The Therapeutic Role of NPS-1034 in Pancreatic Ductal Adenocarcinoma as Monotherapy and in Combination with Chemotherapy
Abstract
:1. Introduction
2. Results
2.1. NPS-1034-Induced Cell Death Decreased the Viability and Clonogenicity of PDAC Cells
2.2. Apoptosis Is the Major Type of Cell Death in PDAC Cells Treated with NPS-1034
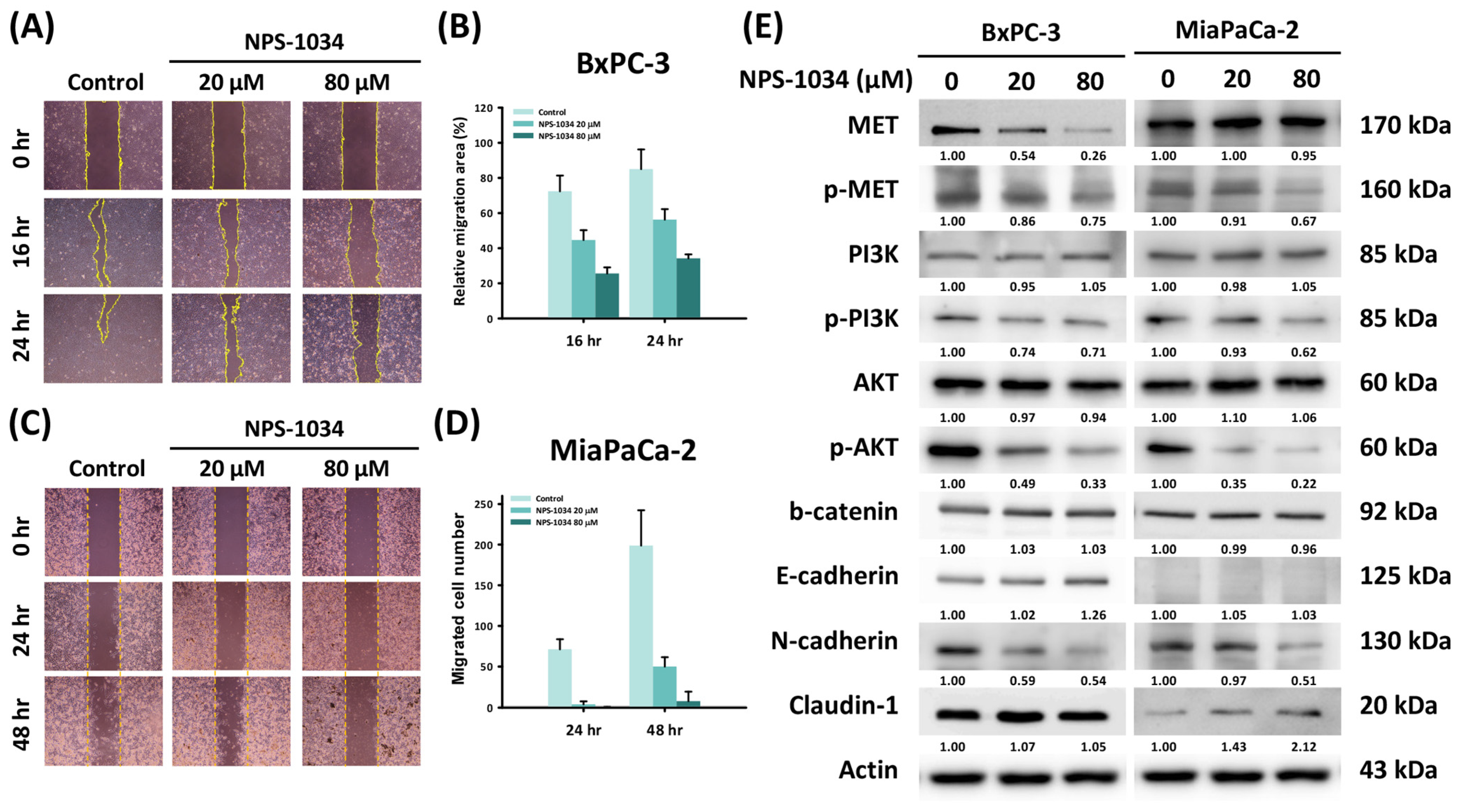
2.3. A 24-Hour NPS-1034 Treatment Suppressed the Migration of PDAC Cells via the Inhibition of MET-Induced EMT
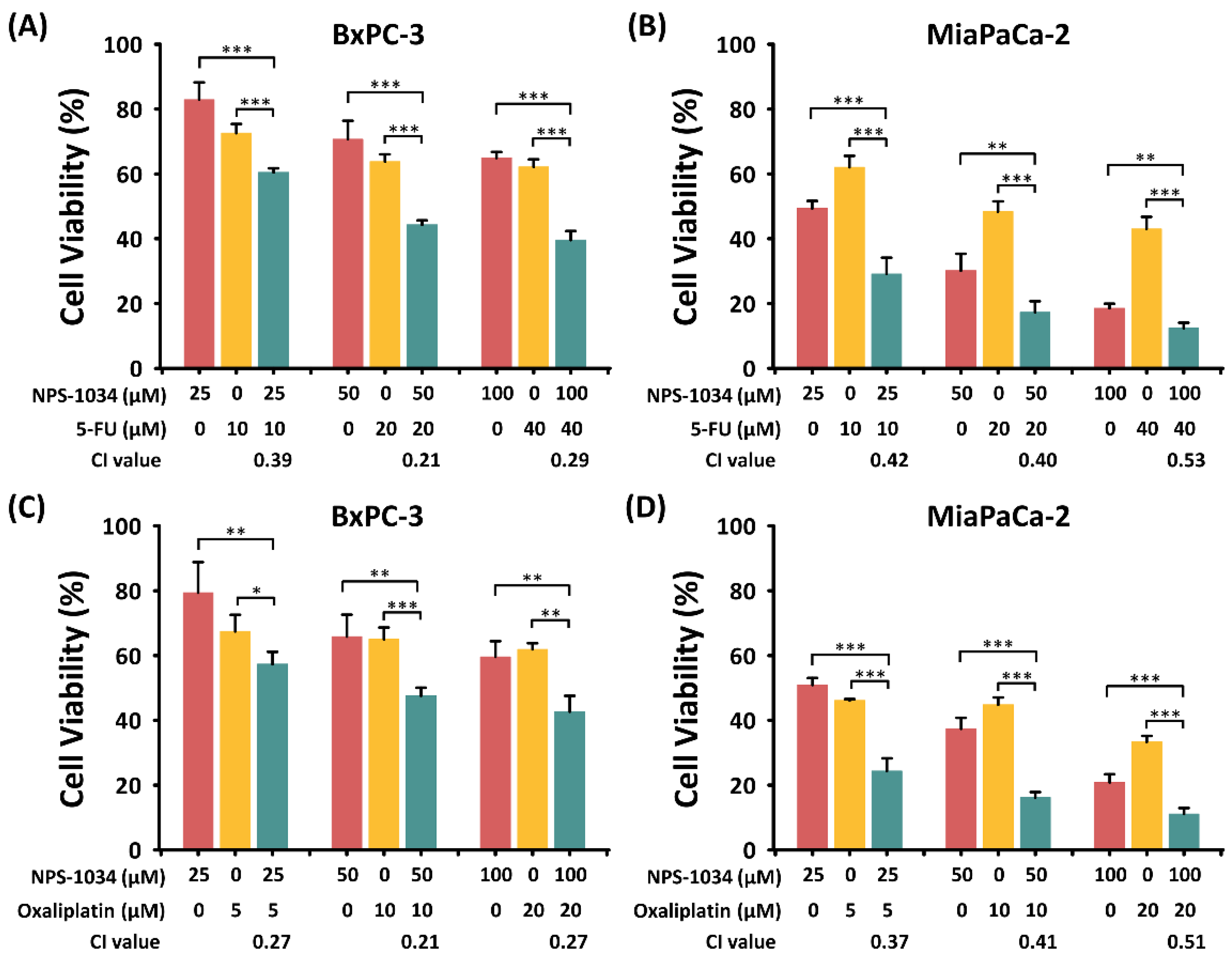
2.4. The Synergistic Effect of NPS-1034 and Common Anti-PDAC Drugs (Fluorouracil or Oxaliplatin) Was Found in PDAC Cells after 24 h of Combined Treatment
2.5. Apoptosis Was Triggered by the Synergistic Effect of Combined Treatment with NPS-1034 and Common Anti-PDAC Drugs (Fluorouracil or Oxaliplatin)
2.6. Next-Generation Sequencing Showed That NPS-1034 Can Modulate Immune Responses by Inducing Type I Interferon and Tumor Necrosis Factor Production in PDAC Cells
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. MTT Assays
4.3. Clonogenic Assay
4.4. Flow Cytometry Analysis
4.5. Hoechst Staining Assay
4.6. Western Blotting
4.7. Wound Healing Assays
4.8. Human Apoptosis Array for Proteome Profiling
4.9. Next-Generation Sequencing
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Vincent, A.; Herman, J.; Schulick, R.; Hruban, R.H.; Goggins, M. Pancreatic cancer. Lancet 2011, 378, 607–620. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Sanagapalli, S.; Stoita, A. Challenges in diagnosis of pancreatic cancer. World J. Gastroenterol. 2018, 24, 2047–2060. [Google Scholar] [CrossRef] [PubMed]
- Suker, M.; Beumer, B.R.; Sadot, E.; Marthey, L.; Faris, J.E.; Mellon, E.A.; El-Rayes, B.F.; Wang-Gillam, A.; Lacy, J.; Hosein, P.J.; et al. FOLFIRINOX for locally advanced pancreatic cancer: A systematic review and patient-level meta-analysis. Lancet Oncol. 2016, 17, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Conroy, T.; Desseigne, F.; Ychou, M.; Bouché, O.; Guimbaud, R.; Bécouarn, Y.; Adenis, A.; Raoul, J.-L.; Gourgou-Bourgade, S.; De La Fouchardière, C.; et al. FOLFIRINOX versus Gemcitabine for Metastatic Pancreatic Cancer. N. Engl. J. Med. 2011, 364, 1817–1825. [Google Scholar] [CrossRef] [PubMed]
