The Effects and Mechanism of ATM Kinase Inhibitors in Toxoplasma gondii
Abstract
1. Introduction
2. Results
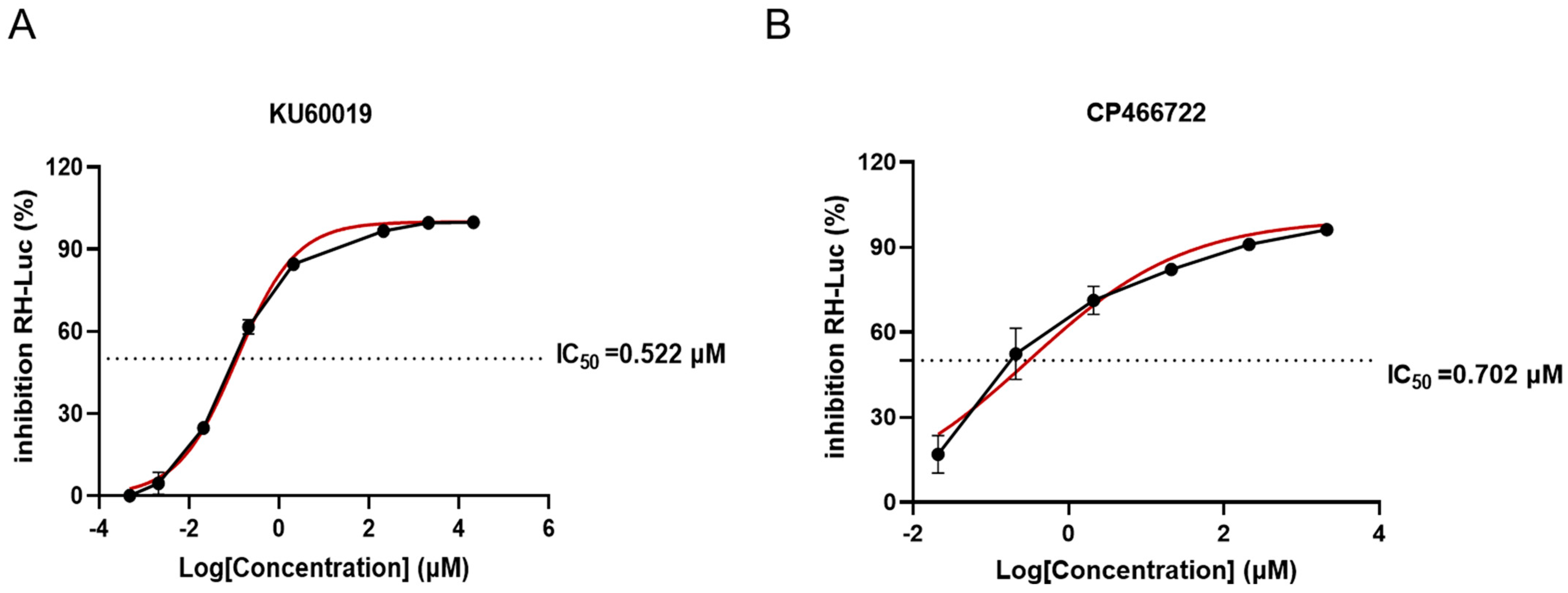
2.1. Evaluation of Anti-T. gondii Activity of ATM Kinase Inhibitors In Vitro
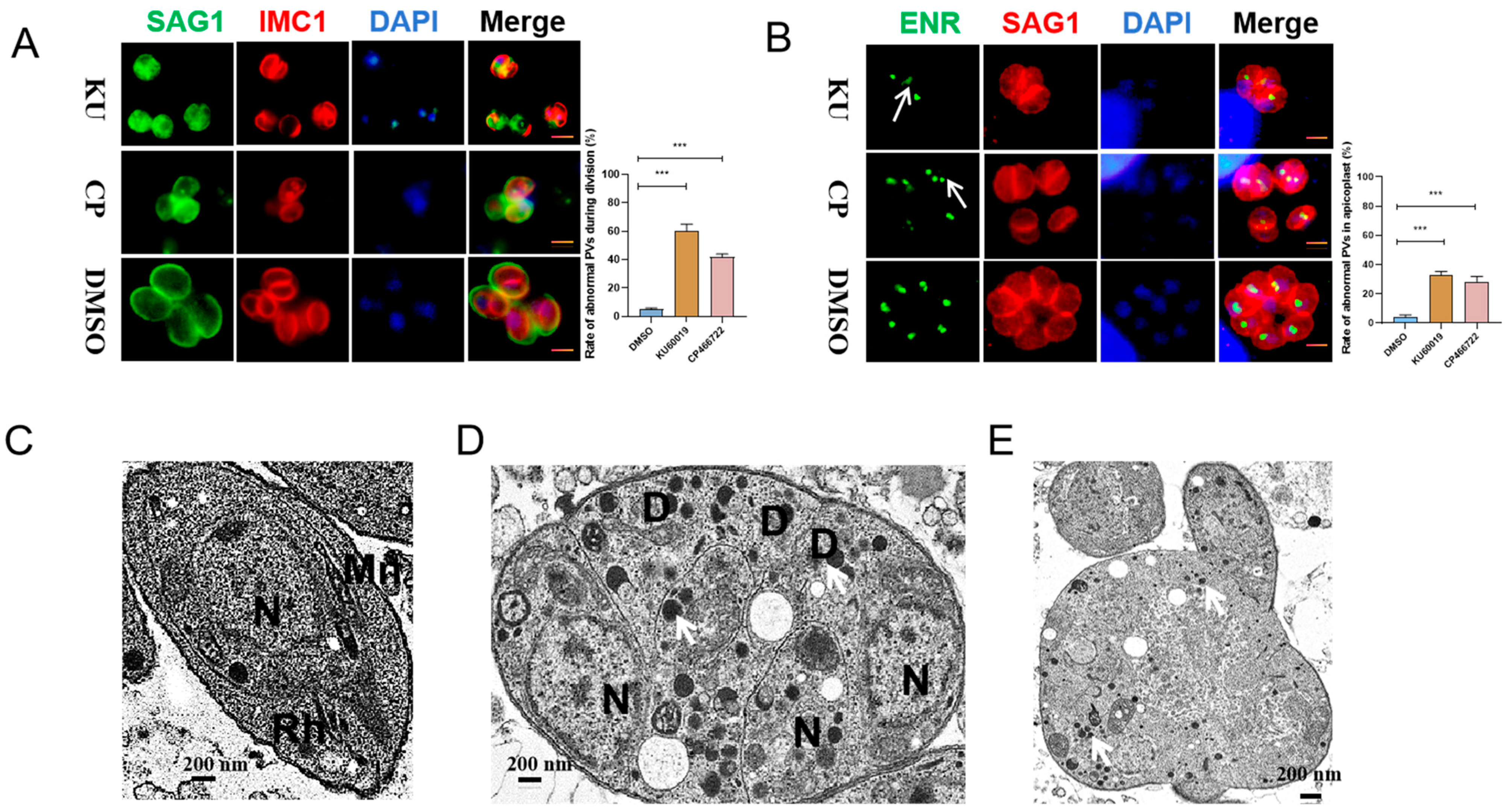
2.2. KU and CP Cause Abnormal Division of T. gondii Tachyzoites
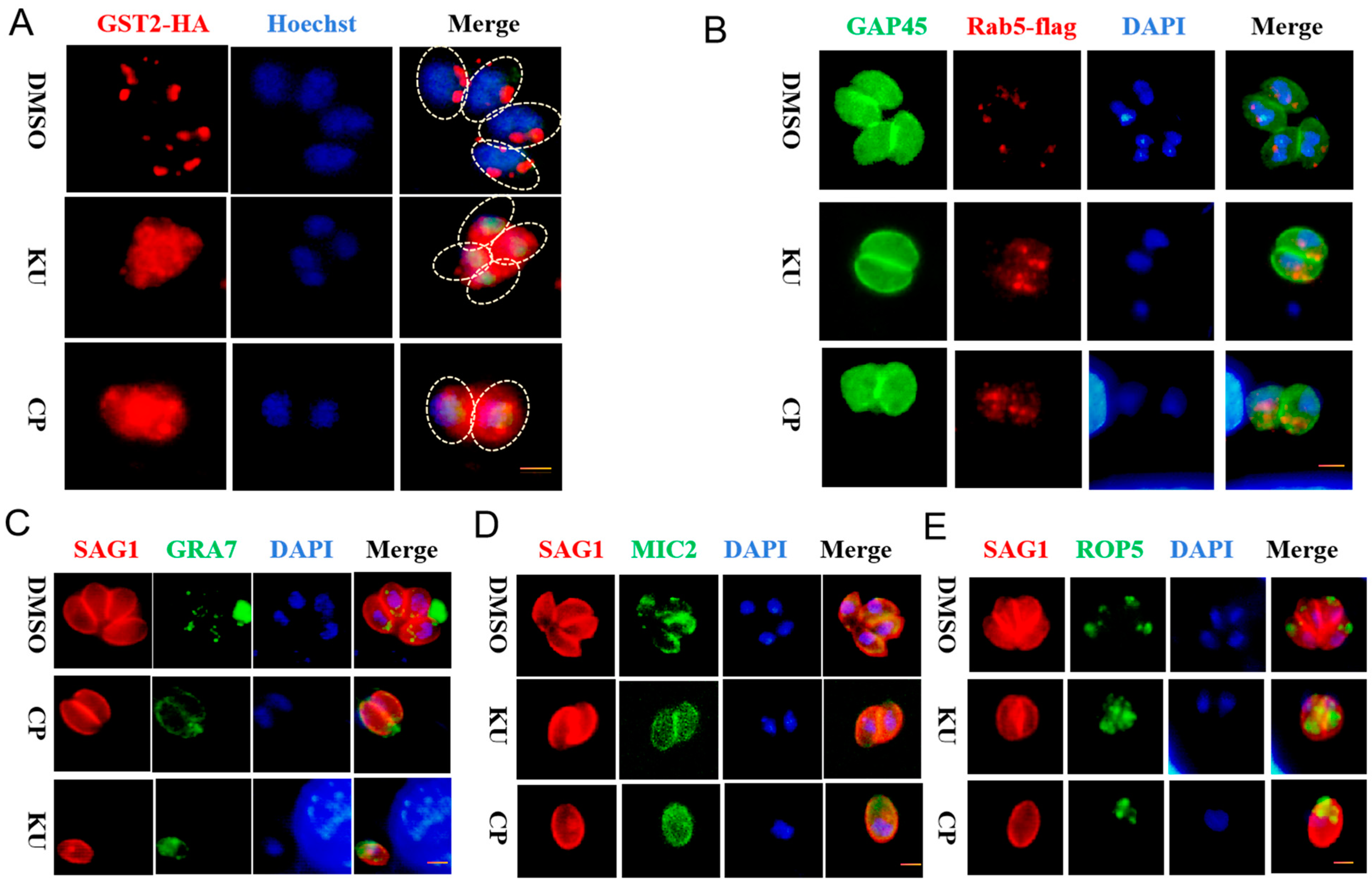
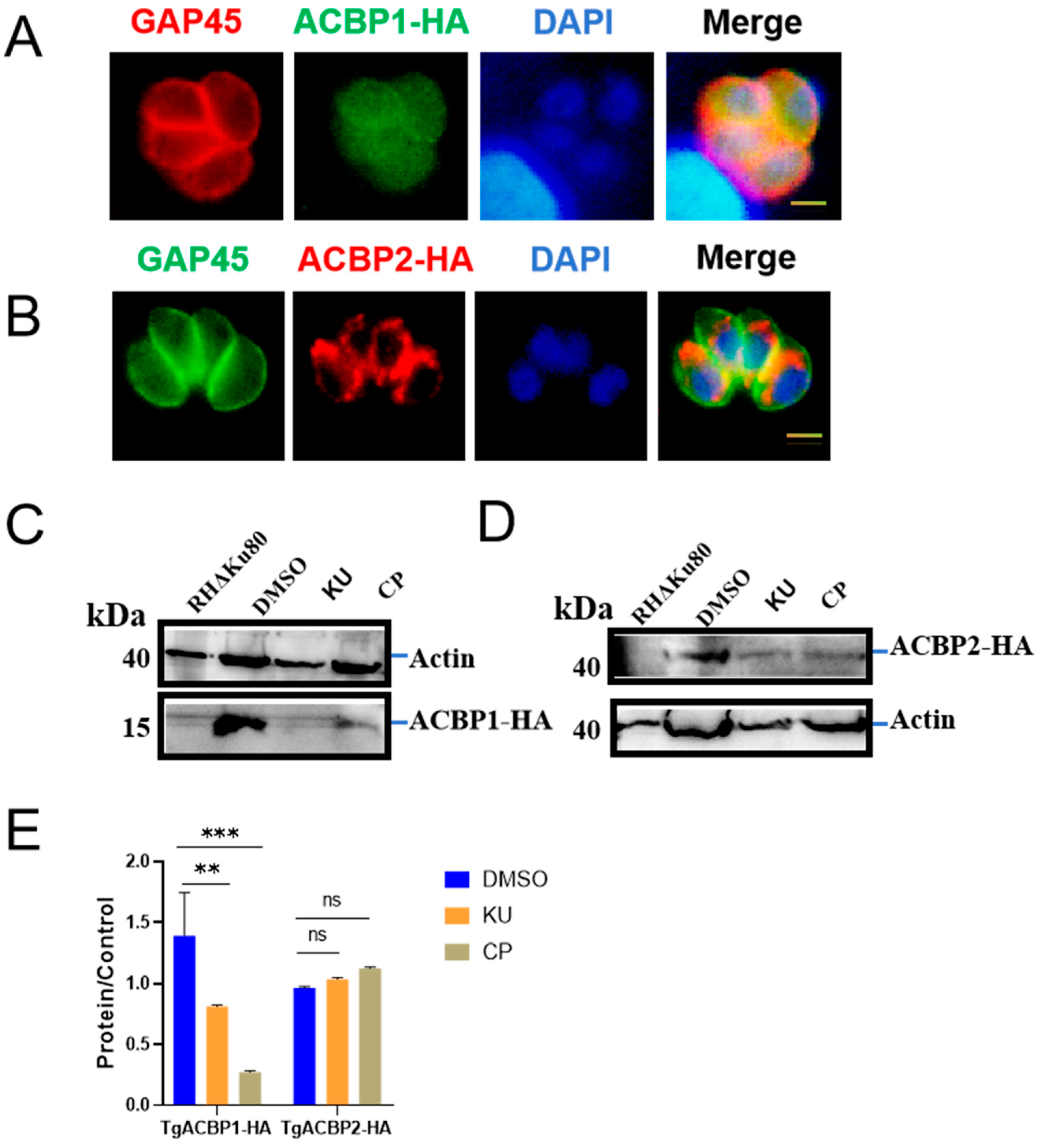
2.3. KU and CP Affect Protein Transport and Fatty Acid Metabolism in T. gondii
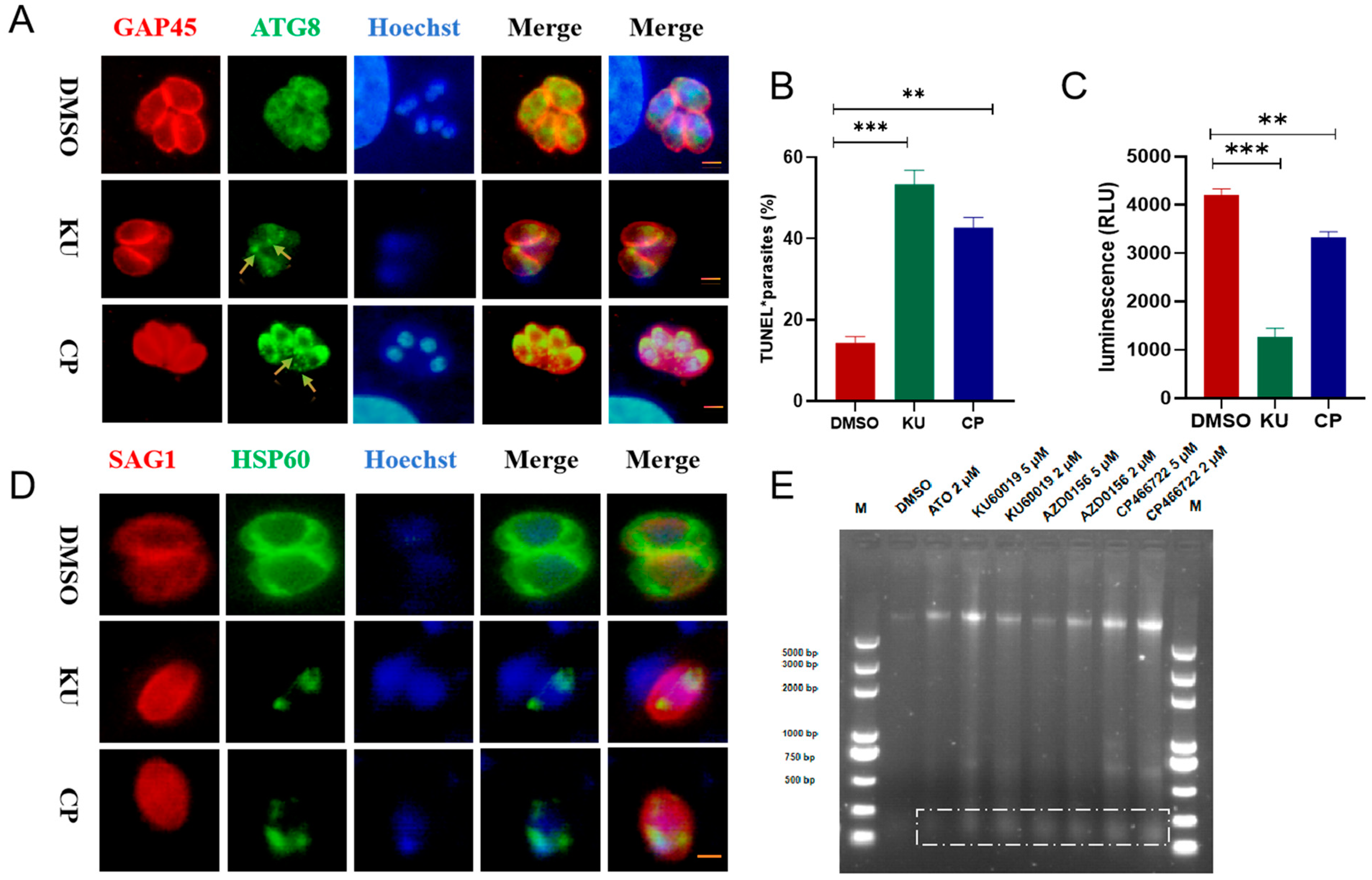
2.4. KU and CP Induced Autophagy and DNA Damage in T. gondii
2.5. The Effect of KU on the Transcription of T. gondii
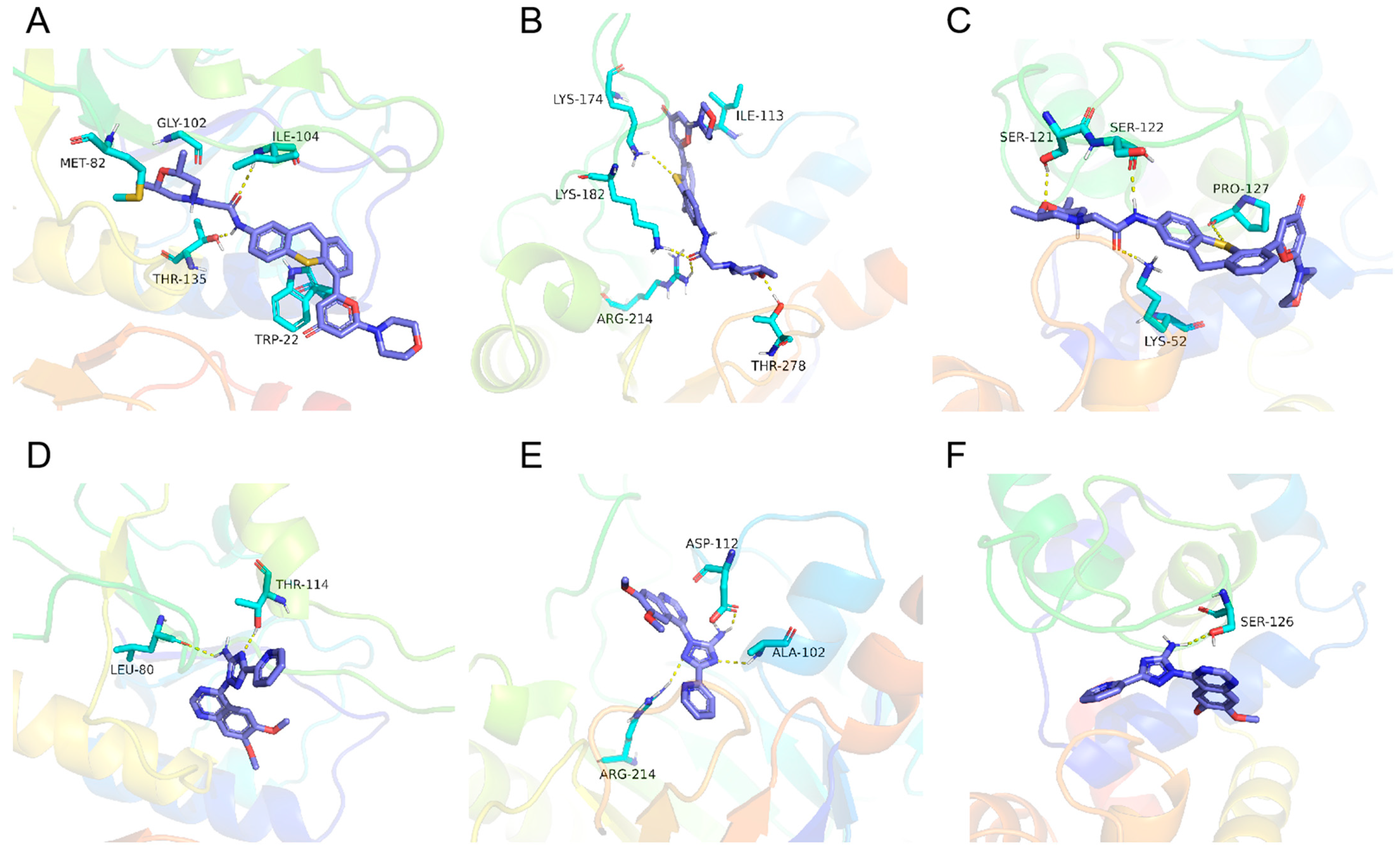
2.6. DARTS Combined with Proteomics to Determine the Binding Protein of KU in T. gondii
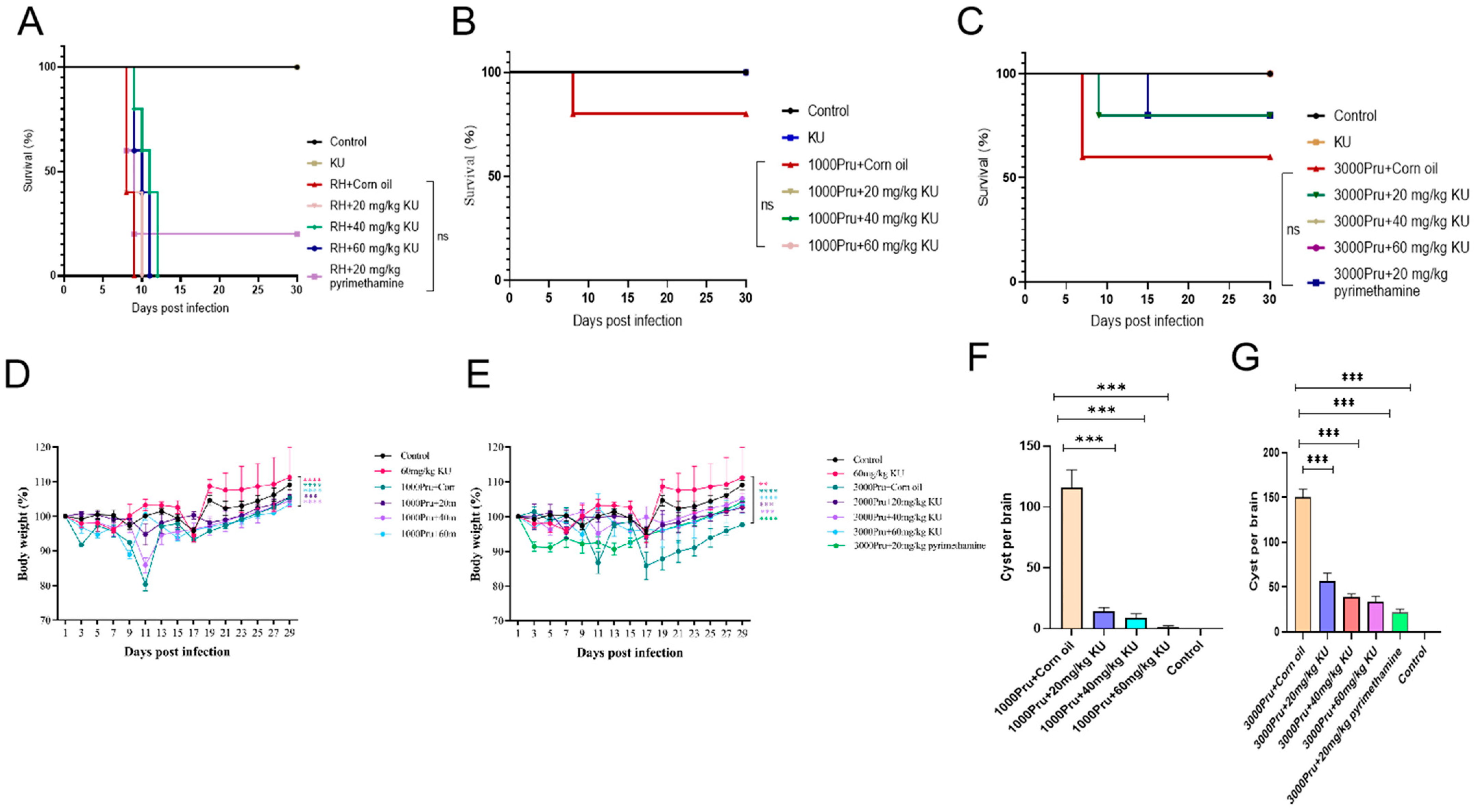
2.7. Evaluation Anti-T. gondii Activity of KU in Mice
3. Discussion
4. Materials and Methods
4.1. Host Cells and Parasites
4.2. Construction of Transgenic Strains
4.3. Drugs
4.4. Cytotoxicity Assay
4.5. Luciferase-Based Growth Inhibition Assays
4.6. Plaque Assays
4.7. Anti-Invasion Assessment of Inhibitors In Vitro
4.8. Anti-Proliferation Activity of Inhibitors
4.9. Immunofluorescence Assays and Western Blot
4.10. Transmission Electron Microscope
4.11. TUNEL Assay
4.12. ATP Content Detection
