Cordycepin Enhances the Therapeutic Efficacy of Doxorubicin in Treating Triple-Negative Breast Cancer
Abstract
:1. Introduction
2. Results
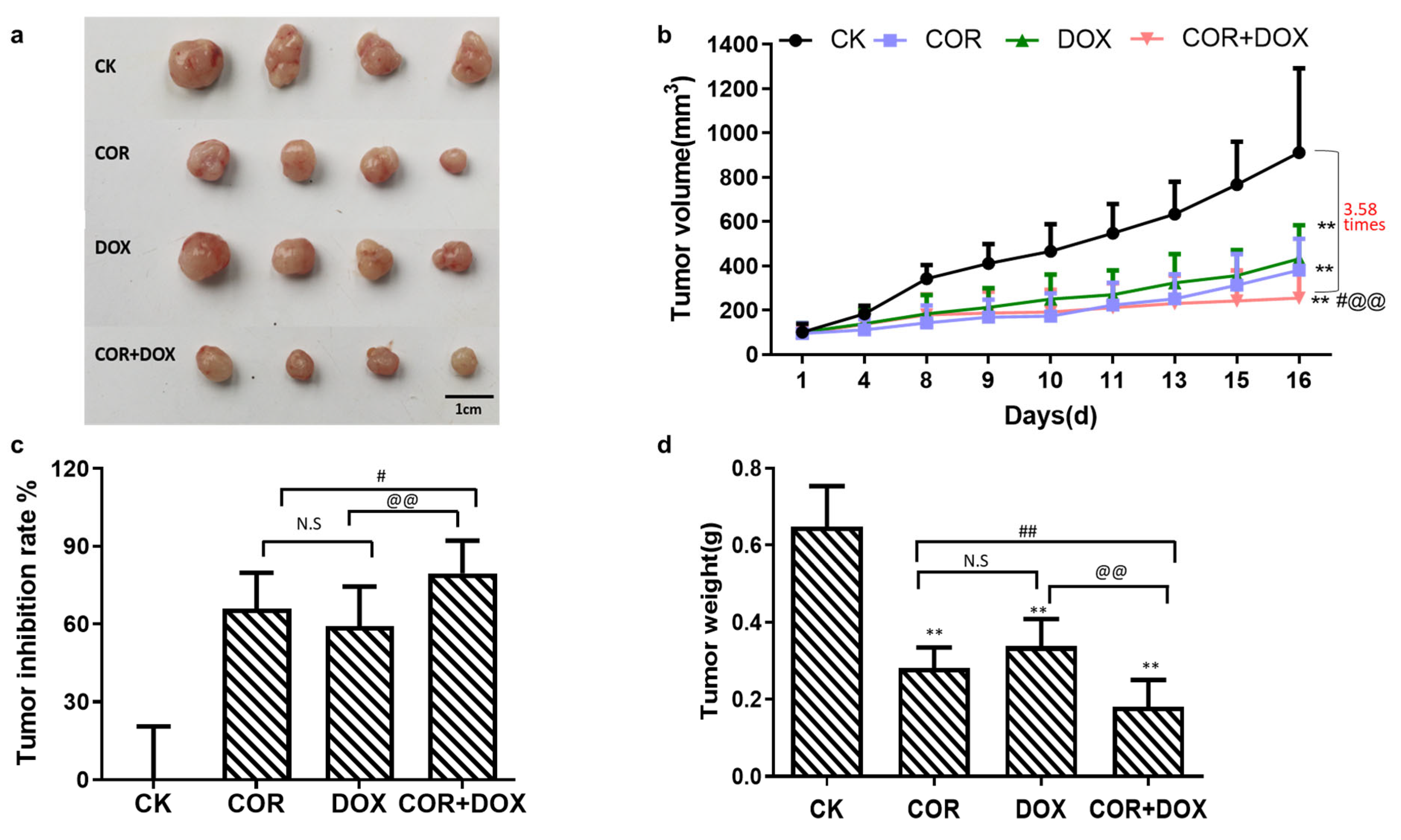
2.1. Cordycepin + Doxorubicin Inhibited the Growth of MDA-MB-231 Xenografts in Nude Mouse Model
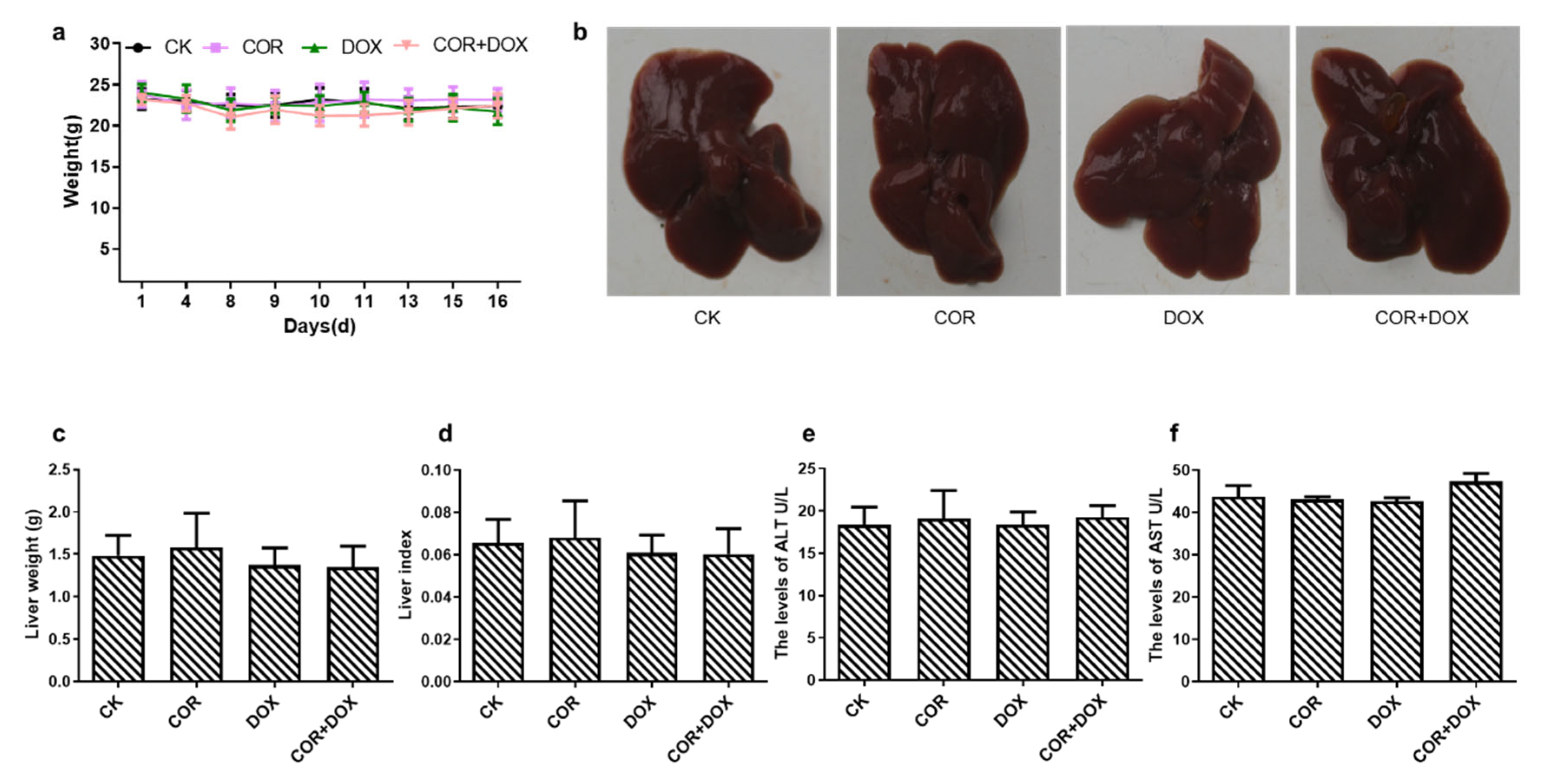
2.2. Cordycepin + Doxorubicin Had No Toxicity in Nude Mice
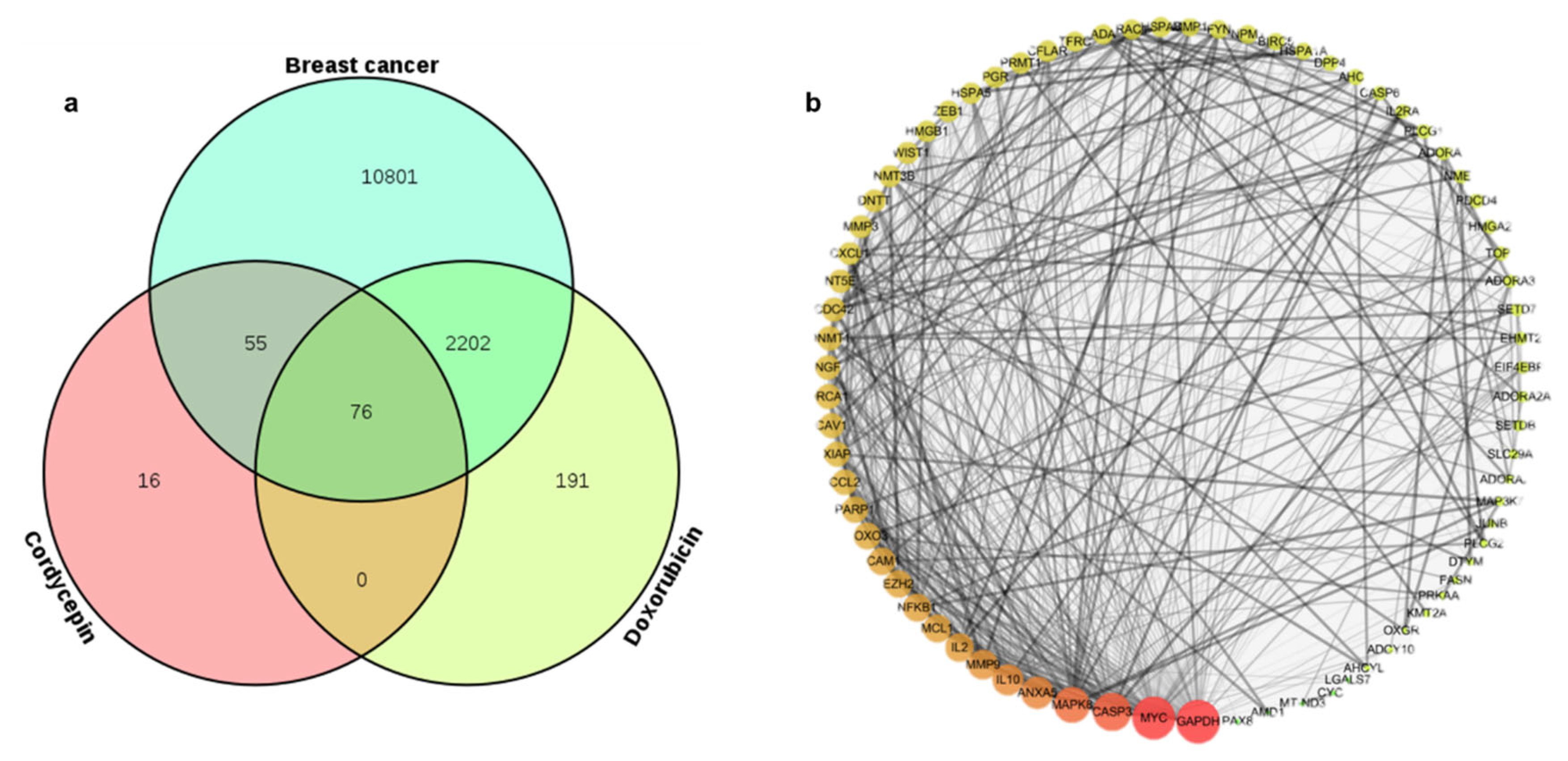
2.3. Identifying Common Molecular Targets of Cordycepin and Doxorubicin in TNBC Cells
2.4. PPI Network Construction
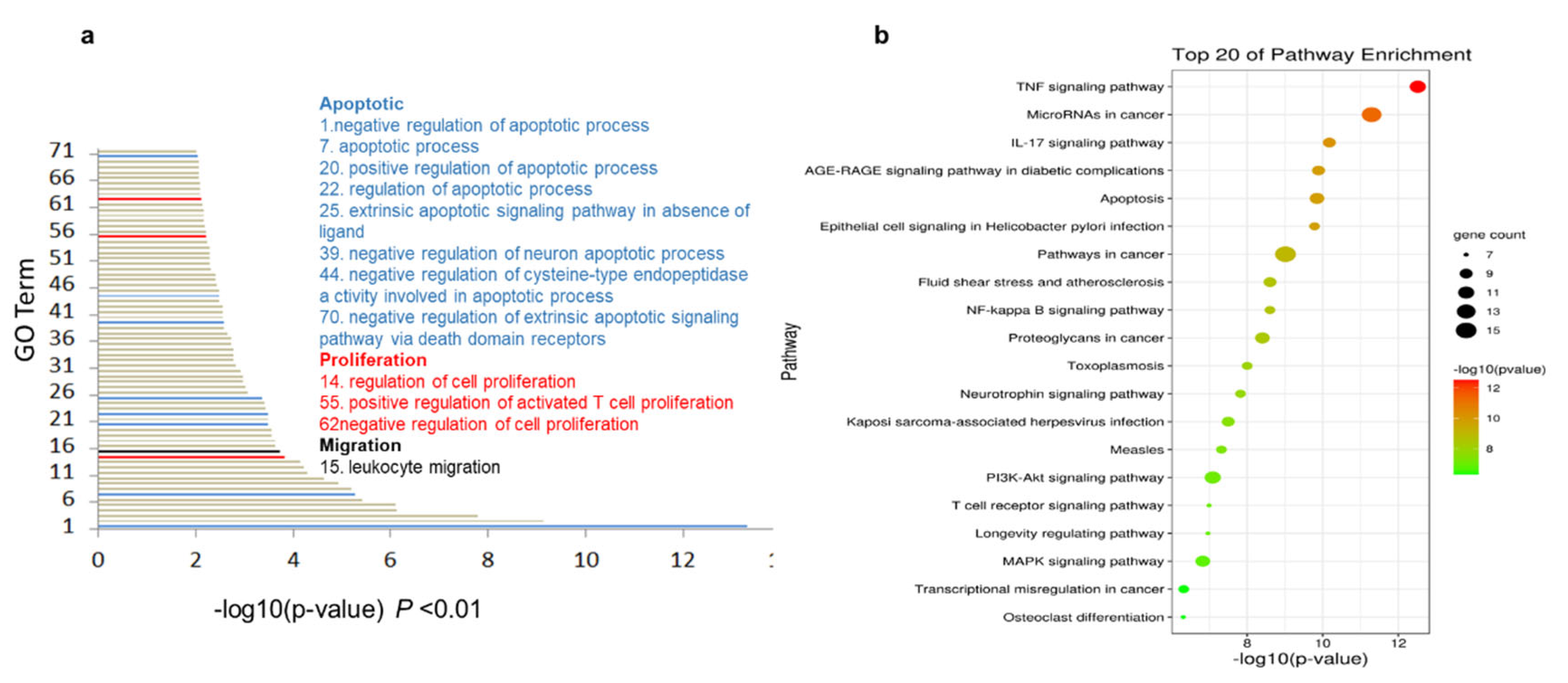
2.5. The Cordycepin + Doxorubicin Drug Combination Affects Multiple Biological Processes
2.6. KEGG Enrichment Analysis Indicates the Central Role of the TNF Pathway in Response to Cordycepin + Doxorubicin Combination Therapy
2.7. Analysis of Molecular Docking between Cordycepin, Doxorubicin, and Target Proteins
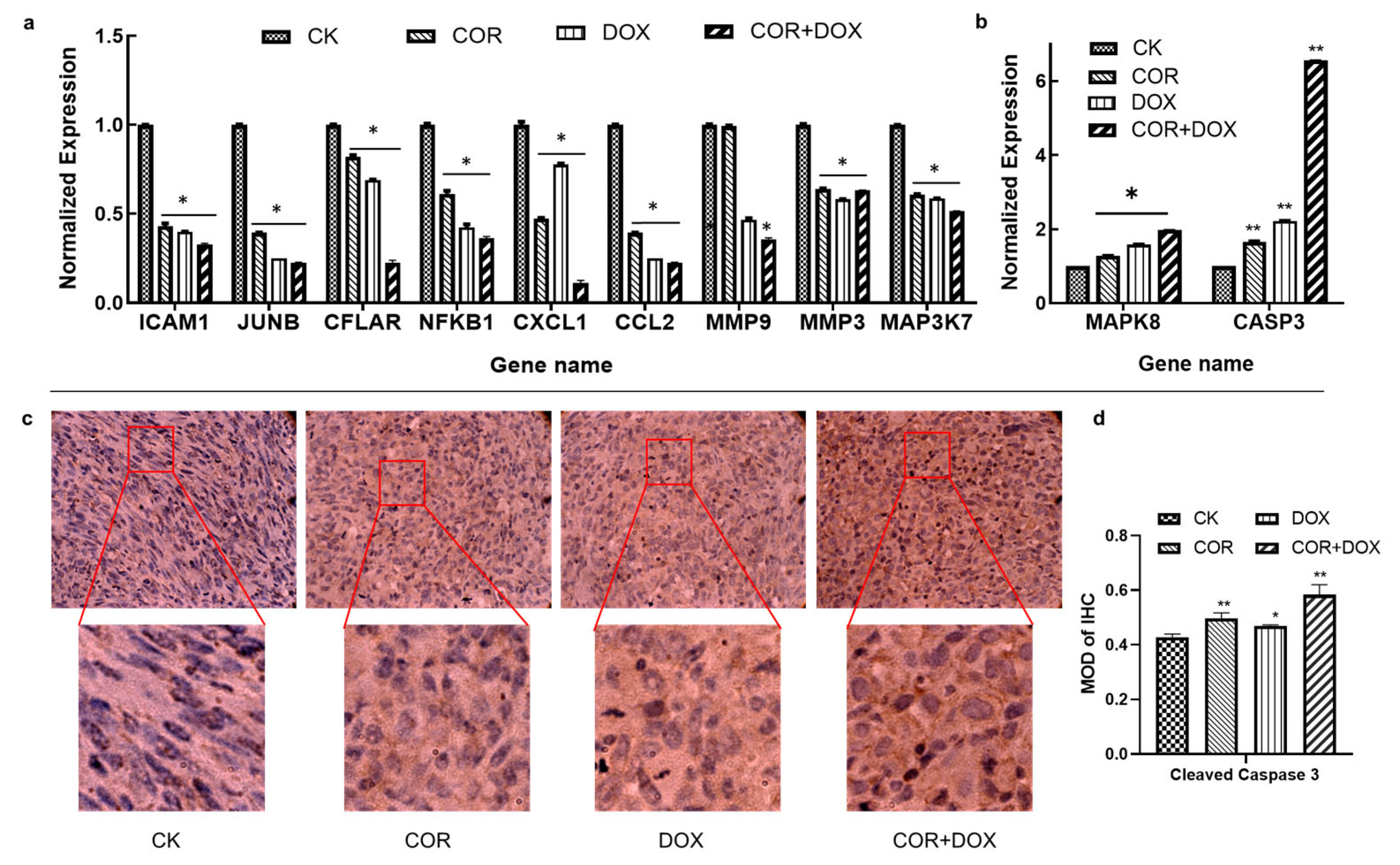
2.8. Cordycepin + Doxorubicin Affect the Expression of Molecules in the TNF Signaling Pathway
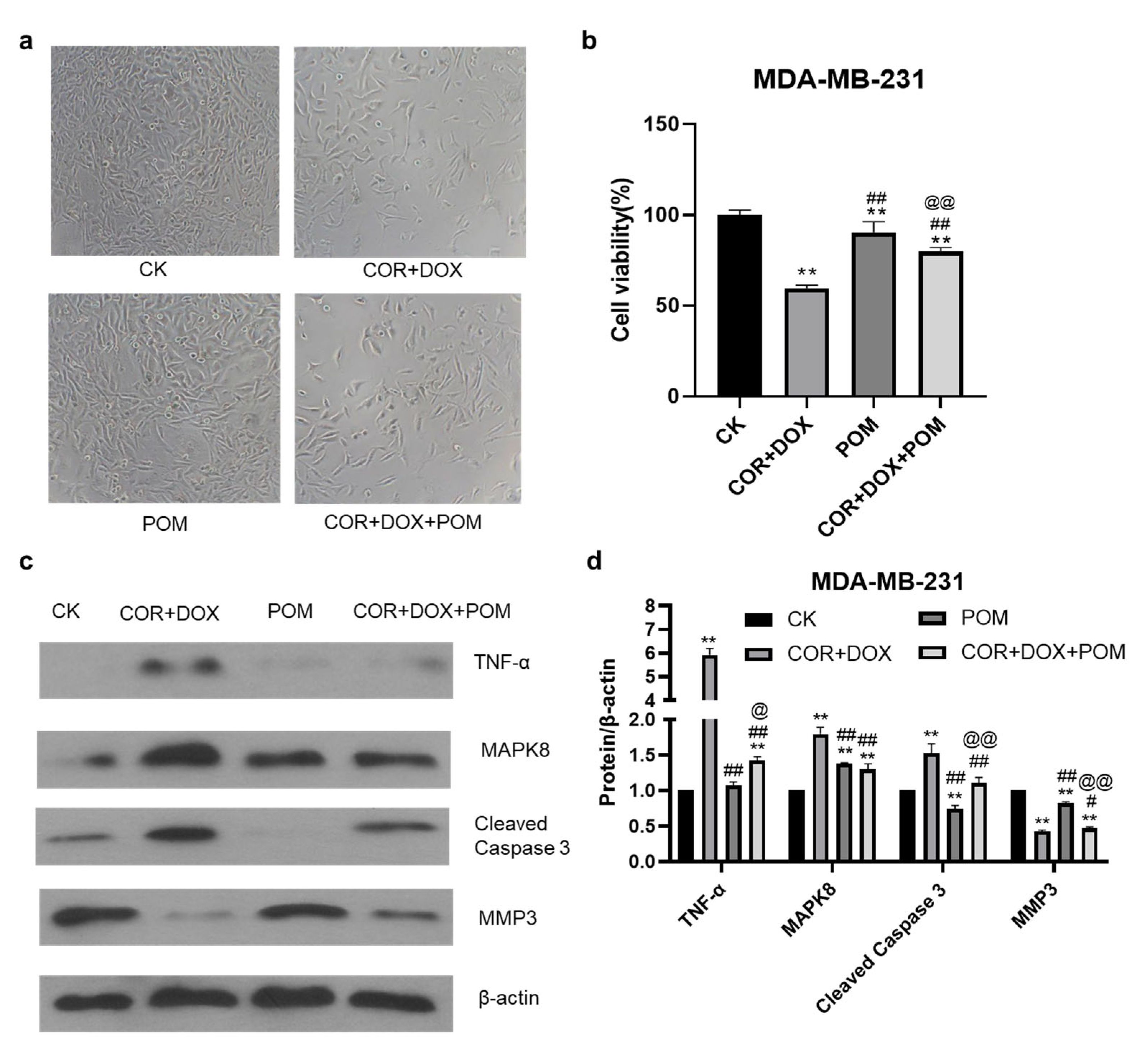
2.9. The TNF-α Inhibitor Pomalidomide Reverses the Inhibitory Effect of Combined Cordycepin + Doxorubicin Treatment on MDA-MB-231 Cells
2.10. The Inhibition of the TNF-α Pathway Inhibitor Eliminates Changes in Protein Abundance Induced by the Combination Treatment of MDA-MB-231 Cells
2.11. Analysis of the Target Pathway Network
3. Discussion
4. Materials and Methods
4.1. Cells, Animals, and Chemical Compounds
4.2. Cell Culture
