Impact of RAAS Receptors and Membrane-Bound Transporter System in the Left Ventricle during the Long-Term Control of Hypertension
Abstract
:1. Introduction
2. Results
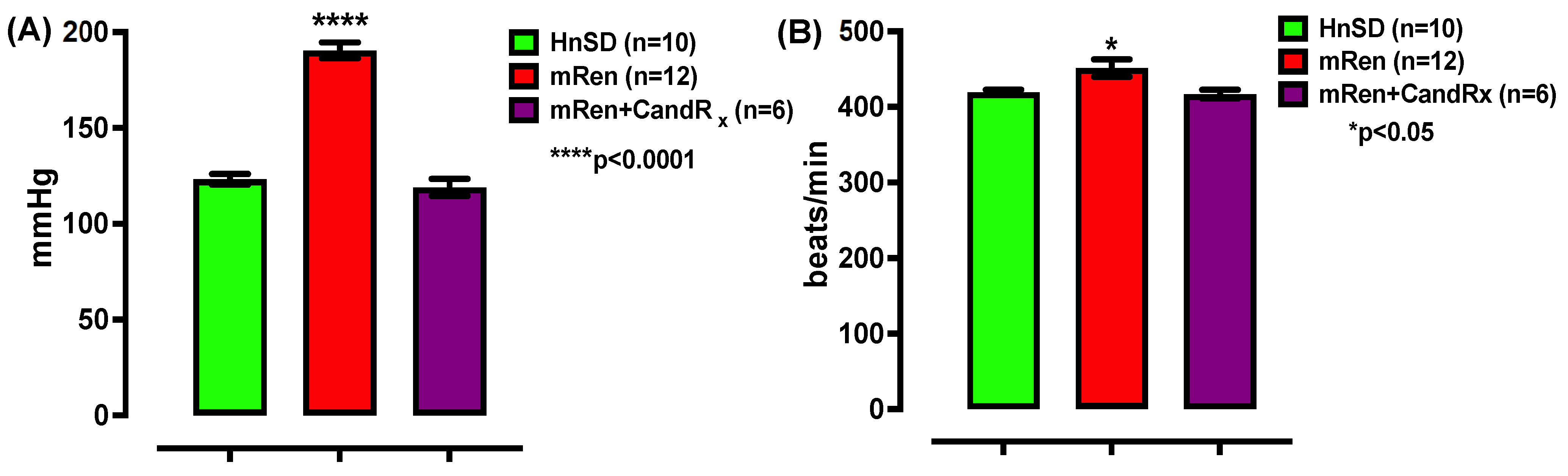
2.1. Blood Pressure
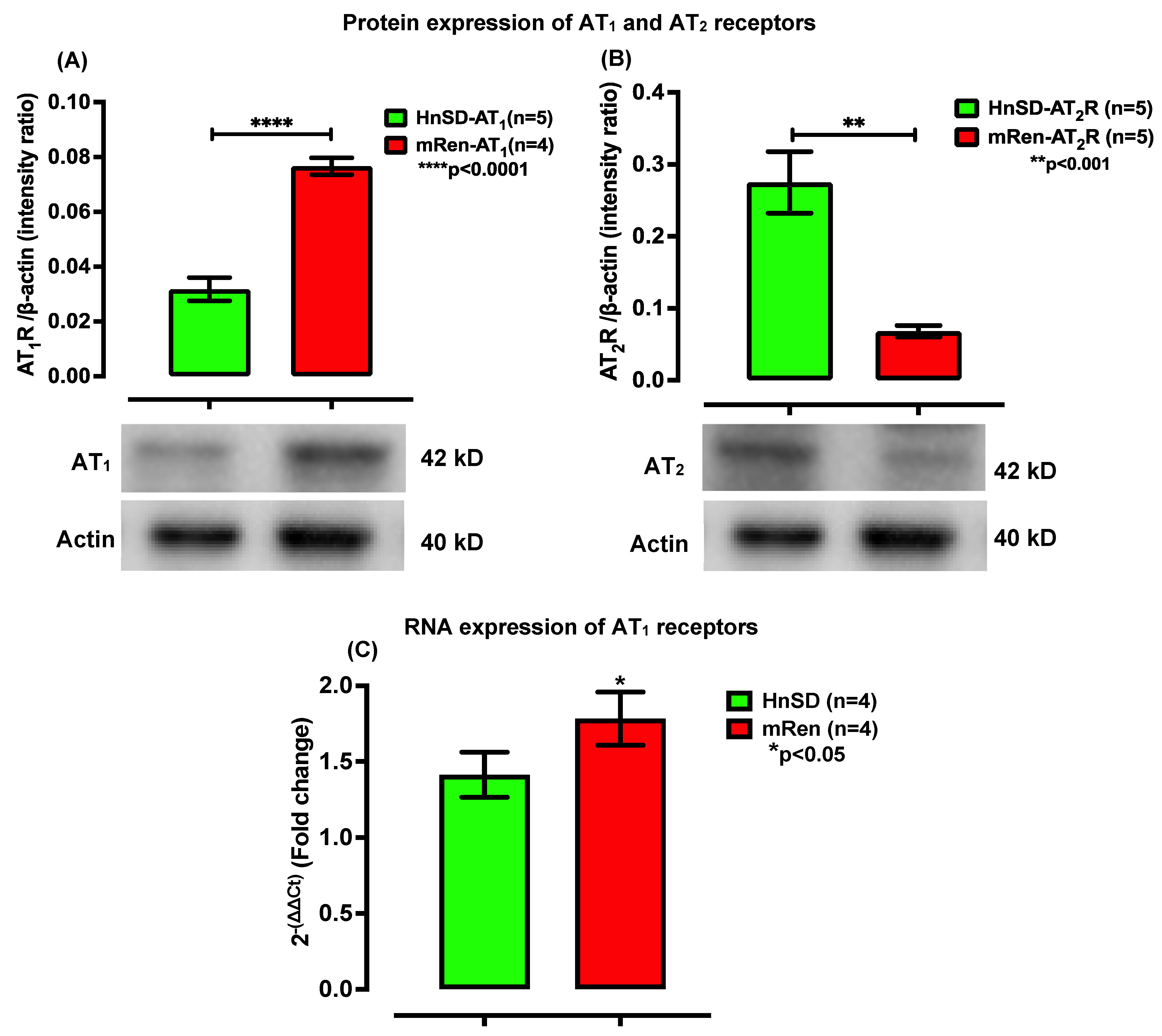
2.2. RAAS Receptor Expression and Gene Transcription Profile in Left Ventricle
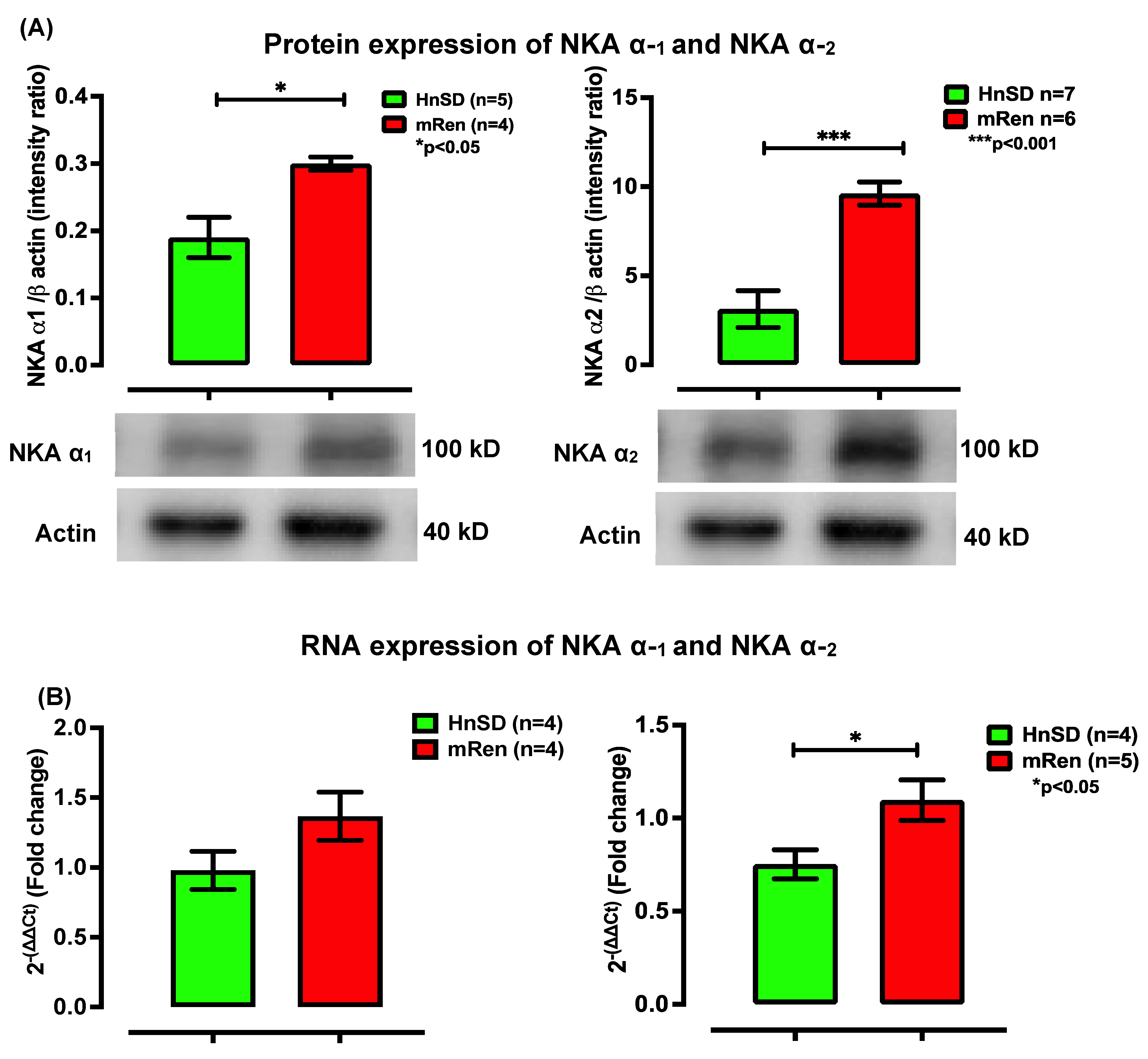
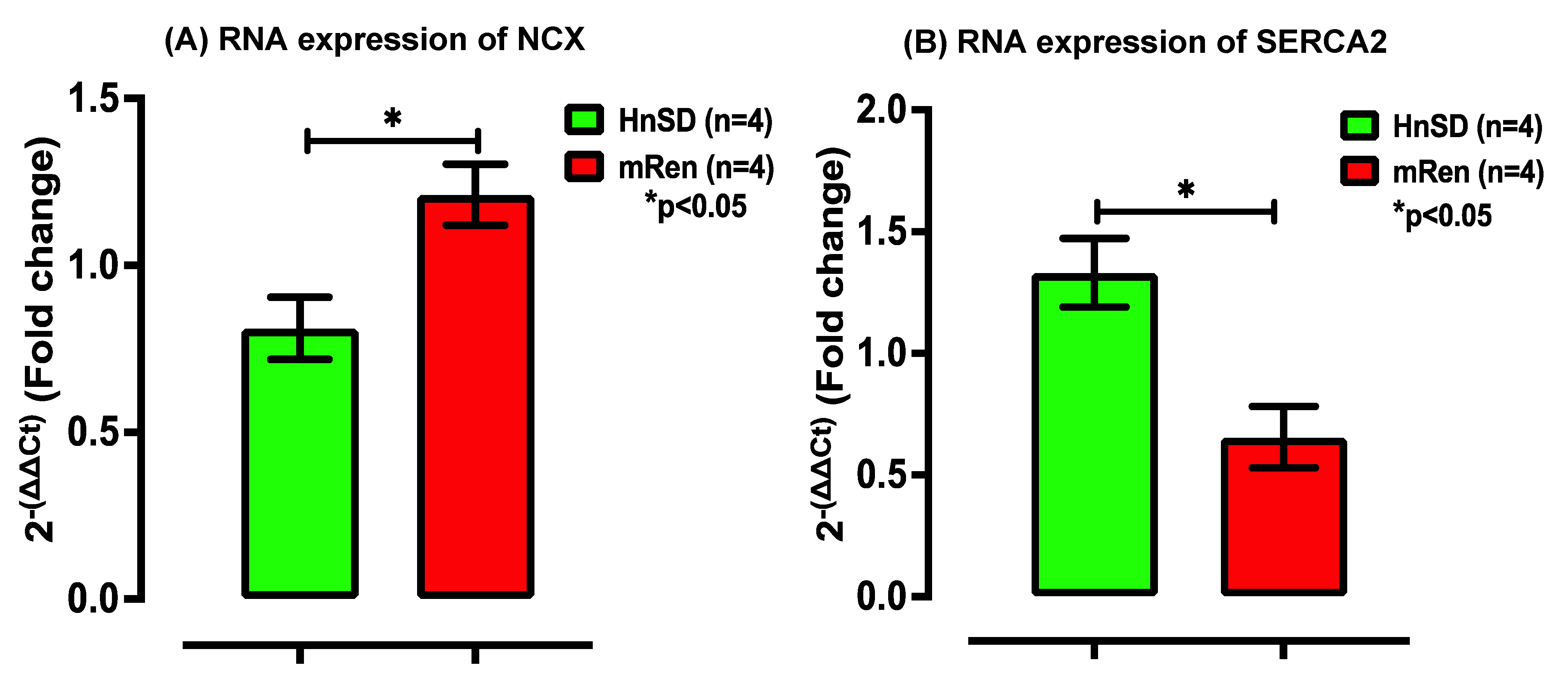
2.3. Na+/K+ Protein Expression and Gene Transcription Profile in Left Ventricle
3. Discussion
4. Conclusions
5. Perspectives
6. Materials and Methods
6.1. Animals Use and Candesartan Treatment
6.2. Non-Invasive Blood Pressure Measurement
6.3. Tissue Harvest
6.4. RAAS Receptor Expression on the Left Ventricle: Western Blotting
6.5. Isolation of RNA Nucleotides: qRT-PCR
6.6. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, J.; Murphy, S.L.; Kochanek, K.D.; Arias, E. Mortality in the United States, 2021. In NCHS Data Brief; U.S. Department of Health & Human Services: Washington, DC, USA, 2022; pp. 1–8. [Google Scholar]
