Neuroprotection by Anethum graveolens (Dill) Seeds and Its Phytocompounds in SH-SY5Y Neuroblastoma Cell Lines and Acellular Assays
Abstract
:1. Introduction
2. Results
2.1. Phytochemical Estimation and Antioxidant Potential of Dill Extract
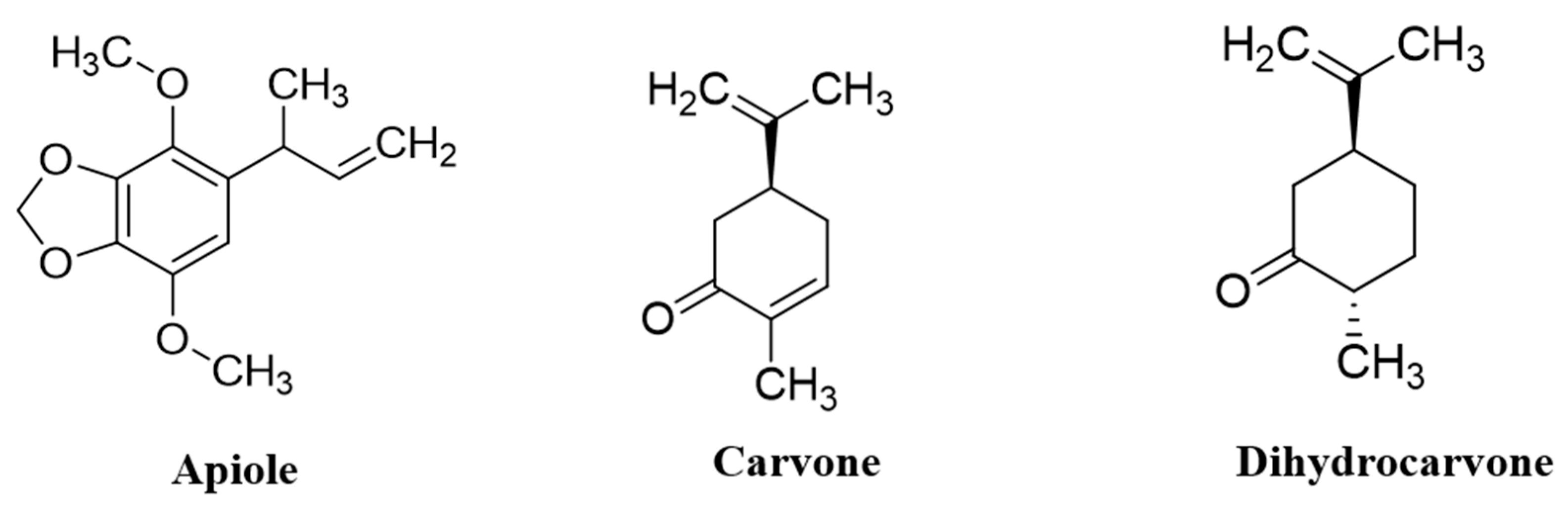
2.2. GC–MS Analysis
2.3. In Vitro Acetylcholinesterase Inhibitory Activity
2.4. Aβ -Fibrilization and Oligomerization Inhibition by Dill
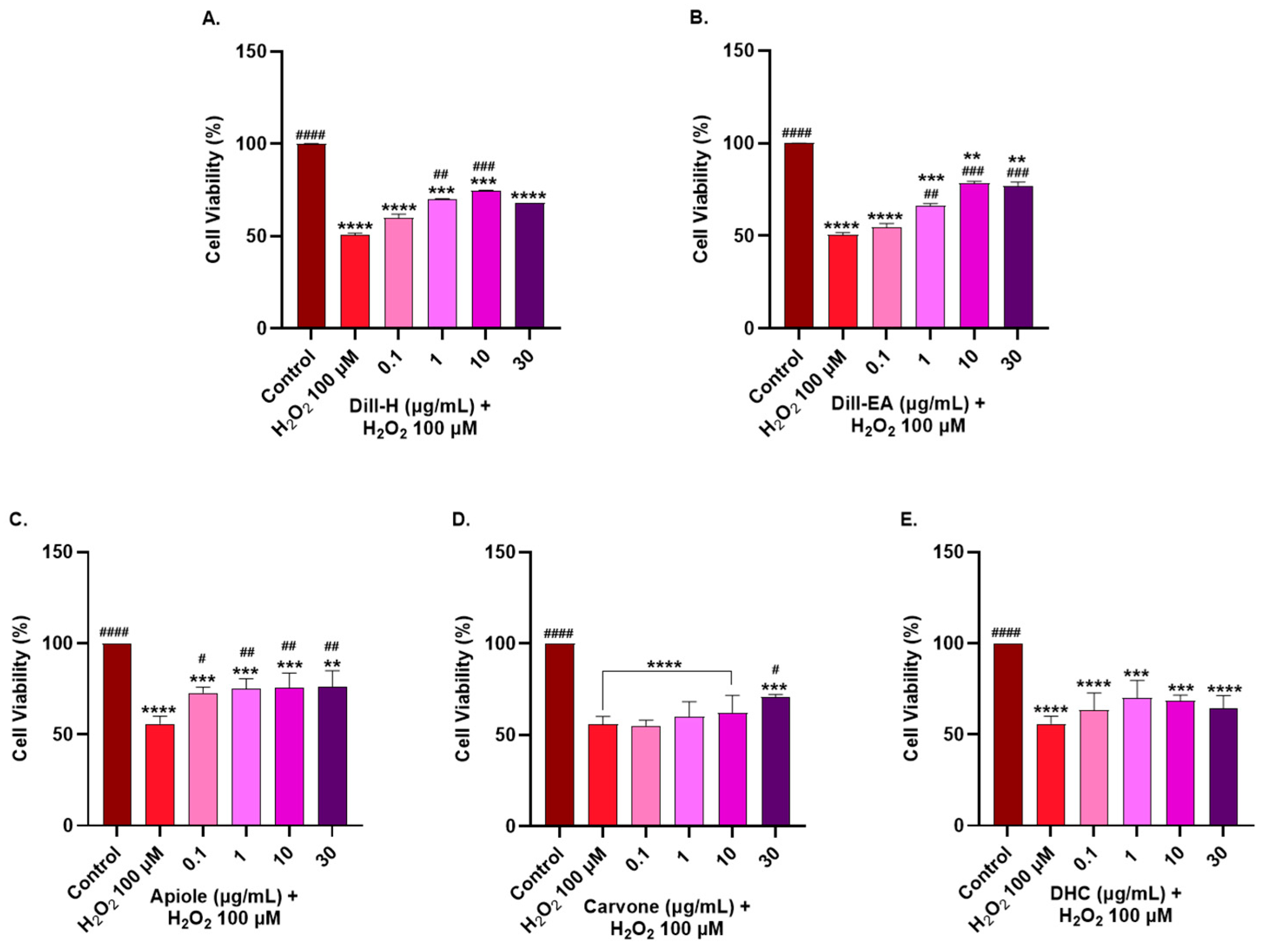
2.5. Cytotoxic Effect of Dill Extracts and Its Bioactive Compounds
2.6. Protective Effect of Dill Extracts and Its Bioactive Compounds against H2O2-Induced Oxidative Stress
2.7. Dill and Its Phytocompounds Mitigated H2O2-Induced ROS Generation
2.8. Dill Extract and Phytocompounds Improved Mitochondrial Membrane Potential
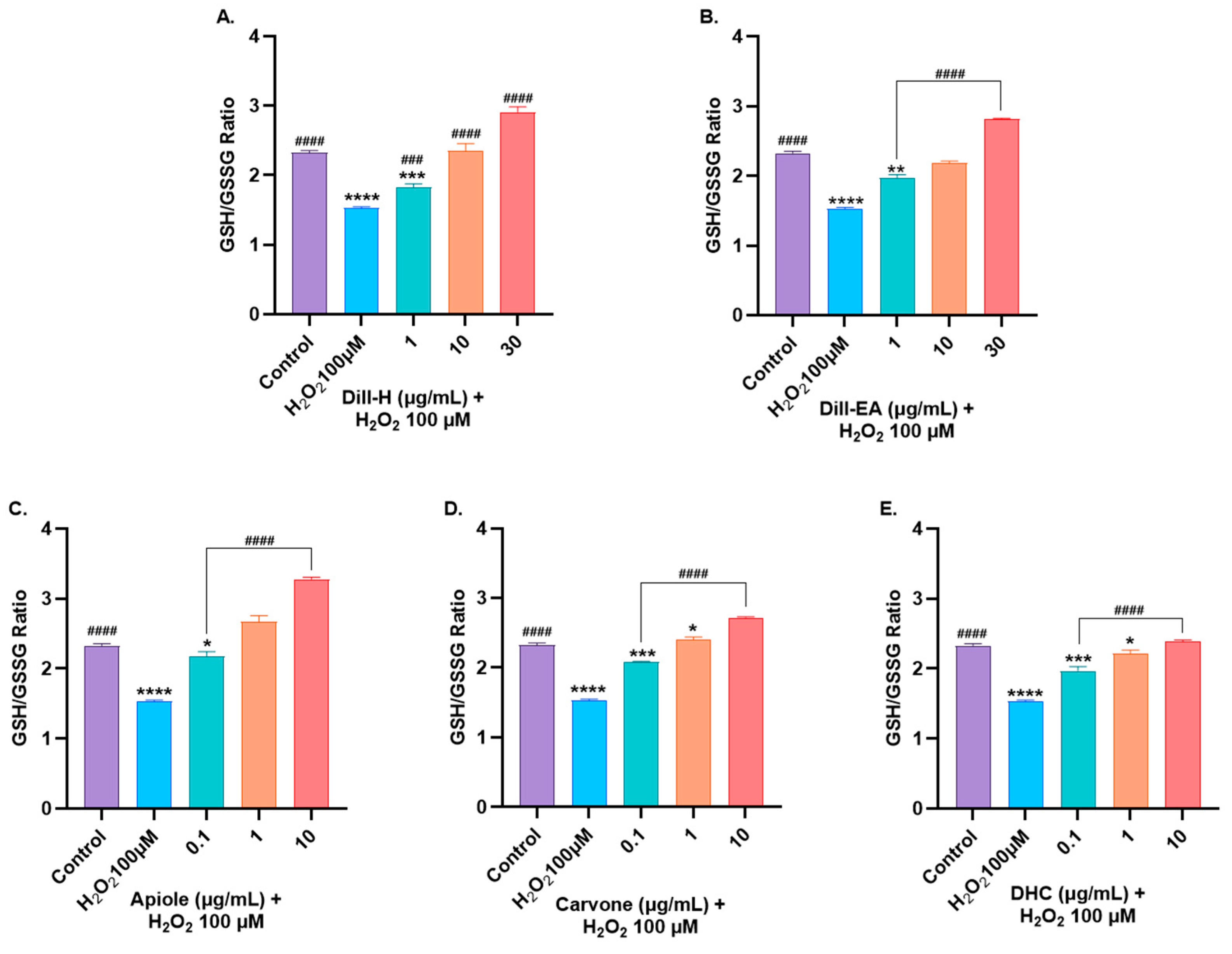
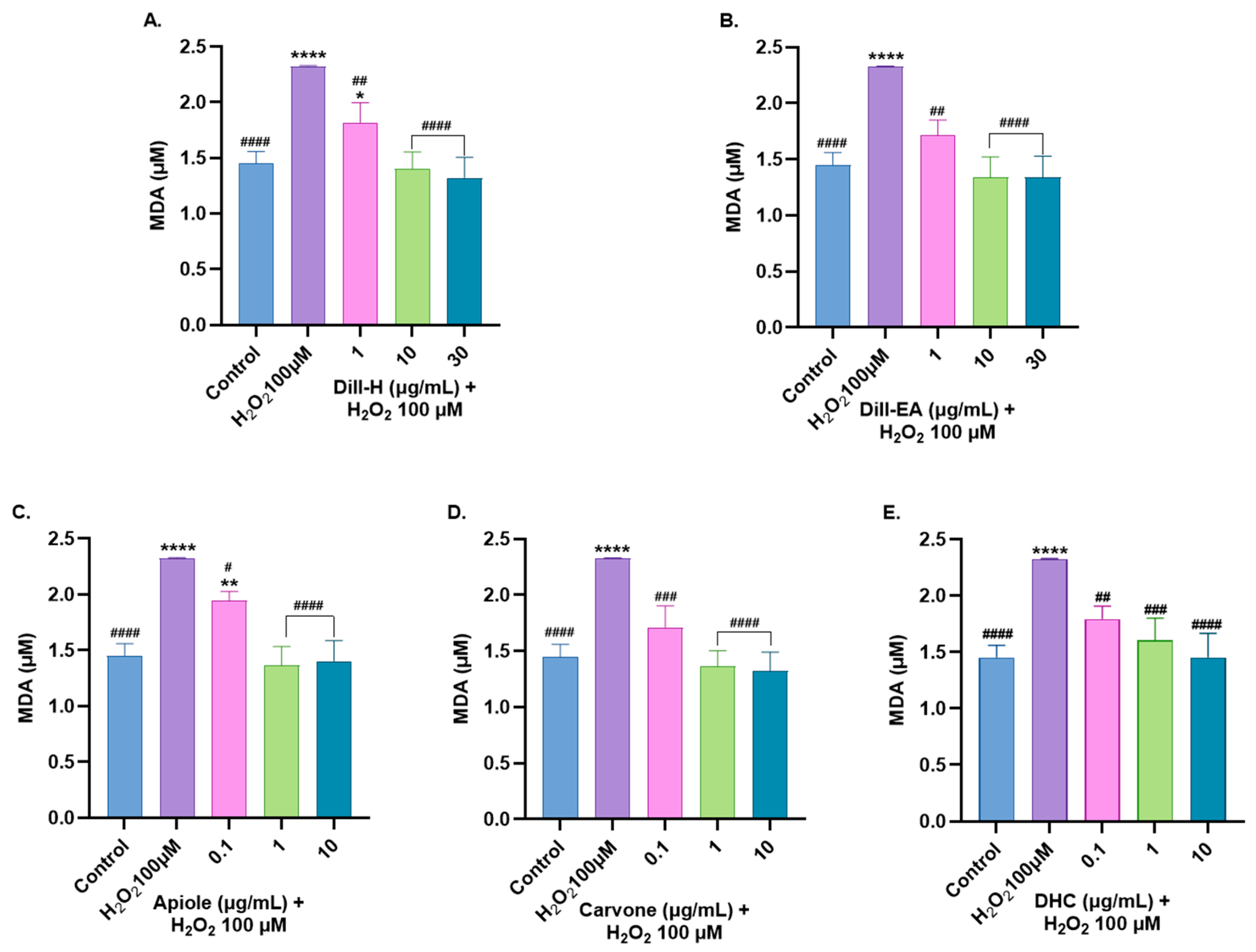
2.9. Dill Extract and Bioactive Compounds Restored Oxidative Stress Markers Altered by H2O2-Induced Oxidative Stress
2.9.1. Restoration of Glutathione Levels
2.9.2. Attenuation of MDA Level by Dill Extract
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Plant Material and Extraction
4.3. Gas Chromatography-Mass Spectrometry (GC-MS) Method
4.4. Determination of Total Phenolic and Flavonoid Content
4.5. Determination of Antioxidant Capacity
- (i)
- 2,2′-Azino-Bis (3-Ethylbenzothiazoline-6-Sulfonic Acid) [ABTS] Radical Scavenging Assay
- (ii)
- Free Radical Scavenging by 2,2-Diphenyl-1-Picrylhydrazylhydrate (DPPH) Radical Assay
- (iii)
- Ferric Reducing Antioxidant Potential (FRAP) Assay
4.6. Acetylcholinesterase Inhibitory Activity
4.7. Anti-Aβ1–42 Oligomerization and Fibrilization Activity
4.8. Cell Culture
4.8.1. Cell Viability Assay
4.8.2. Neuroprotection Assay
4.8.3. Measurement of Intracellular Reactive Oxygen Species (ROS)
4.8.4. Mitochondrial Membrane Potential (ΔΨm) Assay
4.8.5. Antioxidant Parameters in Cell Lysate
Protein Estimation
Estimation of Glutathione
Estimation of Malondialdehyde (MDA)
4.9. Data and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2019 Dementia Forecasting Collaborators. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: An analysis for the Global Burden of Disease Study 2019. Lancet Public Health 2022, 7, e105–e125. [Google Scholar] [CrossRef] [PubMed]
- Bredesen, D.E.; Rao, R.V.; Mehlen, P. Cell death in the nervous system. Nature 2006, 443, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Rubinsztein, D.C. The roles of intracellular protein-degradation pathways in neurodegeneration. Nature 2006, 443, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Guzman-Martinez, L.; Maccioni, R.B.; Andrade, V.; Navarrete, L.P.; Pastor, M.G.; Ramos-Escobar, N. Neuroinflammation as a common feature of neurodegenerative disorders. Front. Pharmacol. 