Cardiac Development and Factors Influencing the Development of Congenital Heart Defects (CHDs): Part I
Abstract
:1. Introduction
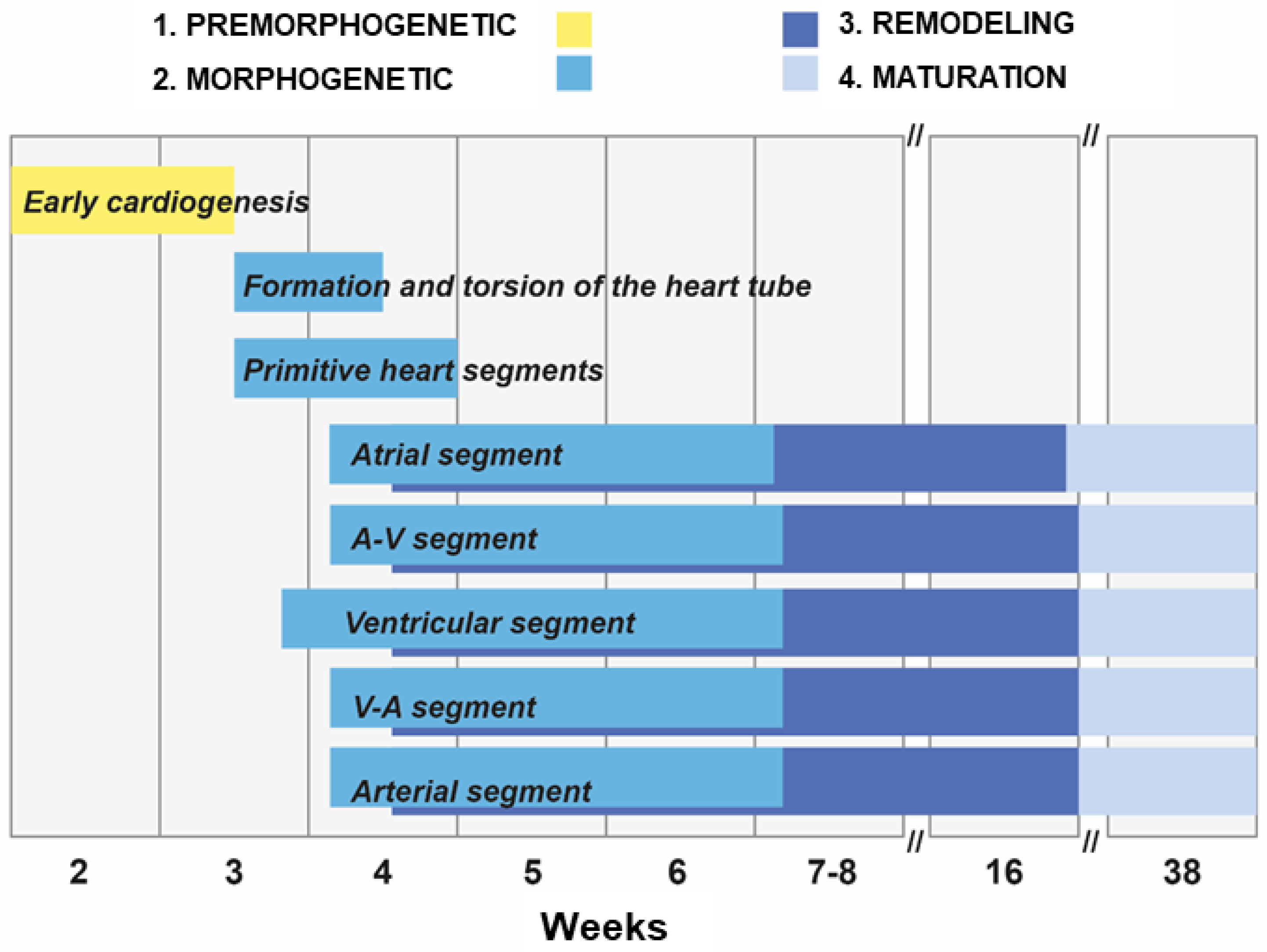
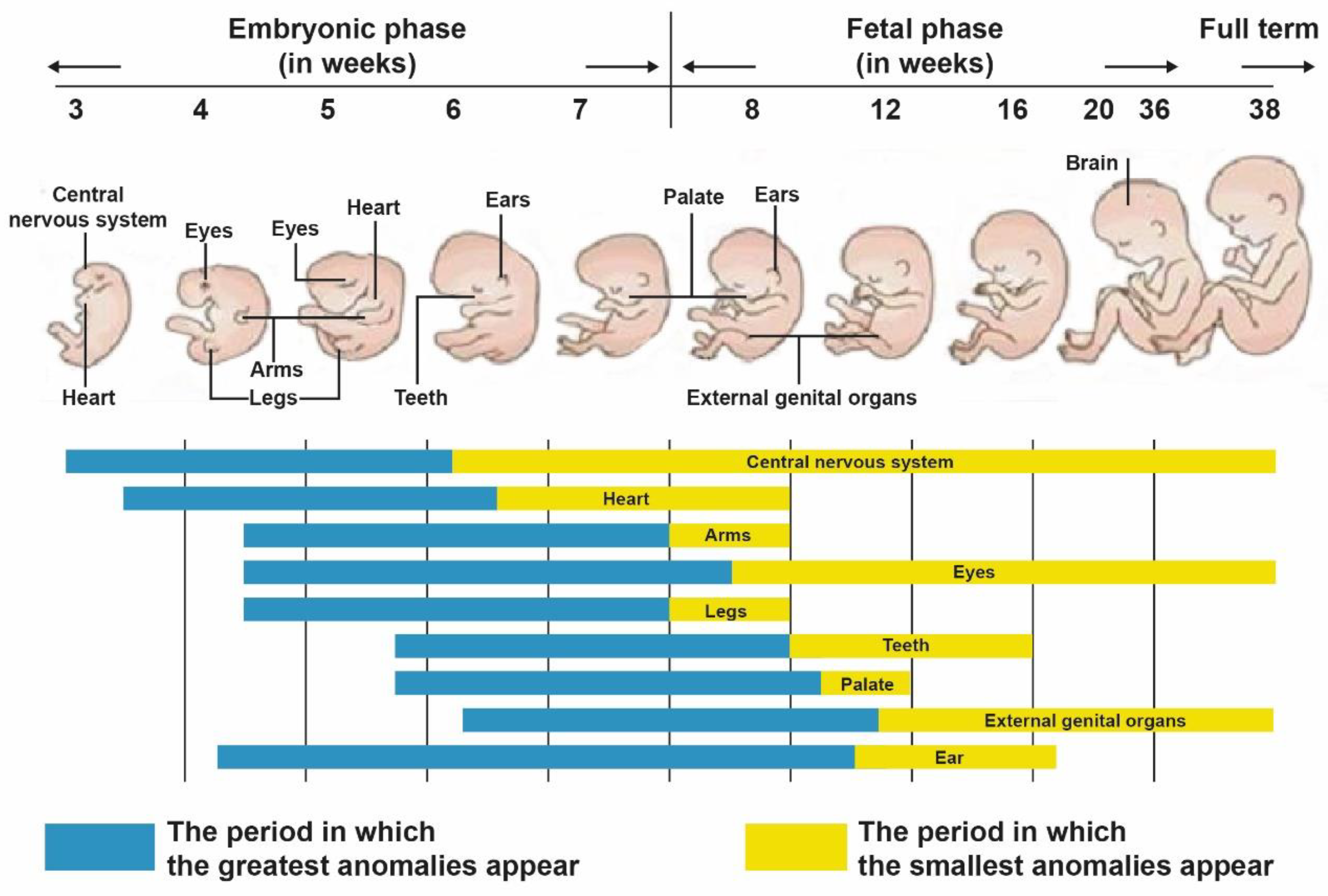
2. Stages of the Human Heart Development
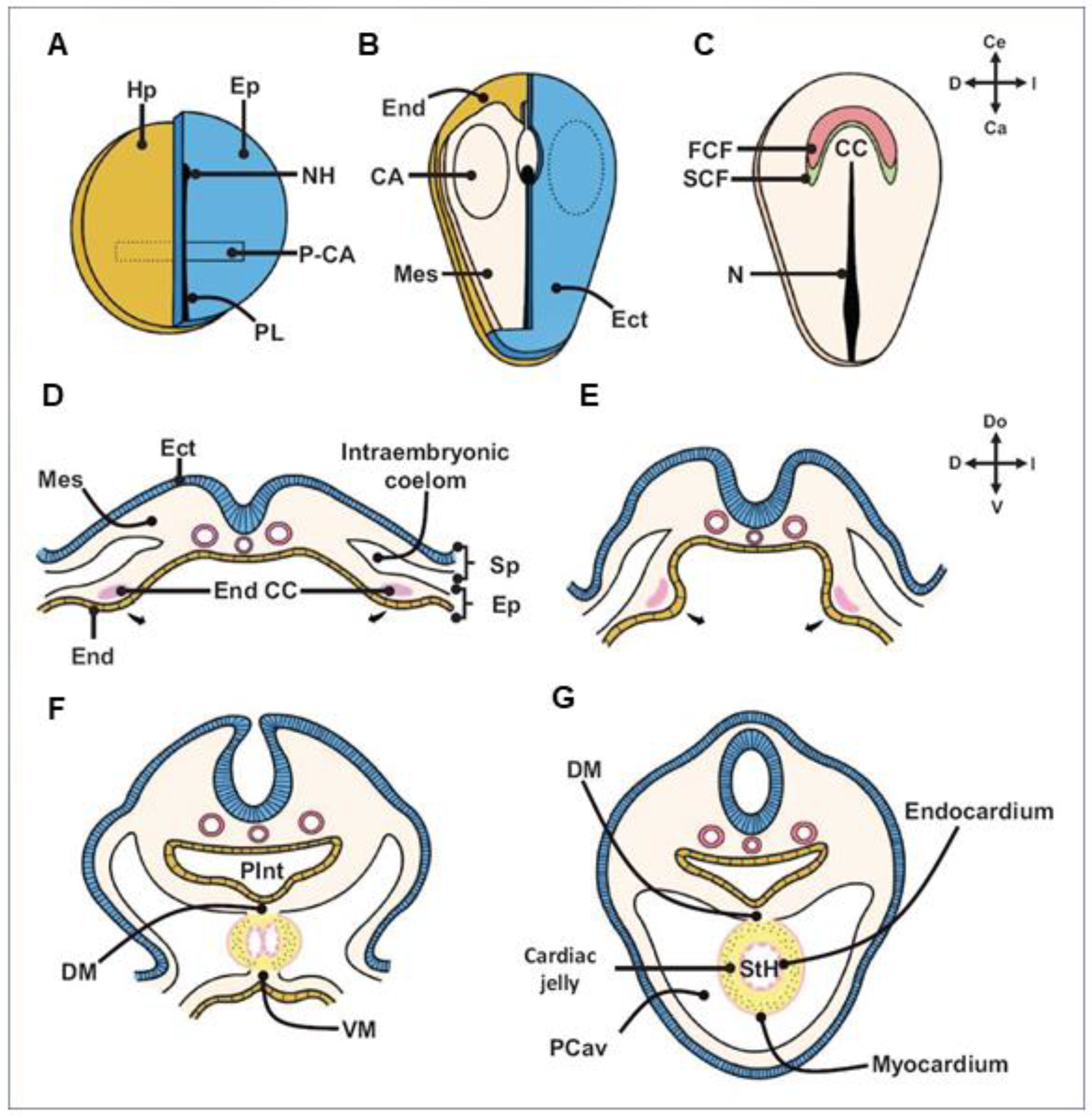
- Early cardiogenesis: It occurs during the premorphogenetic or presomitic stage of the embryo (days 8–18 of development). Early cardiogenesis begins with the organization of the cardiac areas and crescent through gastrulation and ends with the formation of two endocardial tubes that are externally covered by myocardial lineage cells.
- Morphogenetic stage: This stage occurs during weeks 4–8 of embryonic development. It begins with the formation of the straight heart tube, derived from the first heart field (FHF), and ends after the integration of the primordia of all the structures that comprise the four-chambered heart, derived from the second heart field (SHF).
- Septation and remodeling of the heart chambers: This stage begins during midembryonic development (day 30). At this stage, the primordia undergo differential growth and remodeling processes. The valves and septum are formed, and concurrently, the atrial and ventricular cavities acquire their morphological identities.
- Maturation and histodifferentiation: It occurs during the fetal period (weeks 16–38) and involves histological maturation of the ventricular and atrial myocardium and histological differentiation of the ventriculoarterial and atrioventricular valve systems, including the tendinous cords and papillary muscles. Concurrently, the conduction system and coronary vessels are developed [3].
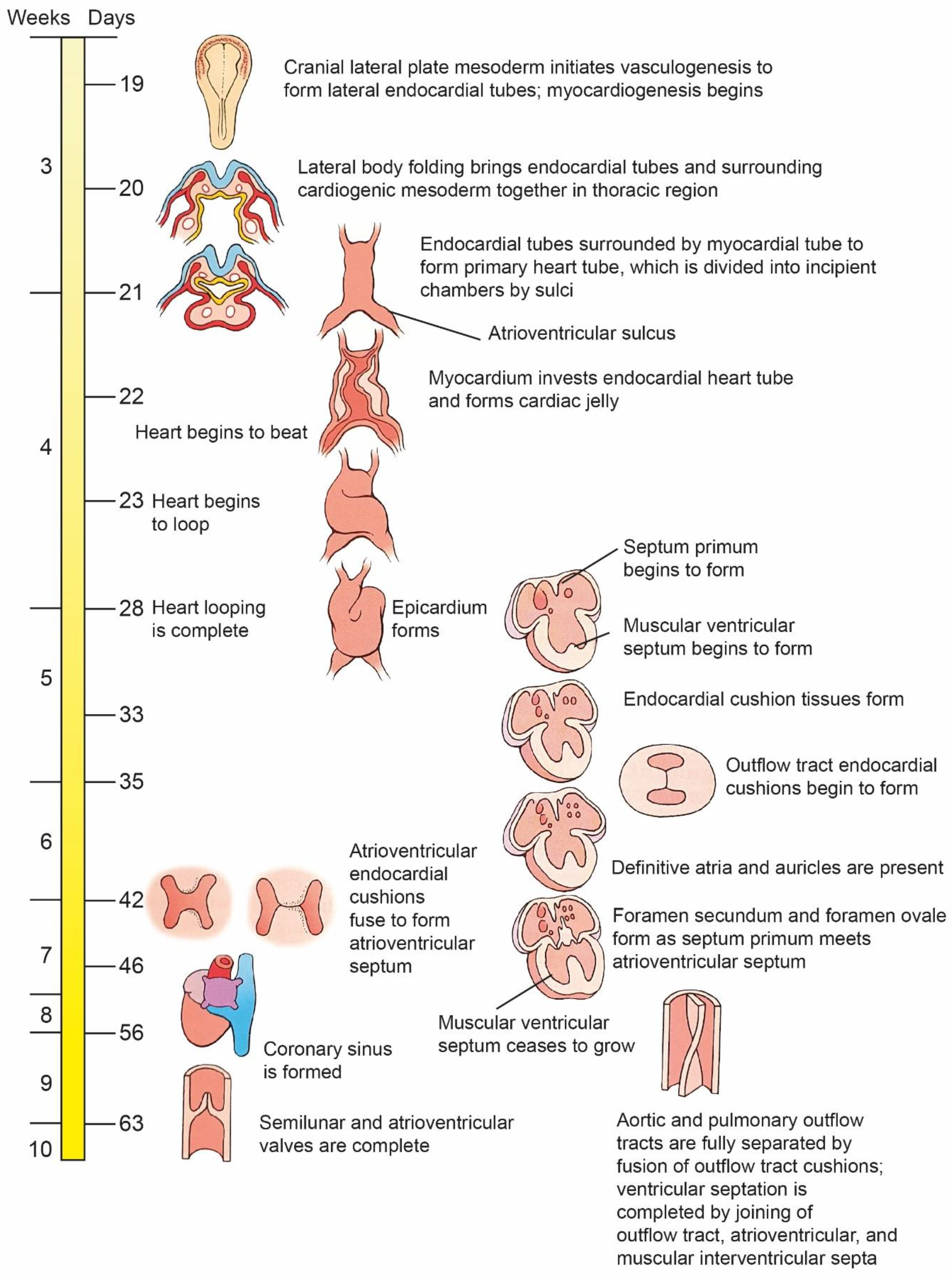
3. Chronology of the Development of the Heart
