Integrated Metabolomics and Transcriptomics Analysis of Anacardic Acid Inhibition of Breast Cancer Cell Viability
Abstract
:1. Introduction
2. Results and Discussion
2.1. AnAc Inhibits TNBC Cell Viability
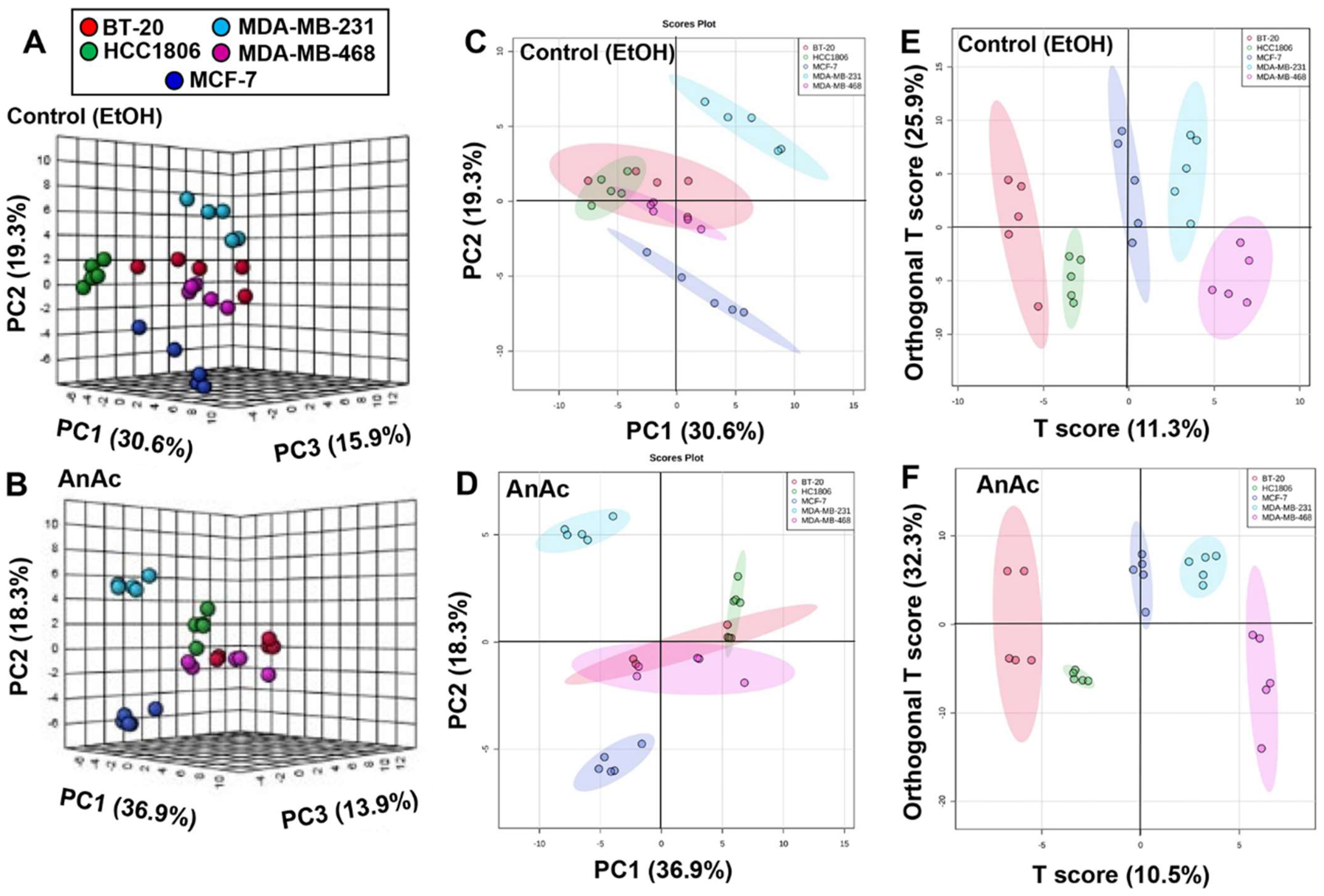
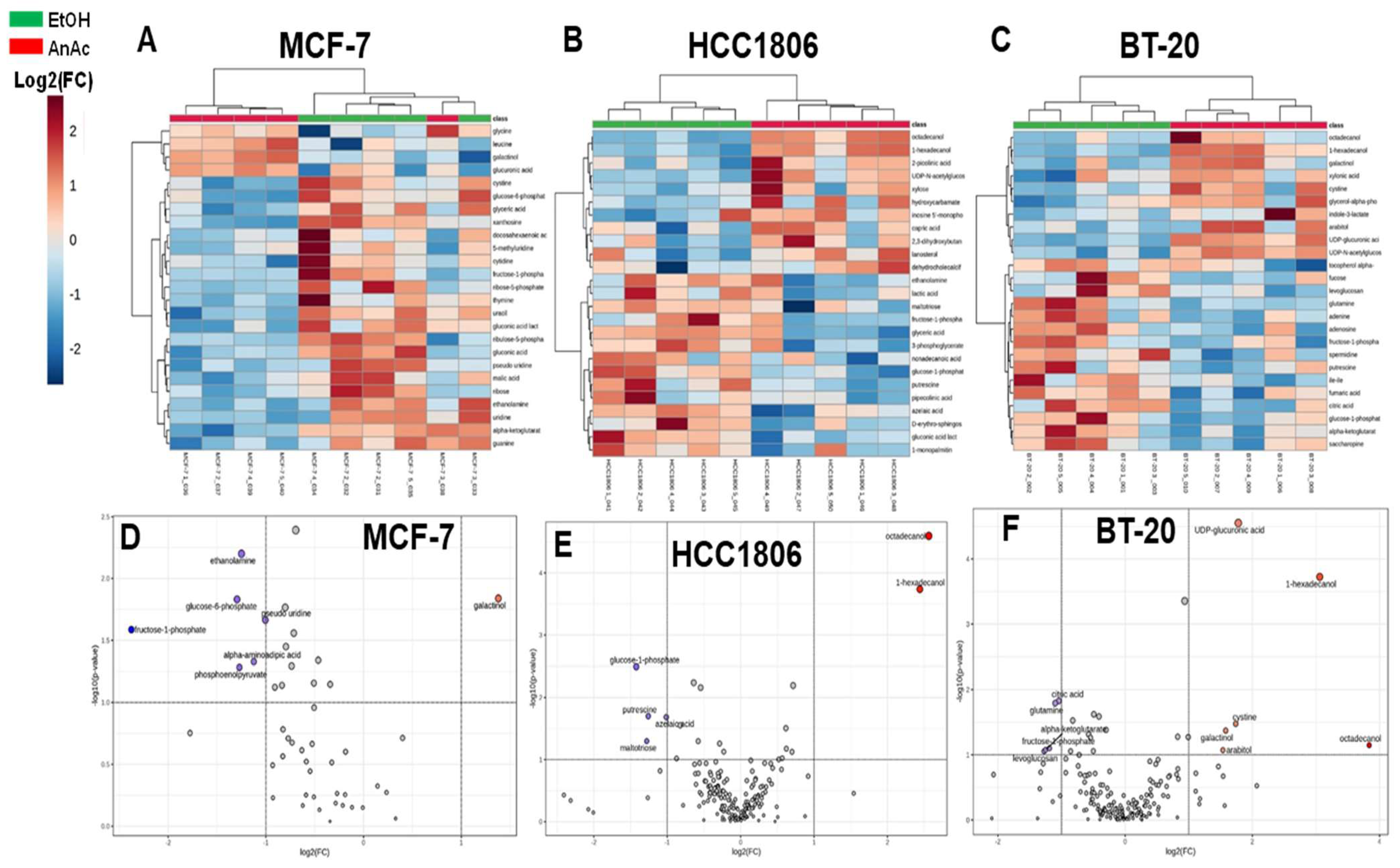
2.2. Metabolome Changes in Response to AnAc in BC Cells
2.3. Pathway Analysis of the Effect of AnAc on Metabolites in BC Cell Lines
2.4. Integration of Multi-Omics Analysis of the Effect of AnAc in MCF-7 and MDA-MB-231 Cells
3. Conclusions
Limitations
4. Materials and Methods
4.1. Materials
4.2. Cell Culture and Treatments
4.3. Metabolomics
4.4. In Silico Analysis
4.5. Integrative Analysis of Metabolomics and Transcriptomics
4.6. Venn Diagram
4.7. Western Blots
4.8. Cell Titer MTT Assay and FluoReporter™ Blue Fluorometric dsDNA (Double-Stranded DNA) Quantification Assay
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Jhaveri, K.; Marmé, F. Current and emerging treatment approaches for hormone receptor-positive/human epidermal growth factor receptor 2-negative metastatic breast cancer. Cancer Treat. Rev. 2024, 123, 102670. [Google Scholar] [CrossRef]
- Nolan, E.; Lindeman, G.J.; Visvader, J.E. Deciphering breast cancer: From biology to the clinic. Cell 2023, 186, 1708–1728. [Google Scholar] [CrossRef]
- Harbeck, N. Risk-adapted adjuvant therapy of luminal early breast cancer in 2020. Curr. Opin. Obs. Gynecol. 2021, 33, 53–58. [Google Scholar] [CrossRef]
- Bianchini, G.; De Angelis, C.; Licata, L.; Gianni, L. Treatment landscape of triple-negative breast cancer—Expanded options, evolving needs. Nat. Rev. Clin. Oncol. 2022, 19, 91–113. [Google Scholar] [CrossRef]
- Zong, Y.; Pegram, M. Research advances and new challenges in overcoming triple-negative breast cancer. Cancer Drug Resist. 2021, 4, 517–542. [Google Scholar] [CrossRef]
- Szymiczek, A.; Lone, A.; Akbari, M.R. Molecular intrinsic versus clinical subtyping in breast cancer: A comprehensive review. Clin. Genet. 2021, 99, 613–637. [Google Scholar] [CrossRef]
- Borri, F.; Granaglia, A. Pathology of triple negative breast cancer. Semin. Cancer Biol. 2021, 72, 136–145. [Google Scholar] [CrossRef]
- Daniels, V.W.; Zoeller, J.J.; van Gastel, N.; McQueeney, K.E.; Parvin, S.; Potter, D.S.; Fell, G.G.; Ferreira, V.G.; Yilma, B.; Gupta, R.; et al. Metabolic perturbations sensitize triple-negative breast cancers to apoptosis induced by BH3 mimetics. Sci. Signal. 2021, 14, eabc7405. [Google Scholar] [CrossRef]
- Davison, C.; Morelli, R.; Knowlson, C.; McKechnie, M.; Carson, R.; Stachtea, X.; McLaughlin, K.A.; Prise, V.E.; Savage, K.; Wilson, R.H.; et al. Targeting nucleotide metabolism enhances the efficacy of anthracyclines and anti-metabolites in triple-negative breast cancer. NPJ Breast Cancer 2021, 7, 38. [Google Scholar] [CrossRef]
- Gong, Y.; Ji, P.; Yang, Y.-S.; Xie, S.; Yu, T.-J.; Xiao, Y.; Jin, M.-L.; Ma, D.; Guo, L.-W.; Pei, Y.-C.; et al. Metabolic-Pathway-Based Subtyping of Triple-Negative Breast Cancer Reveals Potential Therapeutic Targets. Cell Metab. 2021, 33, 51–64.e9. [Google Scholar] [CrossRef]
- Weng, L.; Zhou, J.; Guo, S.; Xu, N.; Ma, R. The molecular subtyping and precision medicine in triple-negative breast cancer—Based on Fudan TNBC classification. Cancer Cell Int. 2024, 24, 120. [Google Scholar] [CrossRef]