2019, 10, 1008. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative stress: A key modulator in neurodegenerative diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.; Yang, H.; Sharma, N.; An, S.S. Trachyspermum ammi Bioactives Promote Neuroprotection by Inhibiting Acetylcholinesterase, Aβ-Oligomerization/Fibrilization, and Mitigating Oxidative Stress In Vitro. Antioxidants 2024, 13, 9. [Google Scholar] [CrossRef] [PubMed]
- Vera, R.R.; Chane-Ming, J. Chemical Composition of Essential Oil of Dill (Anethum graveolens L.) Growing in Reunion Island. J. Essent. Oil Res. 1998, 10, 539–542. [Google Scholar] [CrossRef]
- Dimov, M.; Dobreva, K.; Stoyanova, A. Chemical composition of the dill essential oils (Anethum graveolens L.) from Bulgaria. Bulg. Chem. Commun. 2019, 51, 214–216. [Google Scholar]
- Al-Oqail, M.M.; Farshori, N.N. Antioxidant and Anticancer Efficacies of Anethum graveolens against Human Breast Carcinoma Cells through Oxidative Stress and Caspase Dependency. Biomed Res. Int. 2021, 2021, 5535570. [Google Scholar] [CrossRef]
- Nam, H.-H.; Nan, L.; Choo, B.-K. Anti-Inflammation and Protective Effects of Anethum graveolens L. (Dill Seeds) on Esophageal Mucosa Damages in Reflux Esophagitis-Induced Rats. Foods 2021, 10, 2500. [Google Scholar] [CrossRef]
- Rezaee-Asl, M.; Nikoui, A.B.V.; Bakhtiarian, A.; Nikoui, V.; Sabour, M.; Ostadhadi, S.; Giorgi, M.-S.Y.-N.A.M. Antinociceptive properties of hydro alcoholic extracts of Anethum graveolens L. (dill) seed and aerial parts in mice. Clin. Exp. Pharmacol. 2013, 3, 122. [Google Scholar] [CrossRef]
- Derakhshan, S.; Navidinia, M.; Ahmadi, A. Antibacterial activity of Dill (Anethum graveolens) essential oil and antibiofilm activity of Cumin (Cuminum cyminum) alcoholic extract. Infect. Epidemiol. Microbiol. 2017, 3, 122–126. [Google Scholar]
- Abas, A.-S.M.; Elagib, S.M. Antiparasitic activity of aqueous extract of Anethum graveolens against Entamoeba histolytica: In vitro and in vivo study. Biocatal. Agric. Biotechnol. 2021, 34, 102026. [Google Scholar] [CrossRef]
- Jana, S.; Shekhawat, G.S. Anethum graveolens: An Indian traditional medicinal herb and spice. Pharmacogn. Rev. 2010, 4, 179–184. [Google Scholar] [PubMed]
- Mohammad, I.H. Use of Anethum graveolens in the management of patients with irritable bowel syndrome. Mustansiriya Med. J. 2012, 11, 94–98. [Google Scholar]
- Sadeghi, M.; Kabiri, S.; Amerizadeh, A.; Heshmat-Ghahdarijani, K.; Masoumi, G.; Teimouri-Jervekani, Z.; Amirpour, A. Anethum graveolens L. (Dill) Effect on Human Lipid Profile: An Updated Systematic Review. Curr. Probl. Cardiol. 2022, 47, 101072. [Google Scholar] [CrossRef] [PubMed]
