Idiosyncratic Drug-Induced Liver Injury and Amoxicillin–Clavulanate: Spotlight on Gut Microbiota, Fecal Metabolome and Bile Acid Profile in Patients
Abstract
:1. Introduction
2. Results
2.1. Hematological and Biochemical Alterations Observed in Patients with Idiosyncratic Drug-Induced Liver Injury
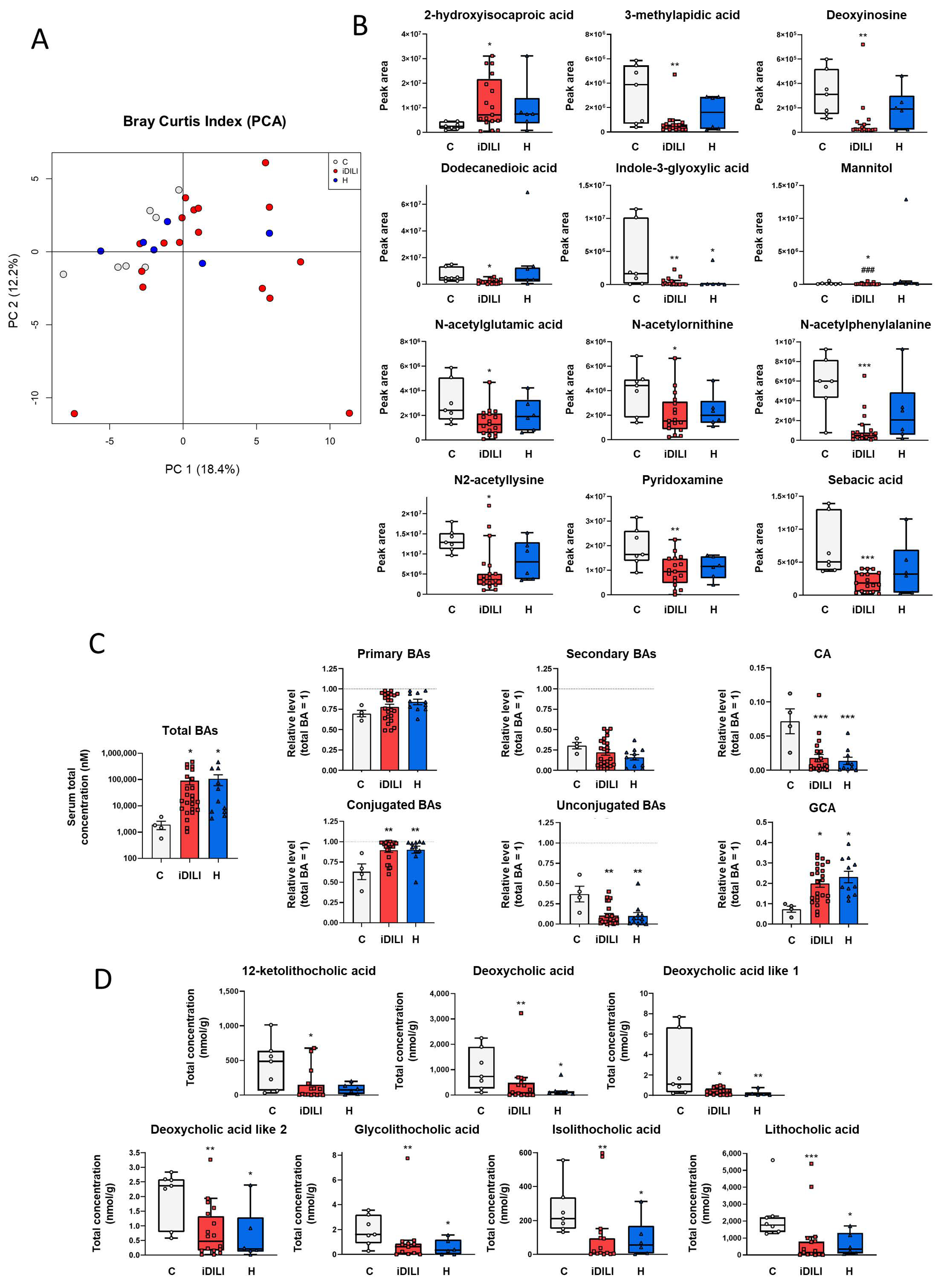
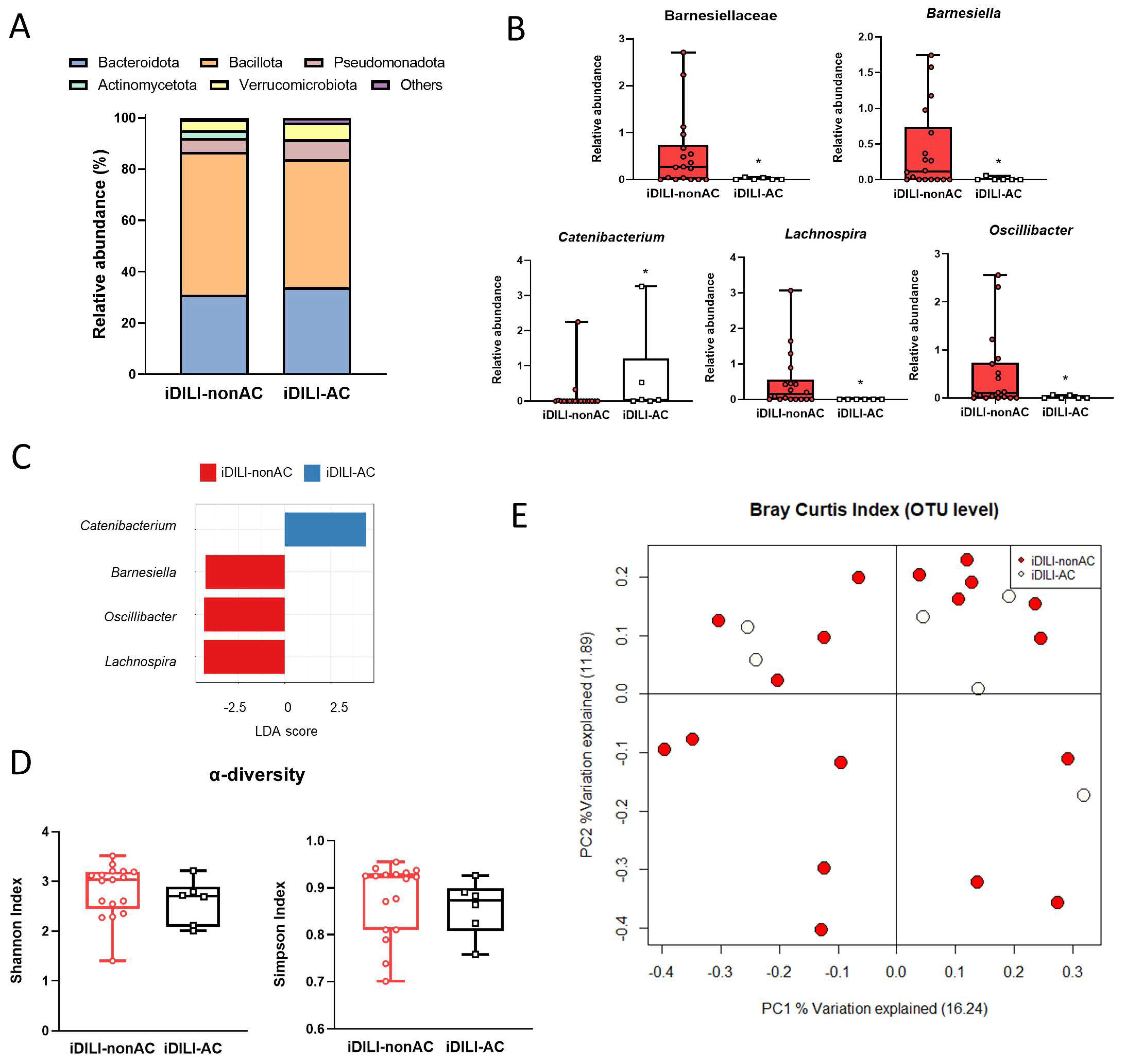
2.2. Analysis of Fecal Gut Microbiota Composition Shows a Specific Profile Associated with Idiosyncratic Drug-Induced Liver Injury
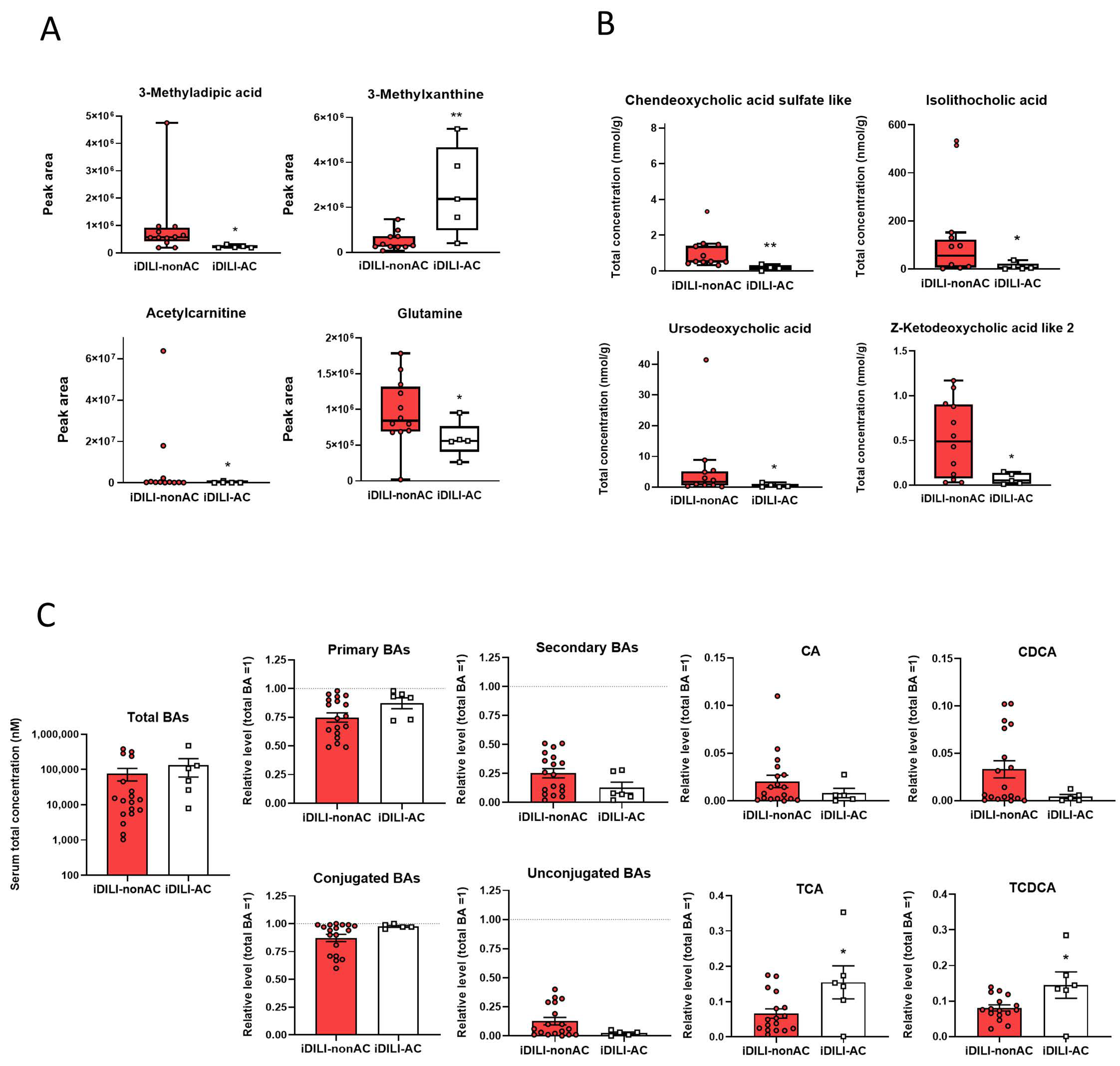
2.3. Idiosyncratic Drug-Induced Liver Injury Is Characterized by a Specific Fecal Metabolome and Bile Acid Profile
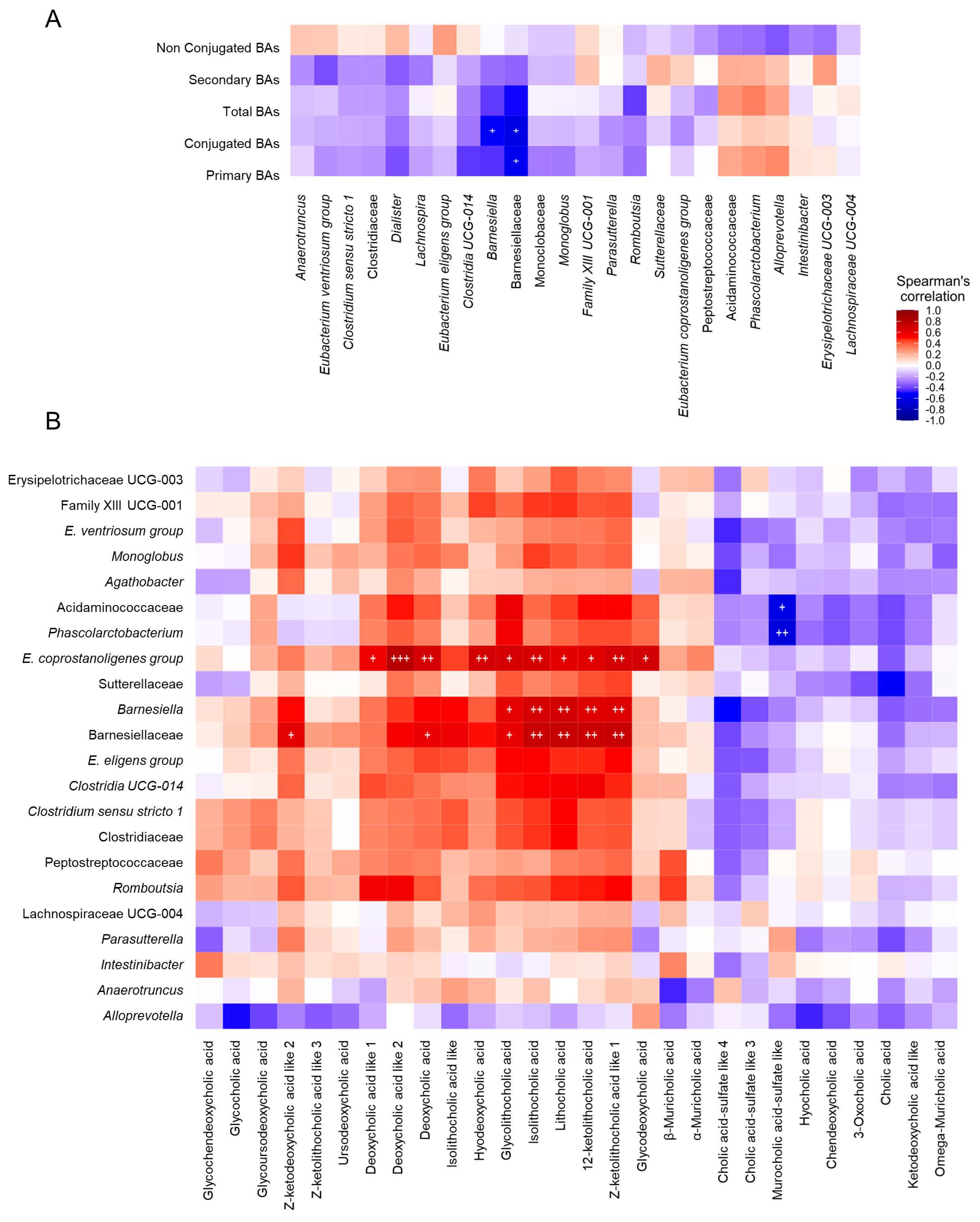
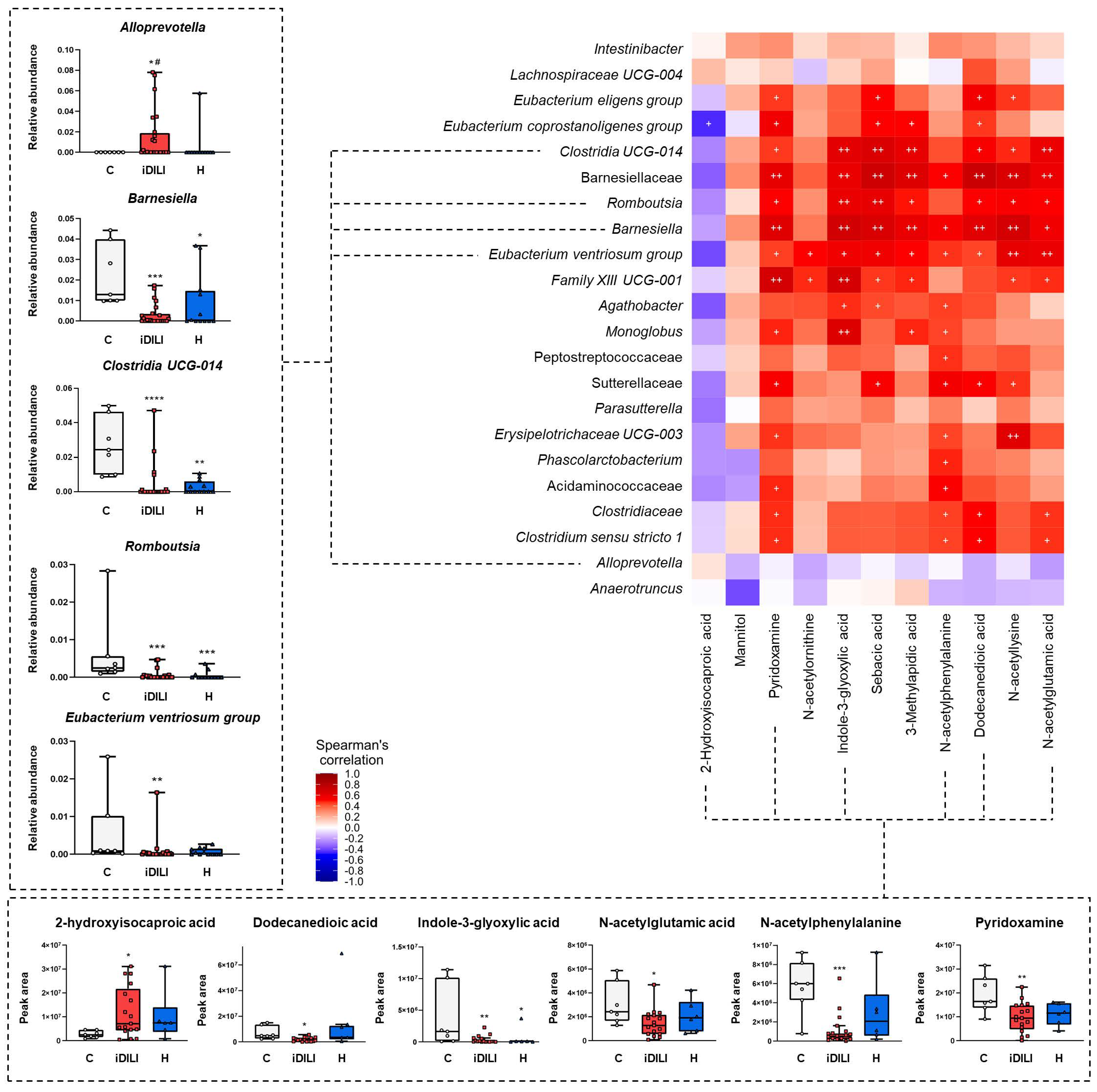
2.4. Correlations among Biochemical Parameters, Gut Microbiota Composition, Fecal Metabolome and Fecal and Serum Bile Acid Profiles in Patients with Idiosyncratic Drug-Induced Liver Injury
2.5. Idiosyncratic Drug-Induced Liver Injury Caused by Amoxicillin–Clavulanate Administration Is Characterized by a Specific Pattern of Gut Microbiota Composition Linked to a Particular Metabolome and Bile Acid Profile
3. Discussion
4. Materials and Methods
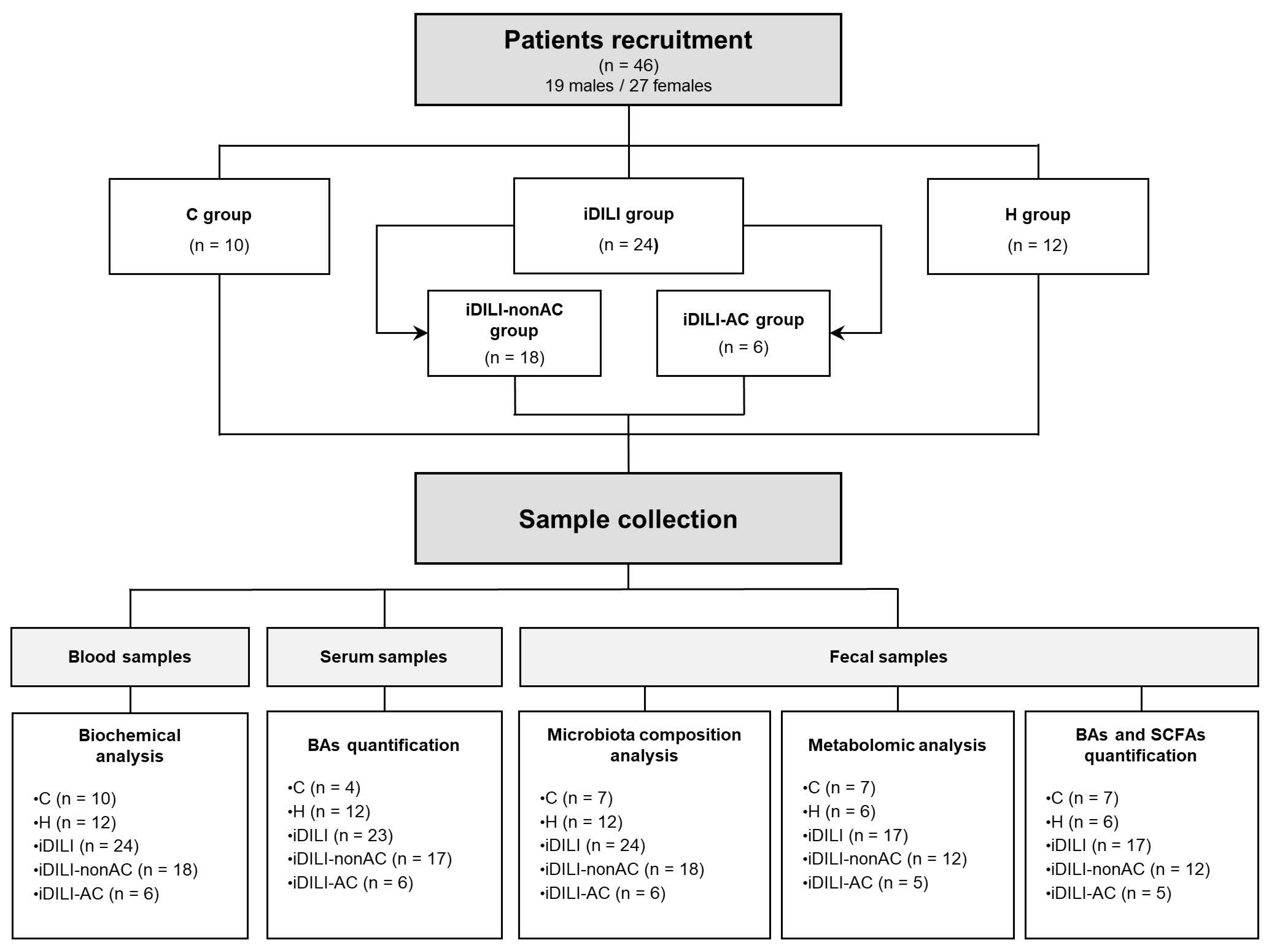
4.1. Participants and Ethical Approval
4.2. Sample Collection
4.3. Fecal Metagenomic Analysis
4.4. Fecal Metabolomic Analysis
4.5. Fecal Short-Chain Fatty Acids (SCFAs) Quantification
4.6. Fecal Bile Acid Quantification
4.7. Serum Bile Acid Quantification
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Andrade, R.J.; Chalasani, N.; Björnsson, E.S.; Suzuki, A.; Kullak-Ublick, G.A.; Watkins, P.B.; Devarbhavi, H.; Merz, M.; Lucena, M.I.; Kaplowitz, N.; et al. Drug-induced liver injury. Nat. Rev. Dis. Primers 2019, 5, 58. [Google Scholar] [CrossRef] [PubMed]
- Niu, M.-W.; Chen, P. Gut microbiota and drug-induced liver injury: An update. Chin. Med. J. 2020, 133, 494–495. [Google Scholar] [CrossRef] [PubMed]
- Björnsson, H.K.; Björnsson, E.S. Drug-induced liver injury: Pathogenesis, epidemiology, clinical features, and practical management. Eur. J. Intern. Med. 2022, 97, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Cortes, M.; Robles-Diaz, M.; Stephens, C.; Ortega-Alonso, A.; Lucena, M.I.; Andrade, R.J. drug induced liver injury: An update. Arch. Toxicol. 2020, 94, 3381–3407. [Google Scholar] [CrossRef] [PubMed]