- Jaman, M.S.; Sayeed, M.A. Ellagic acid, sulforaphane, and ursolic acid in the prevention and therapy of breast cancer: Current evidence and future perspectives. Breast Cancer 2018, 25, 517–528. [Google Scholar] [CrossRef]
- Budisan, L.; Gulei, D.; Zanoaga, O.M.; Irimie, A.I.; Chira, S.; Braicu, C.; Gherman, C.D.; Berindan-Neagoe, I. Dietary Intervention by Phytochemicals and Their Role in Modulating Coding and Non-Coding Genes in Cancer. Int. J. Mol. Sci. 2017, 18, 1178. [Google Scholar] [CrossRef]
- Gerhauser, C. Cancer Chemoprevention and Nutri-Epigenetics: State of the Art and Future Challenges. In Natural Products in Cancer Prevention and Therapy; Pezzuto, J.M., Suh, N., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; Volume 329, pp. 73–132. [Google Scholar]
- Mishra, M.; Seal, S.; Chandra, R. Mango. In Advances in Horticulture Biotechnology; Westville Publishing House: New Delhi, India, 2011; Volume 5, pp. 39–47. [Google Scholar]
- Schultz, D.J.; Wickramasinghe, N.S.; Ivanova, M.M.; Isaacs, S.M.; Dougherty, S.M.; Imbert-Fernandez, Y.; Cunningham, A.R.; Chen, C.; Klinge, C.M. Anacardic acid inhibits estrogen receptor alpha-DNA binding and reduces target gene transcription and breast cancer cell proliferation. Mol. Cancer Ther. 2010, 9, 594–605. [Google Scholar] [CrossRef]
- Schultz, D.J.; Krishna, A.; Vittitow, S.L.; Alizadeh-Rad, N.; Muluhngwi, P.; Rouchka, E.C.; Klinge, C.M. Transcriptomic response of breast cancer cells to anacardic acid. Sci. Rep. 2018, 8, 8063. [Google Scholar] [CrossRef]
- Roongta, U.V.; Pabalan, J.G.; Wang, X.; Ryseck, R.-P.; Fargnoli, J.; Henley, B.J.; Yang, W.-P.; Zhu, J.; Madireddi, M.T.; Lawrence, R.M.; et al. Cancer Cell Dependence on Unsaturated Fatty Acids Implicates Stearoyl-CoA Desaturase as a Target for Cancer Therapy. Mol. Cancer Res. 2011, 9, 1551. [Google Scholar] [CrossRef]
- Göőz, M.; Göőz, P.; Luttrell, L.M.; Raymond, J.R. 5-HT2A Receptor Induces ERK Phosphorylation and Proliferation through ADAM-17 Tumor Necrosis Factor-α-converting Enzyme (TACE) Activation and Heparin-bound Epidermal Growth Factor-like Growth Factor (HB-EGF) Shedding in Mesangial Cells*. J. Biol. Chem. 2006, 281, 21004–21012. [Google Scholar] [CrossRef]
- Chavez, K.J.; Garimella, S.V.; Lipkowitz, S. Triple negative breast cancer cell lines: One tool in the search for better treatment of triple negative breast cancer. Breast Dis. 2010, 32, 35–48. [Google Scholar] [CrossRef]
- Kao, J.; Salari, K.; Bocanegra, M.; Choi, Y.L.; Girard, L.; Gandhi, J.; Kwei, K.A.; Hernandez-Boussard, T.; Wang, P.; Gazdar, A.F.; et al. Molecular profiling of breast cancer cell lines defines relevant tumor models and provides a resource for cancer gene discovery. PLoS ONE 2009, 4, e6146. [Google Scholar] [CrossRef]
- Dai, X.; Cheng, H.; Bai, Z.; Li, J. Breast Cancer Cell Line Classification and Its Relevance with Breast Tumor Subtyping. J. Cancer 2017, 8, 3131–3141. [Google Scholar] [CrossRef]
- Barton, V.N.; D’Amato, N.C.; Gordon, M.A.; Lind, H.T.; Spoelstra, N.S.; Babbs, B.L.; Heinz, R.E.; Elias, A.; Jedlicka, P.; Jacobsen, B.M.; et al. Multiple molecular subtypes of triple-negative breast cancer critically rely on androgen receptor and respond to enzalutamide in vivo. Mol. Cancer Ther. 2015, 14, 769–778. [Google Scholar] [CrossRef]
- Brautigam, K.; Mitzlaff, K.; Uebel, L.; KÖSter, F.; Polack, S.; Pervan, M.; Steinert, G.; Rody, A.; Liedtke, C. Subtypes of Triple-negative Breast Cancer Cell Lines React Differently to Eribulin Mesylate. Anticancer Res. 2016, 36, 2759. [Google Scholar]
