The Total Denervation of the Ischemic Kidney Induces Differential Responses in Sodium Transporters’ Expression in the Contralateral Kidney in Goldblatt Rats
Abstract
1. Introduction
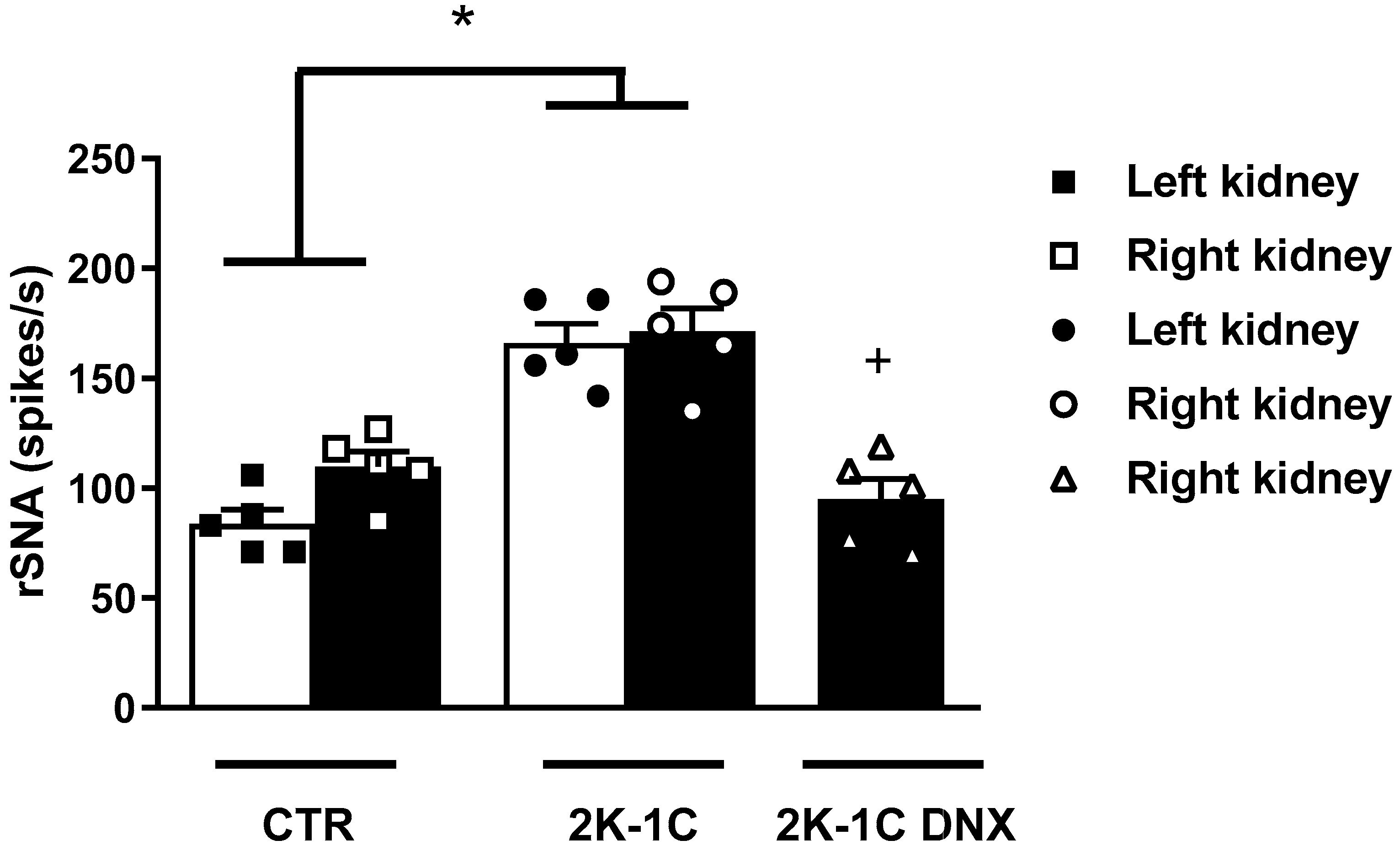
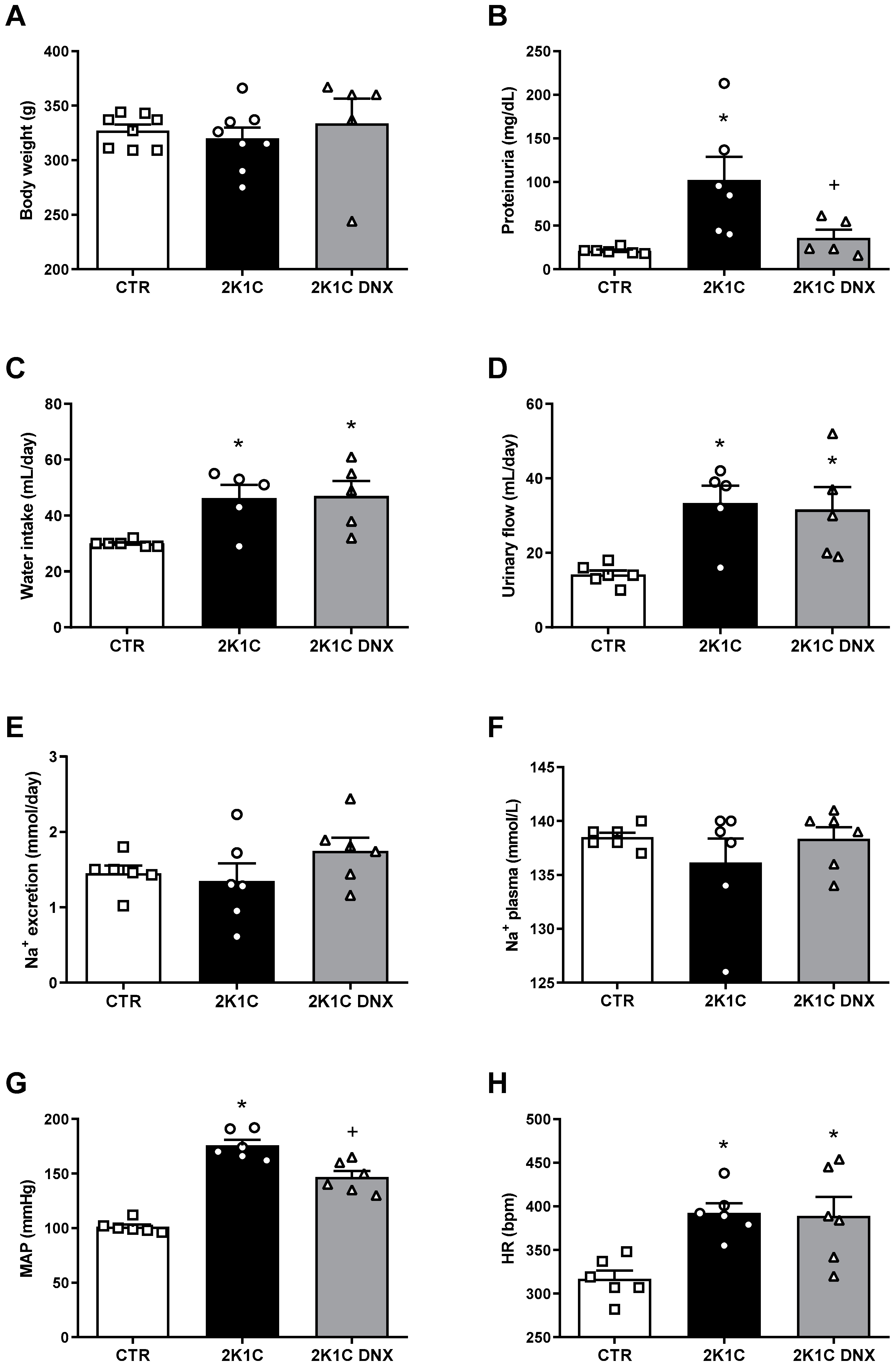
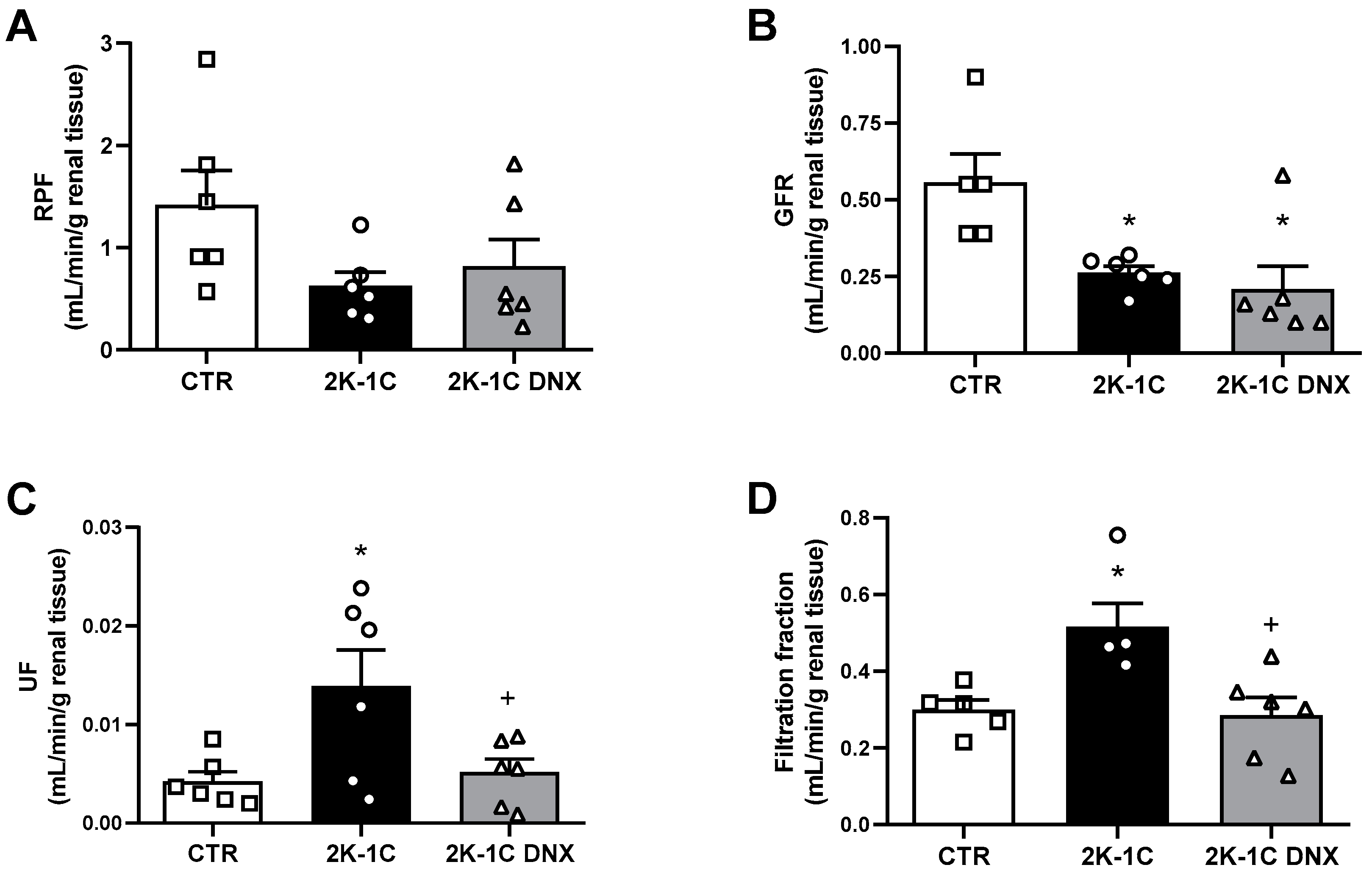
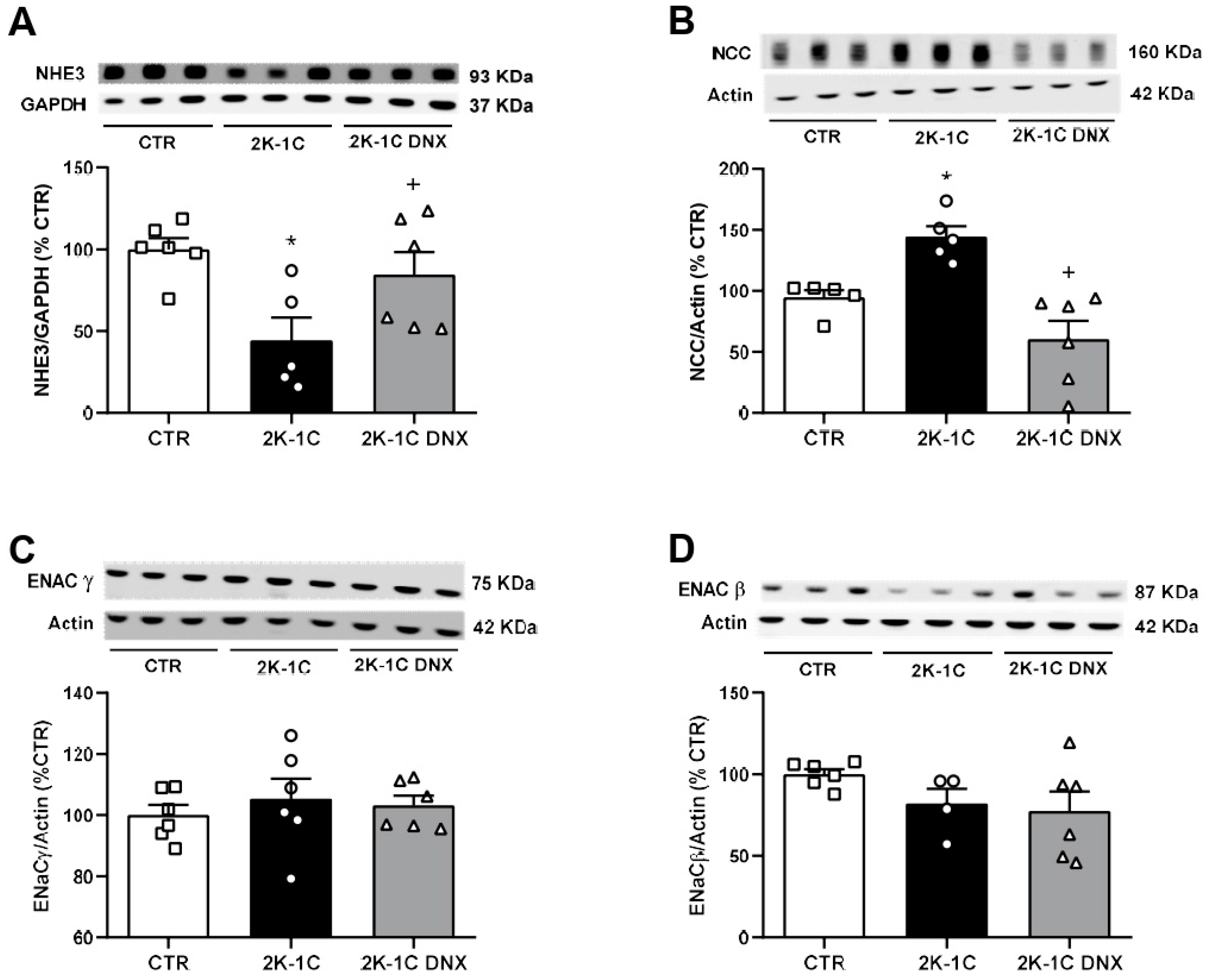
2. Results
2.1. Proteinuria Decreases after DNX in 2K-1C Rats
2.2. GFR and RPF Were Not Affected in Unclipped Kidney by DNX
2.3. Effects of Unilateral Renal Denervation on NHE3, NCC, ENaCβ, and ENaCγ Protein Expressions in Unclipped Kidney
3. Discussion
The Limitations of the Study
4. Material and Methods
4.1. Animals
4.2. Glomerular Filtration Rate (GFR) and Renal Plasma Flow (RPF)
4.3. Blood Pressure (BP) and Renal Sympathetic Nerve Activity (rSNA) Measurements
4.4. Western Blotting
4.5. Statistical Analysis
5. Conclusions
Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Carlström, M.; Braga, V.A. Mechanisms underlying the effects of renal denervation in renovascular hypertension. Hypertens. Res. 2019, 42, 754–757. [Google Scholar] [CrossRef]
- Goldblatt, H.; Lynch, J.; Hanzal, R.F.; Summerville, W.W. Studies on experimental hypertension: I. the production of persistent elevation of systolic blood pressure by means of renal ischemia. J. Exp. Med. 1934, 59, 347–379. [Google Scholar] [CrossRef]
- Nishi, E.E.; Lopes, N.R.; Gomes, G.N.; Perry, J.C.; Sato, A.Y.S.; Naffah-Mazzacoratti, M.G.; Bergamaschi, C.T.; Campos, R.R. Renal denervation reduces sympathetic overactivation, brain oxidative stress, and renal injury in rats with renovascular hypertension independent of its effects on reducing blood pressure. Hypertens. Res. 2019, 42, 628–640. [Google Scholar] [CrossRef]
