Investigating Antiprotozoal Chemotherapies with Novel Proteomic Tools—Chances and Limitations: A Critical Review
Abstract
:1. Introduction
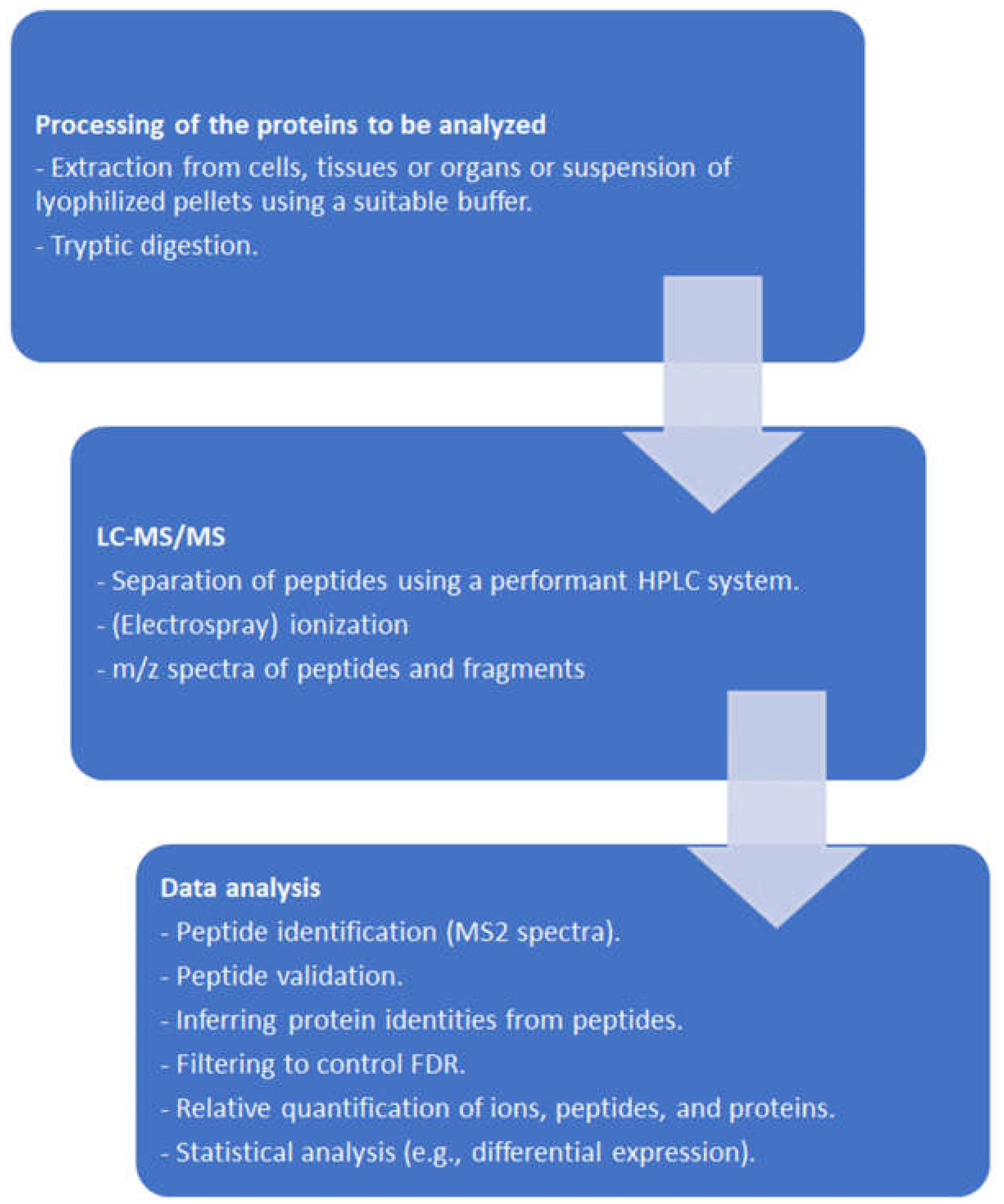
2. Proteomic Tools
2.1. General Remarks
2.2. From Protein Sequencing to Proteomics
2.3. From Mass Spectra to Protein Data
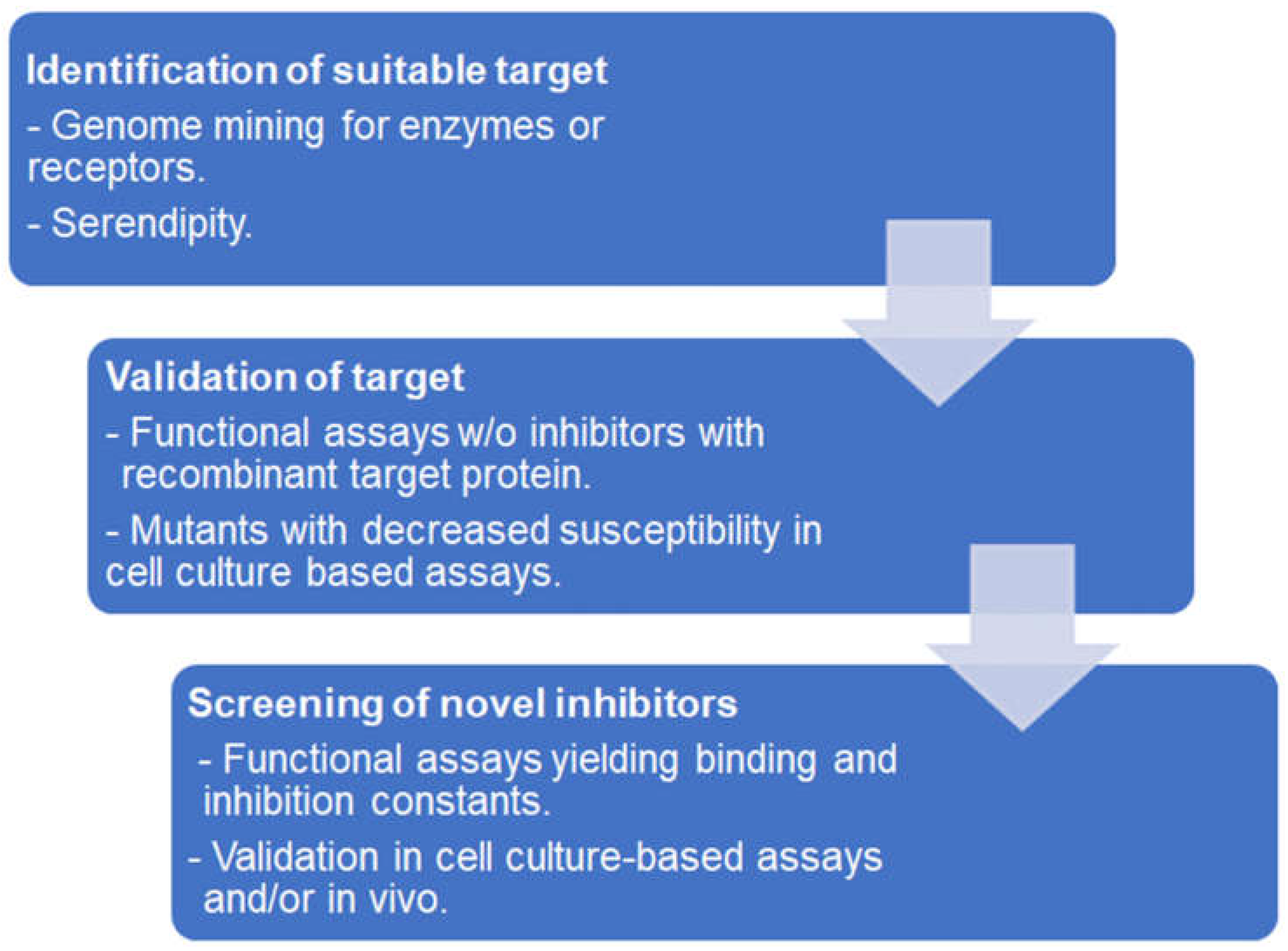
3. Affinity-Based Target Deconvolution
3.1. Functional and Binding Assays Using Isolated Proteins
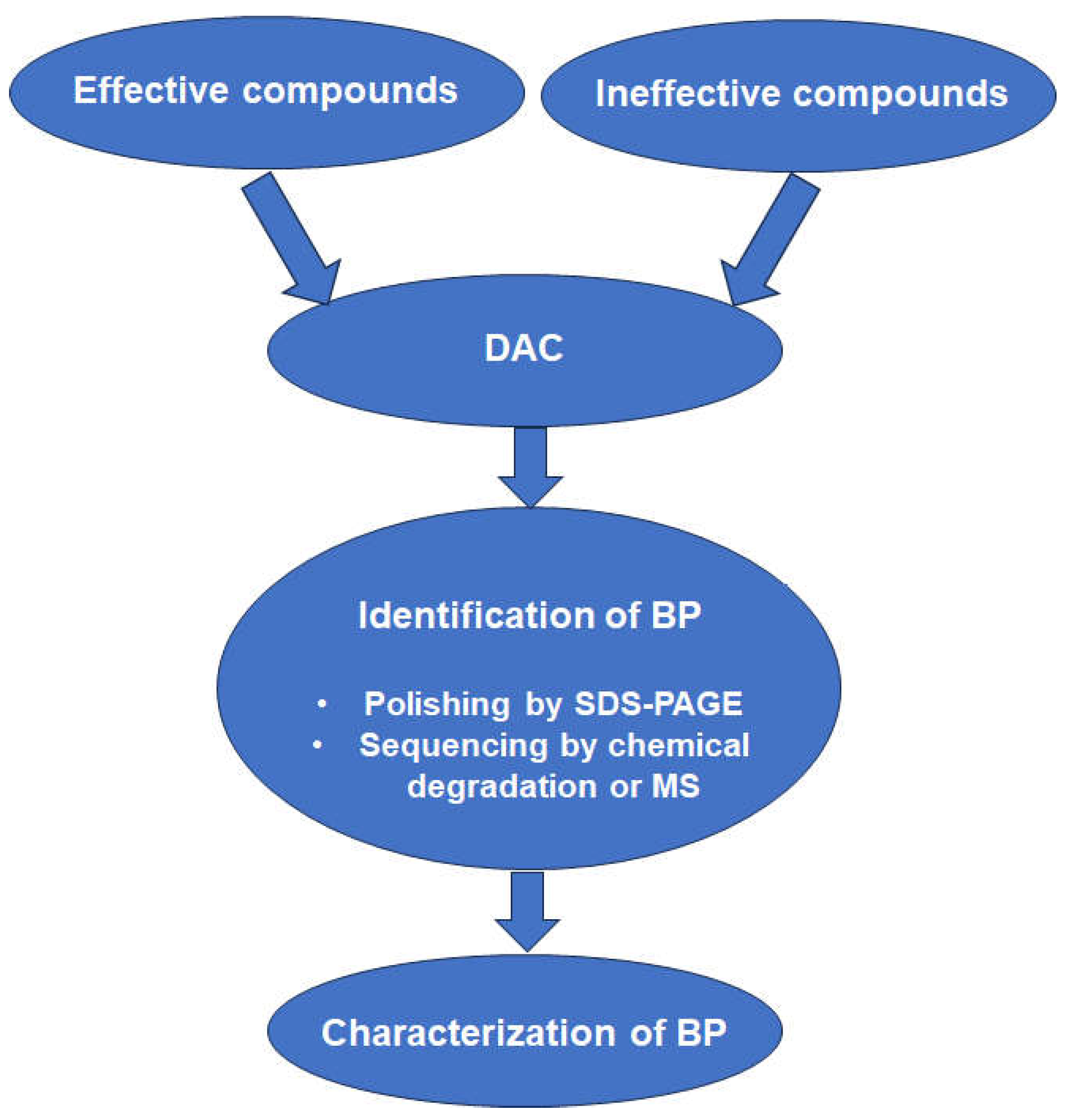
3.2. Affinity Chromatography
3.3. In Situ Binding
3.4. Protein Stability-Based Methods
4. Analysis of Resistant Strains
4.1. General Considerations
4.2. Resistance of Transgenic Strains
4.3. Differential Analysis of the Proteomes of Susceptible vs. Resistant Strains
5. Combining Evidence from Chemoproteomics and Whole-Cell Proteomics
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pillutla, R.C.; Fisher, P.B.; Blume, A.J.; Goldstein, N.I. Target validation and drug discovery using genomic and protein-protein interaction technologies. Expert Opin. Ther. Targets 2002, 6, 517–531. [Google Scholar] [CrossRef] [PubMed]
- Hellerstein, M.K. A critique of the molecular target-based drug discovery paradigm based on principles of metabolic control: Advantages of pathway-based discovery. Metab. Eng. 2008, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, M.A. Target discovery. Nat. Rev. Drug Discov. 2003, 2, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Paananen, J.; Fortino, V. An omics perspective on drug target discovery platforms. Brief. Bioinform. 2020, 21, 1937–1953. [Google Scholar] [CrossRef] [PubMed]
- Tsukidate, T.; Li, Q.; Hang, H.C. Targeted and proteome-wide analysis of metabolite-protein interactions. Curr. Opin. Chem. Biol. 2020, 54, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Dixon, B.R. Giardia duodenalis in humans and animals—Transmission and disease. Res. Vet. Sci. 2021, 135, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Matta, S.K.; Rinkenberger, N.; Dunay, I.R.; Sibley, L.D. Toxoplasma gondii infection and its implications within the central nervous system. Nat. Rev. Microbiol. 2021, 19, 467–480. [Google Scholar] [CrossRef]
- Ryan, U.M.; Feng, Y.; Fayer, R.; Xiao, L. Taxonomy and molecular epidemiology of Cryptosporidium and Giardia—A 50 year perspective (1971–2021). Int. J. Parasitol. 2021, 51, 1099–1119. [Google Scholar] [CrossRef] [PubMed]
- Marin-Garcia, P.J.; Planas, N.; Llobat, L. Toxoplasma gondii in foods: Prevalence, control, and safety. Foods 2022, 11, 2542. [Google Scholar] [CrossRef]
- Nayeri, T.; Moosazadeh, M.; Sarvi, S.; Daryani, A. Neospora caninum infection in aborting bovines and lost fetuses: A systematic review and meta-analysis. PLoS ONE 2022, 17, e0268903. [Google Scholar] [CrossRef]
- Reichel, M.P.; Wahl, L.C.; Ellis, J.T. Research into Neospora caninum—What Have We Learnt in the Last Thirty Years? Pathogens 2020, 9, 505. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, B.M.; Bojang, K.; Whitty, C.J.; Targett, G.A. Malaria. Lancet 2005, 365, 1487–1498. [Google Scholar] [CrossRef] [PubMed]
- Bird, A. Understanding the replication crisis as a base rate fallacy. Br. J. Philos. Sci. 2021, 72, 965–993. [Google Scholar] [CrossRef]
- Edman, P. A method for the determination of amino acid sequence in peptides. Arch. Biochem. 1949, 22, 475. [Google Scholar]
- Rabilloud, T. Paleoproteomics explained to youngsters: How did the wedding of two-dimensional electrophoresis and protein sequencing spark proteomics on: Let there be light. J. Proteom. 2014, 107, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Karas, M.; Bachmann, D.; Hillenkamp, F. Influence of the wavelength in high-irradiance ultraviolet-laser desorption mass-spectrometry of organic-molecules. Anal. Chem. 1985, 57, 2935–2939. [Google Scholar] [CrossRef]
- Shimada, T.; Toyama, A.; Aoki, C.; Aoki, Y.; Tanaka, K.; Sato, T.A. Direct antigen detection from immunoprecipitated beads using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry; a new method for immunobeads-mass spectrometry (iMS). Rapid Commun. Mass Spectrom. 2011, 25, 3521–3526. [Google Scholar] [CrossRef] [PubMed]
- Sedo, O.; Roblickova, A.; Jezek, F.; Gintar, P.; Kamenik, J.; Zdrahal, Z. Discriminatory power of MALDI-TOF MS protein profiling analysis of pork meat and meat products. Food Chem. 2024, 449, 139155. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wu, P.; Zhao, C.; Zheng, F.; Hu, C.; Lu, X.; Xu, G. Protein profiling analysis based on matrix-assisted laser desorption/ionization-Fourier transform ion cyclotron resonance mass spectrometry and its application in typing Streptomyces isolates. Talanta 2020, 208, 120439. [Google Scholar] [CrossRef]
- Horisawa, S.; Iwamoto, K. Identification and typing of strains of wood-rotting basidiomycetes by protein profiling using MALDI-TOF MS. BioTech 2022, 11, 30. [Google Scholar] [CrossRef]
- Chivte, P.; LaCasse, Z.; Seethi, V.D.R.; Bharti, P.; Bland, J.; Kadkol, S.S.; Gaillard, E.R. MALDI-ToF protein profiling as a potential rapid diagnostic platform for COVID-19. J. Mass Spectrom. Adv. Clin. Lab 2021, 21, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Tan, C.; Zenobi, R. Rapid profiling of the glycosylation effects on the binding of SARS-CoV-2 spike protein to angiotensin-converting enzyme 2 using MALDI-MS with high mass detection. Anal. Chem. 2024, 96, 1898–1905. [Google Scholar] [CrossRef] [PubMed]
- Ryan, D.J.; Spraggins, J.M.; Caprioli, R.M. Protein identification strategies in MALDI imaging mass spectrometry: A brief review. Curr. Opin. Chem. Biol. 2019, 48, 64–72. [Google Scholar] [CrossRef] [PubMed]
- James, P.; Quadroni, M.; Carafoli, E.; Gonnet, G. Protein identification by mass profile fingerprinting. Biochem. Biophys. Res. Commun. 1993, 195, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Michalski, A.; Cox, J.; Mann, M. More than 100,000 detectable peptide species elute in single shotgun proteomics runs but the majority is inaccessible to data-dependent LC-MS/MS. J. Proteome Res. 2011, 10, 1785–1793. [Google Scholar] [CrossRef]
- Michalski, A.; Damoc, E.; Lange, O.; Denisov, E.; Nolting, D.; Muller, M.; Viner, R.; Schwartz, J.; Remes, P.; Belford, M.; et al. Ultra high resolution linear ion trap Orbitrap mass spectrometer (Orbitrap Elite) facilitates top down LC MS/MS and versatile peptide fragmentation modes. Mol. Cell Proteom. 2012, 11, O111.013698. [Google Scholar] [CrossRef] [PubMed]
- Shishkova, E.; Hebert, A.S.; Coon, J.J. Now, more than ever, proteomics needs better chromatography. Cell Syst. 2016, 3, 321–324. [Google Scholar] [CrossRef] [PubMed]
- May, J.C.; Goodwin, C.R.; McLean, J.A. Ion mobility-mass spectrometry strategies for untargeted systems, synthetic, and chemical biology. Curr. Opin. Biotechnol. 2015, 31, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Aebersold, R.; Chen, R.; Rush, J.; Goodlett, D.R.; McIntosh, M.W.; Zhang, J.; Brentnall, T.A. Mass spectrometry based targeted protein quantification: Methods and applications. J. Proteome Res. 2009, 8, 787–797. [Google Scholar] [CrossRef]