- Clement, E.; Inuzuka, H.; Nihira, N.T.; Wei, W.; Toker, A. Skp2-dependent reactivation of AKT drives resistance to PI3K inhibitors. Sci. Signal. 2018, 11, eaao3810. [Google Scholar] [CrossRef]
- Carlisle, S.M.; Trainor, P.J.; Doll, M.A.; Stepp, M.W.; Klinge, C.M.; Hein, D.W. Knockout of Human Arylamine N-Acetyltransferase 1 (NAT1) in MDA-MB-231 Breast Cancer Cells Leads to Increased Reserve Capacity, Maximum Mitochondrial Capacity, and Glycolytic Reserve Capacity. Mol. Carcinog. 2018, 57, 1458–1466. [Google Scholar] [CrossRef]
- Tiwary, R.; Yu, W.; Sanders, B.G.; Kline, K. alpha-TEA cooperates with chemotherapeutic agents to induce apoptosis of p53 mutant, triple-negative human breast cancer cells via activating p73. Breast Cancer Res. 2011, 13, R1. [Google Scholar] [CrossRef]
- Kaul, R.; Risinger, A.L.; Mooberry, S.L. Eribulin rapidly inhibits TGF-β-induced Snail expression and can induce Slug expression in a Smad4-dependent manner. Br. J. Cancer 2019, 121, 611–662. [Google Scholar] [CrossRef]
- Stewart, D.A.; Winnike, J.H.; McRitchie, S.L.; Clark, R.F.; Pathmasiri, W.W.; Sumner, S.J. Metabolomics Analysis of Hormone-Responsive and Triple-Negative Breast Cancer Cell Responses to Paclitaxel Identify Key Metabolic Differences. J. Proteome Res. 2016, 15, 3225–3240. [Google Scholar] [CrossRef]
- Fiehn, O.; Wohlgemuth, G.; Scholz, M.; Kind, T.; Lee, D.Y.; Lu, Y.; Moon, S.; Nikolau, B. Quality control for plant metabolomics: Reporting MSI-compliant studies. Plant J. 2008, 53, 691–704. [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.-É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef]
- Bylesjö, M.; Rantalainen, M.; Cloarec, O.; Nicholson, J.K.; Holmes, E.; Trygg, J. OPLS discriminant analysis: Combining the strengths of PLS-DA and SIMCA classification. J. Chemom. 2006, 20, 341–351. [Google Scholar] [CrossRef]
- Goode, G.; Gunda, V.; Chaika, N.V.; Purohit, V.; Yu, F.; Singh, P.K. MUC1 facilitates metabolomic reprogramming in triple-negative breast cancer. PLoS ONE 2017, 12, e0176820. [Google Scholar]
- Chughtai, K.; Jiang, L.; Greenwood, T.R.; Glunde, K.; Heeren, R.M.A. Mass spectrometry images acylcarnitines, phosphatidylcholines, and sphingomyelin in MDA-MB-231 breast tumor models[S]. J. Lipid Res. 2013, 54, 333–344. [Google Scholar] [CrossRef]
- Pereira, F.; Domingues, M.R.; Vitorino, R.; Guerra, I.M.S.; Santos, L.L.; Ferreira, J.A.; Ferreira, R. Unmasking the Metabolite Signature of Bladder Cancer: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 3347. [Google Scholar] [CrossRef]
- Tanaka, M.; Hsuan, C.; Oeki, M.; Shen, W.; Goda, A.; Tahara, Y.; Onodera, T.; Sanematsu, K.; Rikitake, T.; Oki, E.; et al. Identification of characteristic compounds of moderate volatility in breast cancer cell lines. PLoS ONE 2020, 15, e0235442. [Google Scholar] [CrossRef]
- Cífková, E.; Holčapek, M.; Lísa, M.; Vrána, D.; Gatěk, J.; Melichar, B. Determination of lipidomic differences between human breast cancer and surrounding normal tissues using HILIC-HPLC/ESI-MS and multivariate data analysis. Anal. Bioanal. Chem. 2015, 407, 991–1002. [Google Scholar] [CrossRef]
- Tang, X.; Ding, C.K.; Wu, J.; Sjol, J.; Wardell, S.; Spasojevic, I.; George, D.; McDonnell, D.P.; Hsu, D.S.; Chang, J.T.; et al. Cystine addiction of triple-negative breast cancer associated with EMT augmented death signaling. Oncogene 2017, 36, 4235–4242, Erratum in Oncogene 2017, 36, 4379. [Google Scholar] [CrossRef]
