The Role of Tubulin Polymerization-Promoting Protein2 (TPPP2) in Spermatogenesis: A Narrative Review
Abstract
1. Introduction
2. The TPPP Protein Family
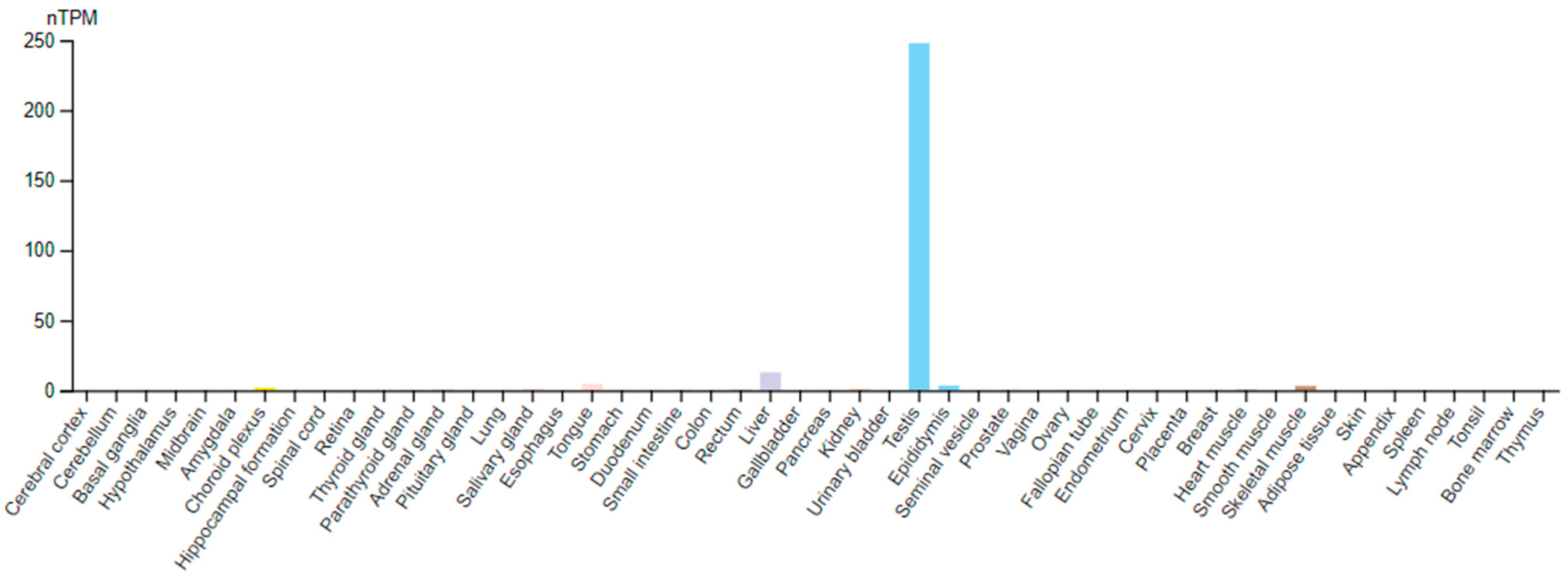
3. TPPP2
4. TPPPs and Flagellum
5. Flagellum, Spermatogenesis, and Its Defects
6. Early Proteomic Data
7. Proteomic Data from Ruminants
8. Bioinformatic Analyses
9. Knockout Mice Models of Oligoasthenozoospermia and Asthenoteratozoospermia TPPP2 Is Involved in Spermiogenesis
10. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, M.; Hu, Z.; Qi, L.; Wang, J.; Zhou, T.; Guo, Y.; Zeng, Y.; Zheng, B.; Wu, Y.; Zhang, P.; et al. Scanning of novel cancer/testis proteins by human testis proteomic analysis. Proteomics 2013, 13, 1200–1210. [Google Scholar] [CrossRef] [PubMed]
- Martins, J.A.M.; Souza, C.E.A.; Silva, F.D.A.; Cadavid, V.G.; Nogueira, F.C.; Domont, G.B.; de Oliveira, J.T.A.; Moura, A.A. Major heparin-binding proteins of the seminal plasma from Morada Nova rams. Small Ruminant Res. 2013, 113, 115–127. [Google Scholar] [CrossRef]
- Aslam, M.K.M.; Kumaresan, A.; Yadav, S.; Mohanty, T.K.; Datta, T.K. Comparative proteomic analysis of high- and low-fertile buffalo bull spermatozoa for identification of fertility-associated proteins. Reprod. Domest. Anim. 2019, 54, 786–794. [Google Scholar] [CrossRef]
- Zhang, R.; Liang, C.; Guo, X.; Bao, P.; Pei, J.; Wu, F.; Yin, M.; Chu, M.; Yan, P. Quantitative phosphoproteomics analyses reveal the regulatory mechanisms related to frozen-thawed sperm capacitation and acrosome reaction in yak (Bos grunniens). Front Physiol. 2022, 13, 1013082. [Google Scholar] [CrossRef] [PubMed]
- Gacem, S.; Castello-Ruiz, M.; Hidalgo, C.O.; Tamargo, C.; Santolaria, P.; Soler, C.; Yániz, J.L.; Silvestre, M.A. Bull sperm SWATH-MS-based proteomics reveals link between high fertility and energy production, motility structures, and sperm-oocyte interaction. J. Proteome Res. 2023, 22, 3607–3624. [Google Scholar] [CrossRef]
- Gòdia, M.; Reverter, A.; González-Prendes, R.; Ramayo-Caldas, Y.; Castelló, A.; Rodríguez-Gil, J.E.; Sánchez, A.; Clop, A. A systems biology framework integrating GWAS and RNA-seq to shed light on the molecular basis of sperm quality in swine. Genet. Sel. Evol. 2020, 52, 72. [Google Scholar] [CrossRef]
- Alagundagi, D.B.; Ghate, S.D.; Shetty, P.; Gollapalli, P.; Shetty, P.; Patil, P. Integrated molecular-network analysis reveals infertility-associated key genes and transcription factors in the non-obstructive azoospermia. Eur. J. Obstet. Gynecol. Reprod. Biol. 2023, 288, 183–190. [Google Scholar] [CrossRef]
- Zhu, F.; Yan, P.; Zhang, J.; Cui, Y.; Zheng, M.; Cheng, Y.; Guo, Y.; Yang, X.; Guo, X.; Zhu, H. Deficiency of TPPP2, a factor linked to oligoasthenozoospermia, causes subfertility in male mice. J. Cell Mol. Med. 2019, 23, 2583–2594. [Google Scholar] [CrossRef]
