Effect of Isoscopoletin on Cytokine Expression in HaCaT Keratinocytes and RBL-2H3 Basophils: Preliminary Study
Abstract
:1. Introduction
2. Results
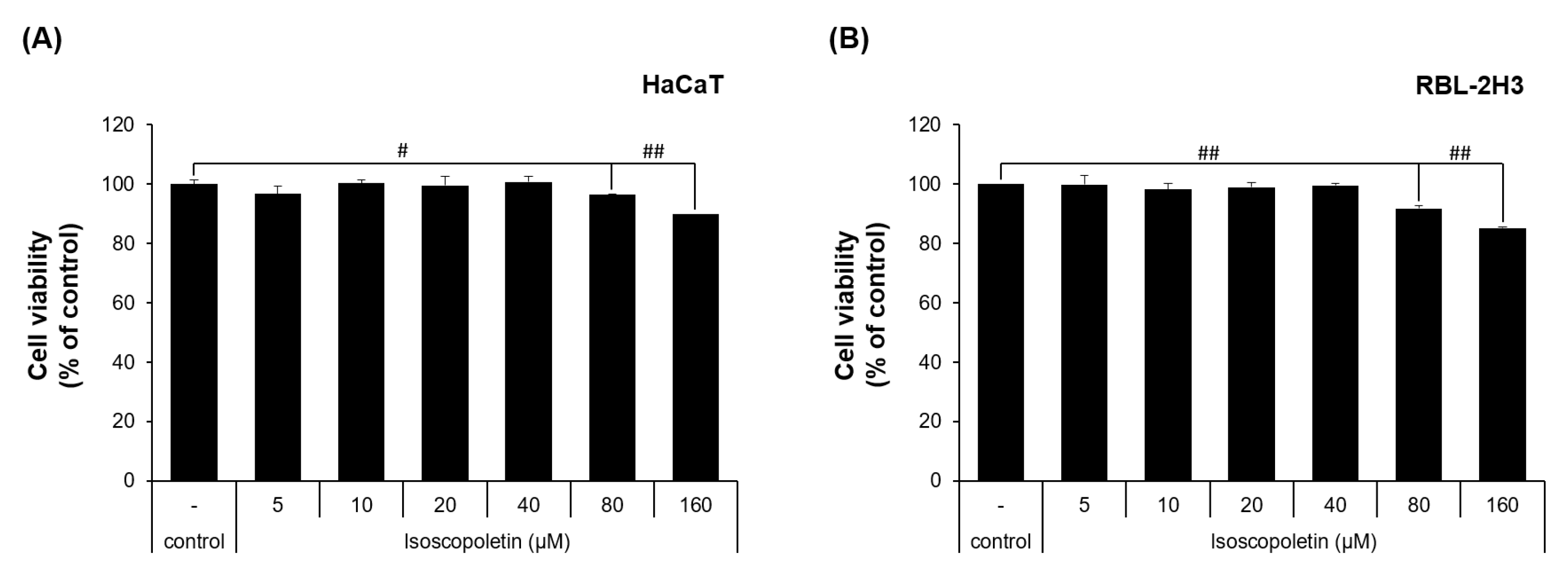
2.1. Effect of Isoscopoletin on the Viability of HaCaT and RBL-2H3 Cells
2.2. Isoscopoletin Inhibits Cytokine and Chemokine Release by HaCaT and RBL-2H3 Cells
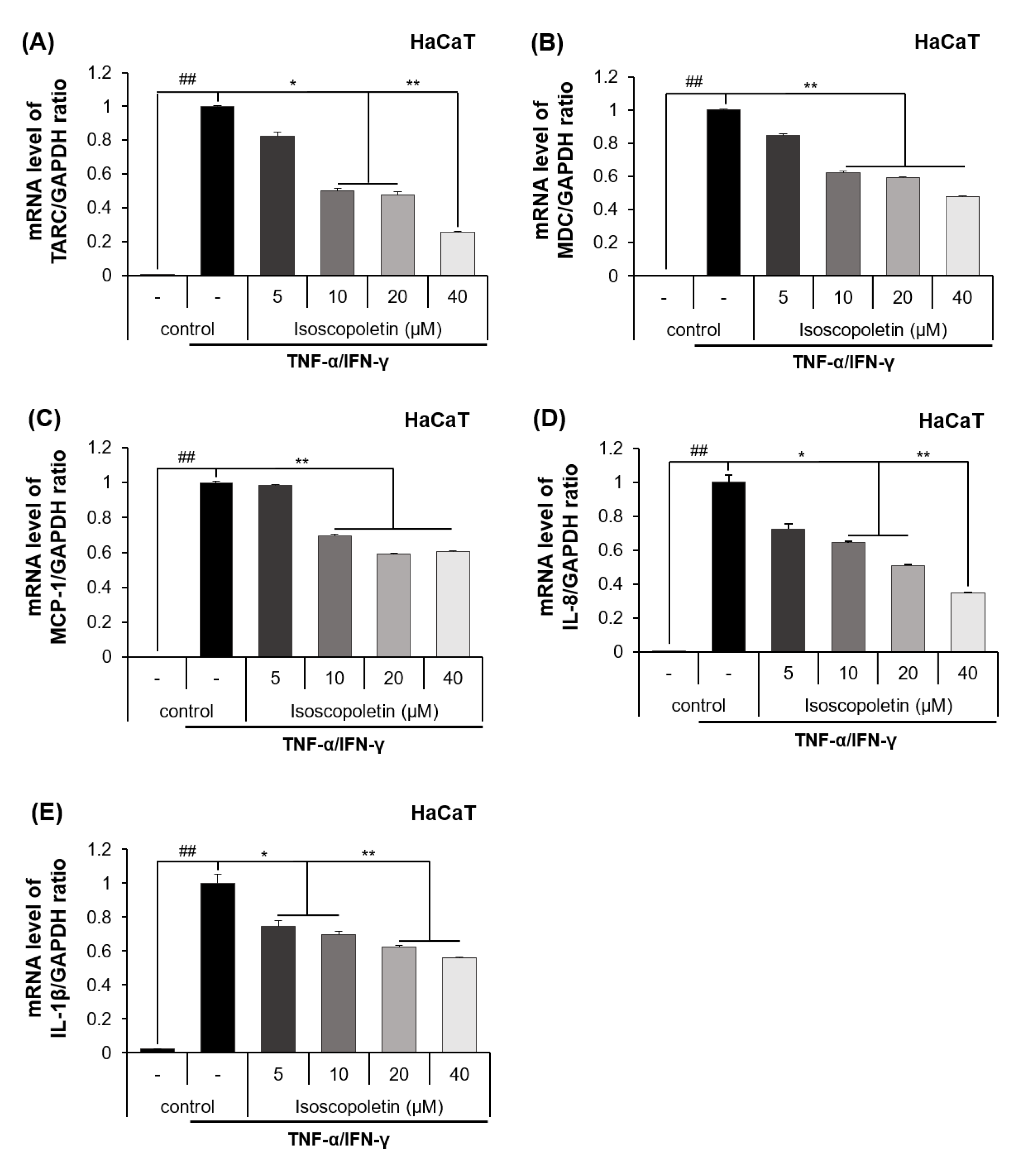
2.3. Isoscopoletin Inhibits Gene Expression Levels of Cytokines and Chemokines in HaCaT Cells
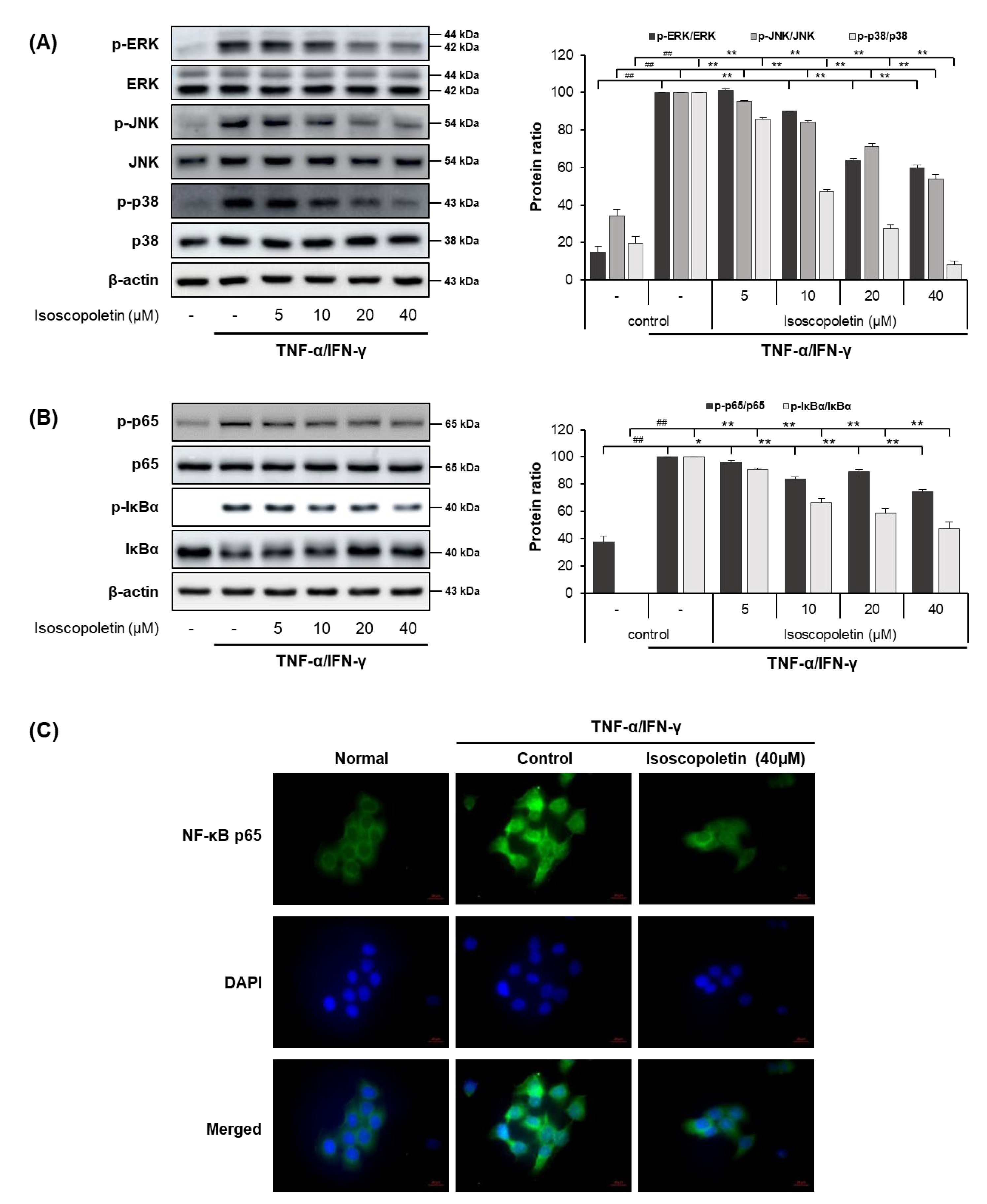
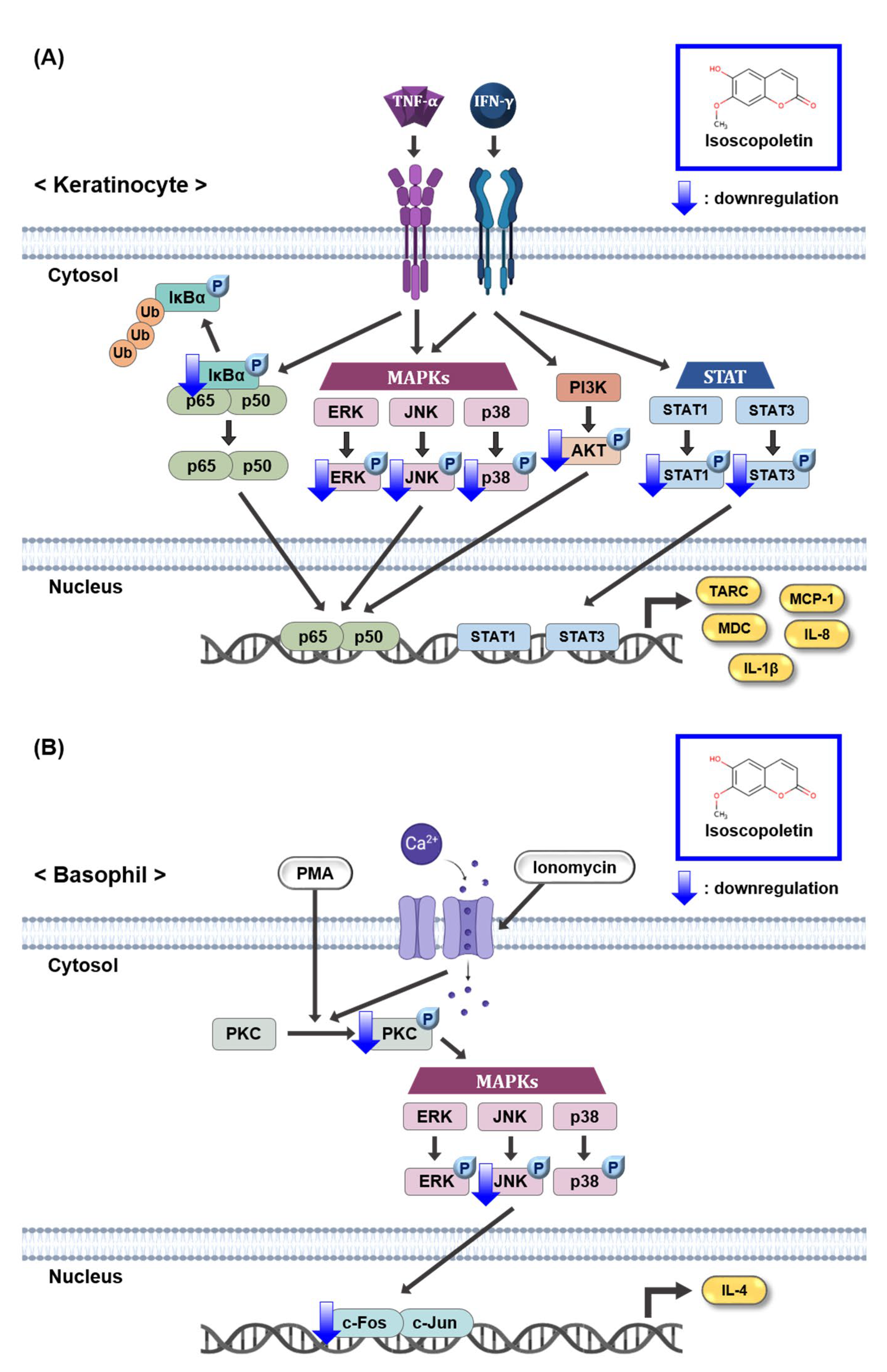
2.4. Isoscopoletin Suppresses Phosphorylation of MAPK and NF-κB Signaling Molecules in HaCaT Cells
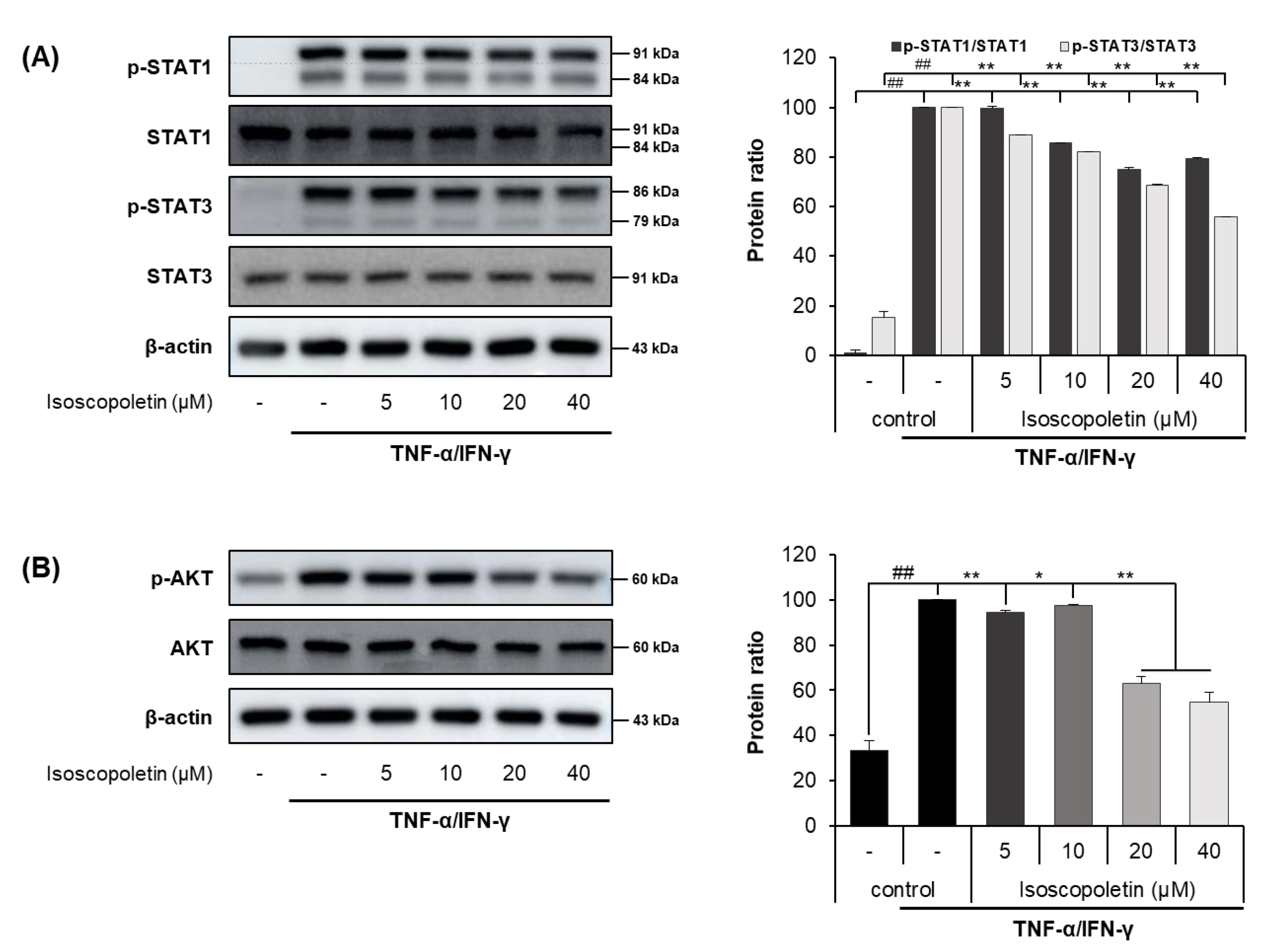
2.5. Isoscopoletin Suppresses Phosphorylation of STAT and AKT Signaling Molecules in HaCaT Cells
2.6. Isoscopoletin Suppresses Activation of PKC, MAPK, and AP-1 Signaling Molecules in RBL-2H3 Cells
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. Cell Viability Assay
4.4. Measurement of Cytokine and Chemokine Secretion
4.5. Reverse Transcription-Quantitative PCR (RT-qPCR)
4.6. Western Blot Analysis
4.7. Immunocytochemistry (ICC) Analysis
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Spergel, J.M.; Paller, A.S. Atopic dermatitis and the atopic march. J. Allergy Clin. Immunol. 2003, 112, S118–S127. [Google Scholar] [CrossRef] [PubMed]
- Weidinger, S.; Novak, N. Atopic dermatitis. Lancet 2016, 387, 1109–1122. [Google Scholar] [CrossRef] [PubMed]
