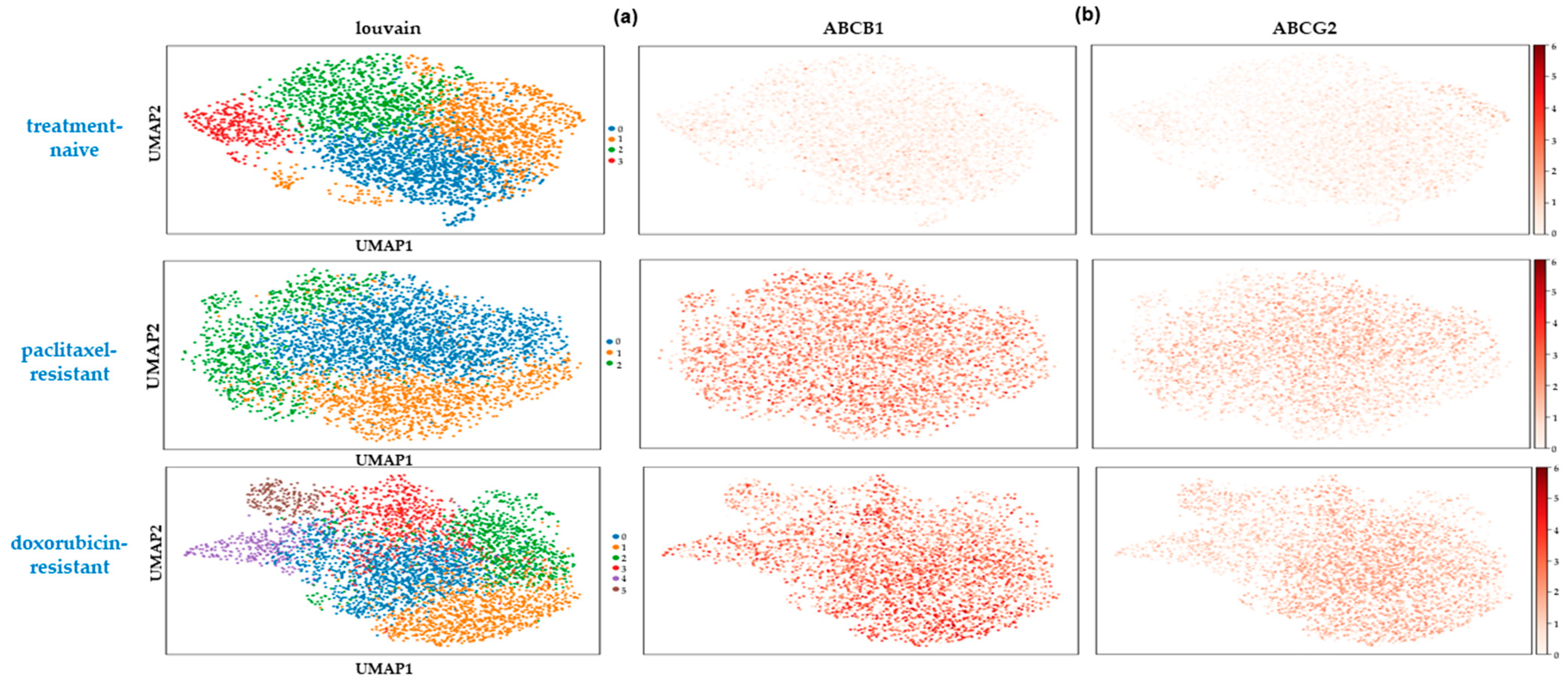
2.4. Rare Drug-Resistant Cells Preexist in the Treatment-Naïve Cell Populations
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Generation of Chemoresistant Cell Lines
4.2. Generation of 3D Spheroid Cultures
4.3. RNA Extraction and Bulk-RNA Seq Experiments
4.4. Sample Preparation and scRNA-Seq Experiments
4.5. Data Analysis
4.5.1. Bulk RNA-Seq Analysis
4.5.2. Targeted Single-Cell RNA-Seq Analysis
4.5.3. STRING Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anders, C.K.; Abramson, V.; Tan, T.; Dent, R. The Evolution of Triple-Negative Breast Cancer: From Biology to Novel Therapeutics. Am. Soc. Clin. Oncol. Educ. Book 2016, 36, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Derakhshan, F.; Reis-Filho, J.S. Pathogenesis of Triple-Negative Breast Cancer. Annu. Rev. Pathol. Mech. Dis. 2022, 17, 181–204. [Google Scholar] [CrossRef]
- Bianchini, G.; De Angelis, C.; Licata, L.; Gianni, L. Treatment Landscape of Triple-Negative Breast Cancer—Expanded Options, Evolving Needs. Nat. Rev. Clin. Oncol. 2021, 19, 91–113. [Google Scholar] [CrossRef] [PubMed]
- Asleh, K.; Riaz, N.; Nielsen, T.O. Heterogeneity of Triple Negative Breast Cancer: Current Advances in Subtyping and Treatment Implications. J. Exp. Clin. Cancer Res. 2022, 41, 265. [Google Scholar] [CrossRef]
- van den Ende, N.S.; Nguyen, A.H.; Jager, A.; Kok, M.; Debets, R.; van Deurzen, C.H.M. Triple-Negative Breast Cancer and Predictive Markers of Response to Neoadjuvant Chemotherapy: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 2969. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, F.; Efferth, T.; Schmidt, F.; Efferth, T. Tumor Heterogeneity, Single-Cell Sequencing, and Drug Resistance. Pharmaceuticals 2016, 9, 33. [Google Scholar] [CrossRef]
- Gunti, S.; Hoke, A.T.K.; Vu, K.P.; London, N.R. Organoid and Spheroid Tumor Models: Techniques and Applications. Cancers 2021, 13, 874. [Google Scholar] [CrossRef]
- Kordias, D.; Kostara, C.E.; Papadaki, S.; Verigos, J.; Bairaktari, E.; Magklara, A. Omics Analysis of Chemoresistant Triple Negative Breast Cancer Cells Reveals Novel Metabolic Vulnerabilities. Cells 2022, 11, 2719. [Google Scholar] [CrossRef]
- Wolf, F.A.; Angerer, P.; Theis, F.J. SCANPY: Large-Scale Single-Cell Gene Expression Data Analysis. Genome Biol. 2018, 19, 15. [Google Scholar] [CrossRef]
- Lambert, A.W.; Fiore, C.; Chutake, Y.; Verhaar, E.R.; Strasser, P.C.; Chen, M.W.; Farouq, D.; Das, S.; Li, X.; Eaton, E.N.; et al. ΔNp63/P73 Drive Metastatic Colonization by Controlling a Regenerative Epithelial Stem Cell Program in Quasi-Mesenchymal Cancer Stem Cells. Dev. Cell 2022, 57, 2714–2730.e8. [Google Scholar] [CrossRef]