4.3. Xenograft Tumor Model
4.4. Analysis of Hepatotoxicity
4.5. Prediction of Relevant Targets
4.6. Protein Interaction Analysis
4.7. GO Analysis and KEGG Analysis
4.8. Molecular Docking
4.9. Quantitative PCR
4.10. Immunohistochemistry
4.11. Assessing Cellular Toxicity Using MTT Assays
4.12. Western Blot Analysis
4.13. Construction of the Compound–Pathway–Target Network
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bryan, B.B.; Schnitt, S.J.; Collins, L.C. Ductal carcinoma in situ with basal-like phenotype: A possible precursor to invasive basal-like breast cancer. Mod. Pathol. 2006, 19, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Mirzania, M.; Safaee, S.R.; Shahi, F.; Jahanzad, I.; Zahedi, G.; Mehdizadeh, R. Treatment Outcomes and Clinicopathologic Characteristics of Triple-Negative Breast Cancer: A Report from Cancer Institute of Iran. Int. J. Hematol. Oncol. Stem Cell Res. 2017, 11, 37–42. [Google Scholar]
- Wang, J.H.; Shi, M.; Ling, R.; Xia, Y.S.; Luo, S.Q.; Fu, X.H.; Xiao, F.; Li, J.P.; Long, X.L.; Wang, J.G.; et al. Adjuvant chemotherapy and radiotherapy in triple-negative breast carcinoma: A prospective randomized controlled multi-center trial. Radiother. Oncol. 2011, 100, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.H.; Williams, G.A.; Sridhara, R.; Chen, G.; McGuinn, W.D., Jr.; Morse, D.; Abraham, S.; Rahman, A.; Liang, C.; Lostritto, R.; et al. United States Food and Drug Administration Drug Approval summary: Gefitinib (ZD1839; Iressa) tablets. Clin. Cancer Res. 2004, 10, 1212–1218. [Google Scholar] [CrossRef]