- Pralea, I.-E.; Moldovan, R.-C.; Țigu, A.-B.; Moldovan, C.-S.; Fischer-Fodor, E.; Iuga, C.-A. Cellular Responses Induced by NCT-503 Treatment on Triple-Negative Breast Cancer Cell Lines: A Proteomics Approach. Biomedicines 2024, 12, 1087. [Google Scholar] [CrossRef]
- Li, L.; Qin, Y.; Chen, Y. The enzymes of serine synthesis pathway in cancer metastasis. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2024, 1871, 119697. [Google Scholar] [CrossRef]
- Metcalf, S.; Petri, B.J.; Kruer, T.; Green, B.; Dougherty, S.; Wittliff, J.L.; Klinge, C.M.; Clem, B.F. Serine synthesis influences tamoxifen response in ER+ human breast carcinoma. Endocr. Relat. Cancer 2021, 28, 27–37. [Google Scholar] [CrossRef]
- Wang, Q.; Liberti, M.V.; Liu, P.; Deng, X.; Liu, Y.; Locasale, J.W.; Lai, L. Rational Design of Selective Allosteric Inhibitors of PHGDH and Serine Synthesis with Anti-tumor Activity. Cell Chem. Biol. 2017, 24, 55–65. [Google Scholar] [CrossRef]
- O’Shaughnessy, J.; Thaddeus Beck, J.; Royce, M. Everolimus-based combination therapies for HR+, HER2− metastatic breast cancer. Cancer Treat. Rev. 2018, 69, 204–214. [Google Scholar] [CrossRef]
- Baselga, J.; Campone, M.; Piccart, M.; Burris, H.A., 3rd; Rugo, H.S.; Sahmoud, T.; Noguchi, S.; Gnant, M.; Pritchard, K.I.; Lebrun, F.; et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N. Engl. J. Med. 2012, 366, 520–529. [Google Scholar] [CrossRef]
- Nye, L.; Khan, S.A. Everolimus for Estrogen Receptor–negative Breast Cancer Prevention: A Journey Begun? Cancer Prev. Res. 2022, 15, 787–789. [Google Scholar] [CrossRef]
- Quek, L.-E.; van Geldermalsen, M.; Guan, Y.F.; Wahi, K.; Mayoh, C.; Balaban, S.; Pang, A.; Wang, Q.; Cowley, M.J.; Brown, K.K.; et al. Glutamine addiction promotes glucose oxidation in triple-negative breast cancer. Oncogene 2022, 41, 4066–4078. [Google Scholar] [CrossRef]
- Vidal, C.M.; Ouyang, C.; Qi, Y.; Mendez-Dorantes, C.; Coblentz, A.; Alva-Ornelas, J.A.; Stark, J.M.; Seewaldt, V.L.; Ann, D.K. Arginine regulates HSPA5/BiP translation through ribosome pausing in triple-negative breast cancer cells. Br. J. Cancer 2023, 129, 444–454. [Google Scholar] [CrossRef]
- Ogretmen, B. Sphingolipid metabolism in cancer signalling and therapy. Nat. Rev. Cancer 2018, 18, 33–50. [Google Scholar] [CrossRef]
- Nagahashi, M.; Miyoshi, Y. Targeting Sphingosine-1-Phosphate Signaling in Breast Cancer. Int. J. Mol. Sci. 2024, 25, 3354. [Google Scholar] [CrossRef] [PubMed]
- Sahin, I.; Eturi, A.; De Souza, A.; Pamarthy, S.; Tavora, F.; Giles, F.J.; Carneiro, B.A. Glycogen synthase kinase-3 beta inhibitors as novel cancer treatments and modulators of antitumor immune responses. Cancer Biol. Ther. 2019, 20, 1047–1056. [Google Scholar] [CrossRef] [PubMed]
- Hemshekhar, M.; Sebastin Santhosh, M.; Kemparaju, K.; Girish, K.S. Emerging Roles of Anacardic Acid and Its Derivatives: A Pharmacological Overview. Basic Clin. Pharmacol. Toxicol. 2012, 110, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.L.N.; Annoni, R.; Silva, P.R.P.; Borelli, P.; Fock, R.A.; Trevisan, M.T.S.; Mauad, T. Acute, subacute toxicity and mutagenic effects of anacardic acids from cashew (Anacardium occidentale Linn.) in mice. J. Ethnopharmacol. 2011, 135, 730–736. [Google Scholar] [CrossRef]
- Sodi, V.L.; Khaku, S.; Krutilina, R.; Schwab, L.P.; Vocadlo, D.J.; Seagroves, T.N.; Reginato, M.J. mTOR/MYC Axis Regulates O-GlcNAc Transferase Expression and O-GlcNAcylation in Breast Cancer. Mol. Cancer Res. 2015, 13, 923–933. [Google Scholar] [CrossRef] [PubMed]