- Shah, S.; Sizemore, G.M. Diverse Roles of Tumor-Stromal PDGFB-to-PDGFRβ Signaling in Breast Cancer Growth and Metastasis. Adv. Cancer Res. 2022, 154, 93–140. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jin, K.; Van Pelt, G.W.; Van Dam, H.; Yu, X.; Mesker, W.E.; Ten Dijke, P.; Zhou, F.; Zhang, L. C-Myb Enhances Breast Cancer Invasion and Metastasis through the Wnt/β-Catenin/Axin2 Pathway. Cancer Res. 2016, 76, 3364–3375. [Google Scholar] [CrossRef] [PubMed]
- Pekarčíková, L.; Knopfová, L.; Beneš, P.; Šmarda, J. C-Myb Regulates NOX1/P38 to Control Survival of Colorectal Carcinoma Cells. Cell Signal. 2016, 28, 924–936. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, Y.; Ma, H.; Gao, Y. he Regulation of MYB Mediated Cisplatin Resistance of Ovarian Cancer Cells Involves MiR-21-Wnt Signaling Axis. Sci. Rep. 2020, 10, 6893. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, W.; Liu, C.; Li, G. MiR-200 Affects Tamoxifen Resistance in Breast Cancer Cells through Regulation of MYB. Sci. Rep. 2019, 9, 18844. [Google Scholar] [CrossRef] [PubMed]
- Metovic, J.; Borella, F.; D’Alonzo, M.; Biglia, N.; Mangherini, L.; Tampieri, C.; Bertero, L.; Cassoni, P.; Castellano, I. FOXA1 in Breast Cancer: A Luminal Marker with Promising Prognostic and Predictive Impact. Cancers 2022, 14, 4699. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fang, Y.; Bao, L.; Wu, F.; Wang, S.; Hao, S. Intercellular Communication Reveals Therapeutic Potential of Epithelial-Mesenchymal Transition in Triple-Negative Breast Cancer. Biomolecules 2022, 12, 1478. [Google Scholar] [CrossRef] [PubMed]
- Mangia, A.; Saponaro, C.; Vagheggini, A.; Opinto, G.; Centonze, M.; Vicenti, C.; Popescu, O.; Pastena, M.; Giotta, F.; Silvestris, N. Should Tumor Infiltrating Lymphocytes, Androgen Receptor, and FOXA1 Expression Predict the Clinical Outcome in Triple Negative Breast Cancer Patients? Cancers 2019, 11, 1393. [Google Scholar] [CrossRef]
- Guiu, S.; Mollevi, C.; Charon-Barra, C.; Boissière, F.; Crapez, E.; Chartron, E.; Lamy, P.J.; Gutowski, M.; Bourgier, C.; Romieu, G.; et al. Prognostic Value of Androgen Receptor and FOXA1 Co-Expression in Non-Metastatic Triple Negative Breast Cancer and Correlation with Other Biomarkers. Br. J. Cancer 2018, 119, 76–79. [Google Scholar] [CrossRef]
- Kumar, U.; Ardasheva, A.; Mahmud, Z.; Coombes, R.C.; Yagüe, E. FOXA1 Is a Determinant of Drug Resistance in Breast Cancer Cells. Breast Cancer Res. Treat. 2021, 186, 317. [Google Scholar] [CrossRef]
- Caldon, C.E.; Sergio, C.M.; Kang, J.; Muthukaruppan, A.; Boersma, M.N.; Stone, A.; Barraclough, J.; Lee, C.S.; Black, M.A.; Miller, L.D.; et al. Cyclin E2 Overexpression Is Associated with Endocrine Resistance but Not Insensitivity to CDK2 Inhibition in Human Breast Cancer Cells. Mol. Cancer Ther. 2012, 11, 1488–1499. [Google Scholar] [CrossRef] [PubMed]
- Tormo, E.; Ballester, S.; Adam-Artigues, A.; Burgués, O.; Alonso, E.; Bermejo, B.; Menéndez, S.; Zazo, S.; Madoz-Gúrpide, J.; Rovira, A.; et al. The MiRNA-449 Family Mediates Doxorubicin Resistance in Triple-Negative Breast Cancer by Regulating Cell Cycle Factors. Sci. Rep. 2019, 9, 5316. [Google Scholar] [CrossRef]