- Gittler, J.K.; Shemer, A.; Suárez-Fariñas, M.; Fuentes-Duculan, J.; Gulewicz, K.J.; Wang, C.Q.; Mitsui, H.; Cardinale, I.; de Guzman Strong, C.; Krueger, J.G.; et al. Progressive activation of T(H)2/T(H)22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis. J. Allergy Clin. Immunol. 2012, 130, 1344–1354. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, T.; Ando, T.; Kimura, M.; Wilson, B.S.; Kawakami, Y. Mast cells in atopic dermatitis. Curr. Opin. Immunol. 2009, 21, 666–678. [Google Scholar] [CrossRef] [PubMed]
- Langan, S.M.; Irvine, A.D.; Weidinger, S. Atopic dermatitis. Lancet 2020, 396, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Werfel, T. The role of leukocytes, keratinocytes, and allergen-specific IgE in the development of atopic dermatitis. J. Investig. Dermatol. 2009, 129, 1878–1891. [Google Scholar] [CrossRef] [PubMed]
- Saeki, H.; Tamaki, K. Thymus and activation regulated chemokine (TARC)/CCL17 and skin diseases. J. Dermatol. Sci. 2006, 43, 75–84. [Google Scholar] [CrossRef]
- Jahnz-Rozyk, K.; Targowski, T.; Paluchowska, E.; Owczarek, W.; Kucharczyk, A. Serum thymus and activation-regulated chemokine, macrophage-derived chemokine and eotaxin as markers of severity of atopic dermatitis. Allergy 2005, 60, 685–688. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, C.; Bang, K.; Gesser, B.; Yoneyama, H.; Matsushima, K.; Larsen, C.G. A Th2 chemokine, TARC, produced by keratinocytes may recruit CLA+CCR4+ lymphocytes into lesional atopic dermatitis skin. J. Investig. Dermatol. 2000, 115, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Sumiyoshi, K.; Nakao, A.; Setoguchi, Y.; Tsuboi, R.; Okumura, K.; Ogawa, H. TGF-beta/Smad signaling inhibits IFN-gamma and TNF-alpha-induced TARC (CCL17) production in HaCaT cells. J. Dermatol. Sci. 2003, 31, 53–58. [Google Scholar] [CrossRef]