- Shuken, S.R. An Introduction to mass spectrometry-based proteomics. J. Proteome Res. 2023, 22, 2151–2171. [Google Scholar] [CrossRef]
- Orsburn, B.C. Single cell proteomics by mass spectrometry reveals deep epigenetic insight and new targets of a class specific histone deacetylase inhibitor. bioRxiv 2024. [Google Scholar] [CrossRef]
- Alfaro, J.A.; Bohlander, P.; Dai, M.; Filius, M.; Howard, C.J.; van Kooten, X.F.; Ohayon, S.; Pomorski, A.; Schmid, S.; Aksimentiev, A.; et al. The emerging landscape of single-molecule protein sequencing technologies. Nat. Methods 2021, 18, 604–617. [Google Scholar] [CrossRef] [PubMed]
- Timp, W.; Timp, G. Beyond mass spectrometry, the next step in proteomics. Sci. Adv. 2020, 6, eaax8978. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Penkauskas, T.; Reiner, J.E.; Kennard, C.; Uline, M.J.; Wang, Q.; Li, S.; Aksimentiev, A.; Robertson, J.W.F.; Liu, C. Engineering biological nanopore approaches toward protein sequencing. ACS Nano 2023, 17, 16369–16395. [Google Scholar] [CrossRef]
- Wang, F.; Zhao, C.; Zhao, P.; Chen, F.; Qiao, D.; Feng, J. MoS(2) nanopore identifies single amino acids with sub-1 Dalton resolution. Nat. Commun. 2023, 14, 2895. [Google Scholar] [CrossRef]
- Daub, H. Quantitative proteomics of kinase inhibitor targets and mechanisms. ACS Chem. Biol. 2015, 10, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Deb, B.; George, I.A.; Sharma, J.; Kumar, P. Phosphoproteomics profiling to identify altered signaling pathways and kinase-targeted cancer therapies. Methods Mol. Biol. 2020, 2051, 241–264. [Google Scholar] [CrossRef] [PubMed]
- Hallal, M.; Braga-Lagache, S.; Jankovic, J.; Simillion, C.; Bruggmann, R.; Uldry, A.C.; Allam, R.; Heller, M.; Bonadies, N. Inference of kinase-signaling networks in human myeloid cell line models by Phosphoproteomics using kinase activity enrichment analysis (KAEA). BMC Cancer 2021, 21, 789. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Haynes, S.E.; Teo, G.C.; Avtonomov, D.M.; Polasky, D.A.; Nesvizhskii, A.I. Fast quantitative analysis of timsTOF PASEF data with MSFragger and IonQuant. Mol. Cell. Proteom. 2020, 19, 1575–1585. [Google Scholar] [CrossRef]
- Tyanova, S.; Temu, T.; Cox, J. The MaxQuant computational platform for mass spectrometry-based shotgun proteomics. Nat. Protoc. 2016, 11, 2301–2319. [Google Scholar] [CrossRef]
- Savitski, M.M.; Wilhelm, M.; Hahne, H.; Kuster, B.; Bantscheff, M. A scalable approach for protein false discovery rate estimation in large proteomic data sets. Mol. Cell Proteom. 2015, 14, 2394–2404. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.L.; Yu, F.; Teo, G.C.; Li, K.; Demichev, V.; Ralser, M.; Nesvizhskii, A.I. MSBooster: Improving peptide identification rates using deep learning-based features. Nat. Commun. 2023, 14, 4539. [Google Scholar] [CrossRef] [PubMed]
- Adams, C.; Gabriel, W.; Laukens, K.; Picciani, M.; Wilhelm, M.; Bittremieux, W.; Boonen, K. Fragment ion intensity prediction improves the identification rate of non-tryptic peptides in timsTOF. Nat. Commun. 2024, 15, 3956. [Google Scholar] [CrossRef] [PubMed]
- Braga-Lagache, S.; Buchs, N.; Iacovache, M.I.; Zuber, B.; Jackson, C.B.; Heller, M. Robust label-free, quantitative profiling of circulating plasma microparticle (MP) associated proteins. Mol. Cell Proteom. 2016, 15, 3640–3652. [Google Scholar] [CrossRef] [PubMed]
- Fabre, B.; Lambour, T.; Bouyssié, D.; Menneteau, T.; Monsarrat, B.; Burlet-Schiltz, O.; Bousquet-Dubouch, M.-P. Comparison of label-free quantification methods for the determination of protein complexes subunits stoichiometry. EuPA Open Proteom. 2014, 4, 82–86. [Google Scholar] [CrossRef]
- Macleod, A.K.; Zang, T.; Riches, Z.; Henderson, C.J.; Wolf, C.R.; Huang, J.T. A targeted in vivo SILAC approach for quantification of drug metabolism enzymes: Regulation by the constitutive androstane receptor. J. Proteome Res. 2014, 13, 866–874. [Google Scholar] [CrossRef]
- Dayon, L.; Sanchez, J.C. Relative protein quantification by MS/MS using the tandem mass tag technology. Methods Mol. Biol. 2012, 893, 115–127. [Google Scholar] [CrossRef]
- Stepath, M.; Zulch, B.; Maghnouj, A.; Schork, K.; Turewicz, M.; Eisenacher, M.; Hahn, S.; Sitek, B.; Bracht, T. Systematic Comparison of Label-Free, SILAC, and TMT Techniques to Study Early Adaption toward Inhibition of EGFR Signaling in the Colorectal Cancer Cell Line DiFi. J. Proteome Res. 2020, 19, 926–937. [Google Scholar] [CrossRef]
- Tyanova, S.; Temu, T.; Sinitcyn, P.; Carlson, A.; Hein, M.Y.; Geiger, T.; Mann, M.; Cox, J. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods 2016, 13, 731–740. [Google Scholar] [CrossRef]
- Heller, M.; Braga, S.; Müller, N.; Müller, J. Transfection with plasmid causing stable expression of a foreign gene affects general proteome pattern in Giardia lamblia trophozoites. Front. Cell Infect. Microbiol. 2020, 10, 602756. [Google Scholar] [CrossRef]
- Uldry, A.C.; Maciel-Dominguez, A.; Jornod, M.; Buchs, N.; Braga-Lagache, S.; Brodard, J.; Jankovic, J.; Bonadies, N.; Heller, M. Effect of sample transportation on the proteome of human circulating blood extracellular vesicles. Int. J. Mol. Sci. 2022, 23, 4515. [Google Scholar] [CrossRef]
- Waduge, P.; Tian, H.; Webster, K.A.; Li, W. Profiling disease-selective drug targets: From proteomics to ligandomics. Drug Discov. Today 2023, 28, 103430. [Google Scholar] [CrossRef]
- Neun, S.; Zurek, P.J.; Kaminski, T.S.; Hollfelder, F. Ultrahigh throughput screening for enzyme function in droplets. Methods Enzymol. 2020, 643, 317–343. [Google Scholar] [CrossRef]
- Yi, Y.; Zankharia, U.; Cassel, J.A.; Lu, F.; Salvino, J.M.; Lieberman, P.M.; Collman, R.G. A high-throughput screening assay for silencing established HIV-1 macrophage infection identifies nucleoside analogs that perturb H3K9me3 on proviral genomes. J. Virol. 2023, 97, e0065323. [Google Scholar] [CrossRef]
- Taoda, Y.; Sugiyama, S.; Seki, T. New designs for HIV-1 integrase inhibitors: A patent review (2018-present). Expert Opin. Ther. Pat. 2023, 33, 51–66. [Google Scholar] [CrossRef]
- Montalbano, A.; Sala, C.; Altadonna, G.C.; Becchetti, A.; Arcangeli, A. High throughput clone screening on overexpressed hERG1 and Kv1.3 potassium channels using ion channel reader (ICR) label free technology. Heliyon 2023, 9, e20112. [Google Scholar] [CrossRef]
- Diaz, G.J.; Daniell, K.; Leitza, S.T.; Martin, R.L.; Su, Z.; McDermott, J.S.; Cox, B.F.; Gintant, G.A. The [3H]dofetilide binding assay is a predictive screening tool for hERG blockade and proarrhythmia: Comparison of intact cell and membrane preparations and effects of altering [K+]o. J. Pharmacol. Toxicol. Methods 2004, 50, 187–199. [Google Scholar] [CrossRef]