- Von Hoff, D.D.; Ramanathan, R.K.; Borad, M.J.; Laheru, D.A.; Smith, L.S.; Wood, T.E.; Korn, R.L.; Desai, N.; Trieu, V.; Iglesias, J.L.; et al. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: A phase I/II trial. J. Clin. Oncol. 2011, 29, 4548–4554. [Google Scholar] [CrossRef] [PubMed]
- Ueno, H.; Ioka, T.; Ikeda, M.; Ohkawa, S.; Yanagimoto, H.; Boku, N.; Fukutomi, A.; Sugimori, K.; Baba, H.; Yamao, K.; et al. Randomized phase III study of gemcitabine plus S-1, S-1 alone, or gemcitabine alone in patients with locally advanced and metastatic pancreatic cancer in Japan and Taiwan: GEST study. J. Clin. Oncol. 2013, 31, 1640–1648. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Li, T.; Du, Y.; Li, M. Pancreatic cancer: Challenges and opportunities. BMC Med. 2018, 16, 214. [Google Scholar] [CrossRef] [PubMed]
- Tempero, M.A. NCCN Guidelines Updates: Pancreatic Cancer. J. Natl. Compr. Cancer Netw. 2019, 17, 603–605. [Google Scholar]
- Delitto, D.; Vertes-George, E.; Hughes, S.J.; Behrns, K.E.; Trevino, J.G. c-Met signaling in the development of tumorigenesis and chemoresistance: Potential applications in pancreatic cancer. World J. Gastroenterol. 2014, 20, 8458–8470. [Google Scholar] [CrossRef]
- Koorstra, J.B.; Karikari, C.A.; Feldmann, G.; Bisht, S.; Rojas, P.L.; Offerhaus, G.J.; Alvarez, H.; Maitra, A. The Axl receptor tyrosine kinase confers an adverse prognostic influence in pancreatic cancer and represents a new therapeutic target. Cancer Biol. Ther. 2009, 8, 618–626. [Google Scholar] [CrossRef]
- Porter, J. Small molecule c-Met kinase inhibitors: A review of recent patents. Expert Opin. Ther. Pat. 2010, 20, 159–177. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Brekken, R.A. Does Axl have potential as a therapeutic target in pancreatic cancer? Expert Opin. Ther. Targets 2018, 22, 955–966. [Google Scholar] [CrossRef]
- Pothula, S.P.; Xu, Z.; Goldstein, D.; Pirola, R.C.; Wilson, J.S.; Apte, M.V. Targeting HGF/c-MET Axis in Pancreatic Cancer. Int. J. Mol. Sci. 2020, 21, 9170. [Google Scholar] [CrossRef]