- Yi, T.H.; Wu, Y.M.; Liu, R.; Zhang, C.J.; Yao, S.J.; Shi, Y.; Li, S.Y. Effects and mechanism of ginsenoside Rg3 combined with tumstatin 19 peptide on apoptosis of HepG2 cells in liver cancer. J. Beijing Univ. Tradit. Chin. Med. 2020, 43, 575–582. [Google Scholar]
- Zhao, Y.; Cai, C.; Liu, M.; Zhao, Y.; Wu, Y.; Fan, Z.; Ding, Z.; Zhang, H.; Wang, Z.; Han, J. Drug-binding albumins forming stabilized nanoparticles for co-delivery of paclitaxel and resveratrol: In vitro/in vivo evaluation and binding properties investigation. Int. J. Biol. Macromol. 2020, 153, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.G.; Shrestha, B.; Lim, S.Y.; Yoon, D.H.; Chang, W.C.; Shin, D.J.; Han, S.K.; Park, S.M.; Park, J.H.; Park, H.I.; et al. Cordycepin inhibits lipopolysaccharide-induced inflammation by the suppression of NF-κB through Akt and p38 inhibition in RAW 264.7 macrophage cells. Eur. J. Pharmacol. 2006, 545, 192–199. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, X.; Liu, J.L.; Tang, Z.S. Advances in research on Antitumor Activities of Cordycepin. Chin. J. Pharm. 2015, 50, 1365–1368. [Google Scholar]
- Ma, P.; Xu, L.; Wang, L.Y.; Chen, N.; Zhang, X.D.; Chen, H.; Li, J.Y. Molecular Detection of Cordycepin-Induced HeLa Cell Apoptosis with Surface-Enhanced Raman Spectroscopy. Appl. Sci. 2019, 9, 3990. [Google Scholar] [CrossRef]
