Nanomedicine in the Treatment of Diabetes
Abstract
:1. Introduction
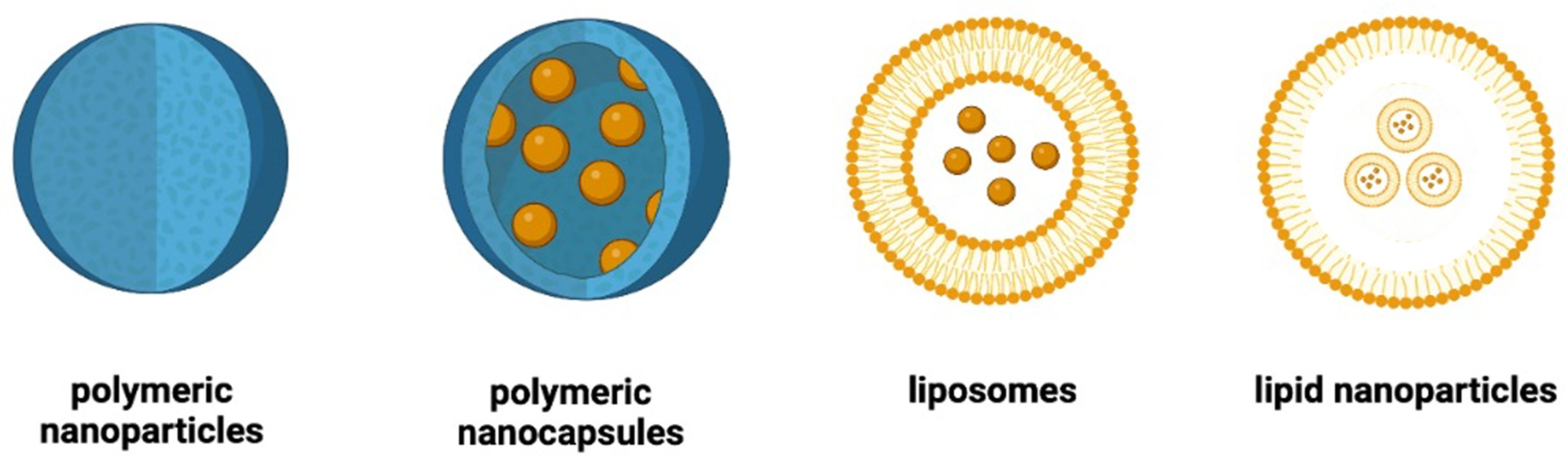
2. Main Types of Nanoparticles Used in the Management of Diabetes
2.1. Polymeric Nanoparticles
2.2. Polymeric Nanocapsules
2.3. Liposomes
2.4. Lipid Nanoparticles
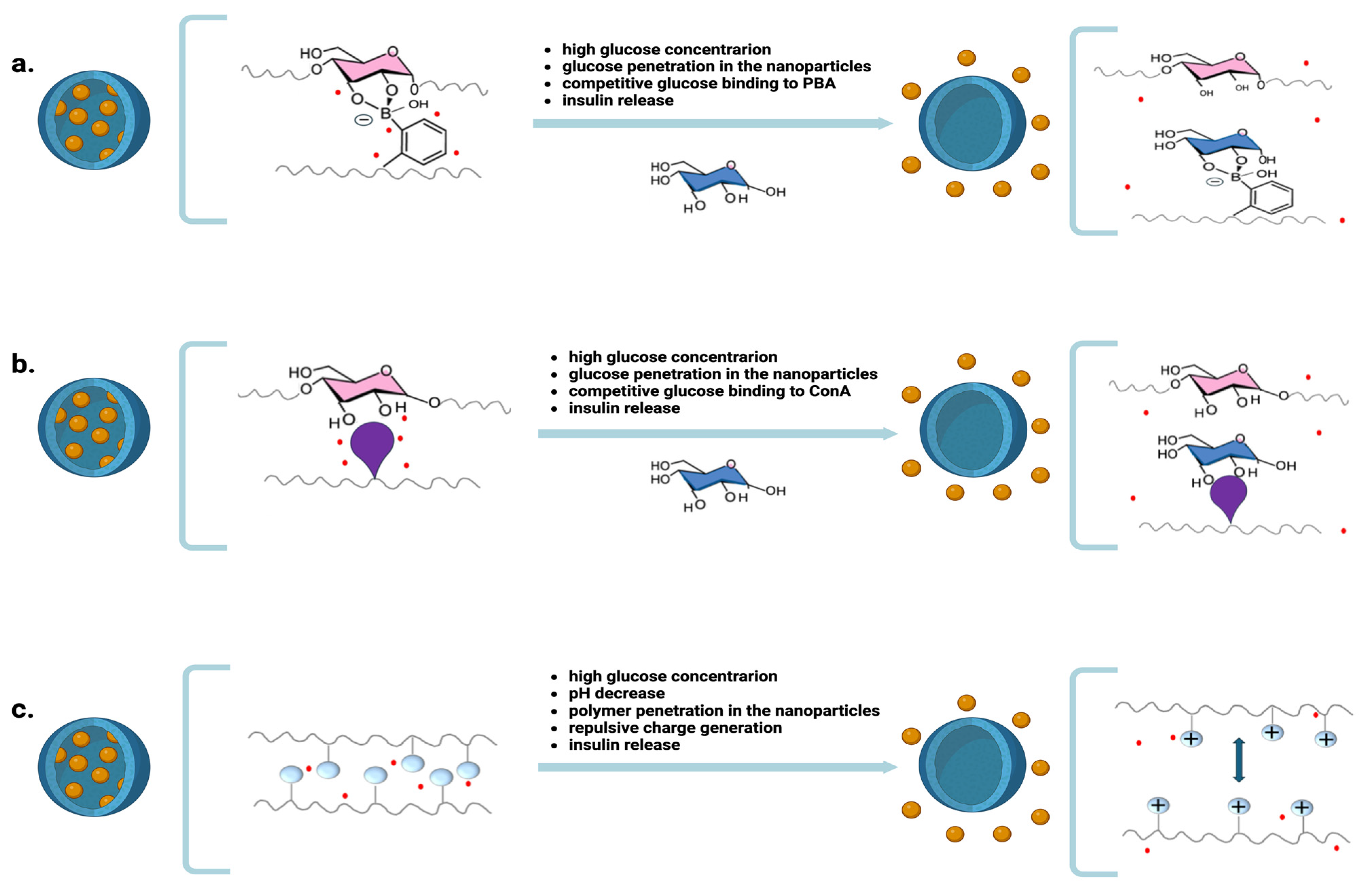
3. Nanoparticles for Releasing Insulin in Response to Blood Glucose Levels
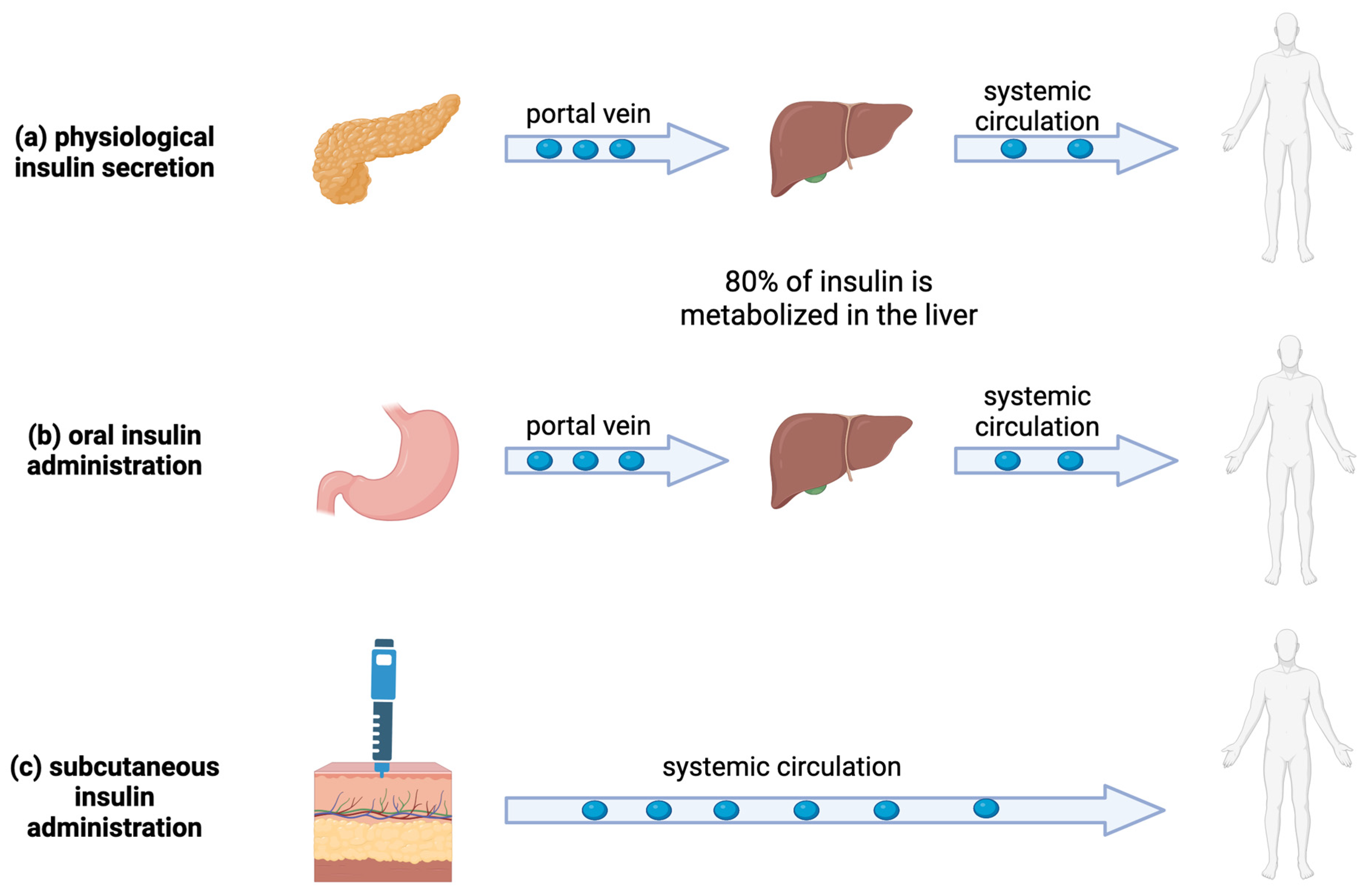
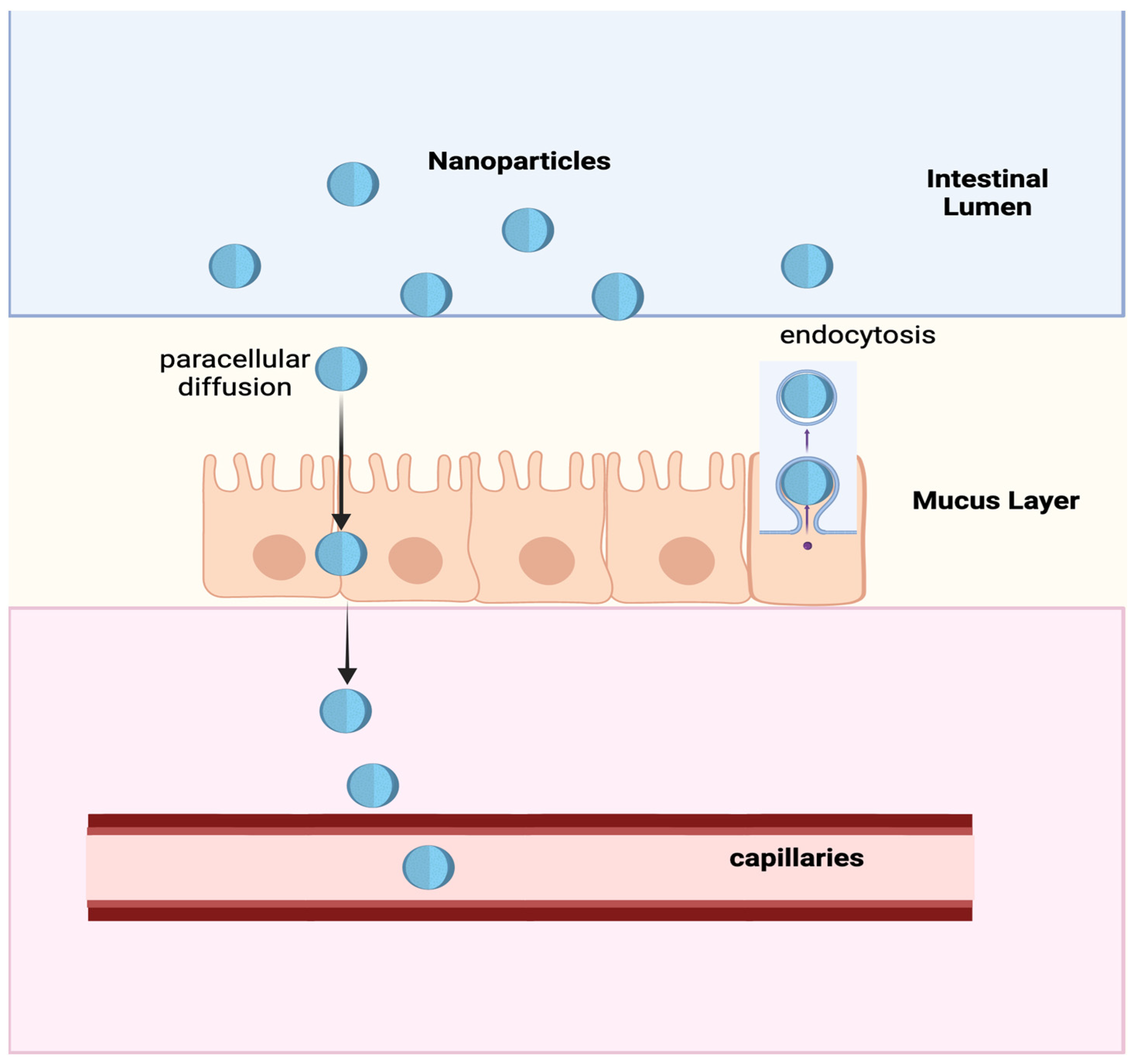
4. Nanoparticles for the Oral Administration of Insulin
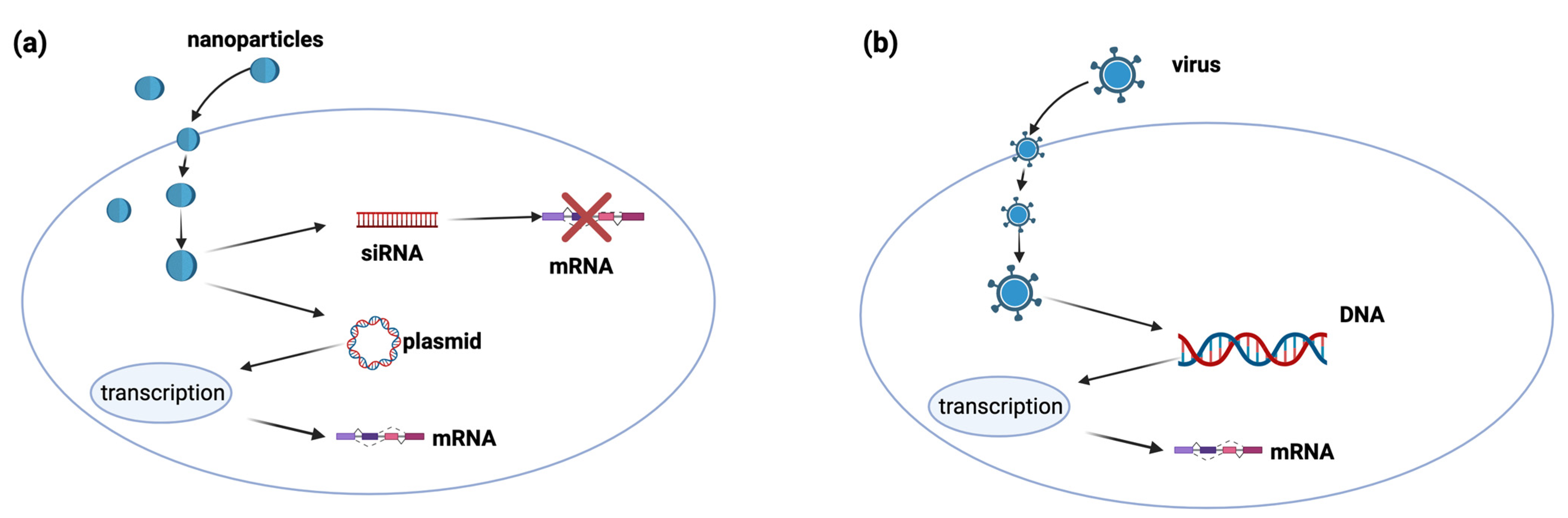
5. Nanoparticles for Gene Therapy
6. Nanoparticles for Cell Therapy
7. Nanoparticles in Diabetic Wound Healing
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2019. Diabetes Care 2019, 42 (Suppl. S1), S13–S28. [Google Scholar] [CrossRef]
- Dabelea, D. The Accelerating Epidemic of Childhood Diabetes. Lancet 2009, 373, 1999–2000. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, S.M.; Di Lorenzo, T.P. A Comprehensive Guide to Antibody and T-cell Responses in Type 1 Diabetes. Tissue Antigens 2003, 62, 359–377. [Google Scholar] [CrossRef] [PubMed]
- Donath, M.Y.; Shoelson, S.E. Type 2 Diabetes as an Inflammatory Disease. Nat. Rev. Immunol. 2011, 11, 98–107. [Google Scholar] [CrossRef]
- The Emerging Risk Factors Collaboration. Diabetes Mellitus, Fasting Blood Glucose Concentration, and Risk of Vascular Disease: A Collaborative Meta-Analysis of 102 Prospective Studies. Lancet 2010, 375, 2215–2222. [Google Scholar] [CrossRef]
- Schulman, R.C.; Moshier, E.L.; Rho, L.; Casey, M.F.; Godbold, J.H.; Mechanick, J.I. Association of Glycemic Control Parameters with Clinical Outcomes in Chronic Critical Illness. Endocr. Pract. 2014, 20, 884–893. [Google Scholar] [CrossRef]
- Pickup, J.C. Insulin-Pump Therapy for Type 1 Diabetes Mellitus. N. Engl. J. Med. 2012, 366, 1616–1624. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.A.; Kovatchev, B.P.; Raghinaru, D.; Lum, J.W.; Buckingham, B.A.; Kudva, Y.C.; Laffel, L.M.; Levy, C.J.; Pinsker, J.E.; Wadwa, R.P.; et al. iDCL Trial Research Group. Six-Month Randomized, Multicenter Trial of Closed-Loop Control in Type. N. Engl. J. Med. 2019, 381, 1707–1717. [Google Scholar] [CrossRef]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an Emerging Platform for Cancer Therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef]
- Betty, Y.S.; Kim, M.D.; Rutka, J.T.; Chan, W.C.W. Nanomedicine. N. Engl. J. Med. 2010, 363, 2434–2443. [Google Scholar] [CrossRef]
- Thatte, A.S.; Billingsley, M.M.; Weissman, D.; Melamed, J.R.; Mitchell, M.J. Emerging Strategies for Nanomedicine in Autoimmunity. Adv. Drug Deliv. Rev. 2024, 207, 115194. [Google Scholar] [CrossRef] [PubMed]