- Katholi, R.E.; Whitlow, P.L.; Winternitz, S.R.; Oparil, S. Importance of the renal nerves in established two-kidney, one clip Goldblatt hypertension. Hypertension 1982, 4, 166–174. [Google Scholar]
- Nishi, E.E.; Bergamaschi, C.T.; Campos, R.R. The crosstalk between the kidney and the central nervous system: The role of renal nerves in blood pressure regulation. Exp. Physiol. 2015, 100, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.; Elam, M.; Rundqvist, B.; Eisenhofer, G.; Herlitz, H.; Lambert, G.; Friberg, P. Increased sympathetic nerve activity in renovascular hypertension. Circulation 1999, 99, 2537–2542. [Google Scholar] [CrossRef]
- Ruilope, L.; Garcia-Robles, R.; Sancho-Rof, J.; Paya, C.; Rodicio, J.L.; Strong, C.G.; Knox, F.G.; Romero, J.C. Effect of furosemide on renal function in the stenotic and contralateral kidneys of patients with renovascular hypertension. Hypertension 1983, 5, V43–V47. [Google Scholar] [CrossRef]
- Navar, L.G.; Zou, L.; Von Thun, A.; Tarng Wang, C.; Imig, J.D.; Mitchell, K.D. Unraveling the Mystery of Goldblatt Hypertension. News Physiol. Sci. 1998, 13, 170–176. [Google Scholar] [CrossRef]
- Himmelstein, S.I.; Klotman, P.E. The role of thromboxane in two-kidney, one-clip Goldblatt hypertension in rats. Am. J. Physiol. 1989, 257, F190–F196. [Google Scholar] [CrossRef]
- Lee, J.; Oh, Y.; Kim, S.W. Altered renal expression of aquaporin-2 water channels in rats with experimental two-kidney, one clip hypertension. J. Korean Med. Sci. 2001, 16, 462–466. [Google Scholar] [CrossRef]