4.13. Transcriptomics Analysis
4.14. DARTS and 4D-Label-Free Quantitative Proteomics
4.15. Molecular Docking
4.16. Anti-T. gondii Activity of KU in Mice
4.17. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Montoya, J.G.; Liesenfeld, O. Toxoplasmosis. Lancet 2004, 363, 1965–1976. [Google Scholar] [CrossRef] [PubMed]
- Dunay, I.R.; Gajurel, K.; Dhakal, R.; Liesenfeld, O.; Montoya, J.G. Treatment of Toxoplasmosis: Historical Perspective, Animal Models, and Current Clinical Practice. Clin. Microbiol. Rev. 2018, 31, e00057-17. [Google Scholar] [CrossRef] [PubMed]
- Neville, A.J.; Zach, S.J.; Wang, X.; Larson, J.J.; Judge, A.K.; Davis, L.A.; Vennerstrom, J.L.; Davis, P.H. Clinically Available Medicines Demonstrating Anti-Toxoplasma Activity. Antimicrob. Agents Chemother. 2015, 59, 7161–7169. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, S.; Serricchio, M.; Striepen, B.; Bütikofer, P. Lipid synthesis in protozoan parasites: A comparison between kinetoplastids and apicomplexans. Prog. Lipid Res. 2013, 52, 488–512. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Cui, X.; Fan, S.; Liu, J.; Zhang, X.; Wu, Y.; Liu, Q. Comprehensive Characterization of Toxoplasma Acyl Coenzyme A-Binding Protein TgACBP2 and Its Critical Role in Parasite Cardiolipin Metabolism. mBio 2018, 9, e01597-18. [Google Scholar] [CrossRef] [PubMed]
