Moderate Prenatal Alcohol Exposure Increases Toll-like Receptor Activity in Umbilical Cord Blood at Birth: A Pilot Study
Abstract
:1. Introduction
2. Results
2.1. Baseline Cytokine Levels
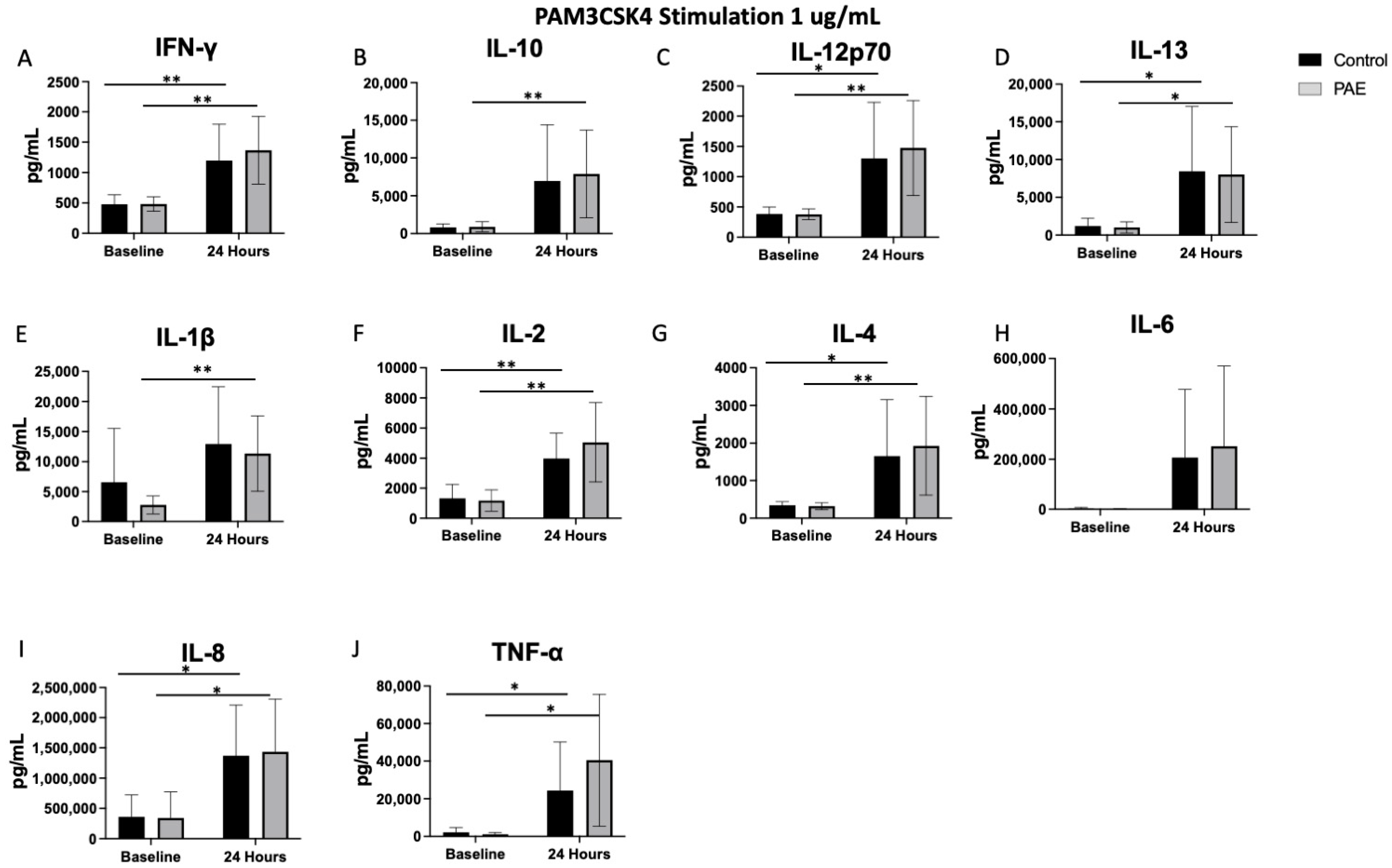
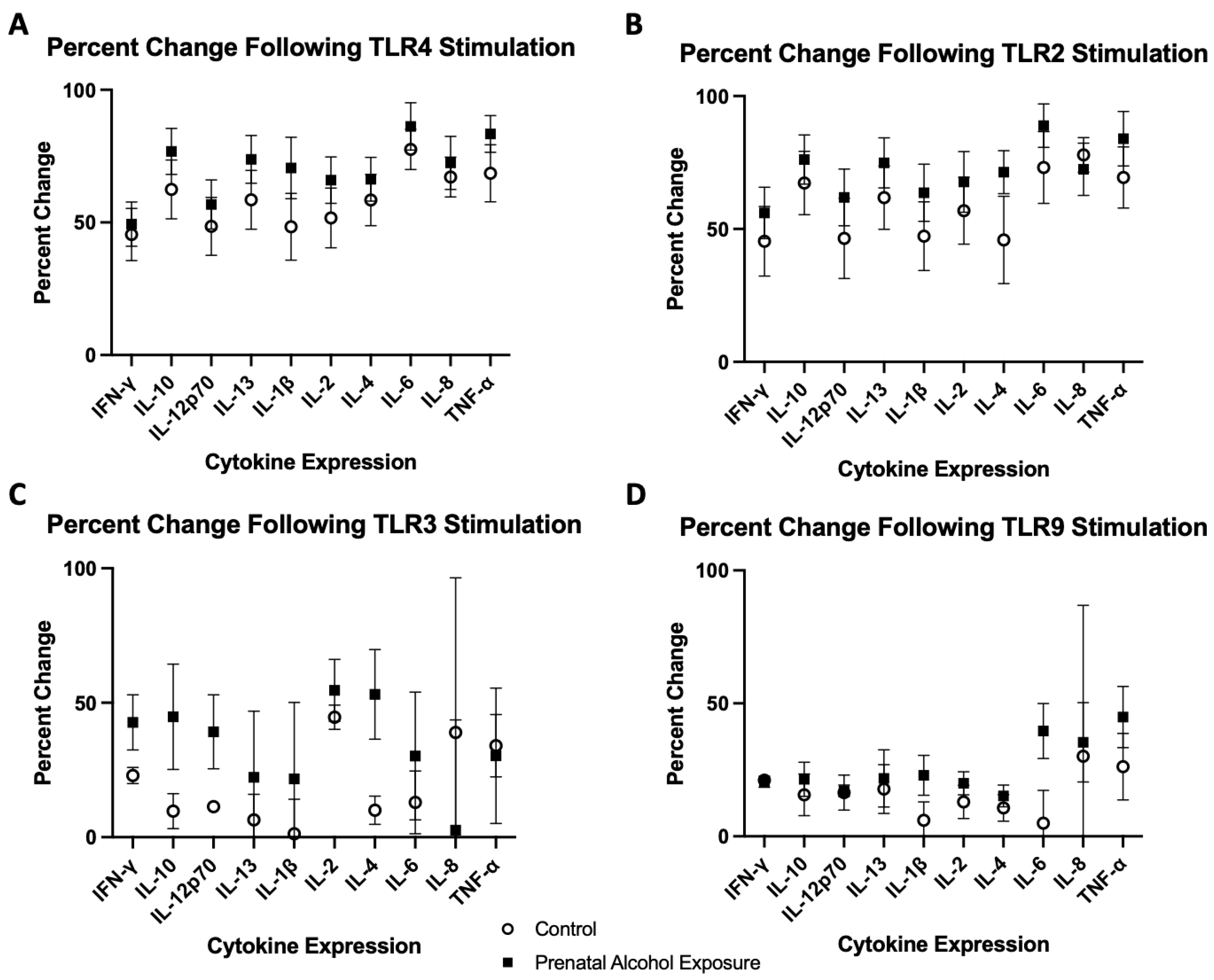
2.2. TLR4 Agonist Stimulation
2.3. TLR2 Agonist Stimulation
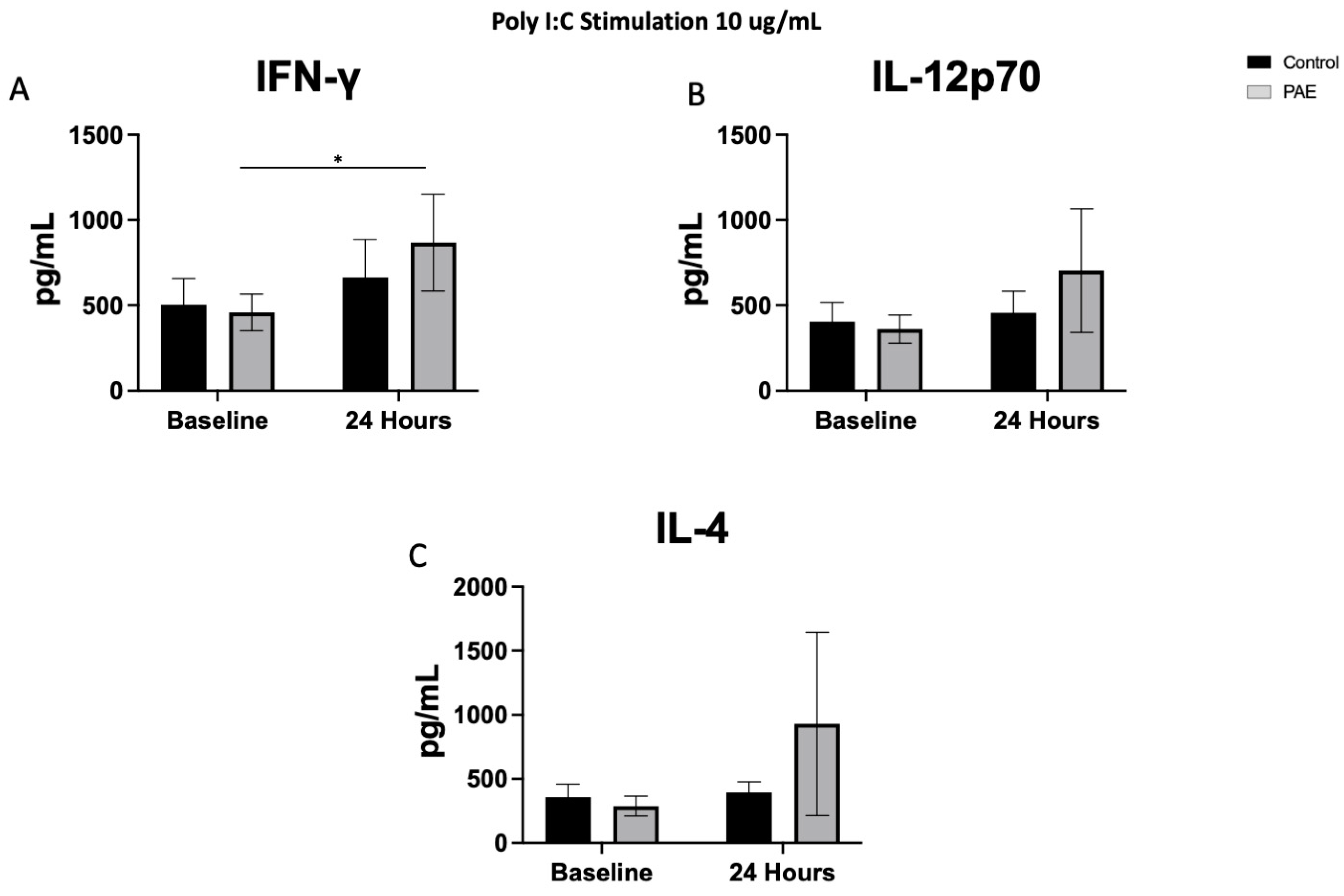
2.4. TLR3 Agonist Stimulation
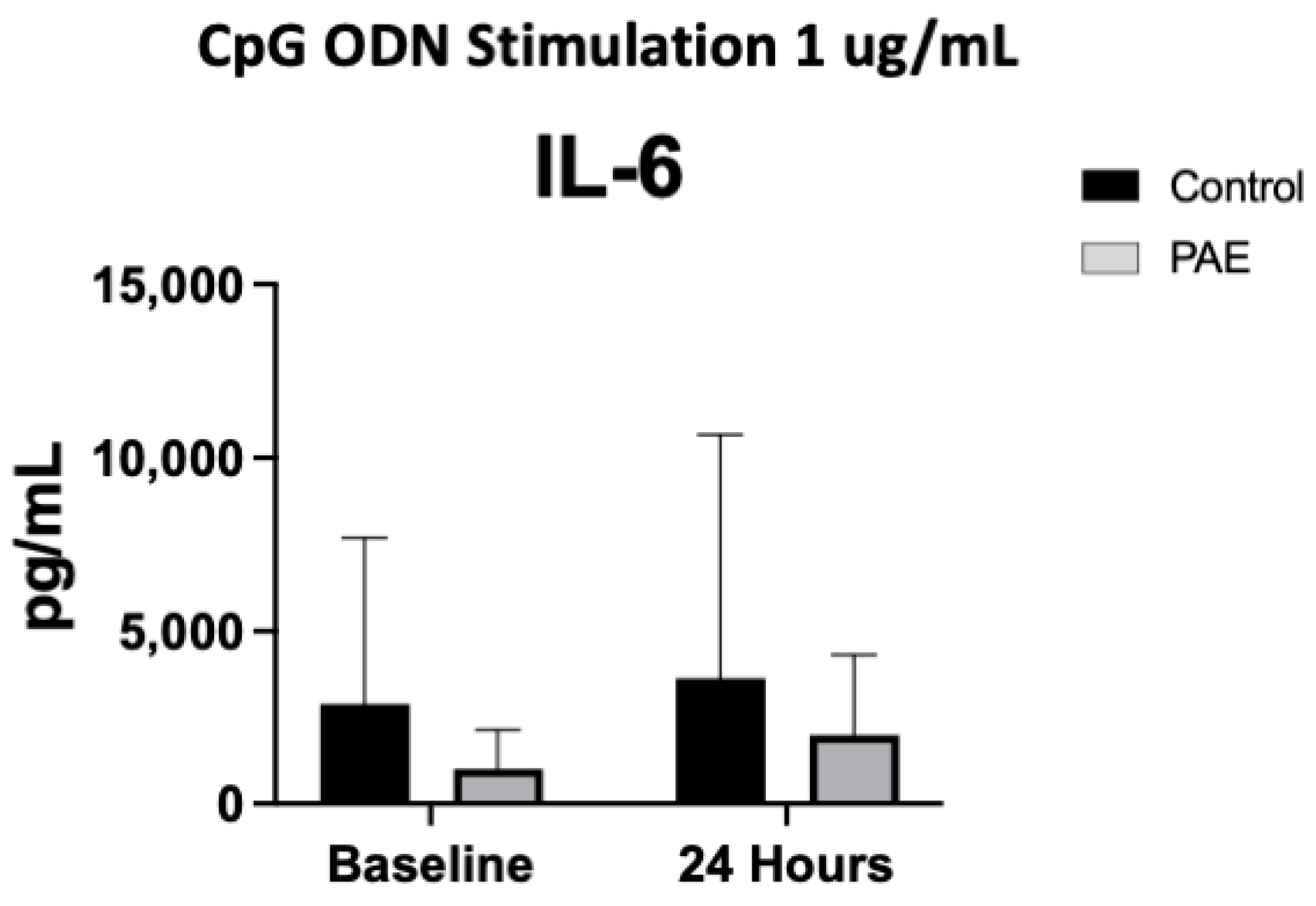
2.5. TLR9 Agonist Stimulation
3. Discussion
4. Material and Methods
4.1. Ethanol, Neurodevelopment, Infant and Child Health Study-2 Cohort
4.2. Peripheral Blood Mononuclear Cell (PBMC) Isolation and Stimulation In Vitro
4.3. Multiplex Immunoassay to Measure Functional Responses of PBMCs
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pruett, D.; Waterman, E.H.; Caughey, A.B. Fetal alcohol exposure: Consequences, diagnosis, and treatment. Obstet. Gynecol. Surv. 2013, 68, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Dejong, K.; Olyaei, A.; Lo, J.O. Alcohol Use in Pregnancy. Clin. Obstet. Gynecol. 2019, 62, 142–155. [Google Scholar] [CrossRef] [PubMed]
- Green, P.P.; McKnight-Eily, L.R.; Tan, C.H.; Mejia, R.; Denny, C.H. Vital Signs: Alcohol-Exposed Pregnancies–United States, 2011–2013. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Burd, L.; Roberts, D.; Olson, M.; Odendaal, H. Ethanol and the placenta: A review. J. Matern. Fetal Neonatal Med. 2007, 20, 361–375. [Google Scholar] [CrossRef] [PubMed]
- Burd, L.; Blair, J.; Dropps, K. Prenatal alcohol exposure, blood alcohol concentrations and alcohol elimination rates for the mother, fetus and newborn. J. Perinatol. 2012, 32, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.F.; Smith, V.C.; Committee on Substance Abuse. Fetal Alcohol Spectrum Disorders. Pediatrics 2015, 136, e1395–e1406. [Google Scholar] [CrossRef] [PubMed]
- May, P.A.; Baete, A.; Russo, J.; Elliott, A.J.; Blankenship, J.; Kalberg, W.O.; Buckley, D.; Brooks, M.; Hasken, J.; Abdul-Rahman, O.; et al. Prevalence and characteristics of fetal alcohol spectrum disorders. Pediatrics 2014, 134, 855–866. [Google Scholar] [CrossRef]