- Wang, W.; Tian, S.; Nie, H.; Tu, C.; Liu, C.; Li, Y.; Li, D.; Yang, X.; Meng, L.; Hu, T.; et al. CFAP65 is required in the acrosome biogenesis and mitochondrial sheath assembly during spermiogenesis. Hum. Mol. Genet. 2021, 30, 2240–2254. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xu, X.; Ni, X.; Pan, J.; Chen, M.; Lin, Y.; Zhao, Z.; Zhang, L.; Ge, N.; Song, G.; et al. Gene-based cancer-testis antigens as prognostic indicators in hepatocellular carcinoma. Heliyon 2023, 9, e13269. [Google Scholar] [CrossRef]
- Hlavanda, E.; Kovács, J.; Oláh, J.; Orosz, F.; Medzihradszky, K.F.; Ovádi, J. Brain-specific p25 protein binds to tubulin and microtubules and induces aberrant microtubule assemblies at substoichiometric concentrations. Biochemistry 2002, 41, 8657–8664. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Tomizawa, K.; Ishiguro, K.; Sato, K.; Omori, A.; Sato, S.; Shiratsuchi, A.; Uchida, T.; Imahori, K. A novel brain-specific 25 kDa protein (p25) is phosphorylated by a Ser/Thr-Pro kinase (TPK II) from tau protein kinase fractions. FEBS Lett. 1991, 289, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Vincze, O.; Tokesi, N.; Oláh, J.; Hlavanda, E.; Zotter, A.; Horvath, I.; Lehotzky, A.; Tirián, L.; Medzihradszky, K.F.; Kovács, J.; et al. Tubulin polymerization promoting proteins (TPPPs): Members of a new family with distinct structures and functions. Biochemistry 2006, 45, 13818–13826. [Google Scholar] [CrossRef] [PubMed]
- Ohno, S. Evolution by Gene Duplication; Springer: Berlin/Heidelberg, Germany, 1970. [Google Scholar]
- Orosz, F. A fish-specific member of the TPPP protein family? J. Mol. Evol. 2012, 75, 55–72. [Google Scholar] [CrossRef] [PubMed]
- Orosz, F. On the tubulin polymerization promoting proteins of zebrafish. Biochem. Biophys. Res. Commun. 2015, 457, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Orosz, F. A new protein superfamily: TPPP-like proteins. PLoS ONE 2012, 7, e49276. [Google Scholar] [CrossRef]
- Orosz, F.; Ovádi, J. TPPP orthologs are ciliary proteins. FEBS Lett. 2008, 582, 3757–3764. [Google Scholar] [CrossRef] [PubMed]
- Orosz, F. On the TPPP-like proteins of flagellated Fungi. Fungal Biol. 2021, 125, 357–367. [Google Scholar] [CrossRef]
- Lehotzky, A.; Lau, P.; Tokesi, N.; Muja, N.; Hudson, L.D.; Ovádi, J. Tubulin polymerization-promoting protein (TPPP/p25) is critical for oligodendrocyte differentiation. Glia 2010, 58, 157–168. [Google Scholar] [CrossRef]
- Kovács, G.G.; László, L.; Kovács, J.; Jensen, P.H.; Lindersson, E.; Botond, G.; Molnar, T.; Perczel, A.; Hudecz, F.; Mezo, G.; et al. Natively unfolded tubulin polymerization promoting protein TPPP/p25 is a common marker of alpha-synucleinopathies. Neurobiol. Dis. 2004, 17, 155–162. [Google Scholar] [CrossRef]
- Orosz, F.; Kovács, G.G.; Lehotzky, A.; Oláh, J.; Vincze, O.; Ovádi, J. TPPP/p25: From unfolded protein to misfolding disease: Prediction and experiments. Biol Cell. 2004, 96, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Staverosky, J.A.; Pryce, B.A.; Watson, S.S.; Schweitzer, R. Tubulin polymerization-promoting protein family member 3, Tppp3, is a specific marker of the differentiating tendon sheath and synovial joints. Dev. Dyn. 2009, 238, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Juneja, S.C. Cellular distribution and gene expression profile during flexor tendon graft repair: A novel tissue engineering approach. J. Tissue Eng. 2013, 4, 2041731413492741. [Google Scholar] [CrossRef] [PubMed]