- Sonda, S.; Hehl, A.B. Lipid biology of Apicomplexa: Perspectives for new drug targets, particularly for Toxoplasma gondii. Trends Parasitol. 2006, 22, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Cui, X.; Liu, J.; Zhang, X.; Zhang, H.; Yang, C.; Liu, Q. Synergistic roles of acyl-CoA binding protein (ACBP1) and sterol carrier protein 2 (SCP2) in Toxoplasma lipid metabolism. Cell. Microbiol. 2019, 21, e12970. [Google Scholar] [CrossRef]
- Golding, S.E.; Rosenberg, E.; Valerie, N.; Hussaini, I.; Frigerio, M.; Cockcroft, X.F.; Chong, W.Y.; Hummersone, M.; Rigoreau, L.; Menear, K.A.; et al. Improved ATM kinase inhibitor KU-60019 radiosensitizes glioma cells, compromises insulin, AKT and ERK prosurvival signaling, and inhibits migration and invasion. Mol. Cancer Ther. 2009, 8, 2894–2902. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.H.; Oh, D.Y. ATM in DNA repair in cancer. Pharmacol. Ther. 2019, 203, 107391. [Google Scholar] [CrossRef]
- O’Connor, M.J. Targeting the DNA Damage Response in Cancer. Mol. Cell. 2015, 60, 547–560. [Google Scholar] [CrossRef]
- Lee, J.H.; Paull, T.T. Cellular functions of the protein kinase ATM and their relevance to human disease. Nat. Rev. Mol. Cell. Biol. 2021, 22, 796–814. [Google Scholar] [CrossRef] [PubMed]