- Ma, S.K.; Bae, E.H.; Kim, I.J.; Choi, C.; Lee, J.; Kim, S.W. Altered renal expression of aquaporin water channels and sodium transporters in rats with two-kidney, one-clip hypertension. Kidney Blood Press Res. 2009, 32, 411–420. [Google Scholar] [CrossRef]
- DiBona, G.F.; Kopp, U.C. Neural control of renal function. Physiol. Rev. 1997, 77, 75–197. [Google Scholar] [CrossRef]
- Esler, M.D.; Krum, H.; Schlaich, M.; Schmieder, R.E.; Böhm, M.; Sobotka, P.A.; Symplicity HTN-2 Investigators. Renal sympathetic denervation for treatment of drug-resistant hypertension: One-year results from the Symplicity HTN-2 randomized, controlled trial. Circulation 2012, 126, 2976–2982. [Google Scholar] [CrossRef]
- Pontes, R.B.; Crajoinas, R.O.; Nishi, E.E.; Oliveira-Sales, E.B.; Girardi, A.C.; Campos, R.R.; Bergamaschi, C.T. Renal nerve stimulation leads to the activation of the Na+/H+ exchanger isoform 3 via angiotensin II type I receptor. Am. J. Physiol. Renal. Physiol. 2015, 308, F848–F856. [Google Scholar] [CrossRef]
- Böhm, M.; Linz, D.; Urban, D.; Mahfoud, F.; Ukena, C. Renal sympathetic denervation: Applications in hypertension and beyond. Nat. Rev. Cardiol. 2013, 10, 465–476. [Google Scholar] [CrossRef]
- Nakagawa, T.; Hasegawa, Y.; Uekawa, K.; Ma, M.; Katayama, T.; Sueta, D.; Toyama, K.; Kataoka, K.; Koibuchi, N.; Maeda, M.; et al. Renal denervation prevents stroke and brain injury via attenuation of oxidative stress in hypertensive rats. J. Am. Heart Assoc. 2013, 2, e000375. [Google Scholar] [CrossRef]
- Lopes, N.R.; Milanez, M.I.O.; Martins, B.S.; Veiga, A.C.; Ferreira, G.R.; Gomes, G.N.; Girardi, A.C.; Carvalho, P.M.; Nogueira, F.N.; Campos, R.R.; et al. Afferent innervation of the ischemic kidney contributes to renal dysfunction in renovascular hypertensive rats. Pflugers Arch. 2020, 472, 325–334. [Google Scholar] [CrossRef]
