Environmentally Relevant Levels of Antiepileptic Carbamazepine Altered Intestinal Microbial Composition and Metabolites in Amphibian Larvae
Abstract
:1. Introduction
2. Results
2.1. Tadpole Body Size and Developmental Stage
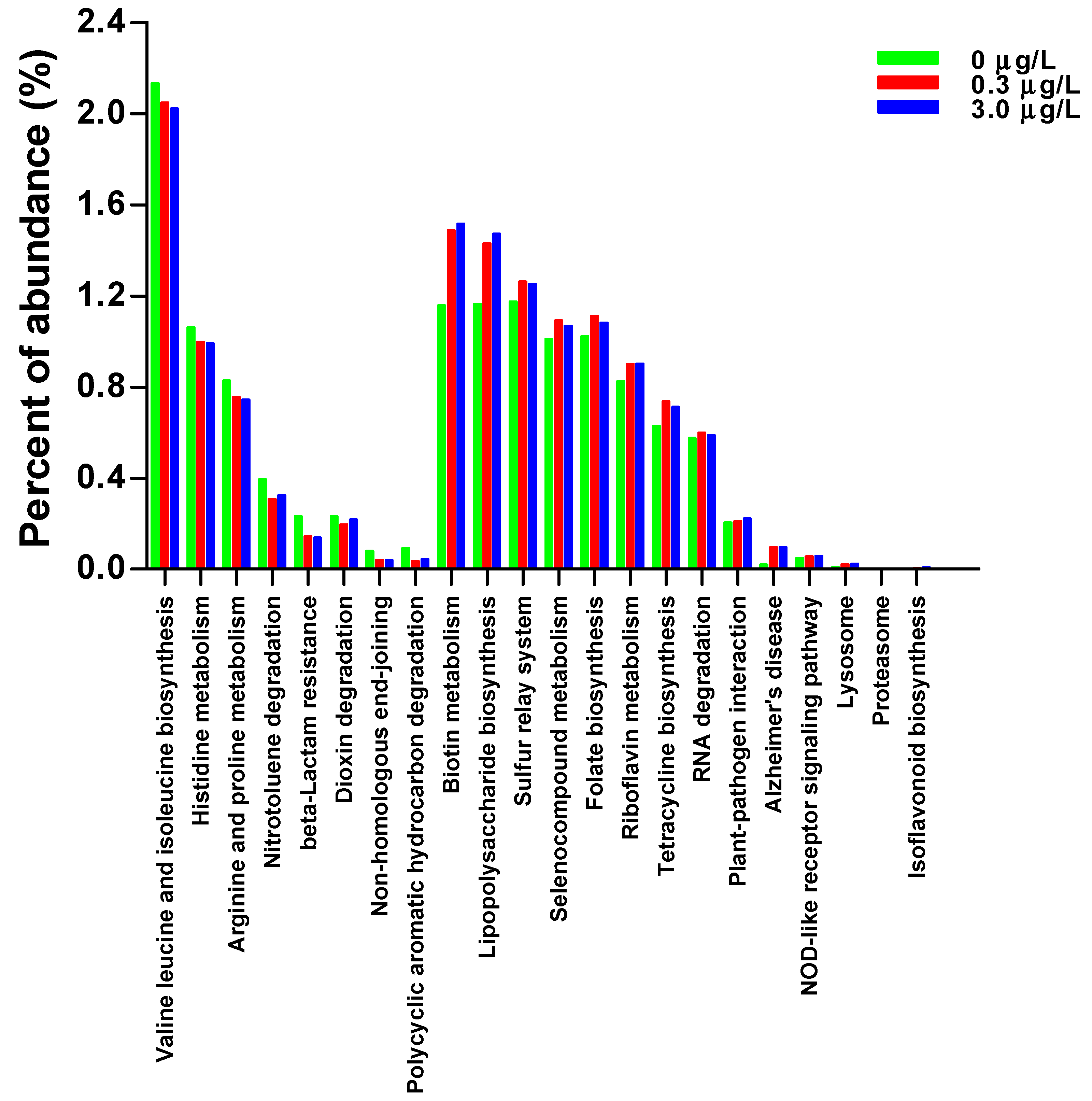
2.2. Tadpole Intestinal Microbiota
2.3. Tadpole Intestinal Metabolite
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fent, K.; Weston, A.A.; Caminada, D. Ecotoxicology of human pharmaceuticals. Aquat. Toxicol. 2006, 76, 122–159. [Google Scholar] [CrossRef]
- Srain, H.S.; Beazley, K.F.; Walker, T.R. Pharmaceuticals and personal care products and their sublethal and lethal effects in aquatic organisms. Environ. Rev. 2021, 29, 142–181. [Google Scholar] [CrossRef]
- Caracciolo, B.; Topp, E.; Grenni, P. Pharmaceuticals in the environment: Biodegradation and effects on natural microbial communities: A review. J. Pharm. Biomed. Anal. 2014, 106, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Naghdi, M.; Taheran, M.; Pulicharla, R.; Rouissi, T.; Brar, S.K.; Verma, M.; Surampalli, R.Y. Pine-wood derived nanobiochar for removal of carbamazepine from aqueous media: Adsorption behavior and influential parameters. Arab. J. Chem. 2019, 12, 5292–5301. [Google Scholar] [CrossRef]
- Liu, J.L.; Wong, M.H. Pharmaceuticals and personal care products (PPCPs): A review on environmental contamination in China. Environ. Int. 2013, 59, 208–224. [Google Scholar] [CrossRef] [PubMed]
- Nkoom, M.; Lu, G.H.; Liu, J.C.; Yang, H.H.; Dong, H.K. Bioconcentration of the antiepileptic drug carbamazepine and its physiological and biochemical effects on Daphnia magna. Ecotoxicol. Environ. Saf. 2019, 172, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Wu, H.H.; Li, L.; Ren, M.; Qie, H.T.; Lin, A.J. A review of distribution and risk of pharmaceuticals and personal care products in the aquatic environment in China. Ecotoxicol. Environ. Saf. 2021, 213, 112044. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.T.; Guo, X.P.; Xu, T.; Yin, D.Q. Effects of carbamazepine on gut microbiota, ARGs and intestinal health in zebrafish. Ecotoxicol. Environ. Saf. 2023, 249, 114473. [Google Scholar] [CrossRef]