- Zaid, A.; Ariel, A. Harnessing Anti-Inflammatory Pathways and Macrophage Nano Delivery to Treat Inflammatory and Fibrotic Disorders. Adv. Drug Deliv. Rev. 2024, 207, 115204. [Google Scholar] [CrossRef] [PubMed]
- Tsokos, G.C.; Nepom, G.T. Gene Therapy in the Treatment of Autoimmune Diseases. J. Clin. Investig. 2000, 106, 181–183. [Google Scholar] [CrossRef] [PubMed]
- Siwakoti, P.; Rennie, C.; Huang, Y.; Li, J.J.; E Tuch, B.; McClements, L.; Xu, X. Challenges with Cell-Based Therapies for Type 1 Diabetes Mellitus. Stem Cell Rev. Rep. 2023, 19, 601–624. [Google Scholar] [CrossRef] [PubMed]
- Lemmerman, L.R.; Das, D.; Higuita-Castro, N.; Mirmira, R.G.; Gallego-Perez, D. Nanomedicine-Based Strategies for Diabetes: Diagnostics, Monitoring, and Treatment. Trends Endocrinol. Metab. 2020, 31, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Ji, K.; Wang, Y.; Gu, Z.; Wang, J. Materials and Carriers Development for Glucose-Responsive Insulin. Acc. Mater. Res. 2022, 3, 960–970. [Google Scholar] [CrossRef]
- De Vos, P.; Faas, M.M.; Strand, B.; Calafiore, R. Alginate-Based Microcapsules for Immunoisolation of Pancreatic Islets. Biomaterials 2006, 27, 5603–5617. [Google Scholar] [CrossRef]
- Khoshnevisan, K.; Sajjadi-Jazi, S.M. Diabetic Stem Cell Therapy and Nanomedicine: Advancements in Treating Diabetes. J. Diabetes Metab. Disord. 2023, 22, 1805–1807. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering Precision Nanoparticles for Drug Delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Afsharzadeh, M.; Hashemi, M.; Mokhtarzadeh, A.; Abnous, K.; Ramezani, M. Recent Advances in Co-Delivery Systems Based on Polymeric Nanoparticle for Cancer Treatment. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1095–1110. [Google Scholar] [CrossRef]
- Purohit, D.; Jalwal, P.; Manchanda, D.; Saini, S.; Verma, R.; Kaushik, D.; Mittal, V.; Kumar, M.; Bhattacharya, T.; Rahman, H.; et al. Nanocapsules: An Emerging Drug Delivery System. Recent Pat. Nanotechnol. 2023, 17, 190–207. [Google Scholar] [CrossRef] [PubMed]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.K.K.; Tam, Y.Y.C.; Chen, S.; Hafez, I.M.; Cullis, P.R. Microfluidic Mixing: A General Method for Encapsulating Macromolecules in Lipid Nanoparticle Systems. J. Phys. Chem. B 2015, 119, 8698–8706. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sun, H.; Mu, T. Materials and Structure of Polysaccharide-Based Delivery Carriers for Oral Insulin: A Review. Carbohydr. Polym. 2024, 323, 121364. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, A.; Ishii, T.; Nishida, J.; Matsumoto, H.; Kataoka, K.; Miyahara, Y. A Synthetic Approach Toward a Self-Regulated Insulin Delivery System. Angew. Chem. Int. Ed. 2012, 51, 2124–2128. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, L.; Yu, H.; Wang, J.; Chen, Z. Organization of Glucose-Responsive Systems and Their Properties. Chem. Rev. 2011, 111, 7855–7875. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.; Fukushima, S.; Harada, A.; Kataoka, K. Design of Environment-Sensitive Supramolecular Assemblies for Intracellular Drug Delivery: Polymeric Micelles That Are Responsive to Intracellular pH Change. Angew. Chem. Int. Ed. 2003, 42, 4640–4643. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, A.; Yoshida, R.; Kataoka, K. Glucose-Responsive Polymer Gel Bearing Phenylborate Derivative as a Glucose-Sensing Moiety Operating at the Physiological pH. Biomacromolecules 2004, 5, 1038–1045. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zhou, S. Responsive Materials for Self- R Egulated Insulin Delivery. Macromol. Biosci. 2013, 13, 1464–1477. [Google Scholar] [CrossRef]
- Brownlee, M.; Cerami, A. A Glucose-Controlled Insulin-Delivery System: Semisynthetic Insulin Bound to Lectin. Science 1979, 206, 1190–1191. [Google Scholar] [CrossRef]
- Bankar, S.B.; Bule, M.V.; Singhal, R.S.; Ananthanarayan, L. Glucose Oxidase—An Overview. Biotechnol. Adv. 2009, 27, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Ferri, S.; Kojima, K.; Sode, K. Review of Glucose Oxidases and Glucose Dehydrogenases: A Bird’s Eye View of Glucose Sensing Enzymes. J. Diabetes Sci. Technol. 2011, 5, 1068–1076. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Dang, T.T.; Ma, M.; Tang, B.C.; Cheng, H.; Jiang, S.; Dong, Y.; Zhang, Y.; Anderson, D.G. Glucose-Responsive Microgels Integrated with Enzyme Nanocapsules for Closed-Loop Insulin Delivery. ACS Nano 2013, 7, 6758–6766. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Cao, S.; Chen, X.; Liu, S.; Tan, H.; Wu, W.; Li, J. Super Long-Term Glycemic Control in Diabetic Rats by Glucose-Sensitive LbL Films Constructed of Supramolecular Insulin Assembly. Biomaterials 2012, 33, 8733–8742. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Yan, X.; Fei, J.; Wang, A.; Cui, Y.; Li, J. Triggered Release of Insulin from Glucose-Sensitive Enzyme Multilayer Shells. Biomaterials 2009, 30, 2799–2806. [Google Scholar] [CrossRef] [PubMed]
- Veiseh, O.; Tang, B.C.; Whitehead, K.A.; Anderson, D.G.; Langer, R. Managing Diabetes with Nanomedicine: Challenges and Opportunities. Nat. Rev. Drug Discov. 2015, 14, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Pang, H.; Huang, X.; Xu, Z.P.; Chen, C.; Han, F.Y. Progress in Oral Insulin Delivery by PLGA Nanoparticles for the Management of Diabetes. Drug Discov. Today 2023, 28, 103393. [Google Scholar] [CrossRef] [PubMed]