- Sichler, S.; Hofner, G.; Nitsche, V.; Niessen, K.V.; Seeger, T.; Worek, F.; Paintner, F.F.; Wanner, K.T. Screening for new ligands of the MB327-PAM-1 binding site of the nicotinic acetylcholine receptor. Toxicol. Lett. 2024, 394, 23–31. [Google Scholar] [CrossRef]
- Loo, C.S.; Lam, N.S.; Yu, D.; Su, X.Z.; Lu, F. Artemisinin and its derivatives in treating protozoan infections beyond malaria. Pharmacol. Res. 2017, 117, 192–217. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y. The discovery of artemisinin (qinghaosu) and gifts from Chinese medicine. Nat. Med. 2011, 17, 1217–1220. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.; Hemphill, A. Toxoplasma gondii infection: Novel emerging therapeutic targets. Expert Opin. Ther. Targets 2023, 27, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Kurganov, B.I. Kinetics of protein aggregation. Quantitative estimation of the chaperone-like activity in test-systems based on suppression of protein aggregation. Biochemistry 2002, 67, 409–422. [Google Scholar] [CrossRef] [PubMed]
- Kassack, M.U. Quantitative comparison of functional screening by measuring intracellular Ca2+ with radioligand binding at recombinant human dopamine receptors. AAPS PharmSci 2002, 4, E31. [Google Scholar] [CrossRef] [PubMed]
- Oostendorp, J.; Meurs, H.; Adriaan Nelemans, S.; Zaagsma, J.; Kauffman, H.F.; Postma, D.S.; Boddeke, H.W.; Biber, K. Cloning, pharmacological characterization, and polymorphism screening of the guinea pig beta(2)-adrenoceptor. Eur. J. Pharmacol. 2002, 457, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, F.; Fisher, F.; Milne, R.; Teran, F.S.; Wiedemar, N.; Wrobel, K.; Edwards, D.; Baumann, H.; Gilbert, I.H.; Baragana, B.; et al. High-throughput screening platform to identify inhibitors of protein synthesis with potential for the treatment of malaria. Antimicrob. Agents Chemother. 2022, 66, e0023722. [Google Scholar] [CrossRef] [PubMed]
- Ojo, K.K.; Larson, E.T.; Keyloun, K.R.; Castaneda, L.J.; Derocher, A.E.; Inampudi, K.K.; Kim, J.E.; Arakaki, T.L.; Murphy, R.C.; Zhang, L.; et al. Toxoplasma gondii calcium-dependent protein kinase 1 is a target for selective kinase inhibitors. Nat. Struct. Mol. Biol. 2010, 17, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.M.; Murphy, R.C.; Geiger, J.A.; DeRocher, A.E.; Zhang, Z.; Ojo, K.K.; Larson, E.T.; Perera, B.G.; Dale, E.J.; He, P.; et al. Development of Toxoplasma gondii calcium-dependent protein kinase 1 (TgCDPK1) inhibitors with potent anti-toxoplasma activity. J. Med. Chem. 2012, 55, 2416–2426. [Google Scholar] [CrossRef] [PubMed]
- Vanichtanankul, J.; Yoomuang, A.; Taweechai, S.; Saeyang, T.; Pengon, J.; Yuvaniyama, J.; Tarnchompoo, B.; Yuthavong, Y.; Kamchonwongpaisan, S. Structural insight into effective inhibitors binding to Toxoplasma gondii dihydrofolate reductase thymidylate synthase. ACS Chem. Biol. 2022, 17, 1691–1702. [Google Scholar] [CrossRef] [PubMed]
- Djapa, L.Y.; Basco, L.K.; Zelikson, R.; Rosowsky, A.; Djaman, J.A.; Yonkeu, J.N.; Bolotin-Fukuhara, M.; Mazabraud, A. Antifolate screening using yeast expressing Plasmodium vivax dihydrofolate reductase and in vitro drug susceptibility assay for Plasmodium falciparum. Mol. Biochem. Parasitol. 2007, 156, 89–92. [Google Scholar] [CrossRef]
- Jelenska, J.; Sirikhachornkit, A.; Haselkorn, R.; Gornicki, P. The carboxyltransferase activity of the apicoplast acetyl-CoA carboxylase of Toxoplasma gondii is the target of aryloxyphenoxypropionate inhibitors. J. Biol. Chem. 2002, 277, 23208–23215. [Google Scholar] [CrossRef]
- Goo, Y.K.; Yamagishi, J.; Ueno, A.; Terkawi, M.A.; Aboge, G.O.; Kwak, D.; Hong, Y.; Chung, D.I.; Igarashi, M.; Nishikawa, Y.; et al. Characterization of Toxoplasma gondii glyoxalase 1 and evaluation of inhibitory effects of curcumin on the enzyme and parasite cultures. Parasit. Vectors 2015, 8, 654. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.S.; Kerscher, S.; Saleh, A.; Brandt, U.; Gross, U.; Bohne, W. The Toxoplasma gondii type-II NADH dehydrogenase TgNDH2-I is inhibited by 1-hydroxy-2-alkyl-4(1H)quinolones. Biochim. Biophys. Acta 2008, 1777, 1455–1462. [Google Scholar] [CrossRef] [PubMed]
- Harada, M.; Nagai, J.; Kurata, R.; Shimizu, K.; Cui, X.; Isagawa, T.; Semba, H.; Ishihara, J.; Yoshida, Y.; Takeda, N.; et al. Establishment of novel high-standard chemiluminescent assay for NTPase in two protozoans and its high-throughput screening. Mar. Drugs 2020, 18, 161. [Google Scholar] [CrossRef] [PubMed]
- Razakantoanina, V.; Florent, I.; Jaureguiberry, G. Plasmodium falciparum: Functional mitochondrial ADP/ATP transporter in Escherichia coli plasmic membrane as a tool for selective drug screening. Exp. Parasitol. 2008, 118, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Walunj, S.B.; Dias, M.M.; Kaur, C.; Wagstaff, K.M.; Dey, V.; Hick, C.; Patankar, S.; Jans, D.A. High-throughput screening to identify inhibitors of Plasmodium falciparum importin alpha. Cells 2022, 11, 1201. [Google Scholar] [CrossRef] [PubMed]
- Radke, J.B.; Melillo, B.; Mittal, P.; Sharma, M.; Sharma, A.; Fu, Y.; Uddin, T.; Gonse, A.; Comer, E.; Schreiber, S.L.; et al. Bicyclic azetidines target acute and chronic stages of Toxoplasma gondii by inhibiting parasite phenylalanyl t-RNA synthetase. Nat. Commun. 2022, 13, 459. [Google Scholar] [CrossRef] [PubMed]
- Bosch, S.S.; Lunev, S.; Batista, F.A.; Linzke, M.; Kronenberger, T.; Domling, A.S.S.; Groves, M.R.; Wrenger, C. Molecular target validation of aspartate transcarbamoylase from Plasmodium falciparum by Torin 2. ACS Infect. Dis. 2020, 6, 986–999. [Google Scholar] [CrossRef] [PubMed]
- Batista, F.A.; Gyau, B.; Vilacha, J.F.; Bosch, S.S.; Lunev, S.; Wrenger, C.; Groves, M.R. New directions in antimalarial target validation. Expert Opin. Drug Discov. 2020, 15, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Jex, A.; Svard, S.G. A chromosome-scale reference genome for Giardia intestinalis WB. Sci. Data 2020, 7, 38. [Google Scholar] [CrossRef]
- Kunz, S.; Balmer, V.; Sterk, G.J.; Pollastri, M.P.; Leurs, R.; Müller, N.; Hemphill, A.; Spycher, C. The single cyclic nucleotide-specific phosphodiesterase of the intestinal parasite Giardia lamblia represents a potential drug target. PLoS Negl. Trop. Dis. 2017, 11, e0005891. [Google Scholar] [CrossRef]
- Müller, J.; Braga, S.; Uldry, A.C.; Heller, M.; Müller, N. Comparative proteomics of three Giardia lamblia strains: Investigation of antigenic variation in the post-genomic era. Parasitology 2020, 147, 1008–1018. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Müller, J.; Kunz, S.; Siderius, M.; Maes, L.; Caljon, G.; Müller, N.; Hemphill, A.; Sterk, G.J.; Leurs, R. 3-nitroimidazo [1,2-b]pyridazine as a novel scaffold for antiparasitics with sub-nanomolar anti-Giardia lamblia activity. Int. J. Parasitol. Drugs Drug Resist. 2022, 19, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Leitsch, D. A review on metronidazole: An old warhorse in antimicrobial chemotherapy. Parasitology 2017, 146, 1167–1178. [Google Scholar] [CrossRef] [PubMed]