- Huttner, A.; Bielicki, J.; Clements, M.N.; Frimodt-Møller, N.; Muller, A.E.; Paccaud, J.-P.; Mouton, J.W. Oral amoxicillin and amoxicillin-clavulanic acid: Properties, indications and usage. Clin. Microbiol. Infect. 2020, 26, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.; Gouveia, C.; Vasques, C.; Faria, C.; Pedroso, A. Drug-induced liver injury caused by amoxicillin/clavulanate. Cureus 2020, 12, e12234. [Google Scholar] [CrossRef]
- Stephens, C.; Robles-Diaz, M.; Medina-Caliz, I.; Garcia-Cortes, M.; Ortega-Alonso, A.; Sanabria-Cabrera, J.; Gonzalez-Jimenez, A.; Alvarez-Alvarez, I.; Slim, M.; Jimenez-Perez, M.; et al. Comprehensive analysis and insights gained from long-term experience of the Spanish DILI Registry. J. Hepatol. 2021, 75, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Win, S.; Than, T.A.; Chen, P.; Kaplowitz, N. Gut microbiota and liver injury (I)—Acute liver injury. In Gut Microbiota and Pathogenesis of Organ Injury; Chen, P., Ed.; Springer: Singapore, 2020; pp. 23–37. ISBN 978-981-15-2385-4. [Google Scholar]
- Kessoku, T.; Kobayashi, T.; Tanaka, K.; Yamamoto, A.; Takahashi, K.; Iwaki, M.; Ozaki, A.; Kasai, Y.; Nogami, A.; Honda, Y.; et al. The role of leaky gut in nonalcoholic fatty liver disease: A novel therapeutic target. Int. J. Mol. Sci. 2021, 22, 8161. [Google Scholar] [CrossRef] [PubMed]
- Miele, L.; Valenza, V.; La Torre, G.; Montalto, M.; Cammarota, G.; Ricci, R.; Mascianà, R.; Forgione, A.; Gabrieli, M.L.; Perotti, G.; et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology 2009, 49, 1877–1887. [Google Scholar] [CrossRef]
- Shanab, A.A.; Scully, P.; Crosbie, O.; Buckley, M.; O’Mahony, L.; Shanahan, F.; Gazareen, S.; Murphy, E.; Quigley, E.M.M. Small intestinal bacterial overgrowth in nonalcoholic steatohepatitis: Association with toll-like receptor 4 expression and plasma levels of interleukin 8. Dig. Dis. Sci. 2011, 56, 1524–1534. [Google Scholar] [CrossRef]