- Haidari, F.; Zakerkish, M.; Borazjani, F.; Angali, K.A.; Foroushani, G.A. The effects of Anethum graveolens (dill) powder supplementation on clinical and metabolic status in patients with type 2 diabetes. Trials 2020, 21, 483. [Google Scholar] [CrossRef] [PubMed]
- Mesripour, A.; Rafieian-Kopaei, M.; Bahrami, B. The effects of Anethum graveolens essence on scopolamine-induced memory impairment in mice. Res. Pharm. Sci. 2016, 11, 145. [Google Scholar] [PubMed]
- Thukham-Mee, W.; Wattanathorn, J. Evaluation of safety and protective effect of combined extract of Cissampelos pareira and Anethum graveolens (PM52) against age-related cognitive impairment. Evid.-Based Complement. Altern. Med. 2012, 2012, 674101. [Google Scholar] [CrossRef]
- Ohnon, W.; Wattanathorn, J.; Thukham-Mee, W.; Muchimapura, S.; Wannanon, P.; Tong-Un, T. The Combined Extract of Black Sticky Rice and Dill Improves Poststroke Cognitive Impairment in Metabolic Syndrome Condition. Oxid. Med. Cell. Longev. 2019, 2019, 9089035. [Google Scholar] [CrossRef]
- Li, Y.; Fan, H.; Sun, J.; Ni, M.; Zhang, L.; Chen, C.; Hong, X.; Fang, F.; Zhang, W.; Ma, P. Circular RNA expression profile of Alzheimer’s disease and its clinical significance as biomarkers for the disease risk and progression. Int. J. Biochem. Cell Biol. 2020, 123, 105747. [Google Scholar] [CrossRef] [PubMed]
- Burlingham, B.T.; Widlanski, T.S. An intuitive look at the relationship of Ki and IC50: A more general use for the Dixon plot. J. Chem. Educ. 2003, 80, 214. [Google Scholar] [CrossRef]
- Sharma, H.; Kim, D.Y.; Shim, K.H.; Sharma, N.; An, S.S.A. Multi-Targeting Neuroprotective Effects of Syzygium aromaticum Bud Extracts and Their Key Phytocompounds against Neurodegenerative Diseases. Int. J. Mol. Sci. 2023, 24, 8148. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.A.; Lagamayo, M.W.; Alejandro, G.J.; An, S.S. Neuroblastoma SH-SY5Y cytotoxicity, anti-amyloidogenic activity and cyclooxygenase inhibition of Lasianthus trichophlebus (Rubiaceae). 3 Biotech 2020, 10, 152. [Google Scholar] [CrossRef] [PubMed]
- Martínez, S.; Fuentes, C.; Carballo, J. Antioxidant Activity, Total Phenolic Content and Total Flavonoid Content in Sweet Chestnut (Castanea sativa Mill.) Cultivars Grown in Northwest Spain under Different Environmental Conditions. Foods 2022, 11, 3519. [Google Scholar] [CrossRef] [PubMed]
- Oshaghi, E.A.; Tavilani, H.; Khodadadi, I.; Goodarzi, M.T. Dill tablet: A potential antioxidant and anti-diabetic medicine. Asian Pac. J. Trop. Biomed. 2015, 5, 720–727. [Google Scholar] [CrossRef]
- Salmanian, S.; Mahoonak, A.R.S.; Alami, M.; Ghorbani, M. Phenolic content, antiradical, antioxidant, and antibacterial properties of hawthorn (Crataegus elbursensis) seed and pulp extract. J. Agric. Sci. Technol. 2014, 16, 343–354. [Google Scholar]