- Easey, K.E.; Dyer, M.L.; Timpson, N.J.; Munafo, M.R. Prenatal alcohol exposure and offspring mental health: A systematic review. Drug Alcohol. Depend. 2019, 197, 344–353. [Google Scholar] [CrossRef]
- Doney, R.; Lucas, B.R.; Jones, T.; Howat, P.; Sauer, K.; Elliott, E.J. Fine motor skills in children with prenatal alcohol exposure or fetal alcohol spectrum disorder. J. Dev. Behav. Pediatr. 2014, 35, 598–609. [Google Scholar] [CrossRef]
- Johnson, S.; Knight, R.; Marmer, D.J.; Steele, R.W. Immune deficiency in fetal alcohol syndrome. Pediatr. Res. 1981, 15, 908–911. [Google Scholar] [CrossRef]
- Gauthier, T.W.; Manar, M.H.; Brown, L.A. Is maternal alcohol use a risk factor for early-onset sepsis in premature newborns? Alcohol 2004, 33, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, T.W.; Ping, X.D.; Harris, F.L.; Wong, M.; Elbahesh, H.; Brown, L.A. Fetal alcohol exposure impairs alveolar macrophage function via decreased glutathione availability. Pediatr. Res. 2005, 57, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, T.W.; Drews-Botsch, C.; Falek, A.; Coles, C.; Brown, L.A. Maternal alcohol abuse and neonatal infection. Alcohol. Clin. Exp. Res. 2005, 29, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, T.W. Prenatal Alcohol Exposure and the Developing Immune System. Alcohol. Res. 2015, 37, 279–285. [Google Scholar] [PubMed]
- Raineki, C.; Bodnar, T.S.; Holman, P.J.; Baglot, S.L.; Lan, N.; Weinberg, J. Effects of early-life adversity on immune function are mediated by prenatal environment: Role of prenatal alcohol exposure. Brain Behav. Immun. 2017, 66, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Bodnar, T.S.; Raineki, C.; Wertelecki, W.; Yevtushok, L.; Plotka, L.; Granovska, I.; Zymak-Zakutnya, N.; Pashtepa, A.; Wells, A.; Honerkamp-Smith, G.; et al. Immune network dysregulation associated with child neurodevelopmental delay: Modulatory role of prenatal alcohol exposure. J. Neuroinflammation 2020, 17, 39. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.W.; Xu, S.M.; An, Q.; Wang, P. A review of risk factors for childhood leukemia. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 3760–3764. [Google Scholar] [PubMed]