- Chen, H.H.; Zha, J.M.; Liang, X.F.; Li, J.S.; Wang, Z.J. Effects of the human antiepileptic drug carbamazepine on the behavior, biomarkers, and heat shock proteins in the Asian clam Corbicula fluminea. Aquat. Toxicol. 2014, 155, 1–8. [Google Scholar] [CrossRef]
- De Lange, H.J.; Noordoven, W.; Murk, A.J.; Lurling, M.; Peeters, E.T.H.M. Behavioural responses of Gammarus pulex (Crustacea, Amphipoda) to low concentrations of pharmaceuticals. Aquat. Toxicol. 2006, 78, 209–216. [Google Scholar] [CrossRef]
- Nassef, M.; Matsumoto, S.; Seki, M.; Khalil, F.; Kang, I.J.; Shimasaki, Y.; Oshima, Y.; Honjo, T. Acute effects of triclosan, diclofenac and carbamazepine on feeding performance of Japanese medaka fish (Oryzias latipes). Chemosphere 2010, 80, 1095–1100. [Google Scholar] [CrossRef] [PubMed]
- Ofoegbu, P.U.; Lourenço, J.; Mendo, S.; Soares, A.M.V.M.; Pestana, J.L.T. Effects of low concentrations of psychiatric drugs (carbamazepine and fluoxetine) on the freshwater planarian, Schmidtea mediterranea. Chemosphere 2019, 217, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Freitas, R.; Almeida, A.; Pires, A.; Velez, C.; Calisto, V.; Schneider, R.J.; Esteves, V.I.; Wrona, F.J.; Figueira, E.; Soares, A.M.V.M. The effects of carbamazepine on macroinvertebrate species: Comparing bivalves and polychaetes biochemical responses. Water Res. 2015, 85, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, P.; Almeida, A.; Calisto, V.; Esteves, V.I.; Schneider, R.J.; Wrona, F.J.; Soares, A.M.V.M.; Figueira, E.; Freitas, R. Physiological and biochemical alterations induced in the mussel Mytilus galloprovincialis after short and long-term exposure to carbamazepine. Water Res. 2017, 117, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Melvin, S.D.; Cameron, M.C.; Lanctôt, C.M. Individual and mixture toxicity of pharmaceuticals naproxen, carbamazepine, and sulfamethoxazole to Australian striped marsh frog tadpoles (Limnodynastes peronii). J. Toxicol. Environ. Health A 2014, 77, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Oetken, M.; Nentwig, G.; Löffler, D.; Ternes, T.; Oehlmann, J. Effects of pharmaceuticals on aquatic invertebrates. part I. the antiepileptic drug carbamazepine. Arch. Environ. Contam. Toxicol. 2005, 49, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Vernouillet, G.; Eullaffroy, P.; Lajeunesse, A.; Blaise, C.; Gagné, F.; Juneau, P. Toxic effects and bioaccumulation of carbamazepine evaluated by biomarkers measured in organisms of different trophic levels. Chemosphere 2010, 80, 1062–1068. [Google Scholar] [CrossRef]