4. Cardiac Loop Formation
5. Differentiation and Remodeling of the Cardiac Chambers
6. Development of the Cardiac Conduction System
7. Development of Large Blood Vessels
8. New Heart Development Research Techniques
9. Impact of Genetic Factors on Heart Development
10. Etiology of Congenital Heart Defects (CHDs)
10.1. Prevalence and Regional Variation of CHDs
10.2. Maternal Factors
10.3. Genetic Factors
10.4. The Influence of Environmental Factors
| Known Defect(s) | |
|---|---|
| Maternal Illness | |
| Phenylketonuria | |
| Pregestational diabetes | Conotruncal defects |
| Laterality and looping | |
| Dextro-looped transposition of the great arteries | |
| Atrioventricular septal defect | |
| Septal defects | |
| Hypoplastic left heart syndrome | |
| Outflow tract defects | |
| Patent ductus arteriosus | |
| Febrile illness | Conotruncal defects |
| Right-sided obstructive defects | |
| Tricuspid atresia | |
| Left-sided obstructive defects | |
| Aortic coarctation | |
| Ventricular septal defects | |
| Influenza | Conotruncal defects |
| Dextro-looped transposition of the great arteries | |
| Right-sided obstructive defects | |
| Left-sided obstructive defects | |
| Aortic coarctation | |
| Ventricular septal defects | |
| Dextro-looped transposition of the great arteries with intact ventricular septum | |
| Tricuspid atresia | |
| Maternal rubella | Ventricular septal defects |
| Patent ductus arteriosus | |
| Pulmonary valve abnormalities | |
| Peripheral pulmonic stenosis | |
| Epilepsy | |
| Maternal therapeutic drug exposure | |
| Anticonvulsants | |
| Indomethacin tocolysis | Patent ductus arteriosus |
| Ibuprofen | Dextro-looped transposition of the great arteries |
| Ventricular septal defects | |
| Bicuspid aortic valve | |