- Kataoka, Y. Thymus and activation-regulated chemokine as a clinical biomarker in atopic dermatitis. J. Dermatol. 2014, 41, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Vilgelm, A.E.; Richmond, A. Chemokines Modulate Immune Surveillance in Tumorigenesis, Metastasis, and Response to Immunotherapy. Front. Immunol. 2019, 10, 333. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.-T.; Chu, C.-Y. Advances in Systemic Treatment for Adults with Moderate-to-Severe Atopic Dermatitis. Dermatologica Sinica 2019, 37, 3–11. [Google Scholar] [CrossRef]
- Hänel, K.H.; Cornelissen, C.; Lüscher, B.; Baron, J.M. Cytokines and the skin barrier. Int. J. Mol. Sci. 2013, 14, 6720–6745. [Google Scholar] [CrossRef] [PubMed]
- Gadina, M.; Gazaniga, N.; Vian, L.; Furumoto, Y. Small molecules to the rescue: Inhibition of cytokine signaling in immune-mediated diseases. J. Autoimmun. 2017, 85, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Giustizieri, M.L.; Mascia, F.; Frezzolini, A.; De Pità, O.; Chinni, L.M.; Giannetti, A.; Girolomoni, G.; Pastore, S. Keratinocytes from patients with atopic dermatitis and psoriasis show a distinct chemokine production profile in response to T cell-derived cytokines. J. Allergy Clin. Immunol. 2001, 107, 871–877. [Google Scholar] [CrossRef]
- Oh, J.H.; Kim, S.H.; Kwon, O.K.; Kim, J.H.; Oh, S.R.; Han, S.B.; Park, J.W.; Ahn, K.S. Purpurin suppresses atopic dermatitis via TNF-α/IFN-γ-induced inflammation in HaCaT cells. Int. J. Immunopathol. Pharmacol. 2022, 36, 3946320221111135. [Google Scholar] [CrossRef]
- Kiu, H.; Nicholson, S.E. Biology and significance of the JAK/STAT signalling pathways. Growth Factors 2012, 30, 88–106. [Google Scholar] [CrossRef] [PubMed]