- Oláh, J.; Lehotzky, A.; Szunyogh, S.; Szénási, T.; Orosz, F.; Ovádi, J. Microtubule-associated proteins with regulatory functions by day and pathological potency at night. Cells 2020, 9, 57. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wu, C.; Huang, W.; Wang, S.; Zhao, E.; Huang, Q.; Xie, Y.; Mao, Y. A novel human gene whose product shares homology with bovine brain-specific protein p25 is expressed in fetal brain but not in adult brain. J. Hum. Genet. 2002, 47, 266–268. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, A.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Proteomics. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef] [PubMed]
- Welch, J.E.; Brown, P.L.; O’Brien, D.A.; Magyar, P.L.; Bunch, D.O.; Mori, C.; Eddy, E.M. Human glyceraldehyde 3-phosphate dehydrogenase-2 gene is expressed specifically in spermatogenic cells. J. Androl. 2000, 21, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Tammana, D.; Tammana, T.V.S. Chlamydomonas FAP265 is a tubulin polymerization promoting protein, essential for flagellar reassembly and hatching of daughter cells from the sporangium. PLoS ONE 2017, 12, e0185108. [Google Scholar] [CrossRef] [PubMed]
- Ikadai, H.; Shaw Saliba, K.; Kanzok, S.M.; McLean, K.J.; Tanaka, T.Q.; Cao, J.; Williamson, K.C.; Jacobs-Lorena, M. Transposon mutagenesis identifies genes essential for Plasmodium falciparum gametocytogenesis. Proc. Natl. Acad. Sci. USA 2013, 110, E1676–E1684. [Google Scholar] [CrossRef]
- Zhang, C.; Li, D.; Meng, Z.; Zhou, J.; Min, Z.; Deng, S.; Shen, J.; Liu, M. Pyp25 is required for male gametocyte exflagellation. Pathog. Dis. 2022, 80, ftac043. [Google Scholar] [CrossRef]
- O’Donnell, L. Mechanisms of spermiogenesis and spermiation and how they are disturbed. Spermatogenesis 2014, 4, e979623. [Google Scholar] [CrossRef]
- Nishimura, H.; L’Hernault, S.W. Spermatogenesis. Curr. Biol. 2017, 27, R988–R994. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, R.; Mieusset, R. The human epididymis: Its function in sperm maturation. Hum. Reprod. Update. 2016, 22, 574–587. [Google Scholar] [CrossRef] [PubMed]
- Okabe, M. The cell biology of mammalian fertilization. Development 2013, 140, 4471–4479. [Google Scholar] [CrossRef] [PubMed]
- Hirohashi, N.; Yanagimachi, R. Sperm acrosome reaction: Its site and role in fertilization. Biol. Reprod. 2018, 99, 127–133. [Google Scholar] [CrossRef]
- Silflow, C.D.; Lefebvre, P.A. Assembly and motility of eukaryotic cilia and flagella. Lessons from Chlamydomonas reinhardtii. Plant Physiol. 2001, 127, 1500–1507. [Google Scholar] [CrossRef] [PubMed]