| Sulfasalazine | |
| Thalidomide | |
| Trimethoprim-sulfonamide | |
| Maternal nontherapeutic drug exposure | |
| Maternal vitamin A | Outflow tract defects |
| Cranial neural crest defects (cardiac and noncardiac) | |
| Pulmonic stenosis | |
| Marijuana | Ventricular septal defects |
| Ebstein’s anomaly | |
| Maternal environmental exposure | |
| Organic solvents | Conotruncal defects |
| Hypoplastic left heart syndrome | |
| Aortic coarctation | |
| Pulmonic stenosis | |
| Dextro-looped transposition of the great arteries with intact ventricular septum | |
| Tetralogy of Fallot | |
| Total anomalous pulmonary venous return | |
| Atrioventricular septal defect | |
| Ebstein’s anomaly | |
| Ventricular septal defects |
10.5. Epigenetic Processes
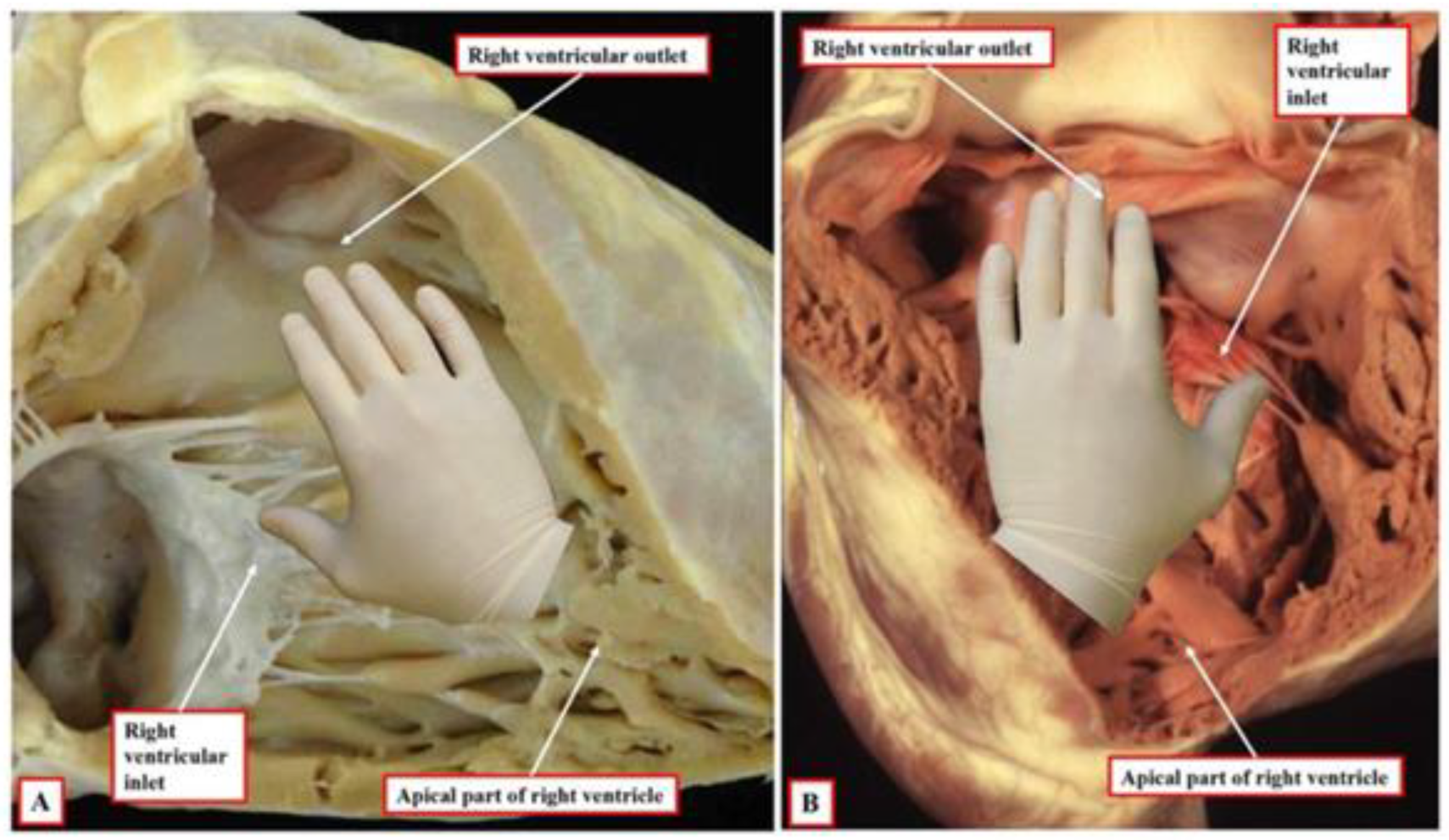
11. Sequential Segmental Analysis of the Heart
- {S, -, -}—situs solitus—Normal visceroatrial relationship, i.e., the presence of both venae cavae and the right atrium on the right side, whereas the left atrium is on the left side. The right atrium is “right-handed”, and the left one is “left-handed”.
- {-, D, -}—D-loop, i.e., right-handed cardiac loop, the right ventricle is “right-handed” and located on the right and anteriorly in relation to the left ventricle. The left ventricle is “left-handed” and situated to the left and inferiorly to the right ventricle due to the right-sided looping of the embryonal heart tube.
- {-, -, S}—normal position of the large blood vessels, i.e., the aorta located posteriorly and to the right of the pulmonary artery trunk. The ventriculovascular junction is concordant, and the cone is located under the pulmonary artery.
- The {l, L, l} cardiotype denotes also a normal heart, but in situs inversus (mirror image).