- Sun, Y.; Zheng, S.; Torossian, A.; Speirs, C.K.; Schleicher, S.; Giacalone, N.J.; Carbone, D.P.; Zhao, Z.; Lu, B. Role of Insulin-Like Growth Factor-1 Signaling Pathway in Cisplatin-Resistant Lung Cancer Cells. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Guix, M.; Faber, A.C.; Wang, S.E.; Olivares, M.G.; Song, Y.; Qu, S.; Rinehart, C.; Seidel, B.; Yee, D.; Arteaga, C.L.; et al. Acquired Resistance to EGFR Tyrosine Kinase Inhibitors in Cancer Cells Is Mediated by Loss of IGF-Binding Proteins. J. Clin. Investig. 2008, 118, 2609–2619. [Google Scholar] [CrossRef] [PubMed]
- Gallego, R.; Codony-Servat, J.; García-Albéniz, X.; Carcereny, E.; Longarón, R.; Oliveras, A.; Tosca, M.; Augé, J.M.; Gascón, P.; Maurel, J. Serum IGF-I, IGFBP-3, and Matrix Metalloproteinase-7 Levels and Acquired Chemo-Resistance in Advanced Colorectal Cancer. Endocr. Relat. Cancer 2009, 16, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Hawsawi, Y.; Humphries, M.P.; Wright, A.; Berwick, A.; Shires, M.; Al-Kharobi, H.; El-Gendy, R.; Jove, M.; Twelves, C.; Speirs, V.; et al. Deregulation of IGF-Binding Proteins -2 and -5 Contributes to the Development of Endocrine Resistant Breast Cancer In Vitro. Oncotarget 2016, 7, 32129–32143. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.; Zhou, Y.; Chui, C.H.; Lam, K.H.; Law, S.; Chan, A.S.; Li, X.; Lam, A.K.; Tang, J.C. Expression of Insulin-Like Growth Factor Binding Protein-5 (IGFBP5) Reverses Cisplatin-Resistance in Esophageal Carcinoma. Cells 2018, 7, 143. [Google Scholar] [CrossRef] [PubMed]
- Tomimaru, Y.; Eguchi, H.; Wada, H.; Noda, T.; Murakami, M.; Kobayashi, S.; Marubashi, S.; Takeda, Y.; Tanemura, M.; Umeshita, K.; et al. Insulin-like Growth Factor-Binding Protein 7 Alters the Sensitivity to Interferon-Based Anticancer Therapy in Hepatocellular Carcinoma Cells. Br. J. Cancer 2010, 102, 1483–1490. [Google Scholar] [CrossRef]
- Okamura, J.; Huang, Y.; Moon, D.; Brait, M.; Chang, X.; Kim, M.S. Downregulation of Insulin-like Growth Factor-Binding Protein 7 in Cisplatin-Resistant Non-Small Cell Lung Cancer. Cancer Biol. Ther. 2012, 13, 148–155. [Google Scholar] [CrossRef]
- Domentean, S.; Paisana, E.; Cascão, R.; Faria, C.C. Role of UBE2C in Brain Cancer Invasion and Dissemination. Int. J. Mol. Sci. 2023, 24, 5792. [Google Scholar] [CrossRef]
- Men, X.; Ma, J.; Wu, T.; Pu, J.; Wen, S.; Shen, J.; Wang, X.; Wang, Y.; Chen, C.; Dai, P. Transcriptome Profiling Identified Differentially Expressed Genes and Pathways Associated with Tamoxifen Resistance in Human Breast Cancer. Oncotarget 2018, 9, 4074–4089. [Google Scholar] [CrossRef] [PubMed]