- Chobufo, M.D.; Gayam, V.; Soluny, J.; Rahman, E.U.; Enoru, S.; Foryoung, J.B.; Agbor, V.N.; Dufresne, A.; Nfor, T. Prevalence and control rates of hypertension in the USA: 2017–2018. Int. J. Cardiol. Hypertens. 2020, 6, 100044. [Google Scholar] [CrossRef] [PubMed]
- Goyal, A.; Cusick, A.S.; Thielemier, B. ACE Inhibitors. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Hill, R.D.; Vaidya, P.N. Angiotensin II Receptor Blockers (ARB). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Aileru, A.A.; De Albuquerque, A.; Hamlyn, J.M.; Manunta, P.; Shah, J.R.; Hamilton, M.J.; Weinreich, D. Synaptic plasticity in sympathetic ganglia from acquired and inherited forms of ouabain-dependent hypertension. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 281, R635–R644. [Google Scholar] [CrossRef] [PubMed]
- Aileru, A.A.; Logan, E.; Callahan, M.; Ferrario, C.M.; Ganten, D.; Diz, D.I. Alterations in sympathetic ganglionic transmission in response to angiotensin II in (mRen2)27 transgenic rats. Hypertension 2004, 43, 270–275. [Google Scholar] [CrossRef]
- Swami Vetha, B.S.; Byrum, R.; Peele, K.; Diz, D.; Aileru, A. Functional Significance of Angiotensin Receptor Type 2 in the Neuroplasticity of Autonomic Ganglia in (mRen2)27 Transgenic Hypertensive Rats. J. Cardiovasc. Pharmacol. 2023, 81, 76–84. [Google Scholar] [CrossRef]