- Jabbar, S.; Reuhl, K.; Sarkar, D.K. Prenatal alcohol exposure increases the susceptibility to develop aggressive prolactinomas in the pituitary gland. Sci. Rep. 2018, 8, 7720. [Google Scholar] [CrossRef] [PubMed]
- Chiappelli, F.; Taylor, A.N. The fetal alcohol syndrome and fetal alcohol effects on immune competence. Alcohol. Alcohol. 1995, 30, 259–262. [Google Scholar] [PubMed]
- Ahluwalia, B.; Wesley, B.; Adeyiga, O.; Smith, D.M.; Da-Silva, A.; Rajguru, S. Alcohol modulates cytokine secretion and synthesis in human fetus: An in vivo and in vitro study. Alcohol 2000, 21, 207–213. [Google Scholar] [CrossRef]
- Terasaki, L.S.; Schwarz, J.M. Effects of Moderate Prenatal Alcohol Exposure during Early Gestation in Rats on Inflammation across the Maternal-Fetal-Immune Interface and Later-Life Immune Function in the Offspring. J. Neuroimmune Pharmacol. 2016, 11, 680–692. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, J.J.; Noor, S.; Davies, S.; Savage, D.; Milligan, E.D. Prenatal alcohol exposure is a risk factor for adult neuropathic pain via aberrant neuroimmune function. J. Neuroinflammation 2017, 14, 254. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Dhapola, R.; Sarma, P.; Medhi, B.; Reddy, D.H. Neuroinflammation in Alzheimer’s Disease: Current Progress in Molecular Signaling and Therapeutics. Inflammation 2023, 46, 1–17. [Google Scholar] [CrossRef]
- Al-Ghraiybah, N.F.; Wang, J.; Alkhalifa, A.E.; Roberts, A.B.; Raj, R.; Yang, E.; Kaddoumi, A. Glial Cell-Mediated Neuroinflammation in Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 10572. [Google Scholar] [CrossRef]
- Dhapola, R.; Hota, S.S.; Sarma, P.; Bhattacharyya, A.; Medhi, B.; Reddy, D.H. Recent advances in molecular pathways and therapeutic implications targeting neuroinflammation for Alzheimer’s disease. Inflammopharmacology 2021, 29, 1669–1681. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Tian, Y.; Zheng, L.; Luu, T.; Kwak-Kim, J. The Update Immune-Regulatory Role of Pro- and Anti-Inflammatory Cytokines in Recurrent Pregnancy Losses. Int. J. Mol. Sci. 2022, 24, 132. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Majid, S.; Ali, M.N.; Taing, S.; Rehman, M.U.; Arafah, A. Cytokine imbalance at materno-embryonic interface as a potential immune mechanism for recurrent pregnancy loss. Int. Immunopharmacol. 2021, 90, 107118. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Li, C.; Wang, X.; Liu, X.; Han, C.; Jin, T.; Liu, S.; Zhang, X.; Zhang, H.; He, X.; et al. Increased Toll-Like Receptors Activity and TLR Ligands in Patients with Autoimmune Thyroid Diseases. Front. Immunol. 2016, 7, 578. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Kawai, T. Toll-like receptor signaling pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef]
- Chaturvedi, A.; Pierce, S.K. How location governs toll-like receptor signaling. Traffic 2009, 10, 621–628. [Google Scholar] [CrossRef]
- Reid, N.; Moritz, K.M.; Akison, L.K. Adverse health outcomes associated with fetal alcohol exposure: A systematic review focused on immune-related outcomes. Pediatr. Allergy Immunol. 2019, 30, 698–707. [Google Scholar] [CrossRef]
- Pascual, M.; Montesinos, J.; Montagud-Romero, S.; Forteza, J.; Rodriguez-Arias, M.; Minarro, J.; Guerri, C. TLR4 response mediates ethanol-induced neurodevelopment alterations in a model of fetal alcohol spectrum disorders. J. Neuroinflammation 2017, 14, 145. [Google Scholar] [CrossRef]
- Fernandez-Lizarbe, S.; Pascual, M.; Guerri, C. Critical role of TLR4 response in the activation of microglia induced by ethanol. J. Immunol. 2009, 183, 4733–4744. [Google Scholar] [CrossRef] [PubMed]
- Kane, C.J.M.; Drew, P.D. Neuroinflammatory contribution of microglia and astrocytes in fetal alcohol spectrum disorders. J. Neurosci. Res. 2021, 99, 1973–1985. [Google Scholar] [CrossRef]
- Granato, A.; Dering, B. Alcohol and the Developing Brain: Why Neurons Die and How Survivors Change. Int. J. Mol. Sci. 2018, 19, 2992. [Google Scholar] [CrossRef]