- Bailey, B.L.; Nguyen, W.; Cowman, A.F.; Sleebs, B.E. Chemo-proteomics in antimalarial target identification and engagement. Med. Res. Rev. 2023, 43, 2303–2351. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, J.; Li, M.; Qiao, Y.; Wang, W.; Ma, L.; Liu, K. Target discovery of bioactive natural products with native-compound-coupled CNBr-activated Sepharose 4B beads (NCCB): Applications, mechanisms and outlooks. Bioorg. Med. Chem. 2023, 96, 117483. [Google Scholar] [CrossRef] [PubMed]
- Lechner, S.; Malgapo, M.I.P.; Gratz, C.; Steimbach, R.R.; Baron, A.; Ruther, P.; Nadal, S.; Stumpf, C.; Loos, C.; Ku, X.; et al. Target deconvolution of HDAC pharmacopoeia reveals MBLAC2 as common off-target. Nat. Chem. Biol. 2022, 18, 812–820. [Google Scholar] [CrossRef]
- O’Carra, P.; Barry, S.; Griffin, T. Spacer arms in affinity chromatography: Use of hydrophilic arms to control or eliminate nonbiospecific adsorption effects. FEBS Lett. 1974, 43, 169–175. [Google Scholar] [CrossRef]
- Spratt, B.G.; Pardee, A.B. Penicillin-binding proteins and cell shape in E. coli. Nature 1975, 254, 516–517. [Google Scholar] [CrossRef] [PubMed]
- Spratt, B.G. Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12. Proc. Natl. Acad. Sci. USA 1975, 72, 2999–3003. [Google Scholar] [CrossRef]
- Curtis, S.J.; Strominger, J.L. Purification of penicillin-binding protein 2 of Escherichia coli. J. Bacteriol. 1981, 145, 398–403. [Google Scholar] [CrossRef]
- Knockaert, M.; Gray, N.; Damiens, E.; Chang, Y.T.; Grellier, P.; Grant, K.; Fergusson, D.; Mottram, J.; Soete, M.; Dubremetz, J.F.; et al. Intracellular targets of cyclin-dependent kinase inhibitors: Identification by affinity chromatography using immobilised inhibitors. Chem. Biol. 2000, 7, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Graves, P.R.; Kwiek, J.J.; Fadden, P.; Ray, R.; Hardeman, K.; Coley, A.M.; Foley, M.; Haystead, T.A. Discovery of novel targets of quinoline drugs in the human purine binding proteome. Mol. Pharmacol. 2002, 62, 1364–1372. [Google Scholar] [CrossRef]
- Morita, M.; Sanai, H.; Hiramoto, A.; Sato, A.; Hiraoka, O.; Sakura, T.; Kaneko, O.; Masuyama, A.; Nojima, M.; Wataya, Y.; et al. Plasmodium falciparum endoplasmic reticulum-resident calcium binding protein is a possible target of synthetic antimalarial endoperoxides, N-89 and N-251. J. Proteome Res. 2012, 11, 5704–5711. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.; Wastling, J.; Sanderson, S.; Müller, N.; Hemphill, A. A novel Giardia lamblia nitroreductase, GlNR1, interacts with nitazoxanide and other thiazolides. Antimicrob. Agents Chemother. 2007, 51, 1979–1986. [Google Scholar] [CrossRef]
- Müller, J.; Rout, S.; Leitsch, D.; Vaithilingam, J.; Hehl, A.; Müller, N. Comparative characterisation of two nitroreductases from Giardia lamblia as potential activators of nitro compounds. Int. J. Parasitol. Drugs Drug Resist. 2015, 5, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.; Müller, N. Nitroreductases of bacterial origin in Giardia lamblia: Potential role in detoxification of xenobiotics. MicrobiologyOpen 2019, 8, e904. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.; Sidler, D.; Nachbur, U.; Wastling, J.; Brunner, T.; Hemphill, A. Thiazolides inhibit growth and induce glutathione-S-transferase Pi (GSTP1)-dependent cell death in human colon cancer cells. Int. J. Cancer 2008, 123, 1797–1806. [Google Scholar] [CrossRef]
- Sidler, D.; Brockmann, A.; Müller, J.; Nachbur, U.; Corazza, N.; Renzulli, P.; Hemphill, A.; Brunner, T. Thiazolide-induced apoptosis in colorectal cancer cells is mediated via the Jun kinase-Bim axis and reveals glutathione-S-transferase P1 as Achilles’ heel. Oncogene 2012, 31, 4095–4106. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.; Hemphill, A. Identification of a host cell target for the thiazolide class of broad-spectrum anti-parasitic drugs. Exp. Parasitol. 2011, 128, 145–150. [Google Scholar] [CrossRef]
- Basto, A.P.; Müller, J.; Rubbiani, R.; Stibal, D.; Giannini, F.; Suss-Fink, G.; Balmer, V.; Hemphill, A.; Gasser, G.; Furrer, J. Characterization of the activities of dinuclear thiolato-bridged arene ruthenium complexes against Toxoplasma gondii. Antimicrob. Agents Chemother. 2017, 61, 10–1128. [Google Scholar] [CrossRef]
- Anghel, N.; Müller, J.; Serricchio, M.; Jelk, J.; Butikofer, P.; Boubaker, G.; Imhof, D.; Ramseier, J.; Desiatkina, O.; Paunescu, E.; et al. Cellular and molecular targets of nucleotide-tagged trithiolato-bridged arene ruthenium complexes in the protozoan parasites Toxoplasma gondii and Trypanosoma brucei. Int. J. Mol. Sci. 2021, 22, 787. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.; Boubaker, G.; Imhof, D.; Hanggeli, K.; Haudenschild, N.; Uldry, A.C.; Braga-Lagache, S.; Heller, M.; Ortega-Mora, L.M.; Hemphill, A. Differential affinity chromatography coupled to mass spectrometry: A suitable tool to identify common binding proteins of a broad-range antimicrobial peptide derived from leucinostatin. Biomedicines 2022, 10, 2675. [Google Scholar] [CrossRef]
- Müller, J.; Anghel, N.; Imhof, D.; Hänggeli, K.; Uldry, A.C.; Braga-Lagache, S.; Heller, M.; Ojo, K.K.; Ortega-Mora, L.M.; Van Voorhis, W.C.; et al. Common molecular targets of a quinoline based bumped kinase inhibitor in Neospora caninum and Danio rerio. Int. J. Mol. Sci. 2022, 23, 2381. [Google Scholar] [CrossRef]
- Ajiboye, J.; Uldry, A.C.; Heller, M.; Naguleswaran, A.; Fan, E.; Van Voorhis, W.C.; Hemphill, A.; Müller, J. Molecular targets of the 5-amido-carboxamide bumped kinase inhibitor BKI-1748 in Cryptosporidium parvum and HCT-8 Host Cells. Int. J. Mol. Sci. 2024, 25, 2707. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.S.; Budin, G.; Tassa, C.; Kister, O.; Weissleder, R. Bioorthogonal approach to identify unsuspected drug targets in live cells. Angew. Chem. Int. Ed. Engl. 2013, 52, 10593–10597. [Google Scholar] [CrossRef] [PubMed]
- Kusza, D.A.; Hunter, R.; Schafer, G.; Smith, M.; Katz, A.A.; Kaschula, C.H. Activity-based proteomic identification of the S-thiolation targets of ajoene in MDA-MB-231 breast cancer cells. J. Agric. Food Chem. 2022, 70, 14679–14692. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Sun, Q.; Xie, Y.; Zheng, Q.; Ding, Y. Virus-like iron-gold heterogeneous nanoparticles for drug target screening. Anal. Chem. 2023, 95, 17187–17192. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.Y.; Corson, T.W. Small molecule target identification using photo-affinity chromatography. Methods Enzymol. 2019, 622, 347–374. [Google Scholar] [CrossRef]