- Schultz, D.J.; Muluhngwi, P.; Alizadeh-Rad, N.; Green, M.A.; Rouchka, E.C.; Waigel, S.J.; Klinge, C.M. Genome-wide miRNA response to anacardic acid in breast cancer cells. PLoS ONE 2017, 12, e0184471. [Google Scholar] [CrossRef]
- Zhang, J.; Li, D. Effect of conjugated linoleic acid on inhibition of prolyl hydroxylase 1 in hearts of mice. Lipids Health Dis. 2012, 11, 22. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, A.R.; Perez Kerkvliet, C.; Truong, T.H.; Hagen, K.M.; Krutilina, R.I.; Parke, D.N.; Oakley, R.H.; Liddle, C.; Cidlowski, J.A.; Seagroves, T.N.; et al. Glucocorticoid Receptors Drive Breast Cancer Cell Migration and Metabolic Reprogramming via PDK4. Endocrinology 2023, 164, bqad083. [Google Scholar] [CrossRef] [PubMed]
- Walter, W.; Thomalla, J.; Bruhn, J.; Fagan, D.H.; Zehowski, C.; Yee, D.; Skildum, A. Altered regulation of PDK4 expression promotes antiestrogen resistance in human breast cancer cells. Springerplus 2015, 4, 689. [Google Scholar] [CrossRef] [PubMed]
- Holder, A.M.; Gonzalez-Angulo, A.M.; Chen, H.; Akcakanat, A.; Do, K.A.; Fraser Symmans, W.; Pusztai, L.; Hortobagyi, G.N.; Mills, G.B.; Meric-Bernstam, F. High stearoyl-CoA desaturase 1 expression is associated with shorter survival in breast cancer patients. Breast Cancer Res Treat 2013, 137, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Tracz-Gaszewska, Z.; Dobrzyn, P. Stearoyl-CoA Desaturase 1 as a Therapeutic Target for the Treatment of Cancer. Cancers 2019, 11, 948. [Google Scholar] [CrossRef]
- Snodgrass, R.G.; Huang, S.; Namgaladze, D.; Jandali, O.; Shao, T.; Sama, S.; Brüne, B.; Hwang, D.H. Docosahexaenoic acid and palmitic acid reciprocally modulate monocyte activation in part through endoplasmic reticulum stress. J. Nutr. Biochem. 2016, 32, 39–45. [Google Scholar] [CrossRef]
- Lemmer, I.L.; Willemsen, N.; Hilal, N.; Bartelt, A. A guide to understanding endoplasmic reticulum stress in metabolic disorders. Mol. Metab. 2021, 47, 101169. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; Chi, Y.; Su, M.; Zhou, J.; Zhou, D.; Zheng, F.; Man, X.; Sun, S.; Huang, J.; Li, H. Potential predictive value of circulating tumor DNA (ctDNA) mutations for the efficacy of immune checkpoint inhibitors in advanced triple-negative breast cancer. Front. Genet. 2023, 14, 1125970. [Google Scholar] [CrossRef] [PubMed]
- El Ansari, R.; Craze, M.L.; Diez-Rodriguez, M.; Nolan, C.C.; Ellis, I.O.; Rakha, E.A.; Green, A.R. The multifunctional solute carrier 3A2 (SLC3A2) confers a poor prognosis in the highly proliferative breast cancer subtypes. Br. J. Cancer 2018, 118, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- van Geldermalsen, M.; Wang, Q.; Nagarajah, R.; Marshall, A.D.; Thoeng, A.; Gao, D.; Ritchie, W.; Feng, Y.; Bailey, C.G.; Deng, N.; et al. ASCT2/SLC1A5 controls glutamine uptake and tumour growth in triple-negative basal-like breast cancer. Oncogene 2016, 35, 3201–3208. [Google Scholar] [CrossRef] [PubMed]
- Morotti, M.; Zois, C.E.; El-Ansari, R.; Craze, M.L.; Rakha, E.A.; Fan, S.-J.; Valli, A.; Haider, S.; Goberdhan, D.C.I.; Green, A.R.; et al. Increased expression of glutamine transporter SNAT2/SLC38A2 promotes glutamine dependence and oxidative stress resistance, and is associated with worse prognosis in triple-negative breast cancer. Br. J. Cancer 2021, 124, 494–505. [Google Scholar] [CrossRef] [PubMed]