- Xin, J.J.; Yan, S.H.; Hong, X.S.; Zhang, H.; Zha, J.M. Environmentally relevant concentrations of carbamazepine induced lipid metabolism disorder of Chinese rare minnow (Gobiocypris rarus) in a gender-specific pattern. Chemosphere 2021, 265, 129080. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.H.; Chen, R.; Wang, M.; Zha, J.M. Carbamazepine at environmentally relevant concentrations caused DNA damage and apoptosis in the liver of Chinese rare minnows (Gobiocypris rarus) by the Ras/Raf/ERK/p53 signaling pathway. Environ. Pollut. 2021, 270, 116245. [Google Scholar] [CrossRef]
- Yan, S.H.; Wang, M.; Zha, J.M.; Zhu, L.F.; Li, W.; Luo, Q.; Sun, J.; Wang, Z.J. Environmentally relevant concentrations of carbamazepine caused endocrine-disrupting effects on nontarget organisms, Chinese rare minnows (Gobiocypris rarus). Environ. Sci. Technol. 2018, 52, 886–894. [Google Scholar] [CrossRef]
- Dumas, T.; Courant, F.; Almunia, C.; Boccard, J.; Rosain, D.; Duporté, G.; Armengaud, J.; Fenet, H.; Gomez, E. An integrated metabolomics and proteogenomics approach reveals molecular alterations following carbamazepine exposure in the male mussel Mytilus galloprovincialis. Chemosphere 2022, 286, 131793. [Google Scholar] [CrossRef]
- Kovacevic, V.; Simpson, A.J.; Simpson, M.J. 1H NMR-based metabolomics of Daphnia magna responses after sub-lethal exposure to triclosan, carbamazepine and ibuprofen. Comp. Biochem. Physiol. D 2016, 19, 199–210. [Google Scholar] [CrossRef]
- O’Rourke, K.; Engelmann, B.; Altenburger, R.; Rolle-Kampczyk, U.; Grintzalis, K. Molecular responses of Daphnids to chronic exposures to pharmaceuticals. Int. J. Mol. Sci. 2023, 24, 4100. [Google Scholar] [CrossRef]
- Orton, F.; Roberts-Rhodes, B.; Whatley, C.; Tyler, C.R. A review of non-destructive biomonitoring techniques to assess the impacts of pollution on reproductive health in frogs and toads. Ecotoxicol. Environ. Saf. 2023, 262, 115163. [Google Scholar] [CrossRef]