- Penarete-Vargas, D.M.; Boisson, A.; Urbach, S.; Chantelauze, H.; Peyrottes, S.; Fraisse, L.; Vial, H.J. A chemical proteomics approach for the search of pharmacological targets of the antimalarial clinical candidate albitiazolium in Plasmodium falciparum using photocrosslinking and click chemistry. PLoS ONE 2014, 9, e113918. [Google Scholar] [CrossRef]
- Ismail, H.M.; Barton, V.; Phanchana, M.; Charoensutthivarakul, S.; Wong, M.H.; Hemingway, J.; Biagini, G.A.; O’Neill, P.M.; Ward, S.A. Artemisinin activity-based probes identify multiple molecular targets within the asexual stage of the malaria parasites Plasmodium falciparum 3D7. Proc. Natl. Acad. Sci. USA 2016, 113, 2080–2085. [Google Scholar] [CrossRef]
- Lubin, A.S.; Rueda-Zubiaurre, A.; Matthews, H.; Baumann, H.; Fisher, F.R.; Morales-Sanfrutos, J.; Hadavizadeh, K.S.; Nardella, F.; Tate, E.W.; Baum, J.; et al. Development of a photo-cross-linkable diaminoquinazoline inhibitor for target identification in Plasmodium falciparum. ACS Infect. Dis. 2018, 4, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Lisauskaite, M.; Nixon, G.L.; Woodley, C.M.; Berry, N.G.; Coninckx, A.; Qie, L.C.; Leung, S.C.; Taramelli, D.; Basilico, N.; Parapini, S.; et al. Design, synthesis and modelling of photoreactive chemical probes for investigating target engagement of plasmepsin IX and X in Plasmodium falciparum. RSC Chem. Biol. 2024, 5, 19–29. [Google Scholar] [CrossRef] [PubMed]
- George, A.L.; Duenas, M.E.; Marin-Rubio, J.L.; Trost, M. Stability-based approaches in chemoproteomics. Expert Rev. Mol. Med. 2024, 26, e6. [Google Scholar] [CrossRef] [PubMed]
- Piazza, I.; Kochanowski, K.; Cappelletti, V.; Fuhrer, T.; Noor, E.; Sauer, U.; Picotti, P. A map of protein-metabolite interactions reveals principles of chemical communication. Cell 2018, 172, 358–372.e323. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Guo, C.W.; Luo, Q.M.; Guo, Z.F.; Chen, L.; Ishihama, Y.; Li, P.; Yang, H.; Gao, W. Thermostability-assisted limited proteolysis-coupled mass spectrometry for capturing drug target proteins and sites. Anal. Chim. Acta 2024, 1312, 342755. [Google Scholar] [CrossRef] [PubMed]
- Luan, C.H.; Light, S.H.; Dunne, S.F.; Anderson, W.F. Ligand screening using fluorescence thermal shift analysis (FTS). Struct. Genom. Drug Discov. Methods Protoc. 2014, 1140, 263–289. [Google Scholar] [CrossRef]
- Jafari, R.; Almqvist, H.; Axelsson, H.; Ignatushchenko, M.; Lundback, T.; Nordlund, P.; Martinez Molina, D. The cellular thermal shift assay for evaluating drug target interactions in cells. Nat. Protoc. 2014, 9, 2100–2122. [Google Scholar] [CrossRef]
- Molina, D.M.; Jafari, R.; Ignatushchenko, M.; Seki, T.; Larsson, E.A.; Dan, C.; Sreekumar, L.; Cao, Y.H.; Nordlund, P. Monitoring drug target engagement in cells and tissues using the cellular thermal shift assay. Science 2013, 341, 84–87. [Google Scholar] [CrossRef]
- Muroi, M.; Osada, H. Two-dimensional electrophoresis-cellular thermal shift assay (2DE-CETSA) for target identification of bioactive compounds. Methods Enzymol. 2022, 675, 425–437. [Google Scholar] [CrossRef]
- Friman, T. Mass spectrometry-based cellular thermal shift assay (CETSA(R)) for target deconvolution in phenotypic drug discovery. Bioorg. Med. Chem. 2020, 28, 115174. [Google Scholar] [CrossRef]
- Sauer, P.; Bantscheff, M. Thermal proteome profiling for drug target identification and probing of protein states. Methods Mol. Biol. 2023, 2718, 73–98. [Google Scholar] [CrossRef] [PubMed]
- George, A.L.; Sidgwick, F.R.; Watt, J.E.; Martin, M.P.; Trost, M.; Marin-Rubio, J.L.; Duenas, M.E. Comparison of quantitative mass spectrometric methods for drug target identification by thermal proteome profiling. J. Proteome Res. 2023, 22, 2629–2640. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Ren, Y.; Li, S.; Hao, P. Identifying drug targets with thermal proteome profiling using IBT-16plex. Rapid Commun. Mass Spectrom. 2024, 38, e9673. [Google Scholar] [CrossRef]
- Savitski, M.M.; Reinhard, F.B.M.; Franken, H.; Werner, T.; Savitski, M.F.; Eberhard, D.; Molina, D.M.; Jafari, R.; Dovega, R.B.; Klaeger, S.; et al. Tracking cancer drugs in living cells by thermal profiling of the proteome. Science 2014, 346, 1255784. [Google Scholar] [CrossRef] [PubMed]
- Ball, K.A.; Webb, K.J.; Coleman, S.J.; Cozzolino, K.A.; Jacobsen, J.; Jones, K.R.; Stowell, M.H.B.; Old, W.M. An isothermal shift assay for proteome scale drug-target identification. Commun. Biol. 2020, 3, 75. [Google Scholar] [CrossRef] [PubMed]
- Zijlmans, D.W.; Hernandez-Quiles, M.; Jansen, P.; Becher, I.; Stein, F.; Savitski, M.M.; Vermeulen, M. STPP-UP: An alternative method for drug target identification using protein thermal stability. J. Biol. Chem. 2023, 299, 105279. [Google Scholar] [CrossRef]
- Kalxdorf, M.; Gunthner, I.; Becher, I.; Kurzawa, N.; Knecht, S.; Savitski, M.M.; Eberl, H.C.; Bantscheff, M. Cell surface thermal proteome profiling tracks perturbations and drug targets on the plasma membrane. Nat. Methods 2021, 18, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hu, L.; Ye, M. Solvent-induced protein precipitation for drug target discovery. Methods Mol. Biol. 2023, 2554, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Paradela, L.S.; Wall, R.J.; Carvalho, S.; Chemi, G.; Corpas-Lopez, V.; Moynihan, E.; Bello, D.; Patterson, S.; Guther, M.L.S.; Fairlamb, A.H.; et al. Multiple unbiased approaches identify oxidosqualene cyclase as the molecular target of a promising anti-leishmanial. Cell Chem. Biol. 2021, 28, 711–721.e718. [Google Scholar] [CrossRef]
- Corpas-Lopez, V.; Wyllie, S. Utilizing thermal proteome profiling to identify the molecular targets of anti-leishmanial compounds. STAR Protoc. 2021, 2, 100704. [Google Scholar] [CrossRef]
- Ibarra-Meneses, A.V.; Corbeil, A.; Wagner, V.; Beaudry, F.; do Monte-Neto, R.L.; Fernandez-Prada, C. Exploring direct and indirect targets of current antileishmanial drugs using a novel thermal proteomics profiling approach. Front. Cell. Infect. Microbiol. 2022, 12, 954144. [Google Scholar] [CrossRef] [PubMed]
- Dziekan, J.M.; Wirjanata, G.; Dai, L.; Go, K.D.; Yu, H.; Lim, Y.T.; Chen, L.; Wang, L.C.; Puspita, B.; Prabhu, N.; et al. Cellular thermal shift assay for the identification of drug-target interactions in the Plasmodium falciparum proteome. Nat. Protoc. 2020, 15, 1881–1921. [Google Scholar] [CrossRef] [PubMed]
- Herneisen, A.L.; Sidik, S.M.; Markus, B.M.; Drewry, D.H.; Zuercher, W.J.; Lourido, S. Identifying the target of an antiparasitic compound in Toxoplasma using thermal proteome profiling. ACS Chem. Biol. 2020, 15, 1801–1807. [Google Scholar] [CrossRef] [PubMed]
- Becher, I.; Andres-Pons, A.; Romanov, N.; Stein, F.; Schramm, M.; Baudin, F.; Helm, D.; Kurzawa, N.; Mateus, A.; Mackmull, M.T.; et al. Pervasive protein thermal stability variation during the cell cycle. Cell 2018, 173, 1495–1507.e1418. [Google Scholar] [CrossRef]