- Christova-Bagdassarian, V.L.; Bagdassarian, K.S.; Atanassova, M. Phenolic profile, antioxidant and antimicrobial activities from the Apiaceae family (dry seeds). Mintage J. Pharm. Med. Sci. 2013, 2, 26–31. [Google Scholar]
- FDA. Substances Added to Food; U.S. Food and Drug Administration: Washington, DC, USA, 2022.
- Bouyahya, A.; Mechchate, H.; Benali, T.; Ghchime, R.; Charfi, S.; Balahbib, A.; Burkov, P.; Shariati, M.A.; Lorenzo, J.M.; El Omari, N. Health Benefits and Pharmacological Properties of Carvone. Biomolecules 2021, 11, 1803. [Google Scholar] [CrossRef]
- Amat-Ur-Rasool, H.; Ahmed, M.; Hasnain, S.; Ahmed, A.; Carter, W.G. In Silico Design of Dual-Binding Site Anti-Cholinesterase Phytochemical Heterodimers as Treatment Options for Alzheimer’s Disease. Curr. Issues Mol. Biol. 2022, 44, 152–175. [Google Scholar] [CrossRef]
- İstİflİ, E.S.; Tepe, A.; Sarikürkcü, C.; Tepe, B. Interaction of certain monoterpenoid hydrocarbons with the receptor binding domain of 2019 novel coronavirus (2019-nCoV), transmembrane serine protease 2 (TMPRSS2), cathepsin B, and cathepsin L (CatB/L) and their pharmacokinetic properties. Turk. J. Biol. 2020, 44, 242–264. [Google Scholar] [CrossRef] [PubMed]
- Song, H.Y.; Yang, J.Y.; Suh, J.W.; Lee, H.S. Acaricidal activities of apiol and its derivatives from Petroselinum sativum seeds against Dermatophagoides pteronyssinus, Dermatophagoides farinae, and Tyrophagus putrescentiae. J. Agric. Food Chem. 2011, 59, 7759–7764. [Google Scholar] [CrossRef] [PubMed]
- Parise-Filho, R.; Pastrello, M.; Camerlingo, C.E.P.; Silva, G.J.; Agostinho, L.A.; de Souza, T.; Magri, F.M.M.; Ribeiro, R.R.; Brandt, C.A.; Polli, M.C. The anti-inflammatory activity of dillapiole and some semisynthetic analogues. Pharm. Biol. 2011, 49, 1173–1179. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Martínez, R.; Arrieta, J.; Cruz-Antonio, L.; Arrieta-Baez, D.; Velázquez-Méndez, A.M.; Sánchez-Mendoza, M.E. Dillapiole, Isolated from Peperomia pellucida, Shows Gastroprotector Activity against Ethanol-Induced Gastric Lesions in Wistar Rats. Molecules 2013, 18, 11327–11337. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.K.; Damião, M.C.F.C.B.; De-Sá-Júnior, P.L.; Pasqualoto, K.F.M.; de Azevedo, R.A.; Câmara, D.A.D.; Costa, A.S.; Figueiredo, C.R.; Matsuo, A.L.; Massaoka, M.H.; et al. Cytotoxic effects of dillapiole on MDA-MB-231 cells involve the induction of apoptosis through the mitochondrial pathway by inducing an oxidative stress while altering the cytoskeleton network. Biochimie 2014, 99, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Benissa, Z.; Dumas, F.; Zouari, S. Chemical Variability and Antioxidant Activity of Stems and Seeds Essential Oils of Pituranthos chloranthus Benth. and Hook Collected from Tunisia. J. Essent. Oil Bear. Plants 2022, 25, 369–379. [Google Scholar] [CrossRef]