- Mai, L.; Liu, Q.; Huang, F.; He, H.; Fan, W. Involvement of Mast Cells in the Pathophysiology of Pain. Front. Cell. Neurosci. 2021, 15, 665066. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Sharma, K.D.; Ashawat, S.M. Pathophysiology and Management of Atopic Dermatitis: A Laconic Review. Curr. Drug Ther. 2020, 15, 321–336. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Alysandratos, K.D.; Angelidou, A.; Delivanis, D.A.; Sismanopoulos, N.; Zhang, B.; Asadi, S.; Vasiadi, M.; Weng, Z.; Miniati, A.; et al. Mast cells and inflammation. Biochim. Biophys. Acta 2012, 1822, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Varricchi, G.; Raap, U.; Rivellese, F.; Marone, G.; Gibbs, B.F. Human mast cells and basophils-How are they similar how are they different? Immunol. Rev. 2018, 282, 8–34. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Shi, Y.; He, X.; Sun, W.; Lv, Y.; Hou, X. Study on screening potential allergenic proteins from infant milk powders based on human mast cell membrane chromatography and histamine release assays. J. Pharm. Anal. 2019, 9, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Iype, J.; Fux, M. Basophils Orchestrating Eosinophils’ Chemotaxis and Function in Allergic Inflammation. Cells 2021, 10, 895. [Google Scholar] [CrossRef] [PubMed]
- Van Beek, A.A.; Knol, E.F.; de Vos, P.; Smelt, M.J.; Savelkoul, H.F.; van Neerven, R.J. Recent developments in basophil research: Do basophils initiate and perpetuate type 2 T-helper cell responses? Int. Arch. Allergy Immunol. 2013, 160, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, S.; Nomura, T.; Common, J.; Kabashima, K. Insights into atopic dermatitis gained from genetically defined mouse models. J. Allergy Clin. Immunol. 2019, 143, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Yamanishi, Y.; Mogi, K.; Takahashi, K.; Miyake, K.; Yoshikawa, S.; Karasuyama, H. Skin-infiltrating basophils promote atopic dermatitis-like inflammation via IL-4 production in mice. Allergy 2020, 75, 2613–2622. [Google Scholar] [CrossRef] [PubMed]