- Nistal, E.; Sáenz de Miera, L.E.; Ballesteros Pomar, M.; Sánchez-Campos, S.; García-Mediavilla, M.V.; Álvarez-Cuenllas, B.; Linares, P.; Olcoz, J.L.; Arias-Loste, M.T.; García-Lobo, J.M.; et al. An altered fecal microbiota profile in patients with non-alcoholic fatty liver disease (NAFLD) associated with obesity. Rev. Esp. Enferm. Dig. 2019, 111, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Juárez-Fernández, M.; Porras, D.; Petrov, P.; Román-Sagüillo, S.; García-Mediavilla, M.V.; Soluyanova, P.; Martínez-Flórez, S.; González-Gallego, J.; Nistal, E.; Jover, R.; et al. The synbiotic combination of Akkermansia muciniphila and quercetin ameliorates early obesity and NAFLD through gut microbiota reshaping and bile acid metabolism modulation. Antioxidants 2021, 10, 2001. [Google Scholar] [CrossRef] [PubMed]
- Juárez-Fernández, M.; Goikoetxea-Usandizaga, N.; Porras, D.; García-Mediavilla, M.V.; Bravo, M.; Serrano-Maciá, M.; Simón, J.; Delgado, T.C.; Lachiondo-Ortega, S.; Martínez-Flórez, S.; et al. Enhanced mitochondrial activity reshapes a gut microbiota profile that delays NASH progression. Hepatology 2023, 77, 1654–1669. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Qian, Y.; Shang, Z.; Sun, X.; Kong, X.; Gao, Y. Antibiotics enhancing drug-induced liver injury assessed for Causality Using Roussel Uclaf Causality Assessment Method: Emerging role of gut microbiota dysbiosis. Front. Med. 2022, 9, 972518. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.-K.; Ai, Y.; Cheng, Z.-L.; Yang, L.; Hou, X.-H. Contribution of gut microbiota to drug-induced liver injury. Hepatobiliary Pancreat. Dis. Int. 2023, 22, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhao, F.; Lin, B.; Feng, J.; Wu, X.; Liu, Y.; Zhao, L.; Zhu, B.; Wei, Y. Gut microbiota participates in antithyroid drug induced liver injury through the lipopolysaccharide related signaling pathway. Front. Pharmacol. 2020, 11, 598170. [Google Scholar] [CrossRef] [PubMed]
- Winston, J.A.; Theriot, C.M. Diversification of host bile acids by members of the gut microbiota. Gut Microbes 2020, 11, 158–171. [Google Scholar] [CrossRef] [PubMed]