- Mousumi, T.; Jakaria, S.; Kazi, S.; Rudolf, K.; Safaei, K.M.; Halim, M.A.; Asaduzzaman, K.M. Cordycepin Downregulates Cdk-2 to Interfere with Cell Cycle and Increases Apoptosis by Generating ROS in Cervical Cancer Cells: In vitro and in silico Study. Curr. Cancer Drug Targets 2019, 19, 152–159. [Google Scholar]
- Wang, C.W.; Wang, Z.; Wang, J.F.; Wu, H.X. Research progress of cordycepin. Fujian Agric. Sci. Technol. 2019, 66–70. [Google Scholar]
- Cai, W.; Ye, Q.; Tang, L.; Yu, B.C.; Zheng, M.H. The effect of cordycepin alone or combined with chemotherapy on the proliferation, migration and apoptosis induction of colon cancer cells in vitro. Chin. J. Clin. (Electron. Ed.) 2011, 5, 4048–4054. [Google Scholar]
- Du, Y.P.; Yu, J.S.; Du, L.; Tang, J.; Feng, W.H. Cordycepin enhances Epstein-Barr virus lytic infection and Epstein-Barr virus-positive tumor treatment efficacy by doxorubicin. Cancer Lett. 2016, 376, 240–248. [Google Scholar] [CrossRef]
- Liu, C.Y.; Qi, M.; Li, L.; Yuan, Y.; Wu, X.P.; Fu, J.S. Natural cordycepin induces apoptosis and suppresses metastasis in breast cancer cells by inhibiting the Hedgehog pathway. Food Funct. 2020, 11, 2107–2116. [Google Scholar] [CrossRef]
- Wu, W.Y.; Li, X.M.; Qi, M.; Hu, X.; Cao, F.H.; Wu, X.P.; Fu, J.S. Cordycepin Inhibits Growth and Metastasis Formation of MDA-MB-231 Xenografts in Nude Mice by Modulating the Hedgehog Pathway. Int. J. Mol. Sci. 2022, 23, 10362. [Google Scholar] [CrossRef]