- Yoon Hee, K.; Ye Rang, C.; Ji Young, K.; Sang Hee, K. Anti-Allergic Effect of 1,2,3,4,6-Penta-O-Galloyl-β-D-Glucose on RBL-2H3 Cells. J. Korean Soc. Food Sci. Nutr. 2016, 45, 613–618. [Google Scholar]
- Huang, Y.; Yang, X.L.; Ni, Y.H.; Xu, Z.M. Geraniol suppresses proinflammatory mediators in phorbol 12-myristate 13-acetate with A23187-induced HMC-1 cells. Drug Des. Dev. Ther. 2018, 12, 2897–2903. [Google Scholar] [CrossRef]
- Penzo, D.; Petronilli, V.; Angelin, A.; Cusan, C.; Colonna, R.; Scorrano, L.; Pagano, F.; Prato, M.; Di Lisa, F.; Bernardi, P. Arachidonic acid released by phospholipase A(2) activation triggers Ca2+-dependent apoptosis through the mitochondrial pathway. J. Biol. Chem. 2004, 279, 25219–25225. [Google Scholar] [CrossRef] [PubMed]
- Chelombitko, M.A.; Firsov, A.M.; Kotova, E.A.; Rokitskaya, T.I.; Khailova, L.S.; Popova, L.B.; Chernyak, B.V.; Antonenko, Y.N. Usnic acid as calcium ionophore and mast cells stimulator. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183303. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Ahn, S.; Lee, D.-C. Inhibitory effect of Alpiniae officinarum Rhizoma extract on degranulation in RBL-2H3 cells. Korean J. Plant Resour. 2015, 28, 321–328. [Google Scholar] [CrossRef]
- Siraganian, R.P.; de Castro, R.O.; Barbu, E.A.; Zhang, J. Mast cell signaling: The role of protein tyrosine kinase Syk, its activation and screening methods for new pathway participants. FEBS Lett. 2010, 584, 4933–4940. [Google Scholar] [CrossRef] [PubMed]