- Almeida, Â.; Freitas, R.; Calisto, V.; Esteves, V.I.; Schneider, R.J.; Soares, A.M.V.M.; Figueira, E. Chronic toxicity of the antiepileptic carbamazepine on the clam Ruditapes philippinarum. Comp. Biochem. Physiol. C 2015, 172–173, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Bars, C.; Hoyberghs, J.; Valenzuela, A.; Buyssens, L.; Ayuso, M.; Van Ginneken, C.; Labro, A.J.; Foubert, K.; Van Cruchten, S.J. Developmental toxicity and biotransformation of two anti-epileptics in zebrafish embryos and early larvae. Int. J. Mol. Sci. 2021, 22, 12696. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.H.; Zlabek, V.; Velisek, J.; Grabic, R.; Machova, J.; Kolarova, J.; Li, P.; Randak, T. Acute toxicity of carbamazepine to juvenile rainbow trout (Oncorhynchus mykiss): Effects on antioxidant responses, hematological parameters and hepatic EROD. Ecotoxicol. Environ. Saf. 2011, 74, 319–327. [Google Scholar] [CrossRef]
- Bókony, V.; Verebélyi, V.; Ujhegyi, N.; Mikó, Z.; Nemesházi, E.; Szederkényi, M.; Orf, S.; Vitányi, E.; Móricz, Á.M. Effects of two little-studied environmental pollutants on early development in anurans. Environ. Pollut. 2020, 260, 114078. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.M.; Cole, S.E. A toxicity and hazard assessment of fourteen pharmaceuticals to Xenopus laevis larvae. Ecotoxicology 2006, 15, 647–656. [Google Scholar] [CrossRef]
- Fu, G.L.; Meng, Q.Y.; Chen, Y.; Xin, J.Z.; Liu, J.H.; Dang, W.; Lu, H.L. Metformin exposure altered intestinal microbiota composition and metabolites in amphibian larvae. Ecotoxicol. Environm. Saf. 2023, 267, 115617. [Google Scholar] [CrossRef]
- Qi, X.Z.; Zhang, Y.; Zhang, Y.L.; Luo, F.; Song, K.G.; Wang, G.X.; Ling, F. Vitamin B12 produced by Cetobacterium somerae improves host resistance against pathogen infection through strengthening the interactions within gut microbiota. Microbiome 2023, 11, 135. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.F.; Qiao, M.; Zhu, D.; Zhu, Y.G. Antibiotic resistance in the collembolan gut microbiome accelerated by the nonantibiotic drug carbamazepine. Environ. Sci. Technol. 2020, 54, 10754–10762. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Ahmed, I.; Fatma, S.; Peres, H. Role of branched-chain amino acids on growth, physiology and metabolism of different fish species: A review. Aquacult. Nutr. 2021, 27, 1270–1289. [Google Scholar] [CrossRef]