- McGill, J.; Meyerholz, D.K.; Edsen-Moore, M.; Young, B.; Coleman, R.A.; Schlueter, A.J.; Waldschmidt, T.J.; Cook, R.T.; Legge, K.L. Fetal exposure to ethanol has long-term effects on the severity of influenza virus infections. J. Immunol. 2009, 182, 7803–7808. [Google Scholar] [CrossRef] [PubMed]
- Hillmer, A.T.; Nadim, H.; Devine, L.; Jatlow, P.; O’Malley, S.S. Acute alcohol consumption alters the peripheral cytokines IL-8 and TNF-alpha. Alcohol 2020, 85, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Yin, Q.; Zhong, Q.; Lv, F.L.; Zhou, Y.; Li, J.Q.; Wang, J.Z.; Su, B.Y.; Yang, Q.W. Heme activates TLR4-mediated inflammatory injury via MyD88/TRIF signaling pathway in intracerebral hemorrhage. J. Neuroinflammation 2012, 9, 46. [Google Scholar] [CrossRef]
- Zheng, C.; Chen, J.; Chu, F.; Zhu, J.; Jin, T. Inflammatory Role of TLR-MyD88 Signaling in Multiple Sclerosis. Front. Mol. Neurosci. 2019, 12, 314. [Google Scholar] [CrossRef]
- Abbott, C.W.; Kozanian, O.O.; Kanaan, J.; Wendel, K.M.; Huffman, K.J. The Impact of Prenatal Ethanol Exposure on Neuroanatomical and Behavioral Development in Mice. Alcohol. Clin. Exp. Res. 2016, 40, 122–133. [Google Scholar] [CrossRef]
- Lin, C.Y.; Chang, Y.C.; Wang, S.T.; Lee, T.Y.; Lin, C.F.; Huang, C.C. Altered inflammatory responses in preterm children with cerebral palsy. Ann. Neurol. 2010, 68, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Zareen, Z.; Strickland, T.; Fallah, L.; McEneaney, V.; Kelly, L.; McDonald, D.; Molloy, E.J. Cytokine dysregulation in children with cerebral palsy. Dev. Med. Child. Neurol. 2021, 63, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Blocking interleukin-1beta in acute and chronic autoinflammatory diseases. J. Intern. Med. 2011, 269, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, N.; Kurata, M.; Yamamoto, T.; Morikawa, S.; Masumoto, J. The role of interleukin-1 in general pathology. Inflamm. Regen. 2019, 39, 12. [Google Scholar] [CrossRef] [PubMed]