- Strehl, C.; Ehlers, L.; Gaber, T.; Buttgereit, F. Glucocorticoids-All-Rounders Tackling the Versatile Players of the Immune System. Front. Immunol. 2019, 10, 1744. [Google Scholar] [CrossRef] [PubMed]
- Carr, W.W. Topical calcineurin inhibitors for atopic dermatitis: Review and treatment recommendations. Pediatr. Drugs 2013, 15, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Guttman-Yassky, E. Efficacy of biologics in atopic dermatitis. Expert Opin. Biol. Ther. 2020, 20, 525–538. [Google Scholar] [CrossRef] [PubMed]
- Kostova, I.; Iossifova, T. Chemical components of Fraxinus species. Fitoterapia 2007, 78, 85–106. [Google Scholar] [CrossRef] [PubMed]
- Hao, D.-C. Chapter 9—Biodiversity, Chemodiversity, and Pharmacotherapy of Ranunculus Medicinal Plants. In Ranunculales Medicinal Plants; Hao, D.-C., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 357–386. [Google Scholar] [CrossRef]
- Zhao, D.D.; Zhao, Q.S.; Liu, L.; Chen, Z.Q.; Zeng, W.M.; Lei, H.; Zhang, Y.L. Compounds from Dryopteris fragrans (L.) Schott with cytotoxic activity. Molecules 2014, 19, 3345–3355. [Google Scholar] [CrossRef] [PubMed]
- Selim, Y.A.; Ouf, N.H. Anti-inflammatory new coumarin from the Ammi majus L. ACS Med. Chem. Lett. 2012, 2, 1. [Google Scholar] [CrossRef] [PubMed]
- Fayyaz, A.; Makwinja, S.; Auriola, S.; Raunio, H.; Juvonen, R.O. Comparison of In Vitro Hepatic Scoparone 7-O-Demethylation between Humans and Experimental Animals. Planta Medica 2018, 84, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Bak, S.G.; Lim, H.J.; Won, Y.S.; Lee, S.; Cheong, S.H.; Lee, S.J.; Bae, E.Y.; Lee, S.W.; Lee, S.J.; Rho, M.C. Regulatory Effects of Lycium barbarum Extract and Isolated Scopoletin on Atopic Dermatitis-Like Skin Inflammation. BioMed Res. Int. 2022, 2022, 2475699. [Google Scholar] [CrossRef] [PubMed]