- Bakhru, S.H.; Furtado, S.; Morello, A.P.; Mathiowitz, E. Oral Delivery of Proteins by Biodegradable Nanoparticles. Adv. Drug Deliv. Rev. 2013, 65, 811–821. [Google Scholar] [CrossRef] [PubMed]
- Rabanel, J.M.; Aoun, V.; Elkin, I.; Mokhtar, M.; Hildgen, P. Drug-Loaded Nanocarriers: Passive Targeting and Crossing of Biological Barriers. Curr. Med. Chem. 2012, 19, 3070–3102. [Google Scholar] [CrossRef]
- Jepson, M.A.; Clark, M.A.; Hirst, B.H. M Cell Targeting by Lectins: A Strategy for Mucosal Vaccination and Drug Delivery. Adv. Drug Deliv. Rev. 2004, 56, 511–525. [Google Scholar] [CrossRef]
- Pridgen, E.M.; Alexis, F.; Kuo, T.T.; Levy-Nissenbaum, E.; Karnik, R.; Blumberg, R.S.; Langer, R.; Farokhzad, O.C. Transepithelial Transport of Fc-Targeted Nanoparticles by the Neonatal Fc Receptor for Oral Delivery. Sci. Transl. Med. 2013, 5, 213ra167. [Google Scholar] [CrossRef]
- Hunt, N.J.; Lockwood, G.P.; Heffernan, S.J.; Daymond, J.; Ngu, M.; Narayanan, R.K.; Westwood, L.J.; Mohanty, B.; Esser, L.; Williams, C.C.; et al. Oral Nanotherapeutic Formulation of Insulin with Reduced Episodes of Hypoglycaemia. Nat. Nanotechnol. 2024, 19, 534–544. [Google Scholar] [CrossRef]
- Barfar, A.; Alizadeh, H.; Masoomzadeh, S.; Javadzadeh, Y. Oral Insulin Delivery: A Review on Recent Advancements and NovelStrategies. Curr. Drug Deliv. 2024, 21, 887–900. [Google Scholar] [CrossRef]
- Sumaila, M.; Marimuthu, T.; Kumar, P.; Choonara, Y.E. Lipopolysaccharide Nanosystems for the Enhancement of Oral Bioavailability. AAPS PharmSciTech 2021, 22, 242. [Google Scholar] [CrossRef]
- Dunbar, C.E.; High, K.A.; Joung, J.K.; Kohn, D.B.; Ozawa, K.; Sadelain, M. Gene Therapy Comes of Age. Science 2018, 359, eaan4672. [Google Scholar] [CrossRef]
- Xu, R.; Li, H.; Lai-yin, T.; Hsiang-fu, K.; Lu, H.; Lam, K.S. Diabetes Gene Therapy: Potential and Challenges. Curr. Gene Ther. 2003, 3, 65–82. [Google Scholar] [CrossRef]
- Wong, M.S.; Hawthorne, W.J.; Manolios, N. Gene Therapy in Diabetes. Self/Nonself 2010, 1, 165–175. [Google Scholar] [CrossRef]
- Mali, S. Delivery Systems for Gene Therapy. Indian J. Hum. Genet. 2013, 19, 3. [Google Scholar] [CrossRef]
- Li, X.; Le, Y.; Zhang, Z.; Nian, X.; Liu, B.; Yang, X. Viral Vector-Based Gene Therapy. Int. J. Mol. Sci. 2023, 24, 7736. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.Y.; Wong, J.L.M.; Sim, Y.J.; Wong, S.S.; Elhassan, S.A.M.; Tan, S.H.; Lim, G.P.L.; Tay, N.W.R.; Annan, N.C.; Bhattamisra, S.K. Type 1 and 2 Diabetes Mellitus: A Review on Current Treatment Approach and Gene Therapy as Potential Intervention. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Chellappan, D.K.; Sivam, N.S.; Teoh, K.X.; Leong, W.P.; Fui, T.Z.; Chooi, K.; Khoo, N.; Yi, F.J.; Chellian, J.; Cheng, L.L.; et al. Gene Therapy and Type 1 Diabetes Mellitus. Biomed. Pharmacother. 2018, 108, 1188–1200. [Google Scholar] [CrossRef]