- Stocker, S.D.; Sullivan, J.B. Deletion of the Transient Receptor Potential Vanilloid 1 Channel Attenuates Sympathoexcitation and Hypertension and Improves Glomerular Filtration Rate in 2-Kidney-1-Clip Rats. Hypertension 2023, 80, 1671–1682. [Google Scholar] [CrossRef]
- Lauar, M.R.; Evans, L.C.; Van Helden, D.; Fink, G.D.; Banek, C.T.; Menani, J.V.; Osborn, J.W. Renal and hypothalamic inflammation in renovascular hypertension: Role of afferent renal nerves. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2023, 325, R411–R422. [Google Scholar] [CrossRef]
- Lincevicius, G.S.; Shimoura, C.G.; Nishi, E.E.; Oliveira, T.; Cespedes, J.G.; Bergamaschi, C.T.; Campos, R.R. Differential effects of renal denervation on arterial baroreceptor function in Goldblatt hypertension model. Auton. Neurosci. 2017, 208, 43–50. [Google Scholar] [CrossRef]
- McAllen, R.M.; Dampney, R.A. Vasomotor neurons in the rostral ventrolateral medulla are organized topographically with respect to type of vascular bed but not body region. Neurosci. Lett. 1990, 110, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Miyajima, E.; Yamada, Y.; Yoshida, Y.; Matsukawa, T.; Shionoiri, H.; Tochikubo, O.; Ishii, M. Muscle sympathetic nerve activity in renovascular hypertension and primary aldosteronism. Hypertension 1991, 17, 1057–1062. [Google Scholar] [CrossRef] [PubMed]
- Tsyrlin, V.A.; Galagudza, M.M.; Kuzmenko, N.V.; Pliss, M.G.; Rubanova, N.S.; Shcherbin, Y.I. Arterial baroreceptor reflex counteracts long-term blood pressure increase in the rat model of renovascular hypertension. PLoS ONE 2013, 8, e64788. [Google Scholar] [CrossRef] [PubMed]