- Munera López, J.; Ganuza, A.; Bogado, S.S.; Muñoz, D.; Ruiz, D.M.; Sullivan, W.J., Jr.; Vanagas, L.; Angel, S.O. Evaluation of ATM Kinase Inhibitor KU-55933 as Potential Anti-Toxoplasma gondii Agent. Front. Cell. Infect. Microbiol. 2019, 9, 26. [Google Scholar] [CrossRef] [PubMed]
- Sidik, S.M.; Huet, D.; Ganesan, S.M.; Huynh, M.H.; Wang, T.; Nasamu, A.S.; Thiru, P.; Saeij, J.P.J.; Carruthers, V.B.; Niles, J.C.; et al. A Genome-wide CRISPR Screen in Toxoplasma Identifies Essential Apicomplexan Genes. Cell 2016, 166, 1423–1435.e12. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Li, C.; Cao, H.; Zhang, H.; Lu, C.; Li, R.; Zhu, Z.; Chen, L.; Zhao, Y. Identification of Adenylate Kinase 5 as a Protein Target of Ginsenosides in Brain Tissues Using Mass Spectrometry-Based Drug Affinity Responsive Target Stability (DARTS) and Cellular Thermal Shift Assay (CETSA) Techniques. J. Agric. Food Chem. 2022, 70, 2741–2751. [Google Scholar] [CrossRef] [PubMed]
- Lomenick, B.; Hao, R.; Jonai, N.; Chin, R.M.; Aghajan, M.; Warburton, S.; Wang, J.; Wu, R.P.; Gomez, F.; Loo, J.A.; et al. Target identification using drug affinity responsive target stability (DARTS). Proc. Natl. Acad. Sci. USA 2009, 106, 21984–21989. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Mann, T.; Striepen, B.; Beckers, C.J.; Roos, D.S.; Murray, J.M. Daughter cell assembly in the protozoan parasite Toxoplasma gondii. Mol. Biol. Cell 2002, 13, 593–606. [Google Scholar] [CrossRef] [PubMed]