12. Ventriculoarterial Relationship
13. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANF | atrial natriuretic factor |
| AV | atrioventricular |
| BMPs | bone morphogenetic proteins |
| cc-TGA | congenitally corrected transposition of the great arteries |
| CHD | congenital heart defect |
| CM | cardiomyocytes |
| CNCCs | neural crest cells |
| DNA | deoxyribonucleic acid |
| D-TGA | transposition of the great arteries |
| DORV | double outlet right ventricle |
| ECCs | endocardial cells |
| EMT | epithelial-mesenchymal transformation |
| FGF | fibroblast growth factor |
| FHF | first heart field |
| LV | left ventricle |
| miRNA | microRNA |
| PDGF | platelet derived growth factor |
| RA | right atrium |
| RNA | ribonucleic acid |
| RV | right ventricle |
| SHF | second heart field |
| SA | situs ambiguus |
| SI | situs inversus |
| SS | situs solitus |
| TF | transcription factor |
| TGA | transposition of the great arteries |
| TGF-β | transforming growth factor beta |
| VA | ventriculoarterial |
| VEGF | vascular endothelial growth factor |
References
- Harvey, R.P.; Rosenthal, N. (Eds.) Heart Development; Academic Press: San Diego, CA, USA, 1999. [Google Scholar]
- Buijtendijk, M.F.J.; Barnett, P.; van den Hoff, M.J.B. Development of the human heart. Am. J. Med. Genet. Part C Semin. Med. Genet. 2020, 184, 7–22. [Google Scholar] [CrossRef] [PubMed]
- Villavicencio-Guzmán, L.; Sánchez-Gómez, C.; Jaime-Cruz, R.; Ramírez-Fuentes, T.C.; Patiño-Morales, C.C.; Salazar-García, M. Human Heart Morphogenesis: A New Vision Based on In Vivo Labeling and Cell Tracking. Life 2023, 13, 165. [Google Scholar] [CrossRef] [PubMed]
- Moorman, A.F.M.; Webb, S.; Brown, N.A.; Lamers, W.; Anderson, R.H. Development of the heart: (1) formation of the cardiac chambers and arterial trunks. Heart 2003, 89, 806–814. [Google Scholar] [CrossRef]
- Sylva, M.; van den Hoff, M.J.; Moorman, A.F. Development of the human heart. Am. J. Med. Genet. Part A 2014, 164A, 1347–1371. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.H.; Webb, S.; Brown, N.A.; Lamers, W.; Moorman, A. Development of the heart: (2) Septation of the atriums and ventricles. Heart 2003, 89, 949–958. [Google Scholar] [CrossRef]
- Kirby, M.L.; Gale, T.F.; Stewart, D.E. Neural crest cells contribute to normal aorticopulmonary septation. Science 1983, 220, 1059–1061. [Google Scholar] [CrossRef] [PubMed]
- Icardo, J.M. Developmental biology of the vertebrate heart. J. Exp. Zool. 1996, 275, 144–161. [Google Scholar] [CrossRef]
- Stoller, J.Z.; Epstein, J.A. Cardiac neural crest. Semin. Cell Dev. Biol. 2005, 16, 704–715. [Google Scholar] [CrossRef]
- Paige, S.L.; Plonowska, K.; Xu, A.; Wu, S.M. Molecular regulation of cardiomyocyte differentiation. Circ. Res. 2015, 116, 341–353. [Google Scholar] [CrossRef]
- Jarrell, D.K.; Lennon, M.L.; Jacot, J.G. Epigenetics and Mechanobiology in Heart Development and Congenital Heart Disease. Diseases 2019, 7, 52. [Google Scholar] [CrossRef]
- Srivastava, D. Genetic regulation of cardiogenesis and congenital heart disease. Annu. Rev. Pathol. 2006, 1, 199–213. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, K.J.; Correa, A.; Feinstein, J.A.; Botto, L.; Britt, A.E.; Daniels, S.R.; Elixson, M.; Warnes, C.A.; Webb, C.L. Noninherited risk factors and congenital cardiovascular defects: Current knowledge. Circulation 2007, 115, 2995–3014. [Google Scholar] [CrossRef] [PubMed]
- Fung, A.; Manlhiot, C.; Naik, S.; Rosenberg, H.; Smythe, J.; Lougheed, J.; Mondal, T.; Chitayat, D.; McCrindle, B.W.; Mital, S. Impact of prenatal risk factors on congenital heart disease in the current era. J. Am. Heart Assoc. 2013, 2, e000064. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhu, B.; Zhuo, L.; He, M.Y.; Xu, Y.; Wang, T.T.; Cai, Q.Q.; Hu, B.; Xu, J.C.; Zhang, W.H. Risk factors for congenital heart disease in Chinese neonates: A Meta analysis. Chin. J. Contemp. Pediatr. 2017, 19, 754–758. [Google Scholar]
- Kalisch-Smith, J.I.; Ved, N.; Sparrow, D.B. Environmental risk factors for congenital heart disease. Cold Spring Harb. Perspect. Biol. 2020, 12, a037234. [Google Scholar] [CrossRef] [PubMed]
- George, R.M.; Firulli, A.B. Epigenetics and Heart Development. Front. Cell Dev. Biol. 2021, 9, 637996. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, B.; Yang, P. Epigenetics in Congenital Heart Disease. J. Am. Heart Assoc. 2022, 11, e025163. [Google Scholar] [CrossRef]
- Boyd, R.; McMullen, H.; Beqaj, H.; Kalfa, D. Environmental Exposures and Congenital Heart Disease. Pediatrics 2022, 149, e2021052151. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Jin, X.; Zhang, Y.; Zheng, J.; Yang, R. Genetic and epigenetic mechanisms in the development of congenital heart diseases. World J. Pediatr. Surg. 2021, 4, e000196. [Google Scholar] [CrossRef]
- Akerberg, B.N.; Pu, W.T. Genetic and Epigenetic Control of Heart Development. Cold Spring Harb. Perspect. Biol. 2020, 12, a036756. [Google Scholar] [CrossRef]
- Flores, B.G.R.; Guzmán, L.V.; García, M.S.; Lazzarini, R. Normal development of the heart: A review of new findings. Bol. Med. Hosp. Infant. Mex. 2023, 80, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Gittenberger-de Groot, A.C.; Poelmann, R.E. Cardiac morphogenesis. In Fetal Cardiology: Embryology, Genetics, Physiology, Echocardiographic Evaluation, Diagnosis and Perinatal Management of Cardiac Diseases, 2nd ed.; Yagel, S., Silverman, N.H., Gembruch, U., Eds.; Informa Healthcare Inc.: London, UK, 2009; pp. 9–17. [Google Scholar]
- Gittenberger-de Groot, A.C.; Calkoen, E.E.; Poelmann, R.E.; Bartelings, M.M.; Jongbloed, M.R. Morphogenesis and molecular considerations on congenital cardiac septal defects. Ann. Med. 2014, 46, 640–652. [Google Scholar] [CrossRef]
- Villavicencio-Guzmán, L.; Salazar-García, M.; Jaime-Cruz, R.; Lazzarini, R.; Toledano-Toledano, F.; Sanchez, C. Incorporation of the first and second heart fields and prospective fate of the straight heart tube via in vivo labeling of chicken embryos. PLoS ONE 2020, 15, e0234069. [Google Scholar]
- Buckingham, M.; Meilhac, S.; Zaffran, S. Building the mammalian heart from two sources of myocardial cells. Nat. Rev. Genet. 2005, 6, 826–835. [Google Scholar] [CrossRef] [PubMed]
- Devine, W.P.; Wythe, J.D.; George, M.; Koshiba-Takeuchi, K.; Bruneau, B.G. Early patterning and specification of cardiac progenitors in gastrulating mesoderm. eLife 2014, 3, e03848. [Google Scholar] [CrossRef]
- Abu-Issa, R.; Waldo, K.; Kirby, M.L. Heart fields: One, two or more? Dev. Biol. 2004, 272, 281–285. [Google Scholar] [CrossRef]
- Abu-Issa, R.; Kirby, M.L. Heart field: From mesoderm to heart tube. Annu. Rev. Cell. Dev. Biol. 2007, 23, 45–68. [Google Scholar] [CrossRef]
- Waldo, K.L.; Kumiski, D.H.; Wallis, K.T.; Stadt, H.A.; Hutson, M.R.; Platt, D.H.; Kirby, M.L. Conotruncal myocardium arises from a secondary heart field. Development 2001, 128, 3179–3188. [Google Scholar] [CrossRef]