- Schadt, H.S.; Wolf, A.; Pognan, F.; Chibout, S.-D.; Merz, M.; Kullak-Ublick, G.A. Bile acids in drug induced liver injury: Key players and surrogate markers. Clin. Res. Hepatol. Gastroenterol. 2016, 40, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Segovia-Zafra, A.; Di Zeo-Sánchez, D.E.; López-Gómez, C.; Pérez-Valdés, Z.; García-Fuentes, E.; Andrade, R.J.; Lucena, M.I.; Villanueva-Paz, M. Preclinical models of idiosyncratic drug-induced liver injury (iDILI): Moving towards prediction. Acta Pharm. Sin. B 2021, 11, 3685–3726. [Google Scholar] [CrossRef]
- Yang, N.; Xu, J.; Wang, X.; Chen, N.; Su, L.; Liu, Y. The spatial landscape of the bacterial community and bile acids in the digestive tract of patients with bile reflux. Front. Microbiol. 2022, 13, 835310. [Google Scholar] [CrossRef]
- Huang, L.; Zheng, J.; Sun, G.; Yang, H.; Sun, X.; Yao, X.; Lin, A.; Liu, H. 5-aminosalicylic acid ameliorates dextran sulfate sodium-induced colitis in mice by modulating gut microbiota and bile acid metabolism. Cell Mol. Life Sci. 2022, 79, 460. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Lordan, C.; Ross, R.P.; Cotter, P.D. Gut microbes from the phylogenetically diverse genus Eubacterium and their various contributions to gut health. Gut Microbes 2020, 12, 1802866. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Coker, O.O.; Chu, E.S.; Fu, K.; Lau, H.C.H.; Wang, Y.-X.; Chan, A.W.H.; Wei, H.; Yang, X.; Sung, J.J.Y.; et al. Dietary cholesterol drives fatty liver-associated liver cancer by modulating gut microbiota and metabolites. Gut 2021, 70, 761–774. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, P.; Li, H.; Jia, W.; Shou, Q.; Zhu, Y.; Mao, L.; Wang, W.; Wu, F.; Chen, X.; Wan, X.; et al. Eicosapentaenoic and docosahexaenoic acids attenuate hyperglycemia through the microbiome-gut-organs axis in db/db mice. Microbiome 2021, 9, 185. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Liu, R.; Su, K.; Gu, Y.; Fang, L.; Fan, Y.; Gao, J.; Ruan, X.; Feng, X. Acupuncture ameliorates breast cancer-related fatigue by regulating the gut microbiota-gut-brain axis. Front. Endocrinol. 2022, 13, 921119. [Google Scholar] [CrossRef] [PubMed]