- Inaba, K. Sperm flagella: Comparative and phylogenetic perspectives of protein components. Mol. Hum. Reprod. 2011, 17, 524–538. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.L.; Tu, C.F.; Tan, Y.Q. Insight on multiple morphological abnormalities of sperm flagella in male infertility: What is new? Asian J. Androl. 2020, 22, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Gilany, K.; Minai-Tehrani, A.; Amini, M.; Agharezaee, N.; Arjmand, B. The challenge of human spermatozoa proteome: A systematic review. J. Reprod. Infertil. 2017, 18, 267–279. [Google Scholar] [PubMed]
- Gilany, K.; Lakpour, N.; Vafakhah, M.; Sadeghi, M.R. The profile of human sperm proteome; a minireview. J. Reprod. Infertil. 2011, 12, 193–199. [Google Scholar]
- Simpson, A.J.; Caballero, O.L.; Jungbluth, A.; Chen, Y.T.; Old, L.J. Cancer/testis antigens, gametogenesis and cancer. Nat. Rev. Cancer 2005, 5, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Andrabi, S.M.H. Factors affecting the quality of cryopreserved buffalo (Bubalus bubalis) bull spermatozoa. Reprod. Domest. Anim. 2009, 44, 552–569. [Google Scholar] [CrossRef] [PubMed]
- Grotter, L.G.; Cattaneo, L.; Marini, P.E.; Kjelland, M.E.; Ferre, L.B. Recent advances in bovine sperm cryopreservation techniques with a focus on sperm post-thaw quality optimization. Reprod. Domest. Anim. 2019, 54, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Hlavanda, E.; Klement, E.; Kókai, E.; Kovács, J.; Vincze, O.; Tökési, N.; Orosz, F.; Medzihradszky, K.F.; Dombrádi, V.; Ovádi, J. Phosphorylation blocks the activity of tubulin polymerization-promoting protein (TPPP): Identification of sites targeted by different kinases. J. Biol. Chem. 2007, 282, 29531–29539. [Google Scholar] [CrossRef] [PubMed]
- Kleinnijenhuis, A.J.; Hedegaard, C.; Lundvig, D.; Sundbye, S.; Issinger, O.G.; Jensen, O.N.; Jensen, P.H. Identification of multiple post-translational modifications in the porcine brain specific p25alpha. J. Neurochem. 2008, 106, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Oláh, J.; Tökési, N.; Vincze, O.; Horváth, I.; Lehotzky, A.; Tirián, L.; Medzihradszky, K.F.; Kovács, J.; Orosz, F.; Kovács, G.G.; et al. Interaction of TPPP/p25 protein with glyceraldehyde-3-phosphate dehydrogenase and their co-localization in Lewy bodies. FEBS Lett. 2006, 580, 5807–5814. [Google Scholar] [CrossRef]
- Liu, S.; Bian, Y.C.; Wang, W.L.; Liu, T.J.; Zhang, T.; Chang, Y.; Xiao, R.; Zhang, C.L. Identification of hub genes associated with spermatogenesis by bioinformatics analysis. Sci. Rep. 2023, 13, 18435. [Google Scholar] [CrossRef]
- Ran, L.; Gao, Z.; Chen, Q.; Cui, F.; Liu, X.; Xue, B. Identification and validation of diagnostic signature genes in non-obstructive azoospermia by machine learning. Aging 2023, 15, 4465–4480. [Google Scholar] [CrossRef]
- Wang, W.; Tu, C.; Nie, H.; Meng, L.; Li, Y.; Yuan, S.; Zhang, Q.; Du, J.; Wang, J.; Gong, F.; et al. Biallelic mutations in CFAP65 lead to severe asthenoterato-spermia due to acrosome hypoplasia and flagellum malformations. J. Med. Genet. 2019, 56, 750–757. [Google Scholar] [CrossRef]