- Snarr, B.S.; Kern, C.B.; Wessels, A. Origin and fate of cardiac mesenchyme. Dev. Dyn. 2008, 237, 2804–2819. [Google Scholar] [CrossRef]
- Van den Berg, G.; Moorman, A.F. Concepts of cardiac development in retrospect. Pediatr. Cardiol. 2009, 30, 580–587. [Google Scholar] [CrossRef]
- Moorman, A.F.; Christoffels, V.M.; Anderson, R.H.; van den Hoff, M.J.B. The heart-forming fields: One or multiple? Phil. Trans. R. Soc. B 2007, 362, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Venema, H.L.; van den Akker, N.M.; Bax, N.A.; Winter, E.M.; Maas, S.; Kekarainen, T.; Hoeben, R.C.; deRuiter, M.C.; Poelmann, R.E. Origin fate and function of epicardium-derived cells (EPDCs) in normal cardiac development. Sci. World J. 2007, 7, 1777–1798. [Google Scholar] [CrossRef] [PubMed]
- Mjaatvedt, C.H.; Nakaoka, T.; Moreno-Rodriguez, R.; Norris, R.A.; Kern, M.J.; Eisenberg, C.A.; Turner, D.; Markwald, P.R. The outflow tract of the heart is recruited from a novel heart-forming field. Dev. Biol. 2001, 238, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Van den Hoff, M.J.; Moorman, A.F. Cardiac neural crest: The holy grail of cardiac abnormalis? Cardiovasc. Res. 2000, 47, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Moorman, A.F.; Christoffels, V.M.; Anderson, R.H. Anatomic substrates for cardiac conduction. Heart Rhythm. 2005, 2, 875–886. [Google Scholar] [CrossRef] [PubMed]
- Keyte, A.; Hutson, M.R. The neural crest in cardiac congenital anomalies. Differentiation 2012, 84, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Velasco, E.; Garcia-Padilla, C.; del Mar Muñoz-Gallardo, M.; Martinez-Amaro, F.J.; Caño-Carrillo, S.; Castillo-Casas, J.M.; Sanchez-Fernandez, C.; Aranega, A.E.; Franco, D. Post-Transcriptional Regulation of Molecular Determinants during Cardiogenesis. Int. J. Mol. Sci. 2022, 23–2839. [Google Scholar]
- Wenink, A.C.; Gittenberger-de Groot, A.C. Pathogenesis of congenital cardiac malformations and mechanisms of cardiac remodeling. Cardiol. Young 2005, 15, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Linask, K.K.; VanAuker, M. A role for the cytoskeleton in heart looping. Sci. World J. 2007, 7, 280–298. [Google Scholar] [CrossRef]
- Angelini, P. Embryology and congenital heart disease. Tex. Heart Inst. J. 1995, 22, 1–12. [Google Scholar]
- Capuani, A.; Uemura, H.; Ho, S.Y.; Anderson, R.H. Anatomic spectrum of abnormal ventriculoarterial connections: Surgical implications. Ann. Thorac. Surg. 1995, 59, 352–360. [Google Scholar]
- Pérez-Pomares, J.M.; de la Pompa, J.L. Signaling during epicardium and coronary vessel development. Circ. Res. 2011, 109, 1429–1442. [Google Scholar] [CrossRef]
- Timmerman, L.A.; Grego-Bessa, J.; Raya, A.; Bertrán, E.; Pérez-Pomares, J.M.; Díez, J.; Aranda, S.; Palomo, S.; McVormick, F.; Izpisua-Belmonte, C.; et al. Notch promotes epithelial-mesenchymal transition during cardiac development and oncogenic transformation. Genes Dev. 2004, 18, 99–115. [Google Scholar] [CrossRef] [PubMed]
- Schoenwolf, G.C.; Bleyl, S.B.; Brauer, P.R.; Francis-West, P.H. Chapter 12—Development of the heart. In Larsenʹs Human Embryology, 5th ed.; Churchill Livingstone: London, UK, 2016; p. 268. [Google Scholar]
- Gittenberger-de Groot, A.C.; Bartelings, M.M.; Poelmann, R.E.; Haak, M.C.; Jongbloed, M.R. Embryology of the heart and its impact on understanding fetal and neonatal heart disease. Semin. Fetal Neonatal. Med. 2013, 18, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Van Weerd, J.H.; Christoffels, V.M. The Formation and Function of the Cardiac Conduction System. Development 2016, 143, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.P.; Aboulhosn, J.A. Introduction to the congenital heart defects: Anatomy of the conduction system. Card. Electrophysiol. Clin. 2017, 9, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.H.; Bamforth, S.D. Morphogenesis of the Mammalian Aortic Arch Arteries. Front. Cell Dev. Biol. 2022, 10, 892900. [Google Scholar] [CrossRef] [PubMed]
- Graham, A.; Hikspoors, J.P.J.M.; Lamers, W.H.; Anderson, R.H.; Bamforth, S.D. Morphogenetic processes in the development and evolution of the arteries of the pharyngeal arches: Their relations to congenital cardiovascular malformations. Front. Cell Dev. Biol. 2023, 11, 1259175. [Google Scholar] [CrossRef]
- Katz, T.C.; Singh, M.K.; Degenhardt, K.; Rivera-Feliciano, J.; Johnson, R.L.; Epstein, J.A.; Tabin, C.J. Distinct compartments of the proepicardial organ give rise to coronary vascular endothelial cells. Dev. Cell. 2012, 22, 639–650. [Google Scholar] [CrossRef]
- Haligheri, G.; Patel, C.R.; Komarlu, R. Prenatal Delineation of Coronary Anatomy in Dextro-Transposition of Great Arteries. J. Cardiovasc. Echogr. 2021, 31, 171–174. [Google Scholar]
- Tukachew, S.V. Indyvidual and age differences in anatomy of main deferent lymphatic vessels of the human heart and their applied significance. Arch. Anat. Embryol. 1988, 45, 20–26. [Google Scholar]
- Lau, K.Y.; Rubinstein, H.; Gantner, C.W.; Hadas, R.; Amadei, G.; Stelzer, Y.; Zernicka-Goetz, M. Mouse embryo model derived exclusively from embryonic stem cells undergoes neurulation and heart development. Cell Stem Cell 2022, 29, 1445–1458.e8. [Google Scholar] [CrossRef]
- Campostrini, G.; Meraviglia, V.; Giacomelli, E.; van Helden, R.W.J.; Yiangou, L.; Davis, R.P.; Bellin, M.; Orlova, V.V.; Mummery, C.L. Generation, functional analysis and applications of isogenic three-dimensional self-aggregating cardiac microtissues from human pluripotent stem cells. Nat. Protoc. 2021, 16, 2213–2256. [Google Scholar] [CrossRef]
- Jaconi, M.E.; Puceat, M. Cardiac Organoids and Gastruloids to Study Physio-Pathological Heart Development. J. Cardiovasc. Dev. Dis. 2021, 8, 178. [Google Scholar] [CrossRef]
- Drakhlis, L.; Biswanath, S.; Farr, C.M.; Lupanow, V.; Teske, J.; Ritzenhoff, K.; Franke, A.; Manstein, F.; Bolesani, E.; Kempf, H.; et al. Human heart-forming organoids recapitulate early heart and foregut development. Nat. Biotechnol. 2021, 39, 737–746. [Google Scholar] [CrossRef]
- Minn, K.T.; Dietmann, S.; Waye, S.E.; Morris, S.A.; Solnica-Krezel, L. Gene expression dynamics underlying cell fate emergence in 2D micropatterned human embryonic stem cell gastruloids. Stem Cell Rep. 2021, 16, 1210–1227. [Google Scholar] [CrossRef]
- Olmsted, Z.T.; Paluh, J.L. A combined human gastruloid model of cardiogenesis and neurogenesis. Science 2022, 25, 104486. [Google Scholar] [CrossRef] [PubMed]
- Van den Brink, S.C.; van Oudenaarden, A. 3D gastruloids: A novel frontier in stem cell-based in vitro modeling of mammalian gastrulation. Trends Cell Biol. 2021, 31, 747–759. [Google Scholar] [CrossRef]