- Dang, J.T.; Mocanu, V.; Park, H.; Laffin, M.; Hotte, N.; Karmali, S.; Birch, D.W.; Madsen, K.L. Roux-En-Y gastric bypass and sleeve gastrectomy induce substantial and persistent changes in microbial communities and metabolic pathways. Gut Microbes 2022, 14, 2050636. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.U.; Aamir, K.; Jusuf, P.R.; Sethi, G.; Sisinthy, S.P.; Ghildyal, R.; Arya, A. Lauric acid ameliorates lipopolysaccharide (lps)-induced liver inflammation by mediating TLR4/MyD88 pathway in Sprague Dawley (SD) Rats. Life Sci. 2021, 265, 118750. [Google Scholar] [CrossRef] [PubMed]
- Park, M.-J.; D’Alecy, L.G.; Anderson, M.A.; Basrur, V.; Feng, Y.; Brady, G.F.; Kim, D.-I.; Wu, J.; Nesvizhskii, A.I.; Lahann, J.; et al. Constitutive release of CPS1 in bile and its role as a protective cytokine during acute liver injury. Proc. Natl. Acad. Sci. USA 2019, 116, 9125–9134. [Google Scholar] [CrossRef] [PubMed]
- Alshanwani, A.R.; Hagar, H.; Shaheen, S.; Alhusaini, A.M.; Arafah, M.M.; Faddah, L.M.; Alharbi, F.M.; Sharma, A.K.; Fayed, A.; Badr, A.M. A promising antifibrotic drug, pyridoxamine attenuates thioacetamide-induced liver fibrosis by combating oxidative stress, advanced glycation end products, and balancing matrix metalloproteinases. Eur. J. Pharmacol. 2022, 923, 174910. [Google Scholar] [CrossRef]
- Funabashi, M.; Grove, T.L.; Wang, M.; Varma, Y.; McFadden, M.E.; Brown, L.C.; Guo, C.; Higginbottom, S.; Almo, S.C.; Fischbach, M.A. A metabolic pathway for bile acid dehydroxylation by the gut microbiome. Nature 2020, 582, 566–570. [Google Scholar] [CrossRef]
- Kakiyama, G.; Pandak, W.M.; Gillevet, P.M.; Hylemon, P.B.; Heuman, D.M.; Daita, K.; Takei, H.; Muto, A.; Nittono, H.; Ridlon, J.M.; et al. Modulation of the fecal bile acid profile by gut microbiota in cirrhosis. J. Hepatol. 2013, 58, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.R.; Haileselassie, Y.; Nguyen, L.P.; Tropini, C.; Wang, M.; Becker, L.S.; Sim, D.; Jarr, K.; Spear, E.T.; Singh, G.; et al. Dysbiosis-induced secondary bile acid deficiency promotes intestinal inflammation. Cell Host Microbe 2020, 27, 659–670.e5. [Google Scholar] [CrossRef] [PubMed]
- Jia, B.; Park, D.; Chun, B.H.; Hahn, Y.; Jeon, C.O. Diet-related alterations of gut bile salt hydrolases determined using a metagenomic analysis of the human microbiome. Int. J. Mol. Sci. 2021, 22, 3652. [Google Scholar] [CrossRef] [PubMed]