- Zitko, J.; Doležal, M. Enoyl acyl carrier protein reductase inhibitors: An updated patent review (2011–2015). Expert Opin. Ther. Pat. 2016, 26, 1079–1094. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, J.; Zhang, H.; Sun, Z.; Ying, Z.; Wu, Y.; Xu, J.; Liu, Q. Toxoplasma gondii glutathione S-transferase 2 plays an important role in partial secretory protein transport. FASEB J. 2021, 35, e21352. [Google Scholar] [CrossRef] [PubMed]
- Xi, L.; Peng, M.; Liu, S.; Liu, Y.; Wan, X.; Hou, Y.; Qin, Y.; Yang, L.; Chen, S.; Zeng, H.; et al. Hypoxia-stimulated ATM activation regulates autophagy-associated exosome release from cancer-associated fibroblasts to promote cancer cell invasion. J. Extracell Vesicles 2021, 10, e12146. [Google Scholar] [CrossRef]
- Toursel, C.; Dzierszinski, F.; Bernigaud, A.; Mortuaire, M.; Tomavo, S. Molecular cloning, organellar targeting and developmental expression of mitochondrial chaperone HSP60 in Toxoplasma gondii. Mol. Biochem. Parasitol. 2000, 111, 319–332. [Google Scholar] [CrossRef]