- Tang, X.-Y.; Wu, S.; Da Wang, D.; Chu, C.; Hong, Y.; Tao, M.; Hu, H.; Xu, M.; Guo, X.; Liu, Y. Human organoids in basic research and clinical applications. Signal Transduct. Target. Ther. 2022, 7, 168. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Chen, Y.; Liu, G.; Han, Z.; Zhao, Z.; Tang, Y. GATA4 transgenic mice as an in vivo model of congenital heart disease. Int. J. Mol. Med. 2015, 35, 1545–1553. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhou, L.; Wang, Q.; You, X.; Li, Y.; Zhao, Y.; Han, X.; Chang, Z.; He, X.; Cheng, C.; et al. NEXN inhibits GATA4 and leads to atrial septal defects in mice and humans. Cardiovasc. Res. 2014, 103, 228–237. [Google Scholar] [CrossRef]
- Lescroart, F.; Meilhac, S.M. Cell lineages, growth and repair of the mouse heart. Results Probl. Cell Differ. 2012, 55, 263–289. [Google Scholar]
- Chen, C.Y.; Croissant, J.; Majesky, M.; Topouzis, S.; McQuinn, T.; Frankovsky, M.J.; Schwartz, R.J. Activation of the cardiac alpha-actin promoter depends upon serum response factor, Tinman homologue, Nkx-2.5, and intact serum response elements. Dev. Genet. 1996, 19, 119–130. [Google Scholar] [CrossRef]
- Qian, L.; Wythe, J.D.; Liu, J.; Cartry, J.; Vogler, G.; Mohapatra, B.; Otway, R.T.; Huang, Y.; King, I.N.; Maillet, M.; et al. Tinman/Nkx2- 5 acts via miR-1 and upstream of Cdc42 to regulate heart function across species. J. Cell Biol. 2011, 193, 1181–1196. [Google Scholar] [CrossRef]
- Stanley, E.G.; Biben, C.; Elefanty, A.; Barnett, L.; Koentgen, F.; Robb, L.; Harvey, R.P. Efficient Cre-mediated deletion in cardiac progenitor cells conferred by a 3’UTR-ires-Cre allele of the homeobox gene Nkx2-5. Int. J. Dev. Biol. 2002, 46, 431–439. [Google Scholar]
- Xu, P.; Johnson, T.L.; Stoller-Conrad, J.R.; Schulz, R.A. Spire, an actin nucleation factor, regulates cell division during Drosophila heart development. PLoS ONE 2012, 7, e30565. [Google Scholar]
- Lien, C.L.; Wu, C.; Mercer, B.; Webb, R.; Richardson, J.A.; Olson, E.N. Control of early cardiac-specific transcription of Nkx2-5 by a GATA-dependent enhancer. Development 1999, 126, 75–84. [Google Scholar] [CrossRef]
- Schwartz, R.J.; Olson, E.N. Building the heart piece by piece: Modularity of cis-elements regulating Nkx2-5 transcription. Development 1999, 126, 4187–4192. [Google Scholar] [CrossRef]
- Tanaka, M.; Chen, Z.; Bartunkova, S.; Yamasaki, N.; Izumo, S. The cardiac homeobox gene Csx/Nkx2.5 lies genetically upstream of multiple genes essential for heart development. Development 1999, 126, 1269–1280. [Google Scholar] [CrossRef]
- Ma, L.; Lu, M.F.; Schwartz, R.J.; Martin, J.F. Bmp2 is essential for cardiac cushion epithelial-mesenchymal transition and myocardial patterning. Development 2005, 132, 5601–5611. [Google Scholar] [CrossRef]
- Searcy, R.D.; Vincent, E.B.; Liberatore, C.M.; Yutzey, K.E. A GATA- -dependent nkx-2.5 regulatory element activates early cardiac gene expression in transgenic mice. Development 1998, 125, 4461–4470. [Google Scholar] [CrossRef]
- Verberne, M.E.; Gittenberger-de Groot, A.C.; Poelmann, R.E. Lineage and development of the parasympathetic nervous system of the embryonic chick heart. Anat. Embryol. 1998, 198, 171–184. [Google Scholar] [CrossRef]
- Zhang, H.; Bradley, A. Mice deficient for BMP2 are nonviable and have defects in amnion/chorion and cardiac development. Development 1996, 122, 2977–2986. [Google Scholar] [CrossRef] [PubMed]
- Lincoln, J.; Yutzey, K.E. Molecular and developmental mechanisms of congenital heart valve disease. Birth Defects Res. Part A Clin. Mol. Teratol. 2011, 91, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Bruneau, B.G. The developmental genetics of congenital heart disease. Nature 2008, 451, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Sugi, Y.; Markwald, R.R. Endodermal growth factors promote endocardial precursor cell formation from precardiac mesoderm. Dev. Biol. 2003, 263, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, L.M.; Eisenberg, C.A. Evaluating the Role of Wnt Signal Transduction in Promoting the Development of the Heart. Sci. World J. 2007, 7, 161–176. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Buckingham, M. The formation of the embryonic mouse heart: Heart fields and myocardial cell lineages. Ann. N. Y. Acad. Sci. 2010, 1188, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Zeisberg, E.M.; Ma, Q.; Juraszek, A.L.; Moses, K.; Schwartz, R.J.; Izumo, S.; Pu, W.T. Morphogenesis of the right ventricle requires myocardial expression of Gata4. J. Clin. Investig. 2005, 115, 1522–1531. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Zhang, Y.; Sethi, I.; Ye, L.; Trembley, M.A.; Cao, Y.; Akerberg, B.N.; Xiao, F.; Zhang, X.; Li, K.; et al. GATA4 Regulates Developing Endocardium Through Interaction with ETS1. Circ. Res. 2022, 131, e152–e168. [Google Scholar] [CrossRef]
- He, A.; Kong, S.W.; Ma, Q.; Pu, W.T. Co-occupancy by multiple cardiac transcription factors identifies transcriptional enhancers active in heart. Proc. Natl. Acad. Sci. USA 2011, 108, 5632–5637. [Google Scholar] [CrossRef]
- He, A.; Gu, F.; Hu, Y.; Ma, Q.; Ye, L.Y.; Akiyama, J.A.; Visel, A.; Pennacchio, L.A.; Pu, W.T. Dynamic GATA4 enhancers shape the chromatin landscape central to heart development and disease. Nat. Commun. 2014, 5, 4907. [Google Scholar] [CrossRef]
- Akerberg, B.N.; Gu, F.; VanDusen, N.J.; Zhang, X.; Dong, R.; Li, K.; Zhang, B.; Zhou, B.; Sethi, I.; Ma, Q.; et al. A reference map of murine cardiac transcription factor chromatin occupancy identifies dynamic and conserved enhancers. Nat. Commun. 2019, 10, 4907. [Google Scholar] [CrossRef] [PubMed]
- Rothenberg, F.; Fisher, S.A.; Watanabe, M. Sculpting the cardiac outflow tract. Birth Defects Res. Part C Embryo Today 2003, 69, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, K.; Miura, H.; Miyagawa-Tomita, S.; Yanazawa, M.; Katoh-Fukui, Y.; Suzuki, R.; Ohuchi, H.; Suehiro, A.; Motegi, Y.; Nakahara, Y.; et al. Mouse Pitx2 deficiency leads to anomalies of the ventral body wall, heart, extra- and periocular mesoderm and right pulmonary isomerism. Development 1999, 126, 5749–5758. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Chang, T.C.; Kang, J.O.; Choudhary, B.; Makita, T.; Tran, C.M.; Burch, J.B.; Eid, H.; Sucov, H.M. Epicardial induction of fetal cardiomyocyte proliferation via a retinoic acid-inducible trophic factor. Dev. Biol. 2002, 250, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Farrell, M.J.; Kirby, M.L. Cell biology of cardiac development. Int. Rev. Cytol. 2001, 202, 99–158. [Google Scholar] [PubMed]
- Hatzopoulos, A.K.; Folkman, J.; Vasile, E.; Eiselen, G.K.; Rosenberg, R.D. Isolation and characterization of endothelial progenitor cells from mouse embryos. Development 1998, 125, 1457–1468. [Google Scholar] [CrossRef] [PubMed]
- Harding, R.; Bocking, A.D. Fetal Growth and Development; Cambridge University Press: Cambridge, UK, 2001. [Google Scholar]