- Machado, J.R.; Soave, D.F.; da Silva, M.V.; de Menezes, L.B.; Etchebehere, R.M.; Monteiro, M.L.; dos Reis, M.A.; Correa, R.R.; Celes, M.R. Neonatal sepsis and inflammatory mediators. Mediators Inflamm. 2014, 2014, 269681. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Dolganiuc, A.; Szabo, G. Toll-like receptors 1 and 6 are involved in TLR2-mediated macrophage activation by hepatitis C virus core and NS3 proteins. J. Leukoc. Biol. 2007, 82, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, M.; Kato, N.; Otsuka, M.; Shao, R.X.; Taniguchi, H.; Kawabe, T.; Omata, M. Interferon-beta is activated by hepatitis C virus NS5B and inhibited by NS4A, NS4B, and NS5A. Hepatol. Int. 2007, 1, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Nascimento, L.; Massari, P.; Wetzler, L.M. The Role of TLR2 in Infection and Immunity. Front. Immunol. 2012, 3, 79. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.H.; Johnson, L.; Limketkai, B.N.; Jusuf, E.; Sun, J.; Kim, B.; Price, J.C.; Woreta, T.A. Trends in the Prevalence of Hepatitis C Infection During Pregnancy and Maternal-Infant Outcomes in the US, 1998 to 2018. JAMA Netw Open 2023, 6, e2324770. [Google Scholar] [CrossRef]
- Lester, S.N.; Li, K. Toll-like receptors in antiviral innate immunity. J. Mol. Biol. 2014, 426, 1246–1264. [Google Scholar] [CrossRef]
- Zhu, K.; Yang, J.; Luo, K.; Yang, C.; Zhang, N.; Xu, R.; Chen, J.; Jin, M.; Xu, B.; Guo, N.; et al. TLR3 signaling in macrophages is indispensable for the protective immunity of invariant natural killer T cells against enterovirus 71 infection. PLoS Pathog. 2015, 11, e1004613. [Google Scholar] [CrossRef]
- Vaure, C.; Liu, Y. A comparative review of toll-like receptor 4 expression and functionality in different animal species. Front. Immunol. 2014, 5, 316. [Google Scholar] [CrossRef]
- Bakhireva, L.N.; Leeman, L.; Roberts, M.; Rodriguez, D.E.; Jacobson, S.W. You Didn’t Drink During Pregnancy, Did You? Alcohol. Clin. Exp. Res. 2021, 45, 543–547. [Google Scholar] [CrossRef]
- Litten, R.Z.; Gardner, M.B. The Basics: Defining How Much Alcohol Is Too Much. Available online: https://www.niaaa.nih.gov/health-professionals-communities/core-resource-on-alcohol/basics-defining-how-much-alcohol-too-much (accessed on 20 January 2024).