- Philips, C.A.; Augustine, P.; Ganesan, K.; Ranade, S.; Chopra, V.; Patil, K.; Shende, S.; Ahamed, R.; Kumbar, S.; Rajesh, S.; et al. The role of gut microbiota in clinical complications, disease severity, and treatment response in severe alcoholic hepatitis. Indian J. Gastroenterol. 2022, 41, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.-H.; Sim, M.; Lee, J.-Y.; Shin, D.-M. Lifestyle and geographic insights into the distinct gut microbiota in elderly women from two different geographic locations. J. Physiol. Anthropol. 2016, 35, 31. [Google Scholar] [CrossRef] [PubMed]
- Mao, B.; Guo, W.; Liu, X.; Cui, S.; Zhang, Q.; Zhao, J.; Tang, X.; Zhang, H. Potential probiotic properties of Blautia producta against lipopolysaccharide-induced acute liver injury. Probiotics Antimicrob. Proteins 2023, 15, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhu, X.; Badawy, S.; Ihsan, A.; Liu, Z.; Xie, C.; Wang, X. Metabolism and mechanism of human cytochrome P450 enzyme 1A2. Curr. Drug Metab. 2021, 22, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Fekete, F.; Mangó, K.; Minus, A.; Tóth, K.; Monostory, K. CYP1A2 mRNA expression rather than genetic variants indicate hepatic CYP1A2 activity. Pharmaceutics 2022, 14, 532. [Google Scholar] [CrossRef] [PubMed]
- Koppula, P.; Zhuang, L.; Gan, B. Cystine transporter SLC7A11/xCT in cancer: Ferroptosis, nutrient dependency, and cancer therapy. Protein Cell 2021, 12, 599–620. [Google Scholar] [CrossRef]
- Amores-Sánchez, M.I.; Medina, M.A. Glutamine, as a precursor of glutathione, and oxidative stress. Mol. Genet. Metab. 1999, 67, 100–105. [Google Scholar] [CrossRef]
- Wang, B.; Wu, G.; Zhou, Z.; Dai, Z.; Sun, Y.; Ji, Y.; Li, W.; Wang, W.; Liu, C.; Han, F.; et al. Glutamine and intestinal barrier function. Amino Acids 2015, 47, 2143–2154. [Google Scholar] [CrossRef] [PubMed]