- Moller, A.C.; Parra, C.; Said, B.; Werner, E.; Flores, S.; Villena, J.; Russo, A.; Caro, N.; Montenegro, I.; Madrid, A. Antioxidant and anti-proliferative activity of essential oil and main components from leaves of Aloysia polystachya harvested in Central Chile. Molecules 2020, 26, 131. [Google Scholar] [CrossRef] [PubMed]
- Milenković, L.; Ilić, Z.S.; Stanojević, L.; Danilović, B.; Šunić, L.; Kevrešan, Ž.; Stanojević, J.; Cvetković, D. Chemical Composition and Bioactivity of Dill Seed (Anethum graveolens L.) Essential Oil from Plants Grown under Shading. Plants 2024, 13, 886. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, F.; Wang, X.; Yao, H.-Y. Evaluation of antioxidant activity of parsley (Petroselinum crispum) essential oil and identification of its antioxidant constituents. Food Res. Int. 2006, 39, 833–839. [Google Scholar] [CrossRef]
- Vinutha, B.; Prashanth, D.; Salma, K.; Sreeja, S.; Pratiti, D.; Padmaja, R.; Radhika, S.; Amit, A.; Venkateshwarlu, K.; Deepak, M. Screening of selected Indian medicinal plants for acetylcholinesterase inhibitory activity. J. Ethnopharmacol. 2007, 109, 359–363. [Google Scholar] [CrossRef]
- Akkapinya, P.; Sattaponpan, C.; Itharat, A. Anti-cholinesterase activity of 5-Theins recipes potentially used for Alzheimer’s disease. Planta Medica 2013, 79, PN3. [Google Scholar] [CrossRef]
- López, M.D.; Pascual-Villalobos, M.J. Are monoterpenoids and phenylpropanoids efficient inhibitors of acetylcholinesterase from stored product insect strains? Flavour Fragr. J. 2015, 30, 108–112. [Google Scholar] [CrossRef]
- Miyazawa, M.; Watanabe, H.; Kameoka, H. Inhibition of acetylcholinesterase activity by monoterpenoids with ap-menthane skeleton. J. Agric. Food Chem. 1997, 45, 677–679. [Google Scholar] [CrossRef]
- López, M.D.; Pascual-Villalobos, M.J. Mode of inhibition of acetylcholinesterase by monoterpenoids and implications for pest control. Ind. Crops Prod. 2010, 31, 284–288. [Google Scholar] [CrossRef]
- Orhan, I.; Kartal, M.; Kan, Y.; Şener, B. Activity of essential oils and individual components against acetyland butyrylcholinesterase. Z. Naturforschung C 2008, 63, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Erdogan Orhan, I.; Senol, F.S.; Ozturk, N.; Celik, S.A.; Pulur, A.; Kan, Y. Phytochemical contents and enzyme inhibitory and antioxidant properties of Anethum graveolens L. (dill) samples cultivated under organic and conventional agricultural conditions. Food Chem. Toxicol. 2013, 59, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Bouzekri, O.; Elgamouz, S.; El Khatabi, K.; Amechrouq, A.; Ajana, M.A.; Bouachrine, M.; Lakhlifi, T.; El Idrissi, M.; Choukrad, M. Chemical composition and in silico acetylcholinesterase inhibitory activity of essential oils of six apiaceae species from South-East Morocco. Biointerface Res. Appl. Chem. 2023, 13, 36. [Google Scholar]