- Hu, X.; Liu, C.Y.; Xue, F.Z.; Wu, W.Y.; Wu, X.P.; Zhang, J.L.; Fu, J.S. Effects of cordycepin combined with doxorubicin on proliferation and metastasis of breast cancer cells. Mycosystema 2021, 40, 3012–3022. [Google Scholar]
- Sha, K.; Yeh, S.Y.; Chang, C.; Nastiuk, K.L.; Krolewski, J.J. TNF signaling mediates an enzalutamide-induced metastatic phenotype of prostate cancer and microenvironment cell co-cultures. Oncotarget 2015, 6, 25726–25740. [Google Scholar] [CrossRef]
- Lee, Y.S.; Dutta, A. MicroRNAs in cancer. Annu. Rev. Pathol. 2009, 4, 199–227. [Google Scholar] [CrossRef]
- Lorena, A.C.; Pedro, M.; José, A.S.T.; Rafael, S.; Manuel, L.C.; Abelardo, A.; Guadalupe, G.M. Natural Plants Compounds as Modulators of Epithelial-to-Mesenchymal Transition. Front. Pharmacol. 2019, 10, 715. [Google Scholar]
- Dong, J.L.; Li, Y.; Xiao, H.W.; Luo, D.; Zhang, S.Q.; Zhu, C.C.; Jiang, M.; Cui, M.; Lu, L.; Fan, S.J. Cordycepin sensitizes breast cancer cells toward irradiation through elevating ROS production involving Nrf2. Toxicol. Appl. Pharmacol. 2019, 364, 12–21. [Google Scholar] [CrossRef]
- Gu, X.X.; Ge, X.Q. Pharmacological effects on the central nervous system and toxicity of cordycepin: Research advances. J. Int. Pharm. Res. 2017, 44, 840–844. [Google Scholar]
- Fan, H.; Zhu, Y.J.; Zhao, Y.F. Effect of cordycepin combined with Gemcitabine on apoptosis of breast cancer cells and its mechanism. Chin. J. Gerontol. 2019, 39, 4846–4850. [Google Scholar]