- Zarei, I.; Koistinen, V.M.; Kokla, M.; Klåvus, A.; Babu, A.F.; Lehtonen, M.; Auriola, S.; Hanhineva, K. Tissue-wide metabolomics reveals wide impact of gut microbiota on mice metabolite composition. Sci. Rep. 2022, 12, 15018. [Google Scholar] [CrossRef]
- Engelking, L.R. Leaks in the tricarboxylic acid (TCA) cycle. In Textbook of Veterinary Physiological Chemistry, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 214–218. [Google Scholar]
- Lu, H.L.; Kang, C.Q.; Meng, Q.Y.; Hu, J.R.; Melvin, S.D. Functional and hepatic metabolite changes in aquatic turtle hatchlings exposed to the anti-androgenic fungicide vinclozolin. Ecotoxicol. Environ. Saf. 2022, 231, 113220. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.H.; Wang, M.; Liang, X.F.; Martyniuk, C.J.; Zha, J.M.; Wang, Z.J. Environmentally relevant concentrations of carbamazepine induce liver histopathological changes and a gender-specific response in hepatic proteome of Chinese rare minnows (Gobiocypris rarus). Environ. Pollut. 2018, 243, 480–491. [Google Scholar] [CrossRef] [PubMed]
- Rabinovitz, S.; Mostofsky, D.I.; Yehuda, S. Anticonvulsant efficiency, behavioral performance and cortisol levels: A comparison of carbamazepine (CBZ) and a fatty acid compound (SR-3). Psychoneuroendocrinology 2004, 29, 113–124. [Google Scholar] [CrossRef]
- Calcagno, E.; Durando, P.; Valdés, M.E.; Franchioni, L.; de los Ángeles Bistoni, M. Effects of carbamazepine on cortisol levels and behavioral responses to stress in the fish Jenynsia multidentate. Physiol. Behav. 2016, 158, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.K.; Li, M.Y.; Yu, L.L.; Tian, F.W.; Zhao, J.X.; Zhai, Q.X. Effects of taurine on gut microbiota homeostasis: An evaluation based on two models of gut dysbiosis. Biomedicines 2023, 11, 1048. [Google Scholar] [CrossRef]
- Tennoune, N.; Andriamihaja, M.; Blachier, F. Production of indole and indole-related compounds by the intestinal microbiota and consequences for the host: The good, the bad, and the ugly. Microorganisms 2022, 10, 930. [Google Scholar] [CrossRef]
- Lv, D.M.; Cao, X.L.; Zhong, L.; Dong, Y.X.; Xu, Z.Y.; Rong, Y.C.; Xu, H.L.; Wang, Z.Y.; Yang, H.; Yin, R.; et al. Targeting phenylpyruvate restrains excessive NLRP3 inflammasome activation and pathological inflammation in diabetic wound healing. Cell Rep. Med. 2023, 4, 101129. [Google Scholar] [CrossRef] [PubMed]