- Wojtunik-Kulesza, K.A.; Targowska-Duda, K.; Klimek, K.; Ginalska, G.; Jóźwiak, K.; Waksmundzka-Hajnos, M.; Cieśla, Ł. Volatile terpenoids as potential drug leads in Alzheimer’s disease. Open Chem. 2017, 15, 332–343. [Google Scholar] [CrossRef]
- da Silva, J.K.R.; Silva, N.N.S.; Santana, J.F.S.; Andrade, E.H.A.; Maia, J.G.S.; Setzer, W.N. Phenylpropanoid-rich essential oils of Piper species from the Amazon and their antifungal and anti-cholinesterase activities. Nat. Prod. Commun. 2016, 11, 1934578X1601101233. [Google Scholar] [CrossRef]
- Grundy, D.L.; Still, C.C. Inhibition of acetylcholinesterases by pulegone-1, 2-epoxide. Pestic. Biochem. Physiol. 1985, 23, 383–388. [Google Scholar] [CrossRef]
- Oshaghi, E.A.; Khodadadi, I.; Tavilani, H.; Goodarzi, M.T. Aqueous Extract of Anethum graveolens L. has Potential Antioxidant and Antiglycation Effects. Iran. J. Med. Sci. 2016, 41, 328–333. [Google Scholar] [PubMed]
- Oshaghi, E.A.; Khodadadi, I.; Mirzaei, F.; Khazaei, M.; Tavilani, H.; Goodarzi, M.T. Methanolic Extract of Dill Leaves Inhibits AGEs Formation and Shows Potential Hepatoprotective Effects in CCl4 Induced Liver Toxicity in Rat. J. Pharm. 2017, 2017, 6081374. [Google Scholar]
- Kotormán, M.; Varga, A.; Kasi, P.B.; Nemcsók, J. Inhibition of the formation of amyloid-like fibrils with spices, especially cloves. Acta Biol. Hung. 2018, 69, 385–394. [Google Scholar] [CrossRef]
- Banerjee, S.; Baghel, D.; Pacheco de Oliveira, A.; Ghosh, A. β-Carotene, a Potent Amyloid Aggregation Inhibitor, Promotes Disordered Aβ Fibrillar Structure. Int. J. Mol. Sci. 2023, 24, 5175. [Google Scholar] [CrossRef]
- Im, D.; Kim, S.; Yoon, G.; Hyun, D.G.; Eom, Y.G.; Lee, Y.E.; Sohn, C.H.; Choi, J.M.; Kim, H.I. Decoding the Roles of Amyloid-β (1–42)’s Key Oligomerization Domains toward Designing Epitope-Specific Aggregation Inhibitors. JACS Au 2023, 3, 1065–1075. [Google Scholar] [CrossRef] [PubMed]
- Gour, N.; Koshti, B.; Kshtriya, V.S. A chemical perspective to the anti-amyloid action of compounds and a nanoparticle based assay for screening amyloid inhibitors. ChemRxiv 2019. [Google Scholar] [CrossRef]
- Necula, M.; Kayed, R.; Milton, S.; Glabe, C.G. Small molecule inhibitors of aggregation indicate that amyloid β oligomerization and fibrillization pathways are independent and distinct. J. Biol. Chem. 2007, 282, 10311–10324. [Google Scholar] [CrossRef] [PubMed]
- Heckmann, M.; Stadlbauer, V.; Drotarova, I.; Gramatte, T.; Feichtinger, M.; Arnaut, V.; Atzmüller, S.; Schwarzinger, B.; Röhrl, C.; Blank-Landeshammer, B.; et al. Identification of Oxidative-Stress-Reducing Plant Extracts from a Novel Extract Library—Comparative Analysis of Cell-Free and Cell-Based In Vitro Assays to Quantitate Antioxidant Activity. Antioxidants 2024, 13, 297. [Google Scholar] [CrossRef]