- Tirián, L.; Hlavanda, E.; Oláh, J.; Horváth, I.; Orosz, F.; Szabó, B.; Kovács, J.; Szabad, J.; Ovádi, J. TPPP/p25 promotes tubulin assemblies and blocks mitotic spindle formation. Proc. Nat. Acad. Sci. USA 2003, 100, 13976–13981. [Google Scholar] [CrossRef]
- Cui, M.; Qu, F.; Wang, L.; Liu, X.; Yu, J.; Tang, Z.; Cheng, D. m5C RNA methyltransferase-related gene NSUN4 stimulates malignant progression of hepatocellular carcinoma and can be a prognostic marker. Cancer Biomark. 2022, 33, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Hou, Y.; Picariello, T.; Craige, B.; Witman, G.B. Proteome of the central apparatus of a ciliary axoneme. J. Cell Biol. 2019, 218, 2051–2070. [Google Scholar] [CrossRef] [PubMed]
- Madeira, F.; Pearce, M.; Tivey, A.R.N.; Basutkar, P.; Lee, J.; Edbali, O.; Madhusoodanan, N.; Kolesnikov, A.; Lopez, R. Search and sequence analysis tools services from EMBL-EBI in 2022. Nucleic. Acids. Res. 2022, 50, W276–W279. [Google Scholar] [CrossRef] [PubMed]
| Paper | Year | Species | Source/Object | Main Findings and Effects |
|---|---|---|---|---|
| Liu [1] | 2013 | human | testis, sperm | |
| Martins [2] | 2013 | sheep | seminal plasma | |
| Aslam [3] | 2019 | buffalo | sperm | TPPP2 is overexpressed 2.2x in low fertility sperm |
| Zhu [8] | 2019 | human, mice | sperm, testis, epididymis | sperm counts and motility of KO mice are lower; inhibition of TPPP2 decreased in vitro fertilization rate; mitochondrial dysfunction |
| Gódia [6] | 2020 | swine | sperm | high positive correlation between TPPP2 and curvilinear velocity of sperms; TPPP2 is the 5th most abundant mRNA in sperm |
| Wang [9] | 2021 | mice | testis, sperm | TPPP2 is downregulated in CFAP65 KO mice |
| Zhang [4] | 2022 | yak | sperm | upregulated in frozen-thawed high motility sperms |
| Alagundagi [7] | 2023 | human | testis | TPPP2 is upregulated 10x in NOA patients |
| Gacem [5] | 2023 | bull | sperm | TPPP2 is upregulated in high fertility bulls |
| Xu [10] | 2023 | human | testis, tumors | TPPP2 is prognostic indicator of tumor (hepatocarcinoma) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orosz, F. The Role of Tubulin Polymerization-Promoting Protein2 (TPPP2) in Spermatogenesis: A Narrative Review. Int. J. Mol. Sci. 2024, 25, 7017. https://doi.org/10.3390/ijms25137017
Orosz F. The Role of Tubulin Polymerization-Promoting Protein2 (TPPP2) in Spermatogenesis: A Narrative Review. International Journal of Molecular Sciences. 2024; 25(13):7017. https://doi.org/10.3390/ijms25137017
Chicago/Turabian StyleOrosz, Ferenc. 2024. "The Role of Tubulin Polymerization-Promoting Protein2 (TPPP2) in Spermatogenesis: A Narrative Review" International Journal of Molecular Sciences 25, no. 13: 7017. https://doi.org/10.3390/ijms25137017
APA StyleOrosz, F. (2024). The Role of Tubulin Polymerization-Promoting Protein2 (TPPP2) in Spermatogenesis: A Narrative Review. International Journal of Molecular Sciences, 25(13), 7017. https://doi.org/10.3390/ijms25137017