- Malabanan, M.M.; Blind, R.D. Inositol polyphosphate multikinase (IPMK) in transcriptional regulation and nuclear inositide metabolism. Biochem. Soc. Trans. 2016, 44, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Jung, O.; Baek, M.-j.; Wooldrik, C.; Johnson, K.R.; Fisher, K.W.; Lou, J.; Ricks, T.J.; Wen, T.; Best, M.D.; Cryns, V.L.; et al. Nuclear phosphoinositide signaling promotes YAP/TAZ-TEAD transcriptional activity in breast cancer. EMBO J. 2024, 43, 1740–1769. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Glodowski, C.R.; Fan, C.; Liu, J.; Mott, K.R.; Kaushik, A.; Vu, H.; Locasale, J.W.; McBrayer, S.K.; DeBerardinis, R.J.; et al. Integrated Metabolic Profiling and Transcriptional Analysis Reveals Therapeutic Modalities for Targeting Rapidly Proliferating Breast Cancers. Cancer Res. 2022, 82, 665–680. [Google Scholar] [CrossRef] [PubMed]
- Schultz, D.J.; Olsen, C.; Cobbs, G.A.; Stolowich, N.J.; Parrott, M.M. Bioactivity of anacardic acid against Colorado potato beetle (Leptinotarsa decemlineata) larvae. J. Agric. Food Chem. 2006, 54, 7522–7529. [Google Scholar] [CrossRef]
- Radde, B.N.; Ivanova, M.M.; Mai, H.X.; Alizadeh-Rad, N.; Piell, K.; Van Hoose, P.; Cole, M.P.; Muluhngwi, P.; Kalbfleisch, T.S.; Rouchka, E.C.; et al. Nuclear respiratory factor-1 and bioenergetics in tamoxifen-resistant breast cancer cells. Exp. Cell Res. 2016, 347, 222–231. [Google Scholar] [CrossRef]
- Petri, B.J.; Piell, K.M.; Wahlang, B.; Head, K.Z.; Andreeva, K.; Rouchka, E.C.; Cave, M.C.; Klinge, C.M. Polychlorinated biphenyls alter hepatic m6A mRNA methylation in a mouse model of environmental liver disease. Environ. Res. 2023, 216 Pt 3, 114686. [Google Scholar] [CrossRef] [PubMed]




| TNBC Cell Line | ATCC Cat. No. | Comments: Patient Source, Subtype, Mutations [22,23,24,25,26] | Medium (+1% Pen/Strep) |
|---|---|---|---|
| MDA-MB-231 | HTB-26 | White, basal B, MSL, claudin-low, p53 mut, BRCA WT, WT PI3K; KRAS G13D [27]; non-LAR (AR-negative) | DMEM with 10% FBS [28] |
| MDA-MB-468 | HTB-132 | Black, basal A, mesenchymal stem-like (MSL), p53 mutated, BRCA WT, PTEN homo deletion; EGFR amplification | DMEM with 10% FBS [29] |
| HCC1806 | CRL-2335 | Black, primary tumor: squamous carcinoma, basal A, basal-like BL2, p53 mut, BRCA WT, non-LAR (AR-negative) | RPMI 1640 with 10% FBS [30] |
| BT-20 | HTB-19 | White, primary tumor: adenocarcinoma, basal A, p53 mutated, BRCA WT, EGFR amplification; PI3CAmut PIK3CA H1047R [27]; RB1 mutated, non-LAR (AR-negative) | MEM with 10% FBS [29] |
| Cell Line | AnAc Cell Viability (MTT) 48 h IC50 µM | AnAc Cell Proliferation (dsDNA Quantification) 48 h IC50 µM | µM AnAc 24 h Treatment for Metabolomics |
|---|---|---|---|
| MCF-7 | 21 ± 6.1 × 10−6 | 38 ± 4.4 × 10−6 | 28 |
| MDA-MB-231 | 38 ± 5.6 × 10−6 | 30 ± 4.3 × 10−6 | 20 |
| MDA-MB-468 | 39 ± 2.2 × 10−6 | 60 ± 8.4 × 10−6 | 20 |
| HCC1806 | 41 ± 6.5 × 10−6 | 48 ±6.2 × 10−6 | 27 |
| BT-20 | 59 ± 5.8 × 10−6 | 79 ± 1.0 × 10−6 | 40 |
| Cells | AnAc-Altered Metabolites | Log2(FC) | p Value |
|---|---|---|---|
| MCF-7 | ribulose-5-phosphate | −1.5615 | 0.0019888 |
| uracil | −1.9264 | 0.0022824 | |
| xanthosine | −1.3499 | 0.0030643 | |
| gluconic acid | −1.6437 | 0.0038966 | |
| ethanolamine | −1.2497 | 0.0063086 | |
| cystine (DL-cystine) | −1.1738 | 0.0080923 | |
| ribose-5-phosphate | −2.0159 | 0.0084623 | |
| galactinol | 1.379 | 0.014507 | |