- Xu, J.; Liu, S.; Yang, X.; Cao, S.; Zhou, Y. Paracrine HGF promotes EMT and mediates the effects of PSC on chemoresistance by activating c-Met/PI3K/Akt signaling in pancreatic cancer in vitro. Life Sci. 2020, 263, 118523. [Google Scholar] [CrossRef]
- Song, X.; Wang, H.; Logsdon, C.D.; Rashid, A.; Fleming, J.B.; Abbruzzese, J.L.; Gomez, H.F.; Evans, D.B.; Wang, H. Overexpression of receptor tyrosine kinase Axl promotes tumor cell invasion and survival in pancreatic ductal adenocarcinoma. Cancer 2011, 117, 734–743. [Google Scholar] [CrossRef]
- Rho, J.K.; Choi, Y.J.; Kim, S.Y.; Kim, T.W.; Choi, E.K.; Yoon, S.J.; Park, B.M.; Park, E.; Bae, J.H.; Choi, C.M.; et al. MET and AXL inhibitor NPS-1034 exerts efficacy against lung cancer cells resistant to EGFR kinase inhibitors because of MET or AXL activation. Cancer Res. 2014, 74, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.S.; Hong, S.W.; Moon, J.H.; Kim, J.S.; Jung, K.A.; Kim, S.M.; Lee, D.H.; Kim, I.; Yoon, S.J.; Lee, C.G.; et al. NPS-1034, a novel MET inhibitor, inhibits the activated MET receptor and its constitutively active mutants. Investig. New Drugs 2014, 32, 389–399. [Google Scholar] [CrossRef]
- Taniguchi, H.; Yamada, T.; Wang, R.; Tanimura, K.; Adachi, Y.; Nishiyama, A.; Tanimoto, A.; Takeuchi, S.; Araujo, L.H.; Boroni, M.; et al. AXL confers intrinsic resistance to osimertinib and advances the emergence of tolerant cells. Nat. Commun. 2019, 10, 259. [Google Scholar] [CrossRef]
- Chen, J.T.; Wang, S.C.; Chen, B.S.; Chang, Y.C.; Yu, C.Y.; Sung, W.W.; Song, T.Y. NPS-1034 Induce Cell Death with Suppression of TNFR1/NF-kappaB Signaling in Testicular Cancer. Medicina 2022, 58, 355. [Google Scholar] [CrossRef] [PubMed]
- Kemik, O.; Purisa, S.; Kemik, A.S.; Tuzun, S. Increase in the circulating level of hepatocyte growth factor in pancreatic cancer patients. Bratisl. Lekárske Listy 2009, 110, 627–629. [Google Scholar]
- Zhu, G.H.; Huang, C.; Qiu, Z.J.; Liu, J.; Zhang, Z.H.; Zhao, N.; Feng, Z.Z.; Lv, X.H. Expression and prognostic significance of CD151, c-Met, and integrin alpha3/alpha6 in pancreatic ductal adenocarcinoma. Dig. Dis. Sci. 2011, 56, 1090–1098. [Google Scholar] [CrossRef] [PubMed]