- Sikdar, S.; Mukherjee, A.; Ghosh, S.; Khuda-Bukhsh, A.R. Condurango glycoside-rich components stimulate DNA damage-induced cell cycle arrest and ROS-mediated caspase-3 dependent apoptosis through inhibition of cell-proliferation in lung cancer, in vitro and in vivo. Environ. Toxicol. Pharmacol. 2014, 37, 300–314. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.S.; Li, H.L.; Piao, X.H.; Yang, Z.Y.; Wang, S.M.; Ge, Y.W. Drug affinity responsive target stability (DARTS) accelerated small molecules target discovery: Principles and application. Biochem. Pharmacol. 2021, 194, 114798. [Google Scholar] [CrossRef]
- Lomenick, B.; Olsen, R.W.; Huang, J. Identification of direct protein targets of small molecules. ACS Chem. Biol. 2011, 6, 34–46. [Google Scholar] [CrossRef]
- Bailey, M.A.; Tang, Y.; Park, H.J.; Fitzgerald, M.C. Comparative Analysis of Protein Folding Stability-Based Profiling Methods for Characterization of Biological Phenotypes. J. Am. Soc. Mass Spectrom. 2023, 34, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Feng, F.; Zhang, W.; Chai, Y.; Guo, D.; Chen, X. Label-free target protein characterization for small molecule drugs: Recent advances in methods and applications. J. Pharm. Biomed. Anal. 2023, 223, 115107. [Google Scholar] [CrossRef]