- Wei, Y.; Bader, D.; Litvin, J. Identification of a novel cardiac-specific transcript critical for cardiac myocyte differentiation. Development 1996, 122, 2779–2789. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.; Chen, J.; Ruiz-Lozano, P.; Zou, Y.; Nemer, M.; Chien, K.R. Control of segmental expression of the cardiac-restricted ankyrin repeat protein gene by distinct regulatory pathways in murine cardiogenesis. Development 1999, 126, 4223–4234. [Google Scholar] [CrossRef]
- Van den Berg, G.; Moorman, A.F. Development of the pulmonary vein and the systemic venous sinus: An interactive 3D overview. PLoS ONE 2011, 6, e22055. [Google Scholar] [CrossRef]
- Zou, Y.; Evans, S.; Chen, J.; Kuo, H.C.; Harvey, R.P.; Chien, K.R. CARP, a cardiac ankyrin repeat protein, is downstream in the Nkx2-5 homeobox gene pathway. Development 1997, 124, 7. [Google Scholar] [CrossRef]
- Searcy, R.D.; Yutzey, K.E. Analysis of Hox gene expression during early avian heart development. Dev. Dyn. 1998, 213, 82–91. [Google Scholar] [CrossRef]
- Wang, D.Z.; Reiter, R.S.; Lin, J.L.; Wang, Q.; Williams, H.S.; Krob, S.L.; Schultheiss, T.M.; Evans, S.; Lin, J.J. Requirement of a novel gene, Xin, in cardiac morphogenesis. Development 1999, 126, 1281–1294. [Google Scholar] [CrossRef]
- Naya, F.J.; Wu, C.; Richardson, J.A.; Overbeek, P.; Olson, E.N. Transcriptional activity of MEF2 during mouse embryogenesis monitored with a MEF2-dependent transgene. Development 1999, 126, 2045–2052. [Google Scholar] [CrossRef] [PubMed]
- McFarlane, C.; Hui, G.Z.; Amanda, W.Z.; Lau, H.Y.; Lokireddy, S.; Xiaojia, G.; Mouly, V.; Butler-Browne, G.; Gluckman, P.D.; Sharma, M.; et al. Human myostatin negatively regulates human myoblast growth and differentiation. Am. J. Physiol. Cell Physiol. 2011, 301, C195–C203. [Google Scholar] [CrossRef] [PubMed]
- Tessadori, F.; Tsingos, E.; Colizzi, E.S.; Kruse, F.; Brink, S.C.V.D.; Boogaard, M.V.D.; Christoffels, V.M.; Merks, R.M.; Bakkers, J. Twisting of the zebrafish heart tube during cardiac looping is a tbx5-dependent and tissue-intrinsic process. eLife 2021, 10, e61733. [Google Scholar] [CrossRef] [PubMed]
- Campione, M.; Steinbeisser, H.; Schweickert, A.; Deissler, K.; van Bebber, F.; Lowe, L.A.; Nowotschin, S.; Viebahn, C.; Haffter, P.; Kuehn, M.R.; et al. The homeobox gene Pitx2: Mediator of asymmetric left-right signaling in vertebrate heart and gut looping. Development 1999, 126, 1225–1234. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, M.; Wirrig, E.; Phelps, A.; Wessels, A. Extracellular matrix and heart development. Birth Defects Res. Part A Clin. Mol. Teratol. 2011, 91, 535–550. [Google Scholar] [CrossRef] [PubMed]
- Erickson, S.L.; O’Shea, K.S.; Ghaboosi, N.; Loverro, L.; Frantz, G.; Bauer, M.; Lu, L.H.; Moore, M.W. ErbB3 is required for normal cerebellar and cardiac development: A comparison with ErbB2- and heregulin-deficient mice. Development 1997, 124, 4999–5011. [Google Scholar] [CrossRef]
- Sugi, Y.; Sasse, J.; Barron, M.; Lough, J. Developmental expression of fibroblast growth factor receptor-1 (cek-1; flg) during heart development. Dev. Dyn. 1995, 202, 115–125. [Google Scholar] [CrossRef]
- Vincentz, J.W.; Firulli, B.A.; Toolan, K.P.; Osterwalder, M.; Pennacchio, L.A.; Firulli, A.B. HAND transcription factors cooperatively specify the aorta and pulmonary trunk. Dev. Biol. 2021, 476, 1–10. [Google Scholar] [CrossRef]
- Moorman, A.F.; Christoffels, V.M. Cardiac chamber formation: Development, genes, and evolution. Physiol. Rev. 2003, 83, 1223–1267. [Google Scholar] [CrossRef] [PubMed]
- Cleves, M.A.; Malik, S.; Yang, S.; Carter, T.C.; Hobbs, C.A. Maternal urinary tract infections and selected cardiovascular malformations. Birth Defects Res. Part A Clin. Mol. Teratol. 2008, 82, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Gruber, P.J.; Kubalak, S.W.; Chien, K.R. Downregulation of atrial markers during cardiac chamber morphogenesis is irreversible in murine embryos. Development 1998, 125, 4427–4438. [Google Scholar] [CrossRef] [PubMed]
- Kastner, P.; Messaddeq, N.; Mark, M.; Wendling, O.; Grondona, J.M.; Ward, S.; Ghyselinck, N.; Chambon, P. Vitamin A deficiency and mutations of RXRα, RXRβ and RARα lead to early differentiation of embryonic ventricular cardiomyocytes. Development 1997, 124, 4749. [Google Scholar] [CrossRef] [PubMed]
- Tran, C.M.; Sucov, H.M. The RXRα gene functions in a non-cell-autonomous manner during mouse cardiac morphogenesis. Development 1998, 125, 1951–1956. [Google Scholar] [CrossRef] [PubMed]
- Ong, L.L.; Kim, N.; Mima, T.; Cohen-Gould, L.; Mikawa, T. Trabecular myocytes of the embryonic heart require N-cadherin for migratory unit identity. Dev. Biol. 1998, 19, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tomanek, R.J. Formation of the coronary vasculature during development. Angiogenesis 2005, 8, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Niessen, K.; Fu, Y.; Chang, L.; Hoodless, P.A.; McFadden, D.; Karsan, A. Slug is a direct Notch target required for initiation of cardiac cushion cellularization. J. Cell Biol. 2008, 182, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lamouille, S.; Derynck, R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009, 19, 156–172. [Google Scholar] [CrossRef]
- Luna-Zurita, L.; Prados, B.; Grego-Bessa, J.; Luxán, G.; del Monte, G.; Benguría, A.; Adams, R.H.; Perez-Pomares, J.M.; de la Pompa, J.L. Integration of a notch-dependent mesenchymal gene program and Bmp2-driven cell invasiveness regulates murine cardiac valve formation. J. Clin. Investig. 2010, 120, 3493–3507. [Google Scholar] [CrossRef]
- Wu, M.; Peng, Z.; Zu, C.; Ma, J.; Lu, S.; Zhong, J.; Zhang, S. Losartan attenuates myocardial endothelial-to-mesenchymal transition in spontaneous hypertensive rats via inhibiting TGF-β/Smad signaling. PLoS ONE 2016, 11, e0155730. [Google Scholar] [CrossRef] [PubMed]
- Christoffels, V.M.; Smits, G.J.; Kisper, A.; Moorman, A.F.M. Development of the pacemaker tissues of the heart. Circ. Res. 2010, 106, 240–254. [Google Scholar] [CrossRef]
- Larsen, W.J. Human Embryology; Churchill Livingstone: New York, NY, USA, 1994; p. 147. [Google Scholar]
- Van der Linde, D.; Konings, E.E.; Slager, M.A.; Witsenburg, M.; Helbing, W.A.; Takkenberg, J.J.; Roos-Hesselink, J.W. Birth prevalence of congenital heart disease worldwide: A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2011, 58, 2241–2247. [Google Scholar] [CrossRef] [PubMed]
- Dolk, H.; Loane, M.; Garne, E. European Surveillance of Congenital Anomalies (EUROCAT) Working Group. Congenital heart defects in Europe: Prevalence and perinatal mortality, 2000 to 2005. Circulation 2011, 123, 841–849. [Google Scholar] [CrossRef]
- Glinianaia, S.V.; Rankin, J.; Wright, C. Congenital anomalies in twins: A register-based study. Hum. Reprod. 2008, 23, 1306–1311. [Google Scholar] [CrossRef]