- Kuroki, M.T.; Fink, G.D.; Osborn, J.W. Comparison of arterial pressure and plasma ANG II responses to three methods of subcutaneous ANG II administration. Am. J. Physiol. Heart Circ. Physiol. 2014, 307, H670–H679. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Aroor, A.R.; Hill, M.A.; Sowers, J.R. Role of Renin-Angiotensin-Aldosterone System Activation in Promoting Cardiovascular Fibrosis and Stiffness. Hypertension 2018, 72, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Lopaschuk, G.D.; Karwi, Q.G.; Tian, R.; Wende, A.R.; Abel, E.D. Cardiac Energy Metabolism in Heart Failure. Circ. Res. 2021, 128, 1487–1513. [Google Scholar] [CrossRef] [PubMed]
- Mullins, J.J.; Peters, J.; Ganten, D. Fulminant hypertension in transgenic rats harbouring the mouse Ren-2 gene. Nature 1990, 344, 541–544. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.; Ganten, D. Adrenal renin expression and its role in ren-2 transgenic rats TGR(mREN2)27. Horm. Metab. Res. 1998, 30, 350–354. [Google Scholar] [CrossRef]
- Zhao, Y.; Bader, M.; Kreutz, R.; Fernandez-Alfonso, M.; Zimmermann, F.; Ganten, U.; Metzger, R.; Ganten, D.; Mullins, J.J.; Peters, J. Ontogenetic regulation of mouse Ren-2d renin gene in transgenic hypertensive rats, TGR(mREN2)27. Am. J. Physiol. 1993, 265, E699–E707. [Google Scholar] [CrossRef] [PubMed]
- Lino, C.A.; da Silva, I.B.; Shibata, C.E.; Monteiro Pde, S.; Barreto-Chaves, M.L. Maternal hyperthyroidism increases the susceptibility of rat adult offspring to cardiovascular disorders. Mol. Cell. Endocrinol. 2015, 416, 1–8. [Google Scholar] [CrossRef]
- Wang, H.; Jessup, J.A.; Zhao, Z.; Da Silva, J.; Lin, M.; MacNamara, L.M.; Ahmad, S.; Chappell, M.C.; Ferrario, C.M.; Groban, L. Characterization of the cardiac renin angiotensin system in oophorectomized and estrogen-replete mRen2.Lewis rats. PLoS ONE 2013, 8, e76992. [Google Scholar] [CrossRef]
- Schoner, W.; Scheiner-Bobis, G. Endogenous and exogenous cardiac glycosides: Their roles in hypertension, salt metabolism, and cell growth. Am. J. Physiol. Cell Physiol. 2007, 293, C509–C536. [Google Scholar] [CrossRef]
- Schoner, W.; Scheiner-Bobis, G. Endogenous cardiac glycosides: Hormones using the sodium pump as signal transducer. Semin. Nephrol. 2005, 25, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P. The Laboratory Rat: Relating Its Age With Human’s. Int. J. Prev. Med. 2013, 4, 624–630. [Google Scholar] [PubMed]
- Heid, C.A.; Stevens, J.; Livak, K.J.; Williams, P.M. Real time quantitative PCR. Genome Res. 1996, 6, 986–994. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Swami Vetha, B.S.; Byrum, R.; Mebane, D.; Katwa, L.C.; Aileru, A. Impact of RAAS Receptors and Membrane-Bound Transporter System in the Left Ventricle during the Long-Term Control of Hypertension. Int. J. Mol. Sci. 2024, 25, 6997. https://doi.org/10.3390/ijms25136997
Swami Vetha BS, Byrum R, Mebane D, Katwa LC, Aileru A. Impact of RAAS Receptors and Membrane-Bound Transporter System in the Left Ventricle during the Long-Term Control of Hypertension. International Journal of Molecular Sciences. 2024; 25(13):6997. https://doi.org/10.3390/ijms25136997
Chicago/Turabian StyleSwami Vetha, Berwin Singh, Rachel Byrum, DaQuan Mebane, Laxmansa C. Katwa, and Azeez Aileru. 2024. "Impact of RAAS Receptors and Membrane-Bound Transporter System in the Left Ventricle during the Long-Term Control of Hypertension" International Journal of Molecular Sciences 25, no. 13: 6997. https://doi.org/10.3390/ijms25136997