- Petrov, P.D.; Soluyanova, P.; Sánchez-Campos, S.; Castell, J.V.; Jover, R. Molecular mechanisms of hepatotoxic cholestasis by clavulanic acid: Role of NRF2 and FXR pathways. Food Chem. Toxicol. 2021, 158, 112664. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.A.; Ignacio, J.R.A.; Winesett, M.P.; Kaiser, G.C.; Lacson, A.G.; Gilbert-Barness, E.; González-Peralta, R.P.; Wilsey, M.J. Vanishing bile duct syndrome: Amoxicillin-clavulanic acid associated intra-hepatic cholestasis responsive to ursodeoxycholic acid. J. Pediatr. Gastroenterol. Nutr. 2005, 41, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Herrero-Herrero, J.-I.; García-Aparicio, J. Corticosteroid therapy in a case of severe cholestasic hepatitis associated with amoxicillin–clavulanate. J. Med. Toxicol. 2010, 6, 420–423. [Google Scholar] [CrossRef] [PubMed]
- Katsinelos, P.; Vasiliadis, T.; Xiarchos, P.; Patakiouta, F.; Christodoulou, K.; Pilpilidis, I.; Eugenidis, N. Ursodeoxycholic acid (UDCA) for the treatment of amoxycillin-clavulanate potassium (Augmentin)-induced intra-hepatic cholestasis: Report of two cases. Eur. J. Gastroenterol. Hepatol. 2000, 12, 365–368. [Google Scholar] [CrossRef] [PubMed]
- Aithal, G.P.; Watkins, P.B.; Andrade, R.J.; Larrey, D.; Molokhia, M.; Takikawa, H.; Hunt, C.M.; Wilke, R.A.; Avigan, M.; Kaplowitz, N.; et al. Case definition and phenotype standardization in drug-induced liver injury. Clin. Pharmacol. Ther. 2011, 89, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Andrade, R.J.; Aithal, G.P.; Björnsson, E.S.; Kaplowitz, N.; Kullak-Ublick, G.A.; Larrey, D.; Karlsen, T.H. EASL Clinical Practice Guidelines: Drug-induced liver injury. J. Hepatol. 2019, 70, 1222–1261. [Google Scholar] [CrossRef] [PubMed]
- Roussel Uclaf Causality Assessment Method (RUCAM) in Drug Induced Liver Injury. In LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012.
- Porras, D.; Nistal, E.; Martínez-Flórez, S.; Olcoz, J.L.; Jover, R.; Jorquera, F.; González-Gallego, J.; García-Mediavilla, M.V.; Sánchez-Campos, S. Functional interactions between gut microbiota transplantation, quercetin, and high-fat diet determine non-alcoholic fatty liver disease development in germ-free mice. Mol. Nutr. Food Res. 2019, 63, e1800930. [Google Scholar] [CrossRef] [PubMed]
- Babraham Bioinformatics—FastQC A Quality Control Tool for High Throughput Sequence Data. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 13 December 2023).