- Jacobson, J.L.; Akkaya-Hocagil, T.; Jacobson, S.W.; Coles, C.D.; Richardson, G.A.; Olson, H.C.; Day, N.L.; Carter, R.C.; Dodge, N.C.; Dang, K.D.; et al. A dose-response analysis of the effects of prenatal alcohol exposure on cognitive development. Alcohol. Clin. Exp. Res. 2024, 48, 623–639. [Google Scholar] [CrossRef]
- Carter, R.C.; Senekal, M.; Dodge, N.C.; Bechard, L.J.; Meintjes, E.M.; Molteno, C.D.; Duggan, C.P.; Jacobson, J.L.; Jacobson, S.W. Maternal Alcohol Use and Nutrition During Pregnancy: Diet and Anthropometry. Alcohol. Clin. Exp. Res. 2017, 41, 2114–2127. [Google Scholar] [CrossRef]
- Jacobson, S.W.; Chiodo, L.M.; Sokol, R.J.; Jacobson, J.L. Validity of maternal report of prenatal alcohol, cocaine, and smoking in relation to neurobehavioral outcome. Pediatrics 2002, 109, 815–825. [Google Scholar] [CrossRef]
- Kable, J.A.; Mukherjee, R.A. Neurodevelopmental disorder associated with prenatal exposure to alcohol (ND-PAE): A proposed diagnostic method of capturing the neurocognitive phenotype of FASD. Eur. J. Med. Genet. 2017, 60, 49–54. [Google Scholar] [CrossRef]
- Pyman, P.; Collins, S.E.; Muggli, E.; Testa, R.; Anderson, P.J. Cognitive and Behavioural Attention in Children with Low-Moderate and Heavy Doses of Prenatal Alcohol Exposure: A Systematic Review and Meta-analysis. Neuropsychol. Rev. 2021, 31, 610–627. [Google Scholar] [CrossRef]
- Bandoli, G.; Hayes, S.; Delker, E. Low to Moderate Prenatal Alcohol Exposure and Neurodevelopmental Outcomes: A Narrative Review and Methodological Considerations. Alcohol. Res. 2023, 43, 1. [Google Scholar] [CrossRef]
- Maxwell, J.R.; DiDomenico, J.; Roberts, M.H.; Marquez, L.E.; Rai, R.; Weinberg, J.; Jacobson, S.W.; Stephen, J.; Bakhireva, L.N. Impact of low-level prenatal alcohol exposure and maternal stress on autonomic regulation. Pediatr. Res. 2024, 95, 350–358. [Google Scholar] [CrossRef]
- Ruyak, S.; Roberts, M.H.; Chambers, S.; Ma, X.; DiDomenico, J.; De La Garza, R., 2nd; Bakhireva, L.N. The effect of the COVID-19 pandemic on substance use patterns and physiological dysregulation in pregnant and postpartum women. Alcohol. Clin. Exp. Res. 2023, 47, 1088–1099. [Google Scholar] [CrossRef]
- Ruyak, S.L.; Roberts, M.H.; Chambers, S.; Ma, X.; DiDomenico, J.; De La Garza, R., 2nd; Bakhireva, L.N. Impulsivity and Alcohol Use during Pregnancy and Postpartum: Insights from Novel Methodological Approaches within the Context of the COVID-19 Pandemic. Behav. Sci. 2023, 13, 600. [Google Scholar] [CrossRef]
- Bakhireva, L.N.; Ma, X.; Wiesel, A.; Wohrer, F.E.; DiDomenico, J.; Jacobson, S.W.; Roberts, M.H. Dose-response effect of prenatal alcohol exposure on perinatal outcomes. Alcohol. Clin. Exp. Res. 2024, 48, 703–714. [Google Scholar] [CrossRef]
- Bakhireva, L.N.; Solomon, E.; Roberts, M.H.; Ma, X.; Rai, R.; Wiesel, A.; Jacobson, S.W.; Weinberg, J.; Milligan, E.D. Independent and Combined Effects of Prenatal Alcohol Exposure and Prenatal Stress on Fetal HPA Axis Development. Int. J. Mol. Sci. 2024, 25, 2690. [Google Scholar] [CrossRef]
- Wohrer, F.; Ngo, H.; DiDomenico, J.; Ma, X.; Roberts, M.H.; Bakhireva, L.N. Potentially modifiable risk and protective factors affecting mental and emotional wellness in pregnancy. Front. Hum. Neurosci. 2024, 18, 1323297. [Google Scholar] [CrossRef]