- Pothula, S.P.; Xu, Z.; Goldstein, D.; Biankin, A.V.; Pirola, R.C.; Wilson, J.S.; Apte, M.V. Hepatocyte growth factor inhibition: A novel therapeutic approach in pancreatic cancer. Br. J. Cancer 2016, 114, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Rodríguez, L.; Abate-Daga, D.; Rojas, A.; González, J.R.; Fillat, C. E-cadherin contributes to the bystander effect of TK/GCV suicide therapy and enhances its antitumoral activity in pancreatic cancer models. Gene Ther. 2011, 18, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Listing, H.; Mardin, W.A.; Wohlfromm, S.; Mees, S.T.; Haier, J. MiR-23a/-24-induced gene silencing results in mesothelial cell integration of pancreatic cancer. Br. J. Cancer 2015, 112, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Park, W.; Chawla, A.; O’Reilly, E.M. Pancreatic Cancer: A Review. JAMA 2021, 326, 851–862. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-J.; Wu, J.-Y.; Wang, J.-M.; Xiang, D.-X. Emerging nanomedicine-based strategies for preventing metastasis of pancreatic cancer. J. Control. Release 2020, 320, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, O.; Akira, S. MDA5/RIG-I and virus recognition. Curr. Opin. Immunol. 2008, 20, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, T.S.; Christman, J.W. The role of nuclear factor-kappa B in cytokine gene regulation. Am. J. Respir. Cell Mol. Biol. 1997, 17, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Snell, L.M.; McGaha, T.L.; Brooks, D.G. Type I Interferon in Chronic Virus Infection and Cancer. Trends Immunol. 2017, 38, 542–557. [Google Scholar] [CrossRef]
- Jin, Z.; El-Deiry, W.S. Overview of cell death signaling pathways. Cancer Biol. Ther. 2005, 4, 147–171. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhang, Y.; Wang, Y.; Zhang, M.; Sun, W.; Dai, T.; Wang, A.; Wu, X.; Zhang, S.; Wang, S.; et al. A Dual Role of Type I Interferons in Antitumor Immunity. Adv. Biosyst. 2020, 4, e1900237. [Google Scholar] [CrossRef] [PubMed]