| glucose-6-phosphate | −1.2953 | 0.014769 | |
| cytidine | −1.1586 | 0.017964 | |
| pseudo uridine | −1.0049 | 0.021683 | |
| fructose-1-phosphate | −2.3767 | 0.02596 | |
| docosahexaenoic acid (DHA) | −2.0523 | 0.027704 | |
| 5-methyluridine | −1.5322 | 0.031222 | |
| thymine | −1.5992 | 0.035159 | |
| alpha-aminoadipic acid | −1.1237 | 0.047021 | |
| glucose | −1.0835 | 0.048064 | |
| HCC1806 | octadecanol | 2.5632 | 2.53 × 10−5 |
| 1-hexadecanol | 2.4413 | 0.00018262 | |
| glucose-1-phosphate | −1.4218 | 0.0032117 | |
| putrescine | −1.2587 | 0.019973 | |
| azelaic acid | −1.0126 | 0.020676 | |
| maltotriose | −1.2791 | 0.050363 | |
| BT-20 | UDP-glucuronic acid | 1.7788 | 2.81 × 10−5 |
| 1-hexadecanol | 3.0591 | 0.00018873 | |
| citric acid | −1.0428 | 0.014918 | |
| glutamine | −1.0999 | 0.01615 | |
| cystine (DL-cystine) | 1.738 | 0.033439 | |
| galactinol | 1.5808 | 0.042584 | |
| MDA-MB-231 | aspartic acid | 0.5855 | 0.0015669 |
| phytosphingosine | −0.67158 | 0.0016425 | |
| sinigrin | −1.6591 | 0.0022074 | |
| lanosterol | −1.1452 | 0.0022373 | |
| 4-hydroxybutyric acid | −0.70683 | 0.003666 | |
| dehydrocholecalciferol (24,25-dihydroxyvitamin D) | −1.2079 | 0.01005 | |
| octadecylglycerol | −0.86493 | 0.013566 | |
| cholesterone | −0.68607 | 0.01592 | |
| inositol-4-monophosphate | −0.94447 | 0.019321 | |
| pyrophosphate | 0.95923 | 0.03129 | |
| xylonic acid | −0.73111 | 0.034291 | |
| lactose | −1.7446 | 0.036909 | |
| MDA-MB-468 | 1-hexadecanol | 1.3145 | 0.00030833 |
| glucuronic acid | 0.60653 | 0.0069837 | |
| xylitol | 0.60827 | 0.034831 | |
| D-erythro-sphingosine | −0.59322 | 0.036251 |
| Metabolomics | RNA-Seq (DEGs) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pathway Maps | Min (p Value) | Min FDR | p-Value | FDR | In Data | Network Objects from Active Data | p-Value | FDR | In Data | Network Objects from Active Data |
| Regulation of lipid metabolism_Fatty acid-dependent regulation of lipidic metabolism | 3.4 × 10−3 | 6.6 × 10−2 | 8.1 × 10−2 | 1.5 × 10−1 | 1 | Linoleic acid | 3.4 × 10−3 | 6.6 × 10−2 | 2 | INSIG, PDK4 |
| Signal transduction_mTORC1 downstream signaling | 1.1 × 10−1 | 1.5 × 10−1 | 1.1 × 10−1 | 1.5 × 10−1 | 1 | D-Ribose 5-phosphate | 1.1 × 10−1 | 1.5 × 10−1 | 1 | SCD |
| Metabolomics | RNA-Seq (DEGs) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pathway Maps | Min (p Value) | Min FDR | p-Value | FDR | In Data | Network Objects from Active Data | p-Value | FDR | In Data | Network Objects from Active Data |
| Signal transduction_ Amino acid-dependent mTORC1 activation | 2.3 × 10−6 | 8.9 × 10−5 | 2.3 × 10−6 | 8.9 × 10−5 | 4 | L-Leucine | 0.13 | 0.28 | 5 | SESN2 (Sestrin 2), SLC3A2, SLC1A5 (ASCT2), IPMK, SLC38A2 |
| Mechanisms of drug resistance in multiple myeloma | 1.0 × 10−4 | 2.7 × 10−3 | 4.7 × 10−2 | 7.4 × 10−2 | 1 | UDP-D-glucuronic acid cytoplasm | 1.0 × 10−4 | 2.7 × 10−3 | 8 | IL-6, HES1, IL-8, MYC, CCND1 (Cyclin D1), HRAS, TCF7L2 (TCF4), FZD2 (Frizzled) |
| Urea cycle | 6.3 × 10−4 | 9.4 × 10−3 | 6.3 × 10−4 | 9.4 × 10−3 | 2 | L-Aspartic acid cytoplasm, Fumaric acid intracellular | 0.64 | 0.751 | 1 | AQP3 (Aquaporin 3) |
| Immune response_ Distinct metabolic pathways in naive and effector CD8+ T cells | 2.6 × 10−3 | 1.7 × 10−2 | 2.6 × 10−3 | 1.7 × 10−2 | 2 | L-Leucine | 4.6 × 10−3 | 3.3 × 10−2 | 7 | 4E-BP1, MYC, SLC3A2, SLC1A5, LCK, SLC38A1, SLC38A2 |