- Ikeda-Imafuku, M.; Wang, L.L.-W.; Rodrigues, D.; Shaha, S.; Zhao, Z.; Mitragotri, S. Strategies to Improve the EPR Effect: A Mechanistic Perspective and Clinical Translation. J. Control. Release 2022, 345, 512–536. [Google Scholar] [CrossRef]
- Liu, Y.; Koziol, J.; Deisseroth, A.; Borgstrom, P. Methods for Delivery of Adenoviral Vectors to Tumor Vasculature. Hum. Gene Ther. 2007, 18, 151–160. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, S.; Li, X.; Zheng, P.; Hu, F.; Zhou, Z. Vaccination with a Co-expression DNA Plasmid Containing GAD65 Fragment Gene and IL-10 Gene Induces Regulatory CD4+ T Cells That Prevent Experimental Autoimmune Diabetes. Diabetes/Metab. Res. Rev. 2016, 32, 522–533. [Google Scholar] [CrossRef]
- Chellappan, D.K.; Yap, W.S.; Suhaimi, N.A.B.A.; Gupta, G.; Dua, K. Current Therapies and Targets for Type 2 Diabetes Mellitus. Panminerva Medica 2018, 60, 117–131. [Google Scholar] [CrossRef]
- Bakay, M.; Pandey, R.; Hakonarson, H. Genes Involved in Type 1 Diabetes: An Update’. Genes 2013, 4, 499–521. [Google Scholar] [CrossRef]
- Kwak, S.H.; Park, K.S. Recent Progress in Genetic and Epigenetic Research on Type 2 Diabetes. Exp. Mol. Med. 2016, 48, e220. [Google Scholar] [CrossRef]
- Neumann, U.H.; Ho, J.S.S.; Chen, S.; Tam, Y.Y.C.; Cullis, P.R.; Kieffer, T.J. Lipid Nanoparticle Delivery of Glucagon Receptor siRNA Improves Glucose Homeostasis in Mouse Models of Diabetes. Mol. Metab. 2017, 6, 1161–1172. [Google Scholar] [CrossRef]
- Oh, S.; Lee, M.; Ko, K.S.; Choi, S.; Kim, S.W. GLP-1 Gene Delivery for the Treatment of Type 2 Diabetes. Mol. Ther. 2003, 7, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Czech, M.P.; Aouadi, M.; Tesz, G.J. RNAi-Based Therapeutic Strategies for Metabolic Disease. Nat. Rev. Endocrinol. 2011, 7, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Koh, J.J.; Ko, K.S.; Lee, M.; Han, S.; Park, J.S.; Kim, S.W. Degradable Polymeric Carrier for the Delivery of IL-10 Plasmid DNA to Prevent Autoimmune Insulitis of NOD Mice. Gene Ther. 2000, 7, 2099–2104. [Google Scholar] [CrossRef]
- Ko, K.S.; Lee, M.; Koh, J.J.; Kim, S.W. Combined Administration of Plasmids Encoding IL-4 and IL-10 Prevents the Development of Autoimmune Diabetes in Nonobese Diabetic Mice. Mol. Ther. 2001, 4, 313–316. [Google Scholar] [CrossRef]