- Welzel, L.; Twele, F.; Schidlitzki, A.; Töllner, K.; Klein, P.; Löscher, W. Network pharmacology for antiepileptogenesis: Tolerability and neuroprotective effects of novel multitargeted combination treatments in nonepileptic vs. post-status epilepticus mice. Epilepsy Res. 2019, 151, 48–66. [Google Scholar] [CrossRef]
- Liu, H.P.; Zeng, L.T.; Yang, K.L.; Zhang, G.M. Network Pharmacology Approach to Explore the Pharmacological Mechanism of Xiaoyao Powder on Anovulatory Infertility. Evid. Based Complement. Altern. Med. 2016, 2016, 2960372. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhou, J.; Zhao, J.; Hou, D.; Zhang, H.; Li, L.; Zou, D.; Hu, J.; Zhang, Y.; Jing, Z. MiR-18a-downregulated RORA inhibits the proliferation and tumorigenesis of glioma using the TNF-α-mediated NF-κB signaling pathway. EBioMedicine 2020, 52, 102651. [Google Scholar] [CrossRef]
- Huang, Q.W.; Huang, T.; Niu, M.L.; Zhang, Y.H.; Li, D.M. Linarin inhibits migration and invasion abilities of human breast cancer MDA-MB-231 cells through IKK/NF-κB signaling pathway. Chin. J. Pathophysiol. 2019, 35, 2194–2200. [Google Scholar]
- Wu, D.; Dong, W.D. The role of TNF-α in the occurrence and development of gastric cancer and its research progress. J. Intractable Dis. 2018, 17, 428–432. [Google Scholar]
- Leva, G.D.; Garofalo, M.; Croce, C.M. MicroRNAs in Cancer. Annu. Rev. Pathol. 2014, 9, 287–314. [Google Scholar] [CrossRef]
- Benchetrit, F.; Ciree, A.; Vives, V.; Warnier, G.; Gey, A.; Sautès-Fridman, C.; Fossiez, F.; Haicheur, N.; Fridman, W.H.; Tartour, E. Interleukin-17 inhibits tumor cell growth by means of a T-cell-dependent mechanism. Blood 2002, 99, 2114–2121. [Google Scholar] [CrossRef]