- Neill, T.; Schaefer, L.; Iozzo, R.V. Decorin as a Multivalent Therapeutic Agent against Cancer. Adv. Drug Deliv. Rev. 2016, 97, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, P.; Natarajan, A.; Sathyanarayanan, A.; Veluswami, S.; Gopisetty, G. The Multifaceted Role of Matricellular Proteins in Health and Cancer, as Biomarkers and Therapeutic Targets. Gene 2022, 815, 146137. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Li, M.; Zhou, Y.; Wang, F.; Li, X.; Wang, L.; Fan, Q. S100A4 Participates in Epithelial-Mesenchymal Transition in Breast Cancer via Targeting MMP2. Tumor Biol. 2016, 37, 2925–2932. [Google Scholar] [CrossRef] [PubMed]
- Hellinger, J.W.; Hüchel, S.; Goetz, L.; Bauerschmitz, G.; Emons, G.; Gründker, C. Inhibition of Cyr61-S100a4 Axis Limits Breast Cancer Invasion. Front. Oncol. 2019, 9, 1074. [Google Scholar] [CrossRef] [PubMed]
- Rezouki, I.; Zohair, B.; Ssi, S.A.; Karkouri, M.; Razzouki, I.; Elkarroumi, M.; Badou, A. High VISTA Expression Is Linked to a Potent Epithelial-Mesenchymal Transition and Is Positively Correlated with PD1 in Breast Cancer. Front. Oncol. 2023, 13, 1154631. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Song, Y.; Ye, M.; Hu, X.; Wang, Z.P.; Zhu, X. The Emerging Role of WISP Proteins in Tumorigenesis and Cancer Therapy. J. Transl. Med. 2019, 17, 28. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Shim, J.S. Targeting Epithelial-Mesenchymal Transition (EMT) to Overcome Drug Resistance in Cancer. Molecules 2016, 21, 965. [Google Scholar] [CrossRef] [PubMed]
- Rašková, M.; Lacina, L.; Kejík, Z.; Venhauerová, A.; Skaličková, M.; Kolář, M.; Jakubek, M.; Rosel, D.; Smetana, K.; Brábek, J. The Role of IL-6 in Cancer Cell Invasiveness and Metastasis—Overview and Therapeutic Opportunities. Cells 2022, 11, 3698. [Google Scholar] [CrossRef]
- Allocati, N.; Masulli, M.; Di Ilio, C.; Federici, L. Glutathione Transferases: Substrates, Inihibitors and pro-Drugs in Cancer and Neurodegenerative Diseases. Oncogenesis 2018, 7, 8. [Google Scholar] [CrossRef]
- Fatima Zattoni, I.; Carani Delabio, L.; de Paula Dutra, J.; Henrique Kita, D.; Scheiffer, G.; Hembecker, M.; da Silva Pereira, G.; Rotuno Moure, V.; Valdameri, G. Targeting Breast Cancer Resistance Protein (BCRP/ABCG2): Functional Inhibitors and Expression Modulators. Eur. J. Med. Chem. 2022, 237, 114346. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Mo, J.; Dong, S.; Liao, Z.; Zhang, B.; Zhu, P. Integrinβ-1 in Disorders and Cancers: Molecular Mechanisms and Therapeutic Targets. Cell Commun. Signal. 2024, 22, 71. [Google Scholar] [CrossRef] [PubMed]
- Marozzi, M.; Parnigoni, A.; Negri, A.; Viola, M.; Vigetti, D.; Passi, A.; Karousou, E.; Rizzi, F. Inflammation, Extracellular Matrix Remodeling, and Proteostasis in Tumor Microenvironment. Int. J. Mol. Sci. 2021, 22, 8102. [Google Scholar] [CrossRef] [PubMed]
- Tune, B.X.J.; Sim, M.S.; Poh, C.L.; Mac Guad, R.; Woon, C.K.; Hazarika, I.; Das, A.; Gopinath, S.C.B.; Rajan, M.; Sekar, M.; et al. Matrix Metalloproteinases in Chemoresistance: Regulatory Roles, Molecular Interactions, and Potential Inhibitors. J. Oncol. 2022, 2022, 3249766. [Google Scholar] [CrossRef]