- Qiang, L.Y.; Cheng, J.P.; Yi, J.; Rotchell, J.M.; Zhu, X.T.; Zhou, J.L. Environmental concentration of carbamazepine accelerates fish embryonic development and disturbs larvae behavior. Ecotoxicology 2016, 25, 1426–1437. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.H.; Meng, Q.Y.; Chen, Y.; Yang, J.M.; Gao, J.F.; Lu, H.L. Exposure to low levels of antidiabetic glibenclamide had no evident adverse effects on intestinal microbial composition and metabolic profiles in amphibian larvae. Environ. Sci. Pollut. Res. 2023, 30, 121196–121206. [Google Scholar] [CrossRef] [PubMed]

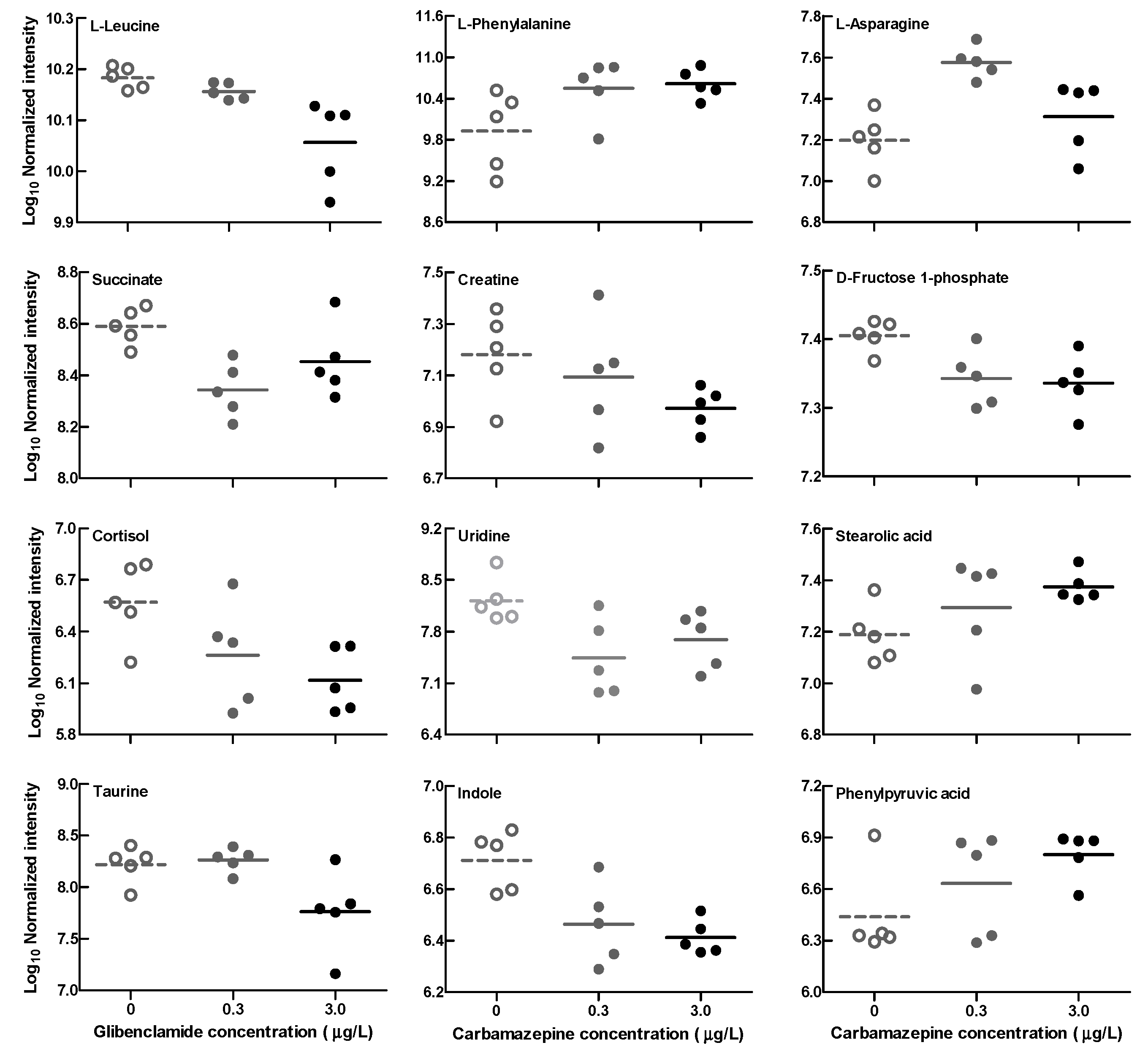

| 0.3 μg/L vs. CTRL | 3.0 μg/L vs. CTRL | |||
|---|---|---|---|---|
| Metabolite | Log2(FC) | p | Log2(FC) | p |
| L-Leucine | −0.09 | −0.40 | ** | |
| L-Phenylalanine | 1.67 | * | 1.62 | * |
| L-Asparagine | 1.22 | ** | 0.42 | |
| Succinate | −0.80 | ** | −0.40 | |
| Creatine | −0.22 | −0.75 | * | |
| D-Fructose 1-phosphate | −0.20 | * | −0.23 | * |
| Cortisol | −0.89 | −1.55 | * | |
| Uridine | −2.11 | * | −1.72 | * |
| Stearolic acid | 0.42 | 0.59 | * | |
| Taurine | 0.09 | −1.18 | * | |
| Indole | −0.79 | * | −1.02 | ** |
| Phenylpyruvic acid | 0.61 | 0.98 | * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dang, W.; Zhang, J.-H.; Cao, Z.-C.; Yang, J.-M.; Lu, H.-L. Environmentally Relevant Levels of Antiepileptic Carbamazepine Altered Intestinal Microbial Composition and Metabolites in Amphibian Larvae. Int. J. Mol. Sci. 2024, 25, 6950. https://doi.org/10.3390/ijms25136950
Dang W, Zhang J-H, Cao Z-C, Yang J-M, Lu H-L. Environmentally Relevant Levels of Antiepileptic Carbamazepine Altered Intestinal Microbial Composition and Metabolites in Amphibian Larvae. International Journal of Molecular Sciences. 2024; 25(13):6950. https://doi.org/10.3390/ijms25136950
Chicago/Turabian StyleDang, Wei, Jin-Hui Zhang, Zi-Chun Cao, Jia-Meng Yang, and Hong-Liang Lu. 2024. "Environmentally Relevant Levels of Antiepileptic Carbamazepine Altered Intestinal Microbial Composition and Metabolites in Amphibian Larvae" International Journal of Molecular Sciences 25, no. 13: 6950. https://doi.org/10.3390/ijms25136950