- Ong, J.; Kinsman, B.J.; Sved, A.F.; Rush, B.M.; Tan, R.J.; Carattino, M.D.; Stocker, S.D. Renal sensory nerves increase sympathetic nerve activity and blood pressure in 2-kidney 1-clip hypertensive mice. J. Neurophysiol. 2019, 122, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Rossi, N.F.; Pajewski, R.; Chen, H.; Littrup, P.J.; Maliszewska-Scislo, M. Hemodynamic and neural responses to renal denervation of the nerve to the clipped kidney by cryoablation in two-kidney, one-clip hypertensive rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 310, R197–R208. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Eriguchi, M.; Tsuruya, K.; Haruyama, N.; Yamada, S.; Tanaka, S.; Suehiro, T.; Noguchi, H.; Masutani, K.; Torisu, K.; Kitazono, T. Renal denervation has blood pressure-independent protective effects on kidney and heart in a rat model of chronic kidney disease. Kidney Int. 2015, 87, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Chade, A.R.; Zhu, X.; Mushin, O.P.; Napoli, C.; Lerman, A.; Lerman, L.O. Simvastatin promotes angiogenesis and prevents microvascular remodeling in chronic renal ischemia. FASEB J. 2006, 20, 1706–1708. [Google Scholar] [CrossRef]
- Al-Qattan, K.K.; Safer, A.M.; Al-Hajri, D.K. Distention of the lateral intercellular spaces (LIS) in the proximal tubule cells of the non-stenosed kidney of the 2K-1C Goldblatt model of hypertension as evidence of pressure diuresis. Anat. Histol. Embryol. 1998, 27, 197–204. [Google Scholar] [CrossRef]
- Granger, J.P.; Alexander, B.T.; Llinas, M. Mechanisms of pressure natriuresis. Curr. Hypertens. Rep. 2002, 4, 152–159. [Google Scholar] [CrossRef]