- Li, F.; Mahato, R.I. RNA Interference for Improving the Outcome of Islet Transplantation. Adv. Drug Deliv. Rev. 2011, 63, 47–68. [Google Scholar] [CrossRef]
- Sheik Abdulazeez, S. Diabetes Treatment: A Rapid Review of the Current and Future Scope of Stem Cell Research. Saudi Pharm. J. 2015, 23, 333–340. [Google Scholar] [CrossRef]
- Bonner-Weir, S.; Baxter, L.A.; Schuppin, G.T.; Smith, F.E. A Second Pathway for Regeneration of Adult Exocrine and Endocrine Pancreas: A Possible Recapitulation of Embryonic Development. Diabetes 1993, 42, 1715–1720. [Google Scholar] [CrossRef]
- Vethe, H.; Bjørlykke, Y.; Ghila, L.M.; Paulo, J.A.; Scholz, H.; Gygi, S.P.; Chera, S.; Ræder, H. Probing the Missing Mature β-Cell Proteomic Landscape in Differentiating Patient iPSC-Derived Cells. Sci. Rep. 2017, 7, 4780. [Google Scholar] [CrossRef]
- Cho, E.Y.; Ryu, J.-Y.; Lee, H.A.R.; Hong, S.H.; Park, H.S.; Hong, K.S.; Park, S.-G.; Kim, H.P.; Yoon, T.-J. Lecithin Nano-Liposomal Particle as a CRISPR/Cas9 Complex Delivery System for Treating Type 2 Diabetes. J. Nanobiotechnol. 2019, 17, 19. [Google Scholar] [CrossRef]
- Luo, Y.-L.; Xu, C.-F.; Li, H.-J.; Cao, Z.-T.; Liu, J.; Wang, J.-L.; Du, X.-J.; Yang, X.-Z.; Gu, Z.; Wang, J. Macrophage-Specific In Vivo Gene Editing Using Cationic Lipid-Assisted Polymeric Nanoparticles. ACS Nano 2018, 12, 994–1005. [Google Scholar] [CrossRef]
- Robertson, R.P. Islet Transplantation as a Treatment for Diabetes—A Work in Progress. N. Engl. J. Med. 2004, 350, 694–705. [Google Scholar] [CrossRef] [PubMed]
- Lanza, R.P.; Hayes, J.L.; Chick, W.L. Encapsulated Cell Technology. Nat. Biotechnol. 1996, 14, 1107–1111. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.T.; Cui, W.; Chaikof, E.L. Layer-by-Layer Assembly of a Conformal Nanothin PEG Coating for Intraportal Islet Transplantation. Nano Lett. 2008, 8, 1940–1948. [Google Scholar] [CrossRef]
- Krol, S.; Del Guerra, S.; Grupillo, M.; Diaspro, A.; Gliozzi, A.; Marchetti, P. Multilayer Nanoencapsulation. New Approach for Immune Protection of Human Pancreatic Islets. Nano Lett. 2006, 6, 1933–1939. [Google Scholar] [CrossRef] [PubMed]
- Chereddy, K.K.; Lopes, A.; Koussoroplis, S.; Payen, V.; Moia, C.; Zhu, H.; Sonveaux, P.; Carmeliet, P.; Rieux, A.D.; Vandermeulen, G.; et al. Combined Effects of PLGA and Vascular Endothelial Growth Factor Promote the Healing of Non-Diabetic and Diabetic Wounds. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1975–1984. [Google Scholar] [CrossRef]
- Contreras, J.L.; Xie, D.; Mays, J.; Smyth, C.A.; Eckstein, C.; Rahemtulla, F.G.; Young, C.J.; Thompson, J.A.; Bilbao, G.; Curiel, D.T.; et al. A Novel Approach to Xenotransplantation Combining Surface Engineering and Genetic Modification of Isolated Adult Porcine Islets. Surgery 2004, 136, 537–547. [Google Scholar] [CrossRef]