- Kolev, M.; Das, M.; Gerber, M.; Baver, S.; Deschatelets, P.; Markiewski, M.M. Inside-Out of Complement in Cancer. Front. Immunol. 2022, 13, 931273. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Roepman, P.; van’t Veer, L.J.; Bernards, R.; de Snoo, F.; Glas, A.M. Biological Functions of the Genes in the Mammaprint Breast Cancer Profile Reflect the Hallmarks of Cancer. Biomark. Insights 2010, 5, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Rébé, C.; Ghiringhelli, F. Interleukin-1β and Cancer. Cancers 2020, 12, 1791. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Jiang, J.; Zheng, X. Interleukin-1 Receptor Antagonist: An Alternative Therapy for Cancer Treatment. Life Sci. 2023, 335, 122276. [Google Scholar] [CrossRef] [PubMed]
- Harrison, M.; Zinovkin, D.; Pranjol, M.Z.I. Endothelin-1 and Its Role in Cancer and Potential Therapeutic Opportunities. Biomedicines 2024, 12, 511. [Google Scholar] [CrossRef]
- Cianfrocca, R.; Rosanò, L.; Tocci, P.; Sestito, R.; Caprara, V.; Di Castro, V.; De Maria, R.; Bagnato, A. Blocking Endothelin-1-Receptor/β-Catenin Circuit Sensitizes to Chemotherapy in Colorectal Cancer. Cell Death Differ. 2017, 24, 1811–1820. [Google Scholar] [CrossRef]
- Lee, H.J.; Hanibuchi, M.; Kim, S.-J.; Yu, H.; Kim, M.S.; He, J.; Langley, R.R.; Lehembre, F.; Regenass, U.; Fidler, I.J. Treatment of Experimental Human Breast Cancer and Lung Cancer Brain Metastases in Mice by Macitentan, a Dual Antagonist of Endothelin Receptors, Combined with Paclitaxel. Neuro Oncol 2016, 18, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, G.; Srivastava, N.; Goyal, M.; Rakha, E.; Lothion-Roy, J.; Mongan, N.P.; Miftakhova, R.R.; Khaiboullina, S.F.; Rizvanov, A.A. Metadherin: A Therapeutic Target in Multiple Cancers. Front. Oncol. 2019, 9, 349. [Google Scholar] [CrossRef]
- Xiao, H.; Zheng, Y.; Ma, L.; Tian, L.; Sun, Q. Clinically-Relevant ABC Transporter for Anti-Cancer Drug Resistance. Front Pharmacol 2021, 12, 648407. [Google Scholar] [CrossRef] [PubMed]
- Nedeljković, M.; Damjanovi, A. Mechanisms of Chemotherapy Resistance in Triple-Negative Breast Cancer-How We Can Rise to the Challenge. Cells 2019, 8, 957. [Google Scholar] [CrossRef] [PubMed]
- Gambardella, G.; Viscido, G.; Tumaini, B.; Isacchi, A.; Bosotti, R.; di Bernardo, D. A Single-Cell Analysis of Breast Cancer Cell Lines to Study Tumour Heterogeneity and Drug Response. Nat. Commun. 2022, 13, 1714. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Gao, R.; Sei, E.; Brandt, R.; Hartman, J.; Hatschek, T.; Crosetto, N.; Foukakis, T.; Navin, N.E. Chemoresistance Evolution in Triple-Negative Breast Cancer Delineated by Single-Cell Sequencing. Cell 2018, 173, 879–893.e13. [Google Scholar] [CrossRef] [PubMed]