- Zhao, D.; Navar, L.G. Acute angiotensin II infusions elicit pressure natriuresis in mice and reduce distal fractional sodium reabsorption. Hypertension 2008, 52, 137–142. [Google Scholar] [CrossRef]
- De Nicola, L.; Blantz, R.C.; Gabbai, F.B. Renal functional reserve in treated and untreated hypertensive rats. Kidney Int. 1991, 40, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Healy, V.; Thompson, C.; Johns, E.J. The adrenergic regulation of proximal tubular Na+/H+ exchanger 3 in the rat. Acta Physiol. 2014, 210, 678–689. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Riquier, A.D.; Yang, L.E.; Leong, P.K.; Maunsbach, A.B.; McDonough, A.A. Acute hypertension provokes acute trafficking of distal tubule Na-Cl cotransporter (NCC) to subapical cytoplasmic vesicles. Am. J. Physiol. Renal. Physiol. 2009, 296, F810–F818. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.E.; Leong, P.K.; McDonough, A.A. Reducing blood pressure in SHR with enalapril provokes redistribution of NHE3, NaPi2, and NCC and decreases NaPi2 and ACE abundance. Am. J. Physiol. Renal. Physiol. 2007, 293, F1197–F1208. [Google Scholar] [CrossRef] [PubMed]
- Rabito, S.F.; Carretero, O.A.; Scicli, A.G. Evidence against a role of vasopressin in the maintenance of high blood pressure in mineralocorticoid and renovascular hypertension. Hypertension 1981, 3, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Percie du Sert, N.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; Emerson, M.; et al. Reporting animal research: Explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol. 2020, 18, e3000411. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimoura, C.G.; Oliveira, T.L.; Lincevicius, G.S.; Crajoinas, R.O.; Oliveira-Sales, E.B.; Varela, V.A.; Gomes, G.N.; Bergamaschi, C.T.; Campos, R.R. The Total Denervation of the Ischemic Kidney Induces Differential Responses in Sodium Transporters’ Expression in the Contralateral Kidney in Goldblatt Rats. Int. J. Mol. Sci. 2024, 25, 6962. https://doi.org/10.3390/ijms25136962
Shimoura CG, Oliveira TL, Lincevicius GS, Crajoinas RO, Oliveira-Sales EB, Varela VA, Gomes GN, Bergamaschi CT, Campos RR. The Total Denervation of the Ischemic Kidney Induces Differential Responses in Sodium Transporters’ Expression in the Contralateral Kidney in Goldblatt Rats. International Journal of Molecular Sciences. 2024; 25(13):6962. https://doi.org/10.3390/ijms25136962
Chicago/Turabian StyleShimoura, Caroline G., Tales L. Oliveira, Gisele S. Lincevicius, Renato O. Crajoinas, Elizabeth B. Oliveira-Sales, Vanessa A. Varela, Guiomar N. Gomes, Cassia T. Bergamaschi, and Ruy R. Campos. 2024. "The Total Denervation of the Ischemic Kidney Induces Differential Responses in Sodium Transporters’ Expression in the Contralateral Kidney in Goldblatt Rats" International Journal of Molecular Sciences 25, no. 13: 6962. https://doi.org/10.3390/ijms25136962
APA StyleShimoura, C. G., Oliveira, T. L., Lincevicius, G. S., Crajoinas, R. O., Oliveira-Sales, E. B., Varela, V. A., Gomes, G. N., Bergamaschi, C. T., & Campos, R. R. (2024). The Total Denervation of the Ischemic Kidney Induces Differential Responses in Sodium Transporters’ Expression in the Contralateral Kidney in Goldblatt Rats. International Journal of Molecular Sciences, 25(13), 6962. https://doi.org/10.3390/ijms25136962