- Jantzie, L.L.; Maxwell, J.R.; Newville, J.C.; Yellowhair, T.R.; Kitase, Y.; Madurai, N.; Ramachandra, S.; Bakhireva, L.N.; Northington, F.J.; Gerner, G.; et al. Prenatal opioid exposure: The next neonatal neuroinflammatory disease. Brain Behav. Immun. 2020, 84, 45–58. [Google Scholar] [CrossRef]
- Grievink, H.W.; Luisman, T.; Kluft, C.; Moerland, M.; Malone, K.E. Comparison of Three Isolation Techniques for Human Peripheral Blood Mononuclear Cells: Cell Recovery and Viability, Population Composition, and Cell Functionality. Biopreserv. Biobank. 2016, 14, 410–415. [Google Scholar] [CrossRef]
- Nombela, I.; Ortega-Villaizan, M.D.M. Nucleated red blood cells: Immune cell mediators of the antiviral response. PLoS Pathog. 2018, 14, e1006910. [Google Scholar] [CrossRef]

| Variable | Healthy Control (N = 10) | PAE (N = 8) | p-Value |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Maternal age | 27.0 ± 6.1 | 27.5 ± 4.9 | 0.85 0 |
| Years of education | 13.3 ± 1.3 | 14.3 ± 2.7 | 0.65 1 |
| Birthweight (grams) | 3028.5 ± 547.8 | 3382.3 ± 286.8 | 0.12 0 |
| N (%) | N (%) | ||
| Marital status: | 0.043 2 | ||
| Single/separated/divorced | 1 (10.0%) | 5 (62.5%) | |
| Married/cohabitating | 9 (90.0%) | 3 (37.5%) | |
| Race: | 0.55 2 | ||
| White | 5 (50.0%) | 4 (50.0%) | |
| Black or African American | 1 (10.0%) | 0 (0.0%) | |
| American Indian | 3 (30.0%) | 1 (12.5%) | |
| Other | 1 (10.0%) | 3 (37.5%) | |
| Education level: | 0.57 2 | ||
| High school or less | 5 (50.0%) | 3 (37.5%) | |
| Some college or vocational school | 4 (40.0%) | 2 (25.0%) | |
| College degree or higher | 1 (10.0%) | 3 (37.5%) | |
| Preterm delivery (<37 weeks) | 1 (10.0%) | 0 (0.0%) | 1.00 2 |
| Infant sex: female | 6 (60.0%) | 2 (25.0%) | 0.19 2 |
| Type of delivery: vaginal | 8 (80.0%) | 8 (100.0%) | 1.00 2 |
| Mean ± SD | Mean ± SD | ||
| AAD a | 0.00 ± 0.00 | 0.45 ± 0.19 | <0.0001 0 |
| AADD a | 0.00 ± 0.00 | 1.82 ± 0.44 | <0.0001 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maxwell, J.R.; Noor, S.; Pavlik, N.; Rodriguez, D.E.; Enriquez Marquez, L.; DiDomenico, J.; Blossom, S.J.; Bakhireva, L.N. Moderate Prenatal Alcohol Exposure Increases Toll-like Receptor Activity in Umbilical Cord Blood at Birth: A Pilot Study. Int. J. Mol. Sci. 2024, 25, 7019. https://doi.org/10.3390/ijms25137019
Maxwell JR, Noor S, Pavlik N, Rodriguez DE, Enriquez Marquez L, DiDomenico J, Blossom SJ, Bakhireva LN. Moderate Prenatal Alcohol Exposure Increases Toll-like Receptor Activity in Umbilical Cord Blood at Birth: A Pilot Study. International Journal of Molecular Sciences. 2024; 25(13):7019. https://doi.org/10.3390/ijms25137019
Chicago/Turabian StyleMaxwell, Jessie R., Shahani Noor, Nathaniel Pavlik, Dominique E. Rodriguez, Lidia Enriquez Marquez, Jared DiDomenico, Sarah J. Blossom, and Ludmila N. Bakhireva. 2024. "Moderate Prenatal Alcohol Exposure Increases Toll-like Receptor Activity in Umbilical Cord Blood at Birth: A Pilot Study" International Journal of Molecular Sciences 25, no. 13: 7019. https://doi.org/10.3390/ijms25137019