- Yu, X.; Ling, J.; Liu, X.; Guo, S.; Lin, Y.; Liu, X.; Su, L. Cordycepin induces autophagy-mediated c-FLIPL degradation and leads to apoptosis in human non-small cell lung cancer cells. Oncotarget 2017, 8, 6691–6699. [Google Scholar] [CrossRef]
- Jiang, X.; Tang, P.C.; Chen, Q.; Zhang, X.; Fan, Y.Y.; Yu, B.C.; Gu, X.X.; Sun, Y.; Ge, X.Q.; Zhang, X.L. Cordycepin Exerts Neuroprotective Effects via an Anti-Apoptotic Mechanism based on the Mitochondrial Pathway in a Rotenone-Induced Parkinsonism Rat Model. CNS Neurol. Disord. Drug Targets 2019, 18, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Lossi, L.; Castagna, C.; Merighim, A. Caspase-3 Mediated Cell Death in the Normal Development of the Mammalian Cerebellum. Int. J. Mol. Sci. 2018, 19, 3999. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, X.H.; Zhou, Y.Y.; Shi, M.Z.; Han, X.Y.; Han, X.H.; Chen, J.J. Potential mechanism of Sophora flavescens against breast cancer via network pharmacology and molecular docking. J. Pharm. Pract. Serv. 2023, 41, 722–732. [Google Scholar]
- Wen, X.; Hu, J.H.; Cheng, M.; Song, Z.X. Mechanism of Solanum nigrum in treatment of breast cancer based on network pharmacology molecular docking and in vitro experimental verification. Nat. Product. Res. Dev. 2023, 35, 1782–1793. [Google Scholar]
- Plumb, J.A. Cell sensitivity assays: The MTT assay. Methods Mol. Med. 1999, 28, 25–30. [Google Scholar]