- Müller, J.; Hemphill, A. In vitro screening technologies for the discovery and development of novel drugs against Toxoplasma gondii. Expert. Opin. Drug Discov. 2024, 19, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Tjorve, K.M.C.; Tjorve, E. The use of Gompertz models in growth analyses, and new Gompertz-model approach: An addition to the Unified-Richards family. PLoS ONE 2017, 12, e0178691. [Google Scholar] [CrossRef] [PubMed]
- Brauner, A.; Fridman, O.; Gefen, O.; Balaban, N.Q. Distinguishing between resistance, tolerance and persistence to antibiotic treatment. Nat. Rev. Microbiol. 2016, 14, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.; Hemphill, A.; Müller, N. Physiological aspects of nitro drug resistance in Giardia lamblia. Int. J. Parasitol. Drugs Drug Resist. 2018, 8, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.; Schlange, C.; Heller, M.; Uldry, A.C.; Braga-Lagache, S.; Haynes, R.K.; Hemphill, A. Proteomic characterization of Toxoplasma gondii ME49 derived strains resistant to the artemisinin derivatives artemiside and artemisone implies potential mode of action independent of ROS formation. Int. J. Parasitol. Drugs Drug Resist. 2022, 21, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wellems, T.E.; Sá, J.M.; Su, X.Z.; Connelly, S.V.; Ellis, A.C. ‘Artemisinin Resistance’: Something New or Old? Something of a Misnomer? Trends Parasitol. 2020, 36, 735–744. [Google Scholar] [CrossRef]
- Yu, X.; Wang, C.; Zhao, Y.; Tang, J.; Zhu, M.; Platon, L.; Culleton, R.; Zhu, G.; Menard, D.; Zhang, Q.; et al. Ring-stage growth arrest: Metabolic basis of artemisinin tolerance in Plasmodium falciparum. iScience 2023, 26, 105725. [Google Scholar] [CrossRef] [PubMed]
- Winzer, P.; Anghel, N.; Imhof, D.; Balmer, V.; Ortega-Mora, L.M.; Ojo, K.K.; Van Voorhis, W.C.; Müller, J.; Hemphill, A. Neospora caninum: Structure and Fate of Multinucleated Complexes Induced by the Bumped Kinase Inhibitor BKI-1294. Pathogens 2020, 9, 382. [Google Scholar] [CrossRef] [PubMed]
- Winzer, P.; Müller, J.; Imhof, D.; Ritler, D.; Uldry, A.C.; Braga-Lagache, S.; Heller, M.; Ojo, K.K.; Van Voorhis, W.C.; Ortega-Mora, L.M.; et al. Neospora caninum: Differential Proteome of Multinucleated Complexes Induced by the Bumped Kinase Inhibitor BKI-1294. Microorganisms 2020, 8, 801. [Google Scholar] [CrossRef] [PubMed]
- Jerlström-Hultqvist, J.; Stadelmann, B.; Birkestedt, S.; Hellman, U.; Svärd, S.G. Plasmid vectors for proteomic analyses in Giardia: Purification of virulence factors and analysis of the proteasome. Eukaryot. Cell 2012, 11, 864–873. [Google Scholar] [CrossRef] [PubMed]
- Meissner, M.; Breinich, M.S.; Gilson, P.R.; Crabb, B.S. Molecular genetic tools in Toxoplasma and Plasmodium: Achievements and future needs. Curr. Opin. Microbiol. 2007, 10, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Carucci, D.J. Technologies for the study of gene and protein expression in Plasmodium. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2002, 357, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Donald, R.G.; Roos, D.S. Stable molecular transformation of Toxoplasma gondii: A selectable dihydrofolate reductase-thymidylate synthase marker based on drug-resistance mutations in malaria. Proc. Natl. Acad. Sci. USA 1993, 90, 11703–11707. [Google Scholar] [CrossRef] [PubMed]
- Howe, D.K.; Sibley, L.D. Development of molecular genetics for Neospora caninum: A complementary system to Toxoplasma gondii. Methods 1997, 13, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Ullu, E.; Tschudi, C.; Chakraborty, T. RNA interference in protozoan parasites. Cell Microbiol. 2004, 6, 509–519. [Google Scholar] [CrossRef]
- Sidik, S.M.; Hackett, C.G.; Tran, F.; Westwood, N.J.; Lourido, S. Efficient genome engineering of Toxoplasma gondii using CRISPR/Cas9. PLoS ONE 2014, 9, e100450. [Google Scholar] [CrossRef]
- Shen, B.; Brown, K.M.; Lee, T.D.; Sibley, L.D. Efficient gene disruption in diverse strains of Toxoplasma gondii using CRISPR/CAS9. MBio 2014, 5, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Sidik, S.M.; Huet, D.; Ganesan, S.M.; Huynh, M.H.; Wang, T.; Nasamu, A.S.; Thiru, P.; Saeij, J.P.; Carruthers, V.B.; Niles, J.C.; et al. A Genome-wide CRISPR screen in Toxoplasma Identifies essential apicomplexan genes. Cell 2016, 166, 1423–1435.e1412. [Google Scholar] [CrossRef] [PubMed]
- Castaneda-Barba, S.; Top, E.M.; Stalder, T. Plasmids, a molecular cornerstone of antimicrobial resistance in the One Health era. Nat. Rev. Microbiol. 2024, 22, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Leitsch, D.; Williams, C.F.; Hrdy, I. Redox pathways as drug targets in microaerophilic parasites. Trends Parasitol. 2018, 34, 576–589. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.; Heller, M.; Uldry, A.C.; Braga, S.; Müller, N. Nitroreductase activites in Giardia lamblia: ORF 17150 encodes a quinone reductase with nitroreductase activity. Pathogens 2021, 10, 129. [Google Scholar] [CrossRef] [PubMed]
- Palmer, A.C.; Kishony, R. Opposing effects of target overexpression reveal drug mechanisms. Nat. Commun. 2014, 5, 4296. [Google Scholar] [CrossRef] [PubMed]
- Hanggeli, K.P.A.; Hemphill, A.; Müller, N.; Heller, M.; Uldry, A.C.; Braga-Lagache, S.; Müller, J.; Boubaker, G. Comparative proteomic analysis of Toxoplasma gondii RH wild-type and four SRS29B (SAG1) knock-out clones reveals significant differences between individual strains. Int. J. Mol. Sci. 2023, 24, 454. [Google Scholar] [CrossRef] [PubMed]
- Freisleben, F.; Behrmann, L.; Thaden, V.; Muschhammer, J.; Bokemeyer, C.; Fiedler, W.; Wellbrock, J. Downregulation of GLI3 expression mediates chemotherapy resistance in acute myeloid leukemia. Int. J. Mol. Sci. 2020, 21, 5084. [Google Scholar] [CrossRef] [PubMed]
- de Koning, H.P. Drug resistance in protozoan parasites. Emerg. Top. Life Sci. 2017, 1, 627–632. [Google Scholar] [CrossRef]
- Gardner, M.J.; Hall, N.; Fung, E.; White, O.; Berriman, M.; Hyman, R.W.; Carlton, J.M.; Pain, A.; Nelson, K.E.; Bowman, S.; et al. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature 2002, 419, 498–511. [Google Scholar] [CrossRef]
- Cooper, R.A.; Carucci, D.J. Proteomic approaches to studying drug targets and resistance in Plasmodium. Curr. Drug Targets Infect. Disord. 2004, 4, 41–51. [Google Scholar] [CrossRef]
- Wang, S.; Huang, F.; Yan, H.; Yin, J.; Xia, Z. A review of malaria molecular markers for drug resistance in Plasmodium falciparum and Plasmodium vivax in China. Front. Cell Infect. Microbiol. 2023, 13, 1167220. [Google Scholar] [CrossRef] [PubMed]
- Pandit, K.; Surolia, N.; Bhattacharjee, S.; Karmodiya, K. The many paths to artemisinin resistance in Plasmodium falciparum. Trends Parasitol. 2023, 39, 1060–1073. [Google Scholar] [CrossRef] [PubMed]