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME Allows Analysis of High-Throughput Community Sequencing Data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Doneanu, C.; Fang, J.; Alelyunas, Y.; Yu, Y.Q.; Wrona, M.; Chen, W. An HS-MRM assay for the quantification of host-cell proteins in protein biopharmaceuticals by liquid chromatography ion mobility QTOF mass spectrometry. J. Vis. Exp. 2018, 134, 55325. [Google Scholar] [CrossRef]
- Johnsen, L.G.; Skou, P.B.; Khakimov, B.; Bro, R. Gas chromatography—Mass spectrometry data processing made easy. J. Chromatogr. A 2017, 1503, 57–64. [Google Scholar] [CrossRef] [PubMed]
- García-Cañaveras, J.C.; Donato, M.T.; Castell, J.V.; Lahoz, A. Targeted profiling of circulating and hepatic bile acids in human, mouse, and rat using a UPLC-MRM-MS-validated method. J. Lipid Res. 2012, 53, 2231–2241. [Google Scholar] [CrossRef] [PubMed]

| C | iDILI | H | p Value | |
|---|---|---|---|---|
| Glucose (mg/dL) | 88 ± 4 | 109 ± 6 | 111 ± 9 | 0.184 |
| Urea (mg/dL) | 33 ± 3 | 45 ± 7 | 36 ± 8 | 0.275 |
| Creatinine (mg/dL) | 0.80 ± 0.03 | 0.89 ± 0.08 | 0.98 ± 0.15 | 0.930 |
| ALT (U/L) | 22 ± 3 | 741 ± 200 ***# | 1349 ± 282 *** | <0.001 |
| AST (U/L) | 22 ± 2 | 487 ± 142 ***## | 1048 ± 236 *** | <0.001 |
| ALP (U/L) | 70 ± 6 | 246 ± 33 ** | 192 ± 24 *** | 0.004 |
| GGT (U/L) | 22 ± 4 | 283 ± 48 *** | 473 ± 70 *** | <0.001 |
| TBL (mg/dL) | 0.50 ± 0.05 | 5.59 ± 1.22 ** | 8.90 ± 2.27 *** | 0.002 |
| Albumin (g/dL) | 4.71 ± 0.04 | 3.58 ± 0.13 *** | 3.66 ± 0.23 * | 0.006 |
| Platelets (103/µL) | 230 ± 17 | 242 ± 16 | 220 ± 27 | 0.631 |
| Leukocytes (103/µL) | 5.5 ± 0.3 | 6.9 ± 0.6 | 8.2 ± 1.1 | 0.160 |
| Neutrophils (103/µL) | 2.9 ± 0.2 | 3.6 ± 0.3 # | 5.9 ± 0.9 * | 0.027 |
| INR | 0.98 ± 0.03 | 1.30 ± 0.17 | 1.38 ± 0.17 ** | 0.024 |
| Demographic Parameters | Data |
|---|---|
| Age, mean ± SEM | 58 ± 2 |
| Sex, n (%) | |
| Men | 19 (43) |
| Women | 27 (57) |
| Samples recruitment, n/total (%) | Data |
| Healthy controls | 10/46 (21.7) |
| non-iDILI acute hepatitis | 12/46 (26.1) |
| iDILI | 24/46 (52.2) |
| iDILI-nonAC | 18/24 (75) |
| iDILI-AC | 6/24 (25) |
| iDILI liver damage, n/total (%) | Data |
| Hepatocellular | 9/24 (37.5) |
| Cholestatic | 11/24 (45.8) |
| Mixed | 4/24 (16.7) |
| iDILI causal agent, n/total (%) | Data |
| Antibiotics | 14/24 (58.3) |
| Amoxicillin–clavulanate | 6/14 (43) |
| NSAIDs | 5/24 (20.8) |
| Statins | 3/24 (12.5) |
| Antifungals | 1/24 (4.2) |
| Chemotherapeutics | 1/24 (4.2) |
| Non-iDILI acute hepatitis causal agent, n/total (%) | Data |
| Autoimmune hepatitis | 9/12 (75) |
| Hepatitis C | 1/12 (8.3) |
| Hepatitis B | 2/12 (16.7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Román-Sagüillo, S.; Quiñones Castro, R.; Juárez-Fernández, M.; Soluyanova, P.; Stephens, C.; Robles-Díaz, M.; Jorquera Plaza, F.; González-Gallego, J.; Martínez-Flórez, S.; García-Mediavilla, M.V.; et al. Idiosyncratic Drug-Induced Liver Injury and Amoxicillin–Clavulanate: Spotlight on Gut Microbiota, Fecal Metabolome and Bile Acid Profile in Patients. Int. J. Mol. Sci. 2024, 25, 6863. https://doi.org/10.3390/ijms25136863
Román-Sagüillo S, Quiñones Castro R, Juárez-Fernández M, Soluyanova P, Stephens C, Robles-Díaz M, Jorquera Plaza F, González-Gallego J, Martínez-Flórez S, García-Mediavilla MV, et al. Idiosyncratic Drug-Induced Liver Injury and Amoxicillin–Clavulanate: Spotlight on Gut Microbiota, Fecal Metabolome and Bile Acid Profile in Patients. International Journal of Molecular Sciences. 2024; 25(13):6863. https://doi.org/10.3390/ijms25136863
Chicago/Turabian StyleRomán-Sagüillo, Sara, Raisa Quiñones Castro, María Juárez-Fernández, Polina Soluyanova, Camilla Stephens, Mercedes Robles-Díaz, Francisco Jorquera Plaza, Javier González-Gallego, Susana Martínez-Flórez, María Victoria García-Mediavilla, and et al. 2024. "Idiosyncratic Drug-Induced Liver Injury and Amoxicillin–Clavulanate: Spotlight on Gut Microbiota, Fecal Metabolome and Bile Acid Profile in Patients" International Journal of Molecular Sciences 25, no. 13: 6863. https://doi.org/10.3390/ijms25136863