- Homey, B.; Steinhoff, M.; Ruzicka, T.; Leung, D.Y. Cytokines and chemokines orchestrate atopic skin inflammation. J. Allergy Clin. Immunol. 2006, 118, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Harada, A.; Sekido, N.; Akahoshi, T.; Wada, T.; Mukaida, N.; Matsushima, K. Essential involvement of interleukin-8 (IL-8) in acute inflammation. J. Leukoc. Biol. 1994, 56, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Sabbatino, F.; Liguori, L.; Polcaro, G.; Salvato, I.; Caramori, G.; Salzano, F.A.; Casolaro, V.; Stellato, C.; Col, J.D.; Pepe, S. Role of Human Leukocyte Antigen System as A Predictive Biomarker for Checkpoint-Based Immunotherapy in Cancer Patients. Int. J. Mol. Sci. 2020, 21, 7295. [Google Scholar] [CrossRef] [PubMed]
- Yamaoka, K.; Saharinen, P.; Pesu, M.; Holt, V.E., 3rd; Silvennoinen, O.; O’Shea, J.J. The Janus kinases (Jaks). Genome Biol. 2004, 5, 253. [Google Scholar] [CrossRef] [PubMed]
- Jorgovanovic, D.; Song, M.; Wang, L.; Zhang, Y. Roles of IFN-γ in tumor progression and regression: A review. Biomark. Res. 2020, 8, 49. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Kim, E.; Lee, K.; Lee, D.C. Cinnamaldehyde derivatives inhibit degranulation and inflammatory mediator production in rat basophilic leukemia cells. Int. Immunopharmacol. 2016, 38, 342–348. [Google Scholar] [CrossRef]



| Cell Line | Gene | Direction | Sequence (5′→3′) |
|---|---|---|---|
| Human HaCaT | TARC | Forward | CAC GCA GCT CGA GGG ACC AAT GTG |
| Reverse | TCA AGA CCT CTC AAG GCT TTG CAG G | ||
| MDC | Forward | AGG ACA GAG CAT GGC TCG CCT ACA GA | |