- Roskoski, R. The Role of Small Molecule Platelet-Derived Growth Factor Receptor (PDGFR) Inhibitors in the Treatment of Neoplastic Disorders. Pharmacol. Res. 2018, 129, 65–83. [Google Scholar] [CrossRef] [PubMed]
- Dorso, M.; Patel, P.T.; Pankov, A.; Boyer, J.A.; Soni, R.K.; Del Priore, I.S.; Hayatt, O.; Kulick, A.; Hagen, C.J.; De Stanchina, E.; et al. A Druggable FOXA1-Glucocorticoid Receptor Transcriptional Axis Drives Tumor Growth in a Subset of Non-Small Cell Lung Cancer. Cancer Res. Commun. 2023, 3, 1788–1799. [Google Scholar] [CrossRef] [PubMed]
- Son, S.; Shin, J.M.; Shin, S.; Kim, C.H.; Lee, J.A.; Ko, H.; Lee, E.S.; Jung, J.M.; Kim, J.; Park, J.H. Repurposing Macitentan with Nanoparticle Modulates Tumor Microenvironment to Potentiate Immune Checkpoint Blockade. Biomaterials 2021, 276, 121058. [Google Scholar] [CrossRef]
- Prieto-Vila, M.; Usuba, W.; Takahashi, R.-U.; Shimomura, I.; Sasaki, H.; Ochiya, T.; Yamamoto, Y. Single-Cell Analysis Reveals a Preexisting Drug-Resistant Subpopulation in the Luminal Breast Cancer Subtype. Cancer Res. 2019, 79, 4412–4425. [Google Scholar] [CrossRef]
- Afgan, E.; Baker, D.; Batut, B.; Van Den Beek, M.; Bouvier, D.; Ech, M.; Chilton, J.; Clements, D.; Coraor, N.; Grüning, B.A.; et al. The Galaxy Platform for Accessible, Reproducible and Collaborative Biomedical Analyses: 2018 Update. Nucleic Acids Res. 2018, 46, W537–W544. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-Based Genome Alignment and Genotyping with HISAT2 andHISAT-Genotype. Nat. Biotechnol. 2019, 37, 907. [Google Scholar] [CrossRef] [PubMed]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq—A Python Framework to Work with High-Throughput Sequencing Data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Franceschini, A.; Wyder, S.; Forslund, K.; Heller, D.; Huerta-Cepas, J.; Simonovic, M.; Roth, A.; Santos, A.; Tsafou, K.P.; et al. STRING v10: Protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015, 43, D447–D452. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foutadakis, S.; Kordias, D.; Vatsellas, G.; Magklara, A. Identification of New Chemoresistance-Associated Genes in Triple-Negative Breast Cancer by Single-Cell Transcriptomic Analysis. Int. J. Mol. Sci. 2024, 25, 6853. https://doi.org/10.3390/ijms25136853
Foutadakis S, Kordias D, Vatsellas G, Magklara A. Identification of New Chemoresistance-Associated Genes in Triple-Negative Breast Cancer by Single-Cell Transcriptomic Analysis. International Journal of Molecular Sciences. 2024; 25(13):6853. https://doi.org/10.3390/ijms25136853
Chicago/Turabian StyleFoutadakis, Spyros, Dimitrios Kordias, Giannis Vatsellas, and Angeliki Magklara. 2024. "Identification of New Chemoresistance-Associated Genes in Triple-Negative Breast Cancer by Single-Cell Transcriptomic Analysis" International Journal of Molecular Sciences 25, no. 13: 6853. https://doi.org/10.3390/ijms25136853