| Aminoacyl-tRNA biosynthesis in mitochondrion | 3.1 × 10- | 1.7 × 10−2 | 3.1 × 10- | 1.7 × 10−2 | 2 | L-Aspartic acid cytoplasm, L-Leucine cytoplasm | 0.90 | 0.90 | 1 | GARS1 |
| Aminoacyl-tRNA biosynthesis in cytoplasm | 4.5 × 10−3 | 2.1 × 10−2 | 4.5 × 10- | 2.1 × 10−2 | 2 | L-Aspartic acid cytoplasm, L-Leucine cytoplasm | 0.93 | 0.93 | 1 | GARS1 |
| Neurophysiological process_Role of CDK5 in presynaptic signaling | 2.9 × 10−2 | 7.5 × 10−2 | 2.9 × 10−2 | 7.53 × 10−2 | 1 | Inositol 4-phosphate intracellular | 0.55 | 0.7 | 1 | SYT1 (Synaptotagmin I) |
| Prolactin signaling in Prostate Cancer | 3.4 × 10−2 | 7.5 × 10−2 | 3.4 × 10−2 | 7.5 × 10−2 | 1 | L-Aspartic acid cytoplasm | 0.61 | 0.73 | 1 | CCND1 (Cyclin D1) |
| Regulation of CFTR gating (normal and CF) | 3.5 × 10−2 | 7.5 × 10−2 | 3.5 × 10−2 | 7.5 × 10−2 | 1 | Pyrophosphate cytoplasm | 0.62 | 0.74 | 1 | PDE4D |
| Development_Thrombospondin 1 signaling | 3.7 × 10−2 | 7.5 × 10−2 | 3.7 × 10−2 | 7.5 × 10−2 | 1 | Pyrophosphate cytoplasm | 7.9 × 10−2 | 0.19 | 3 | VLDLR, VEGF-A, VEGFR-2 |
| Dysregulation of Adiponectin secretion from adipocytes in obesity, type 2 diabetes and metabolic syndrome X | 3.9 × 10−2 | 7.5 × 10−2 | 3.9 × 10−2 | 7.5 × 10−2 | 1 | Fumaric acid intracellular | 0.66 | 0.76 | 1 | FKHR |
| Neurophysiological process_Glucose-excited neurons of arcuate nucleus (rodent model) | 4.1 × 10−2 | 7.5 × 10−2 | 4.1 × 10−2 | 7.5 × 10−2 | 1 | Fumaric acid intracellular | 0.68 | 0.77 | 1 | GLUT3 |
| Neurophysiological process_Glucose-inhibited neurons of ventromedial and arcuate nuclei | 4.2 × 10−2 | 7.5 × 10−2 | 4.2 × 10−2 | 7.5 × 10−2 | 1 | Pyrophosphate cytoplasm | 0.68 | 0.771 | 1 | GLUT3 |
| Sulfur metabolism | 4.3 × 10−2 | 7.5 × 10−2 | 4.3 × 10−2 | 7.5 × 10−2 | 1 | L-Cystathionine intracellular | 0.69 | 0.77 | 1 | CTH |
| Disruption of methionine metabolism in induction and progression of HCC | 4.3 × 10−2 | 7.5 × 10−2 | 4.3 × 10−2 | 7.5 × 10−2 | 1 | L-Cystathionine intracellular | 0.69 | 0.771 | 1 | c-Myc |
| L-Alanine and L-cysteine metabolism | 4.7 × 10−2 | 7.5 × 10−2 | 4.7 × 10−22 | 7.5 × 10−2 | 1 | L-Cystathionine intracellular | 0.37 | 0.541 | 2 | CTH, GPT2 |
| Neurophysiological process_Circadian rhythm | 4.8 × 10−2 | 7.5 × 10−2 | 4.8 × 10−2 | 7.53 × 10−2 | 1 | Pyrophosphate cytoplasm | 0.73 | 0.79 | 1 | REV-ERBalpha (NR1D1) |
| Metabolism of L-cysteine, D-cysteine and L-cystine | 5.0 × 10−2 | 0.11 | 10.11 | 0.11 | 1 | L-Cystathionine intracellular | 5.0 × 10−2 | 0.15 | 7 | ASCT1 (SLC1A4), CTH, SLC3A2, SLC38A4, SLC7A11, SLC38A1, SLC38A2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piell, K.M.; Poulton, C.C.; Stanley, C.G.; Schultz, D.J.; Klinge, C.M. Integrated Metabolomics and Transcriptomics Analysis of Anacardic Acid Inhibition of Breast Cancer Cell Viability. Int. J. Mol. Sci. 2024, 25, 7044. https://doi.org/10.3390/ijms25137044
Piell KM, Poulton CC, Stanley CG, Schultz DJ, Klinge CM. Integrated Metabolomics and Transcriptomics Analysis of Anacardic Acid Inhibition of Breast Cancer Cell Viability. International Journal of Molecular Sciences. 2024; 25(13):7044. https://doi.org/10.3390/ijms25137044
Chicago/Turabian StylePiell, Kellianne M., Claire C. Poulton, Christian G. Stanley, David J. Schultz, and Carolyn M. Klinge. 2024. "Integrated Metabolomics and Transcriptomics Analysis of Anacardic Acid Inhibition of Breast Cancer Cell Viability" International Journal of Molecular Sciences 25, no. 13: 7044. https://doi.org/10.3390/ijms25137044