| Reverse | TAA TGG CAG GGA GGT AGG GCT CCT GA | ||
| MCP-1 | Forward | TCT GTG CCT GCT GCT CAT AG | |
| Reverse | CAG ATC TCC TTG GCC ACA AT | ||
| IL-8 | Forward | ATG ACT TCC AAG CTG GCC GTG GCT | |
| Reverse | TTA TGA ATT CTC AGC CCT CTT CAA AAA | ||
| IL-1β | Forward | CTG TCG TGC GTG TTG AAA GA | |
| Reverse | TTC TGC TTG AGA GGT GCT GA | ||
| GAPDH | Forward | CGG AGT CAA CGG ATT TGG TCG | |
| Reverse | AGC CTT CTC CAT GGT GGT GAA G |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, D.-Y.; Park, J.-W.; Kim, S.-H.; Oh, S.-R.; Han, S.-B.; Kwon, O.-K.; Ahn, K.-S. Effect of Isoscopoletin on Cytokine Expression in HaCaT Keratinocytes and RBL-2H3 Basophils: Preliminary Study. Int. J. Mol. Sci. 2024, 25, 6908. https://doi.org/10.3390/ijms25136908
Seo D-Y, Park J-W, Kim S-H, Oh S-R, Han S-B, Kwon O-K, Ahn K-S. Effect of Isoscopoletin on Cytokine Expression in HaCaT Keratinocytes and RBL-2H3 Basophils: Preliminary Study. International Journal of Molecular Sciences. 2024; 25(13):6908. https://doi.org/10.3390/ijms25136908
Chicago/Turabian StyleSeo, Da-Yun, Ji-Won Park, Seung-Ho Kim, Sei-Ryang Oh, Sang-Bae Han, Ok-Kyoung Kwon, and Kyung-Seop Ahn. 2024. "Effect of Isoscopoletin on Cytokine Expression in HaCaT Keratinocytes and RBL-2H3 Basophils: Preliminary Study" International Journal of Molecular Sciences 25, no. 13: 6908. https://doi.org/10.3390/ijms25136908
APA StyleSeo, D.-Y., Park, J.-W., Kim, S.-H., Oh, S.-R., Han, S.-B., Kwon, O.-K., & Ahn, K.-S. (2024). Effect of Isoscopoletin on Cytokine Expression in HaCaT Keratinocytes and RBL-2H3 Basophils: Preliminary Study. International Journal of Molecular Sciences, 25(13), 6908. https://doi.org/10.3390/ijms25136908