- Matsuoka, S.; Ballif, B.A.; Smogorzewska, A.; McDonald, E.R., 3rd; Hurov, K.E.; Luo, J.; Bakalarski, C.E.; Zhao, Z.; Solimini, N.; Lerenthal, Y.; et al. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science 2007, 316, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Jiang, Y.Y.; Chen, H.; Wu, Y.C.; Zhang, L. Tanshinone I attenuates the malignant biological properties of ovarian cancer by inducing apoptosis and autophagy via the inactivation of PI3K/AKT/mTOR pathway. Cell Prolif. 2020, 53, e12739. [Google Scholar] [CrossRef]
- Janetka, J.W.; Hopper, A.T.; Yang, Z.; Barks, J.; Dhason, M.S.; Wang, Q.; Sibley, L.D. Optimizing Pyrazolopyrimidine Inhibitors of Calcium Dependent Protein Kinase 1 for Treatment of Acute and Chronic Toxoplasmosis. J. Med. Chem. 2020, 63, 6144–6163. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, P.; Liu, Y.; Li, J.; Yang, D.; Liu, Z.; Jiang, L. Anti-Toxoplasma gondii Effects of Lipopeptide Derivatives of Lycosin-I. Toxins 2023, 15, 477. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, X.; Fu, Y.; Liu, J.; Xue, Y.; Liu, Q. Progesterone Can Directly Inhibit the Life Activities of Toxoplasma gondii In Vitro through the Progesterone Receptor Membrane Component (PGRMC). Int. J. Mol. Sci. 2022, 23, 3843. [Google Scholar] [CrossRef]