- Buder, B.; Alexander, M.; Krishnan, R.; Chapman, D.W.; Lakey, J.R. Encapsulated Islet Transplantation: Strategies and Clinical Trials. Immune Netw. 2013, 13, 235. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, P.; Gorain, B.; Low, S.Y.; Tan, S.A.; Ling, E.C.S.; Lim, Y.K.; Chin, C.M.; Lee, P.Y.; Lee, C.M.; Ooi, C.H.; et al. Nanotechnology based approaches for anti-diabetic drugs delivery. Diabetes Res. Clin. Pract. 2018, 136, 52–77. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-J.; Fan, J.; Zhou, J.; Ren, Y.-T.; Shen, C.; Che, G.-W. Diabetes Mellitus and Risk of Bronchopleural Fistula After Pulmonary Resections: A Meta-Analysis. Ann. Thorac. Surg. 2016, 102, 328–339. [Google Scholar] [CrossRef]
- Blanco-Fernandez, B.; Castaño, O.; Mateos-Timoneda, M.Á.; Engel, E.; Pérez-Amodio, S. Nanotechnology Approaches in Chronic Wound Healing. Adv. Wound Care 2021, 10, 234–256. [Google Scholar] [CrossRef]
- Haque, S.T.; Saha, S.K.; Haque, M.E.; Biswas, N. Nanotechnology-Based Therapeutic Applications: In Vitro and in Vivo Clinical Studies for Diabetic Wound Healing. Biomater. Sci. 2021, 9, 7705–7747. [Google Scholar] [CrossRef]
- Qi, X.; Huan, Y.; Si, H.; Zou, J.; Mu, Z. Study of the Effect Epidermal Growth Factor Nanoparticles in the Treatment of Diabetic Rat Ulcer Skin and Regeneration. J. Nanosci. Nanotechnol. 2021, 21, 3028–3034. [Google Scholar] [CrossRef]
- Chu, Y.; Yu, D.; Wang, P.; Xu, J.; Li, D.; Ding, M. Nanotechnology Promotes the Full-Thickness Diabetic Wound Healing Effect of Recombinant Human Epidermal Growth Factor in Diabetic Rats: rhEGF Nanoparticles Treat the Wounds in Diabetic Rats. Wound Repair Regen. 2010, 18, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Hajimiri, M.; Shahverdi, S.; Esfandiari, M.A.; Larijani, B.; Atyabi, F.; Rajabiani, A.; Dehpour, A.R.; Amini, M.; Dinarvand, R. Preparation of Hydrogel Embedded Polymer-Growth Factor Conjugated Nanoparticles as a Diabetic Wound Dressing. Drug Dev. Ind. Pharm. 2016, 42, 707–719. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Wu, Y.; Liu, J.; Yuan, X.; Gao, J. A Comprehensive Review of the Application of Nanoparticles in Diabetic Wound Healing: Therapeutic Potential and Future Perspectives. Int. J. Nanomed. 2022, 17, 6007–6029. [Google Scholar] [CrossRef] [PubMed]
- Meng, B.; Li, J.; Cao, H. Antioxidant and antiinflammatory activities of curcumin on diabetes mellitus and its complications. Curr Pharm Des. 2013, 19, 2101–2113. [Google Scholar] [PubMed]