- Platon, L.; Menard, D. Plasmodium falciparum ring-stage plasticity and drug resistance. Trends Parasitol. 2024, 40, 118–130. [Google Scholar] [CrossRef]
- Müller, J.; Ley, S.; Felger, I.; Hemphill, A.; Müller, N. Identification of differentially expressed genes in a Giardia lamblia WB C6 clone resistant to nitazoxanide and metronidazole. J. Antimicrob. Chemother. 2008, 62, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Townson, S.M.; Laqua, H.; Upcroft, P.; Boreham, P.F.; Upcroft, J.A. Induction of metronidazole and furazolidone resistance in Giardia. Trans. R. Soc. Trop. Med. Hyg. 1992, 86, 521–522. [Google Scholar] [CrossRef]
- Müller, J.; Sterk, M.; Hemphill, A.; Müller, N. Characterization of Giardia lamblia WB C6 clones resistant to nitazoxanide and to metronidazole. J. Antimicrob. Chemother. 2007, 60, 280–287. [Google Scholar] [CrossRef]
- Brown, D.M.; Upcroft, J.A.; Edwards, M.R.; Upcroft, P. Anaerobic bacterial metabolism in the ancient eukaryote Giardia duodenalis. Int. J. Parasitol. 1998, 28, 149–164. [Google Scholar] [CrossRef]
- Müller, J.; Braga, S.; Heller, M.; Müller, N. Resistance formation to nitro drugs in Giardia lamblia: No common markers identified by comparative proteomics. Int. J. Parasitol. Drugs Drug Resist. 2019, 9, 112–119. [Google Scholar] [CrossRef]
- Ansell, B.R.; Baker, L.; Emery, S.J.; McConville, M.J.; Svard, S.G.; Gasser, R.B.; Jex, A.R. Transcriptomics indicates active and passive metronidazole resistance mechanisms in three seminal Giardia lines. Front. Microbiol. 2017, 8, 398. [Google Scholar] [CrossRef]
- Emery, S.J.; Baker, L.; Ansell, B.R.E.; Mirzaei, M.; Haynes, P.A.; McConville, M.J.; Svard, S.G.; Jex, A.R. Differential protein expression and post-translational modifications in metronidazole-resistant Giardia duodenalis. Gigascience 2018, 7, giy024. [Google Scholar] [CrossRef] [PubMed]
- Koncarevic, S.; Bogumil, R.; Becker, K. SELDI-TOF-MS analysis of chloroquine resistant and sensitive Plasmodium falciparum strains. Proteomics 2007, 7, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Doliwa, C.; Xia, D.; Escotte-Binet, S.; Newsham, E.L.; Sanya, J.S.; Aubert, D.; Randle, N.; Wastling, J.M.; Villena, I. Identification of differentially expressed proteins in sulfadiazine resistant and sensitive strains of Toxoplasma gondii using difference-gel electrophoresis (DIGE). Int. J. Parasitol. Drugs Drug Resist. 2013, 3, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Scheele, S.; Geiger, J.A.; DeRocher, A.E.; Choi, R.; Smith, T.R.; Hulverson, M.A.; Vidadala, R.S.R.; Barrett, L.K.; Maly, D.J.; Merritt, E.A.; et al. Toxoplasma calcium-dependent protein kinase 1 inhibitors: Probing activity and resistance using cellular thermal shift assays. Antimicrob. Agents Chemother. 2018, 62, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Burata, O.E.; Yeh, T.J.; Macdonald, C.B.; Stockbridge, R.B. Still rocking in the structural era: A molecular overview of the small multidrug resistance (SMR) transporter family. J. Biol. Chem. 2022, 298, 102482. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lin, Q. Chemical proteomics approach reveals the direct targets and the heme-dependent activation mechanism of artemisinin in Plasmodium falciparum using an artemisinin-based activity probe. Microb. Cell 2016, 3, 230–231. [Google Scholar] [CrossRef] [PubMed]
- Rios-Barros, L.V.; Silva-Moreira, A.L.; Horta, M.F.; Gontijo, N.F.; Castro-Gomes, T. How to get away with murder: The multiple strategies employed by pathogenic protozoa to avoid complement killing. Mol. Immunol. 2022, 149, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Prucca, C.G.; Lujan, H.D. Antigenic variation in Giardia lamblia. Cell Microbiol. 2009, 11, 1706–1715. [Google Scholar] [CrossRef]
- Müller, N.; Gottstein, B. Antigenic variation and the murine immune response to Giardia lamblia. Int. J. Parasitol. 1998, 28, 1829–1839. [Google Scholar] [CrossRef]
- Müller, J.; Vermathen, M.; Leitsch, D.; Vermathen, P.; Müller, N. Metabolomic profiling of wildtype and transgenic Giardia lamblia Strains by (1)H HR-MAS NMR Spectroscopy. Metabolites 2020, 10, 53. [Google Scholar] [CrossRef]

| Protein Target for Inhibitor Screenings | Pathogen | Methodology | Reference |
|---|---|---|---|
| Protein biosynthesis | P. falciparum | Luciferase assay | [65] |
| Calcium-dependent protein kinase 1 | T. gondii | Kinase assay Cocrystallization | [66,67] |
| Dihydrofolate reductase thymidylate synthase | T. gondii | Functional assay | [68] |
| Dihydrofolate reductase | P. vivax | Heterologous expression in yeast Growth assay | [69] |
| Acetyl-CoA carboxylase | T. gondii | Functional assay | [70] |
| Glyoxalase 1 | T. gondii | Functional assay | [71] |
| Type-II NADH dehydrogenase | T. gondii | Functional assay | [72] |
| Nucleoside triphosphate hydrolase | N. caninum, T. gondii | Chemoluminescence assay | [73] |
| Mitochondrial ADP/ATP | P. falciparum | Heterologous expression in E. coli Radioactive uptake assay | [74] |
| Importin alpha binding to nuclear localization signal | P. falciparum | Alpha screen binding assay | [75] |
| Phenylalanyl t-RNA synthetase | T. gondii | Functional assay | [76] |
| Aspartate transcarbamoylase | P. falciparum | Functional assay, protein interference assay | [77,78] |
| Antimalarial | Methodology | Remarks | Reference |
|---|---|---|---|
| Kinase inhibitors | Cell-free extracts from various cell types and organisms. DAC with active and inactive purines. SDS-PAGE followed by digestion of binding proteins and microsequencing of the peptides. | Detection of known kinases by Western blotting. Some of the peptide sequences match to other kinases and other proteins. | [91] |
| Quinolines | Cell-free extracts of infected human erythrocytes. DAC with ATP as a control, elution with various quinoline antimalarials, SDS-PAGE followed by Edman mixed peptide sequencing. | Human aldehyde dehydrogenase 1 and quinone reductase 2 major BP. Validated as potential target by FA. | [92] |
| Endoperoxides | P. falciparum trophozoite lysates. AC with an artemisinin analog, followed by 2-D SDS-PAGE and MALDI-TOF MS. | Identification of 9 P. falciparum BPs. Major BP is a calcium-binding protein. | [93] |
| Organism | Ligand | Methodology | Remarks | Reference |
|---|---|---|---|---|
| G. lamblia | Thiazolide | AC, elution with ligand, SDS-PAGE followed by LC-MS/MS. | Nitroreductase NR1 major BP. Validated as a potential target by FA and in subsequent studies. | [94,95,96] |
| H. sapiens Caco2 | Thiazolide | AC, elution with ligand, SDS-PAGE followed by LC-MS/MS. | Human GSTP1 major BP. Validated as a potential target by FA and in subsequent studies. | [97,98] |
| H. sapiens Fibroblasts | Thiazolide | AC, elution with ligand, SDS-PAGE followed by LC-MS/MS. | Human quinone reductase 1 major BP in N. caninum infected cells. Validation by FA. | [99] |