- Asle-Rousta, M.; Amini, R.; Aghazadeh, S. Carvone suppresses oxidative stress and inflammation in the liver of immobilised rats. Arch. Physiol. Biochem. 2023, 129, 597–602. [Google Scholar] [CrossRef]
- Ogaly, H.A.; Aldulmani, S.A.A.; Al-Zahrani, F.A.M.; Abd-Elsalam, R.M. D-Carvone Attenuates CCl(4)-Induced Liver Fibrosis in Rats by Inhibiting Oxidative Stress and TGF-ß 1/SMAD3 Signaling Pathway. Biology 2022, 11, 739. [Google Scholar] [CrossRef]
- Rajeshwari, T.; Raja, B. D-carvone, a monoterpene reverses alterations in heart rate, nitric oxide, aortic lipids and enzymatic antioxidant status in nitric oxide deficient hypertensive rats. Int. Lett. Nat. Sci. 2015, 5, 18–30. [Google Scholar] [CrossRef]
- Sharma, N.; Tan, M.A.; An, S.S.A. Mechanistic aspects of Apiaceae family spices in ameliorating Alzheimer’s disease. Antioxidants 2021, 10, 1571. [Google Scholar] [CrossRef] [PubMed]
- Vásquez-Garzón, V.R.; Arellanes-Robledo, J.; García-Román, R.; Aparicio-Rautista, D.I.; Villa-Treviño, S. Inhibition of reactive oxygen species and pre-neoplastic lesions by quercetin through an antioxidant defense mechanism. Free. Radic. Res. 2009, 43, 128–137. [Google Scholar] [CrossRef]
- Zitka, O.; Skalickova, S.; Gumulec, J.; Masarik, M.; Adam, V.; Hubalek, J.; Trnkova, L.; Kruseova, J.; Eckschlager, T.; Kizek, R. Redox status expressed as GSH:GSSG ratio as a marker for oxidative stress in paediatric tumour patients. Oncol. Lett. 2012, 4, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.E.; Younis, N.S. Ameliorative effect of D-carvone against hepatic ischemia-reperfusion-induced injury in rats. Life 2022, 12, 1502. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Dhiman, C.; Kothiyal, P. Evaluation of Anethum graveolens extract on memory impaired mice. Indo Am. J. Pharm. Sci. 2017, 4, 1965–1975. [Google Scholar]
- Koppula, S.; Choi, D.K. Anethum Graveolens linn (Umbelliferae) extract attenuates stress-induced urinary biochemical changes and improves cognition in scopolamineinduced amnesic rats. Trop. J. Pharm. Res. 2011, 10, 1. [Google Scholar] [CrossRef]
- Mohammadali, S.; Heshami, N.; Komaki, A.; Tayebinia, H.; Oshaghi, E.A.; Karimi, J.; Hashemnia, M.; Khodadadi, I. Dill tablet and Ocimum basilicum aqueous extract: Promising therapeutic agents for improving cognitive deficit in hypercholesterolemic rats. J. Food Biochem. 2020, 44, e13485. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-Y.; Kim, H.-S.; Hong, S.S.; Sul, D.; Hwang, K.W.; Lee, D. The neuroprotective effects of traditional oriental herbal medicines against β-amyloid-induced toxicity. Pharm. Biol. 2009, 47, 976–981. [Google Scholar] [CrossRef]