| Target | Binding Energy (KJ/mol) | Inhib Constant (μmol/L) | ||
|---|---|---|---|---|
| Cordycepin | Doxorubicin | Cordycepin | Doxorubicin | |
| CFLAR | −7.05 | −6.88 | 6.77 | 9.05 |
| NFKB1 | −5.22 | −5.56 | 1.48 × 102 | 84.44 |
| CASP3 | −5.21 | −7.7 | 1.58 × 102 | 2.28 |
| MAP3K7 | −5.96 | −6.92 | 42.84 | 8.44 |
| ICAM1 | −5.22 | −5.86 | 1.44 × 103 | 50.42 |
| JUNB | −3.45 | −2.14 | 2.98 × 103 | 2.71 × 104 |
| MMP9 | −4.25 | −6.48 | 7.62 × 102 | 17.93 |
| CXCL1 | −4.34 | −5.13 | 6.57 × 102 | 1.73 × 102 |
| MAPK8 | −5.37 | −5.67 | 1.16 × 102 | 69.51 |
| MMP3 | −6.75 | −8.92 | 11.24 | 0.29 |
| CCL2 | −4.43 | −4.84 | 5.63 × 102 | 2.82 × 102 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, H.; Li, X.; Wu, W.; Liu, C.; Shao, Y.; Wu, X.; Fu, J. Cordycepin Enhances the Therapeutic Efficacy of Doxorubicin in Treating Triple-Negative Breast Cancer. Int. J. Mol. Sci. 2024, 25, 7077. https://doi.org/10.3390/ijms25137077
Huang H, Li X, Wu W, Liu C, Shao Y, Wu X, Fu J. Cordycepin Enhances the Therapeutic Efficacy of Doxorubicin in Treating Triple-Negative Breast Cancer. International Journal of Molecular Sciences. 2024; 25(13):7077. https://doi.org/10.3390/ijms25137077
Chicago/Turabian StyleHuang, Haichen, Xiaomin Li, Wenya Wu, Chengyi Liu, Yunhe Shao, Xiaoping Wu, and Junsheng Fu. 2024. "Cordycepin Enhances the Therapeutic Efficacy of Doxorubicin in Treating Triple-Negative Breast Cancer" International Journal of Molecular Sciences 25, no. 13: 7077. https://doi.org/10.3390/ijms25137077