- Huet, D.; Rajendran, E.; van Dooren, G.G.; Lourido, S. Identification of cryptic subunits from an apicomplexan ATP synthase. Elife 2018, 7, e38097. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yang, X.; Liu, A.; Li, Y.; Niu, Z.; Lyu, C.; Liang, X.; Xia, N.; Cui, J.; Li, M.; et al. The beta subunit of AMP-activated protein kinase is critical for cell cycle progression and parasite development in Toxoplasma gondii. Cell. Mol. Life Sci. 2022, 79, 532. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, Y.; Ying, Z.; Wang, F.; Yin, M.; Pei, Y.; Liu, J.; Liu, Q. The Effects and Mechanism of ATM Kinase Inhibitors in Toxoplasma gondii. Int. J. Mol. Sci. 2024, 25, 6947. https://doi.org/10.3390/ijms25136947
Xue Y, Ying Z, Wang F, Yin M, Pei Y, Liu J, Liu Q. The Effects and Mechanism of ATM Kinase Inhibitors in Toxoplasma gondii. International Journal of Molecular Sciences. 2024; 25(13):6947. https://doi.org/10.3390/ijms25136947
Chicago/Turabian StyleXue, Yangfei, Zhu Ying, Fei Wang, Meng Yin, Yanqun Pei, Jing Liu, and Qun Liu. 2024. "The Effects and Mechanism of ATM Kinase Inhibitors in Toxoplasma gondii" International Journal of Molecular Sciences 25, no. 13: 6947. https://doi.org/10.3390/ijms25136947
APA StyleXue, Y., Ying, Z., Wang, F., Yin, M., Pei, Y., Liu, J., & Liu, Q. (2024). The Effects and Mechanism of ATM Kinase Inhibitors in Toxoplasma gondii. International Journal of Molecular Sciences, 25(13), 6947. https://doi.org/10.3390/ijms25136947