- Leclerc, M.; Dudonné, S.; Calon, F. Can Natural Products Exert Neuroprotection without Crossing the Blood–Brain Barrier? Int. J. Mol. Sci. 2021, 22, 3356. [Google Scholar] [CrossRef]
- Sembiring, E.N.; Elya, B.; Sauriasari, R. Phytochemical screening, total flavonoid and total phenolic content and antioxidant activity of different parts of Caesalpinia bonduc (L.) Roxb. Pharmacogn. J. 2018, 10, 123–127. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.; Sharma, N.; An, S.S.A. Black Pepper (Piper nigrum) Alleviates Oxidative Stress, Exerts Potential Anti-Glycation and Anti-AChE Activity: A Multitargeting Neuroprotective Agent against Neurodegenerative Diseases. Antioxidants 2023, 12, 1089. [Google Scholar] [CrossRef] [PubMed]
- Aktumsek, A.; Zengin, G.; Guler, G.O.; Cakmak, Y.S.; Duran, A. Antioxidant potentials and anticholinesterase activities of methanolic and aqueous extracts of three endemic Centaurea L. species. Food Chem. Toxicol. 2013, 55, 290–296. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]




| Vmax (μmole/min/mg) | Km (mM) | Ki (µg/mL) | Type of Inhibition | |
|---|---|---|---|---|
| No inhibitor | 2.975 | 23.43 | ||
| Dill-H (100 μg/mL) | 3.472 | 31.94 | 463.36 | Competitive |
| Dill-H (200 μg/mL) | 3.710 | 39.14 | 464.68 | |
| Dill-EA (100 μg/mL) | 2.921 | 27.39 | 495.05 | Competitive |
| Dill-EA (200 μg/mL) | 3.512 | 38.21 | 497.58 | |
| Apiole (100 μg/mL) | 3.551 | 31.02 | 382.18 | Competitive |
| Apiole (200 μg/mL) | 3.712 | 34.59 | 382.81 | |
| Carvone (100 μg/mL) | 2.900 | 25.12 | 270.31 | Competitive |
| Carvone (200 μg/mL) | 3.141 | 25.28 | 270.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, H.; Yang, H.; Sharma, N.; An, S.S.A. Neuroprotection by Anethum graveolens (Dill) Seeds and Its Phytocompounds in SH-SY5Y Neuroblastoma Cell Lines and Acellular Assays. Int. J. Mol. Sci. 2024, 25, 7104. https://doi.org/10.3390/ijms25137104
Sharma H, Yang H, Sharma N, An SSA. Neuroprotection by Anethum graveolens (Dill) Seeds and Its Phytocompounds in SH-SY5Y Neuroblastoma Cell Lines and Acellular Assays. International Journal of Molecular Sciences. 2024; 25(13):7104. https://doi.org/10.3390/ijms25137104
Chicago/Turabian StyleSharma, Himadri, Hyewon Yang, Niti Sharma, and Seong Soo A. An. 2024. "Neuroprotection by Anethum graveolens (Dill) Seeds and Its Phytocompounds in SH-SY5Y Neuroblastoma Cell Lines and Acellular Assays" International Journal of Molecular Sciences 25, no. 13: 7104. https://doi.org/10.3390/ijms25137104
APA StyleSharma, H., Yang, H., Sharma, N., & An, S. S. A. (2024). Neuroprotection by Anethum graveolens (Dill) Seeds and Its Phytocompounds in SH-SY5Y Neuroblastoma Cell Lines and Acellular Assays. International Journal of Molecular Sciences, 25(13), 7104. https://doi.org/10.3390/ijms25137104