- Shen, Y.-I.; Cho, H.; Papa, A.E.; Burke, J.A.; Chan, X.Y.; Duh, E.J.; Gerecht, S. Engineered Human Vascularized Constructs Accelerate Diabetic Wound Healing. Biomaterials 2016, 102, 107–119. [Google Scholar] [CrossRef]
- Kamar, S.S.; Abdel-Kader, D.H.; Rashed, L.A. Beneficial Effect of Curcumin Nanoparticles-Hydrogel on Excisional Skin Wound Healing in Type-I Diabetic Rat: Histological and Immunohistochemical Studies. Ann. Anat.-Anat. Anz. 2019, 222, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Gourishetti, K.; Keni, R.; Nayak, P.G.; Jitta, S.R.; Bhaskaran, N.A.; Kumar, L.; Kumar, N.; Nandakumar, K.; Shenoy, R.R. Sesamol-Loaded PLGA Nanosuspension for Accelerating Wound Healing in Diabetic Foot Ulcer in Rats. Int. J. Nanomed. 2020, 15, 9265–9282. [Google Scholar] [CrossRef]
- Lopes Rocha Correa, V.; Martins, J.A.; De Souza, T.R.; De Castro Nunes Rincon, G.; Miguel, M.P.; De Menezes, L.B.; Amaral, A.C. Melatonin Loaded Lecithin-Chitosan Nanoparticles Improved the Wound Healing in Diabetic Rats. Int. J. Biol. Macromol. 2020, 162, 1465–1475. [Google Scholar] [CrossRef]
- Bairagi, U.; Mittal, P.; Singh, J.; Mishra, B. Preparation, characterization, and in vivo evaluation of nano formulations of ferulic acid in diabetic wound healing. Drug Dev. Ind. Pharm. 2018, 44, 1783–1796. [Google Scholar] [CrossRef]
- Arantes, V.T.; Faraco, A.A.G.; Ferreira, F.B.; Oliveira, C.A.; Martins-Santos, E.; Cassini-Vieira, P.; Barcelos, L.S.; Ferreira, L.A.; Goulart, G.A. Retinoic acid-loaded solid lipid nanoparticles surrounded by chitosan film support diabetic wound healing in in vivo study. Colloids Surf. B Biointerfaces 2020, 188, 110749. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andreadi, A.; Lodeserto, P.; Todaro, F.; Meloni, M.; Romano, M.; Minasi, A.; Bellia, A.; Lauro, D. Nanomedicine in the Treatment of Diabetes. Int. J. Mol. Sci. 2024, 25, 7028. https://doi.org/10.3390/ijms25137028
Andreadi A, Lodeserto P, Todaro F, Meloni M, Romano M, Minasi A, Bellia A, Lauro D. Nanomedicine in the Treatment of Diabetes. International Journal of Molecular Sciences. 2024; 25(13):7028. https://doi.org/10.3390/ijms25137028
Chicago/Turabian StyleAndreadi, Aikaterini, Pietro Lodeserto, Federica Todaro, Marco Meloni, Maria Romano, Alessandro Minasi, Alfonso Bellia, and Davide Lauro. 2024. "Nanomedicine in the Treatment of Diabetes" International Journal of Molecular Sciences 25, no. 13: 7028. https://doi.org/10.3390/ijms25137028
APA StyleAndreadi, A., Lodeserto, P., Todaro, F., Meloni, M., Romano, M., Minasi, A., Bellia, A., & Lauro, D. (2024). Nanomedicine in the Treatment of Diabetes. International Journal of Molecular Sciences, 25(13), 7028. https://doi.org/10.3390/ijms25137028