- Doherty, M.R.; Parvani, J.G.; Tamagno, I.; Junk, D.J.; Bryson, B.L.; Cheon, H.J.; Stark, G.R.; Jackson, M.W. The opposing effects of interferon-beta and oncostatin-M as regulators of cancer stem cell plasticity in triple-negative breast cancer. Breast Cancer Res. 2019, 21, 54. [Google Scholar] [CrossRef] [PubMed]
- Abele, R.; Tampé, R. The ABCs of Immunology: Structure and Function of TAP, the Transporter Associated with Antigen Processing. Physiology 2004, 19, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Lanier, L.L. Natural Killer Cells and Cancer. In Advances in Cancer Research; Academic Press: Cambridge, MA, USA, 2003; Volume 90, pp. 127–156. [Google Scholar]
- Petry, S. Mechanisms of Mitotic Spindle Assembly. Annu. Rev. Biochem. 2016, 85, 659–683. [Google Scholar] [CrossRef] [PubMed]
- Hammond, C.M.; Strømme, C.B.; Huang, H.; Patel, D.J.; Groth, A. Histone chaperone networks shaping chromatin function. Nat. Rev. Mol. Cell Biol. 2017, 18, 141–158. [Google Scholar] [CrossRef] [PubMed]
- Blaauboer, A.; Sideras, K.; van Eijck, C.H.J.; Hofland, L.J. Type I interferons in pancreatic cancer and development of new therapeutic approaches. Crit. Rev. Oncol. Hematol. 2021, 159, 103204. [Google Scholar] [CrossRef] [PubMed]
- Vitale, G.; van Eijck, C.H.; van Koetsveld Ing, P.M.; Erdmann, J.I.; Speel, E.J.; van der Wansem Ing, K.; Mooij, D.M.; Colao, A.; Lombardi, G.; Croze, E.; et al. Type I interferons in the treatment of pancreatic cancer: Mechanisms of action and role of related receptors. Ann. Surg. 2007, 246, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Tomimaru, Y.; Eguchi, H.; Wada, H.; Tomokuni, A.; Kobayashi, S.; Marubashi, S.; Takeda, Y.; Tanemura, M.; Umeshita, K.; Mori, M.; et al. Synergistic antitumor effect of interferon-ß with gemcitabine in interferon-α-non-responsive pancreatic cancer cells. Int. J. Oncol. 2011, 38, 1237–1243. [Google Scholar] [CrossRef]
- Bernhard, H.; Jäger-Arand, E.; Bernhard, G.; Heike, M.; Klein, O.; Riemann, J.F.; Meyer zum Büschenfelde, K.H.; Dippold, W.; Knuth, A. Treatment of advanced pancreatic cancer with 5-fluorouracil, folinic acid and interferon alpha-2A: Results of a phase II trial. Br. J. Cancer 1995, 71, 102–105. [Google Scholar] [CrossRef]