| T. gondii | Ruthenium complex | DAC with mock column only; elution by pH shift; SDS-PAGE followed by LC-MS/MS. | Translation elongation factor 1 alpha and two ribosomal proteins identified as binding proteins. | [100] |
| T. gondii T. brucei | Ruthenium complex | Comparative DAC with two ineffective complexes in two pathogens, elution with pH shift, LC-MS/MS on entire eluates. | 128 specific T. gondii BPs and 46 specific T. brucei BPs. Major T. brucei BP mitochondrial ATP synthase subunit validated by FA. | [101] |
| T. gondii M. musculus splenocytes | Antimicrobial peptide | Comparative DAC with ineffective peptide, elution with pH shift, LC-MS/MS on entire eluates. | Several hundred BPs in eluates from both organisms, suggesting common modes of action. | [102] |
| N. caninum D. rerio | Bumped kinase inhibitor with quinoline core | Comparative DAC with quinine, elution with pH shift, LC-MS/MS on entire eluates. | 12 specific N. caninum BPs and 13 specific D. rerio BPs. Many BPs in both organisms in quinine eluates, as well. Majority involved in RNA binding or modification. | [103] |
| C. parvum H. sapiens HCT-8 cells | Bumped kinase inhibitor with quinoline core | Comparative DAC with quinine, elution with pH shift, LC-MS/MS on entire eluates. | No specific binding proteins in C. parvum, 25 specific BPs in host cells; 29 C. parvum and 224 host cell BPs also in quinine eluates. Common targets in RNA binding or modification. | [104] |
| Organism | Methodology | Remarks | Reference |
|---|---|---|---|
| Leishmania donovani | Classical CETSA-MS on promastigotes with an inhibitor of sterol biosynthesis. | Oxidosqualene cyclase identified as a target of this inhibitor. | [129,130] |
| L. infantum | Classical CETSA-MS on cell-free extracts of amphotericin B, antimony, or miltefosine susceptible and resistant lines incubated with the respective drugs. | Up to several hundred proteins with altered melting profiles depending on the compound. Sb tends to stabilize ribosomal proteins. | [131] |
| P. falciparum | Comparison of classical and isothermal CETSA on intraerythrocytic stages using pyrimethamine as a proof of concept. | Conceptual study. No data on novel binding proteins directly available. | [132] |
| T. gondii | Classical CETSA-MS with calcium egress inhibitor ENH1 as ligand. | 82 proteins with enhanced thermal stability identified, including calcium-dependent protein kinase 1. | [133] |
| Methodology | Advantages | Inconveniences |
|---|---|---|
| AC—elution with ligand | Well established. Does not need sophisticated equipment. Fast. | Modification of original ligand necessary to create column matrix. Identification of major binding proteins after PAGE, resulting in low yields and bias. Cell-free extracts. |
| DAC—unspecific elution | See above. LC-MS/MS if elution with compatible solvent. | Needs an ineffective control compound with similar structure. Cell-free extracts. |
| Affinity labeling | Interaction occurs intracellularly, therefore, under physiological conditions. Fast. | Modification of original ligand necessary to create compound for affinity labeling. Polishing of labeled proteins by PAGE, therefore, low yields and bias. Label may interfere with subsequent MS. |
| TPP | Flexible, since interaction of proteomes and ligands is investigated under physiological conditions or in cell-free extracts. Unmodified compounds may be used. | Time and cost intensive. Use of isobaric labels. Large data volumes need appropriate bioinformatic tools. |
| Organism | Drug | Methodology | Remarks | Reference |
|---|---|---|---|---|
| G. lamblia | Metronidazole | Comparison of three resistant cell lines created by increasing drug concentrations plus UV irradiation with susceptible parental strains. Analysis of proteomes and post-translational modifications by broad panel of proteome analytical methods. | 265, 171, and 76 differentially expressed proteins depending on the strains. High isolate-dependent variability of adaptation mechanisms. | [171] |
| G. lamblia | Nitazoxanide Metronidazole | Comparison of a strain generated by increasing nitazoxanide concentrations and two metronidazole-resistant strains from study quoted above with their corresponding wildtypes. All resistant strains were resistant to both drugs and were grown in the presence of either drug prior to analysis by shotgun LC/MS-MS. | 225, 248, and 304 differentially expressed proteins in the presence of nitazoxanide, 510, 287, and 216 in the presence of metronidazole. No common markers for nitro resistance. Common pattern of antigenic variation in all metronidazole-resistant vs. susceptible strains. Strategies of coping with nitro reduction strain and drug dependence. | [169] |
| P. falciparum | Chloroquine | Comparison of two clinical isolates resistant to chloroquine with two susceptible isolates using SELDI-TOF. | Study focused on the methodology. One of the susceptible strains and both resistant strains are resistant to pyrimethamine, and one resistant strain is resistant to quinine and sulfadoxine; 10 “marker proteins” identified. | [172] |
| T. gondii | Sulfadizine | Resistant clinical isolates, susceptible reference strains. Comparison of proteomes by DIGE-MS. | 31 unique differential proteins were identified. | [173] |
| T. gondii | Artemisone Artemiside | Generation of resistant strains by treating the reference strain ME49 with increasing concentrations. Whole-cell-shotgun LC/MS-MS. | 215 proteins downregulated in the artemisone-resistant strain, 8 proteins in the artemiside-resistant strain. | [139] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Müller, J.; Boubaker, G.; Müller, N.; Uldry, A.-C.; Braga-Lagache, S.; Heller, M.; Hemphill, A. Investigating Antiprotozoal Chemotherapies with Novel Proteomic Tools—Chances and Limitations: A Critical Review. Int. J. Mol. Sci. 2024, 25, 6903. https://doi.org/10.3390/ijms25136903
Müller J, Boubaker G, Müller N, Uldry A-C, Braga-Lagache S, Heller M, Hemphill A. Investigating Antiprotozoal Chemotherapies with Novel Proteomic Tools—Chances and Limitations: A Critical Review. International Journal of Molecular Sciences. 2024; 25(13):6903. https://doi.org/10.3390/ijms25136903
Chicago/Turabian StyleMüller, Joachim, Ghalia Boubaker, Norbert Müller, Anne-Christine Uldry, Sophie Braga-Lagache, Manfred Heller, and Andrew Hemphill. 2024. "Investigating Antiprotozoal Chemotherapies with Novel Proteomic Tools—Chances and Limitations: A Critical Review" International Journal of Molecular Sciences 25, no. 13: 6903. https://doi.org/10.3390/ijms25136903
APA StyleMüller, J., Boubaker, G., Müller, N., Uldry, A.-C., Braga-Lagache, S., Heller, M., & Hemphill, A. (2024). Investigating Antiprotozoal Chemotherapies with Novel Proteomic Tools—Chances and Limitations: A Critical Review. International Journal of Molecular Sciences, 25(13), 6903. https://doi.org/10.3390/ijms25136903