- Wagener, D.J.; Wils, J.A.; Kok, T.C.; Planting, A.; Couvreur, M.L.; Baron, B. Results of a randomised phase II study of cisplatin plus 5-fluorouracil versus cisplatin plus 5-fluorouracil with alpha-interferon in metastatic pancreatic cancer: An EORTC gastrointestinal tract cancer group trial. Eur. J. Cancer 2002, 38, 648–653. [Google Scholar] [CrossRef]
- Sung, W.W.; Wang, Y.C.; Lin, P.L.; Cheng, Y.W.; Chen, C.Y.; Wu, T.C.; Lee, H. IL-10 promotes tumor aggressiveness via upregulation of CIP2A transcription in lung adenocarcinoma. Clin. Cancer Res. 2013, 19, 4092–4103. [Google Scholar] [CrossRef]
- Sung, W.W.; Wang, Y.C.; Cheng, Y.W.; Lee, M.C.; Yeh, K.T.; Wang, L.; Wang, J.; Chen, C.Y.; Lee, H. A polymorphic -844T/C in FasL promoter predicts survival and relapse in non-small cell lung cancer. Clin. Cancer Res. 2011, 17, 5991–5999. [Google Scholar] [CrossRef]
- Chen, S.L.; Huang, Y.H.; Wei, T.Y.; Huang, K.M.; Ho, S.H.; Bih, L.I. Motor and bladder dysfunctions in patients with vertebral fractures at the thoracolumbar junction. Eur. Spine J. 2012, 21, 844–849. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luan, Y.-Z.; Wang, C.-C.; Yu, C.-Y.; Chang, Y.-C.; Sung, W.-W.; Tsai, M.-C. The Therapeutic Role of NPS-1034 in Pancreatic Ductal Adenocarcinoma as Monotherapy and in Combination with Chemotherapy. Int. J. Mol. Sci. 2024, 25, 6919. https://doi.org/10.3390/ijms25136919
Luan Y-Z, Wang C-C, Yu C-Y, Chang Y-C, Sung W-W, Tsai M-C. The Therapeutic Role of NPS-1034 in Pancreatic Ductal Adenocarcinoma as Monotherapy and in Combination with Chemotherapy. International Journal of Molecular Sciences. 2024; 25(13):6919. https://doi.org/10.3390/ijms25136919
Chicago/Turabian StyleLuan, Yu-Ze, Chi-Chih Wang, Chia-Ying Yu, Ya-Chuan Chang, Wen-Wei Sung, and Ming-Chang Tsai. 2024. "The Therapeutic Role of NPS-1034 in Pancreatic Ductal Adenocarcinoma as Monotherapy and in Combination with Chemotherapy" International Journal of Molecular Sciences 25, no. 13: 6919. https://doi.org/10.3390/ijms25136919