- Rankin, J.; Pattenden, S.; Abramsky, L.; Boyd, P.; Jordan, H.; Stone, D.; Vrijheid, M.; Wellesley, D.; Dolk, H. Prevalence of congenital anomalies in five British regions, 1991–99. Arch. Dis. Child. Fetal Neonatal Ed. 2005, 90, F374–F379. [Google Scholar] [CrossRef]
- Mamasoula, C.; Addor, M.C.; Carbonell, C.C.; Dias, C.M.; Echevarría-Gonzalez-de- Garibay, L.J.; Gatt, M.; Khoshnood, B.; Klungsoyr, K.; Randall, K.; Stoianova, S.; et al. Prevalence of congenital heart defects in Europe, 2008–2015: A registry-based study. Brith Defects Res. 2022, 114, 1404–1416. [Google Scholar] [CrossRef] [PubMed]
- Kordjalik, P.; Tobota, Z.; Respondek-Liberska, M. Selected data from the Polish National Prenatal Cardiac Pathology Registry from the year 2016. Prenat. Cardio. 2017, 7, 7–11. [Google Scholar] [CrossRef]
- Hoffman, J.I.E.; Kaplan, S. The incidence of congenital heart disease. J. Am. Coll. Cardiol. 2002, 39, 1890–1900. [Google Scholar] [CrossRef]
- Bernier, P.L.; Stefanescu, A.; Samoukovic, G.; Tchervenkov, C.I. The challenge of congenital heart disease worldwide: Epidemiologic and demographic facts. Semin. Thorac. Cardiovasc. Surg. Pediatr. Card. Surg. Annu. 2010, 13, 26–34. [Google Scholar] [CrossRef]
- Hoffman, J.I.E. The global burden of congenital heart disease. Cardiovasc. J. Afr. 2013, 24, 141–145. [Google Scholar] [CrossRef]
- Abqari, S.; Gupta, A.; Shahab, T.; Rabbani, M.U.; Ali, S.M.; Firdaus, U. Profile and risk factors for congenital heart defects: A study in a tertiary care hospital. Ann. Pediatr. Cardiol. 2016, 9, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Nora, J.J.; Nora, A.H. Recurrence risks in children having one parent with a congenital heart disease. Circulation 1976, 53, 701–702. [Google Scholar] [CrossRef] [PubMed]
- Mamasoula, C.; Bigirumurame, T.; Chadwick, T.; Addor, M.C.; Carbonell, C.C.; Dias, C.M.; Echevarría-Gonzalez-de-Garibay, L.J.; Gatt, M.; Khoshnood, B.; Klungsoyr, K.; et al. Maternal age and the prevalence of congenital heart defects in Europe, 1995–2015: A register-based study. Brith Defects Res. 2023, 115, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Rodriguez, R.A.; Krug, E.L. Cardiovascular development. In Charlene à McQueen, Comprehensive Toxicology; Academic Press: Oxford, UK, 2010; Volume 6, pp. 3–33. [Google Scholar]
- Campbell, M. Genetic and environmental factors in congenital heart disease. Q. J. Med. 1949, 18, 379–391. [Google Scholar] [PubMed]
- Campbell, M. Incidence of cardiac malformations at birth and later, and neonatal mortality. Br. Heart J. 1973, 35, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Blue, G.M.; Kirk, E.P.; Giannoulatou, E.; Sholler, G.F.; Dunwoodie, S.L.; Harvey, R.P.; Winlaw, D.S. Advances in the genetics of congenital heart disease: A Clinician’s guide. J. Am. Coll. Cardiol. 2017, 69, 859–870. [Google Scholar] [CrossRef] [PubMed]
- Gelb, B.D. History of our understanding of the causes of congenital heart disease. Circ. Cardiovasc. Genet. 2015, 8, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Lalani, S.R. Other genomic disorders and congenital heart disease. Am. J. Med. Genet. Part C Semin. Med. Genet. 2020, 184, 107–115. [Google Scholar] [CrossRef]
- Yuan, S.; Zaidi, S.; Brueckner, M. Congenital Heart Disease: Emerging themes linking genetics and development. Curr. Opin. Genet. Dev. 2013, 23, 352–359. [Google Scholar] [CrossRef]
- Wang, X.; Li, P.; Chen, S.; Xi, L.; Guo, Y.; Guo, A.; Sun, K. Influence of genes and the environment in familial congenital heart defects. Mol. Med. Rep. 2014, 9, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Hartman, R.J.; Rasmussen, S.A.; Botto, L.D.; Riehle-Colarusso, T.; Martin, C.L.; Cragan, J.D.; Shin, M.; Correa, A. The Contribution of Chromosomal Abnormalities to Congenital Heart Defects: A Population-Based Study. Pediatr. Cardiol. 2011, 32, 1147–1157. [Google Scholar] [CrossRef] [PubMed]
- Findley, T.O.; Northrup, H. The current state of prenatal detection of genetic conditions in congenital heart defects. Transl. Pediatr. 2021, 10, 2157–2170. [Google Scholar] [CrossRef]
- Akhirome, E.; Walton, N.A.; Nogee, J.M.; Jay, P.Y. The complex genetic basis of congenital heart defects. Circ. J. 2017, 81, 629–634. [Google Scholar] [CrossRef]
- Gu, Q.; Li, Y.; Cui, Z.L.; Luo, X.P. Homocysteine, folate, vitamin B12 and B6 in mothers of children with neural tube defects in Xinjiang, China. Acta Pediatr. 2012, 101, e486–e490. [Google Scholar] [CrossRef]
- Mitchell, L.E.; Long, J.; Garbarini, J.; Paluru, P.; Goldmuntz, E. Variants of folate metabolism genes and risk of left-sided cardiac defects. Birth Defects Res. Part A Clin. Mol. Teratol. 2010, 88, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xiao, A. Epigenetic regulation in neural crest development. Birth Defects Res. Part A Clin. Mol. Teratol. 2011, 91, 788–796. [Google Scholar] [CrossRef]
- Yelbuz, T.M.; Waldo, K.L.; Kumiski, D.H.; Stadt, H.A.; Wolfe, R.R.; Leatherbury, L.; Kirby, M.L. Shortened outflow tract leads to altered cardiac looping after neural crest ablation. Circulation 2002, 106, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Szymanski, P.; Klisiewicz, A.; Lubiszewska, B.; Lipczynska, M.; Konka, M.; Kusmierczyk, M.; Hoffman, P. Functional anatomy of tricuspid regurgitation in patients with systemic right ventricles. J. Am. Soc. Echocardiogr. 2010, 23, 504–510. [Google Scholar] [CrossRef]
- Shaw, G.M.; O’Malley, C.D.; Wasserman, C.R.; Tolarova, M.M.; Lammer, E.J. Maternal periconceptional use of multivitamins and reduced risk for conotruncal heart defects and limb deficiencies among offspring. Am. J. Med. Genet. 1995, 59, 536–545. [Google Scholar] [CrossRef]
- Chen, L.; Yang, T.; Chen, L.; Wang, L.; Wang, T.; Zhao, L.; Ye, Z.; Zhang, S.; Luo, L.; Zheng, Z.; et al. Risk of congenital heart defects in offspring exposed to maternal diabetes mellitus: An updated systematic review and meta-analysis. Arch. Gynecol. Obstet. 2019, 300, 1491–1506. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.; Gaborit, B.; Lenoir, M.; Collod-Beroud, G.; Stefanovic, S. Maternal Pre-Existing Diabetes: A Non-Inherited Risk Factor for Congenital Cardiopathies. Int. J. Mol. Sci. 2023, 24, 16258. [Google Scholar] [CrossRef] [PubMed]
- Corrigan, N.; Brazil, D.P.; McAuliffe, F. Fetal cardiac effects of maternal hyperglycemia during pregnancy. Birth Defects Res. Part A Clin. Mol. Teratol. 2009, 85, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Herdt-Losavio, M.; Gensburg, L.; Marshall, E.; Druschel, C. Maternal asthma, asthma medication use, and the risk of congenital heart defects. Birth Defects Res. Part A Clin. Mol. Teratol. 2009, 85, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Grewal, J.; Carmichael, S.L.; Ma, C.; Lammer, E.J.; Shaw, G.M. Maternal periconceptional smoking and alcohol consumption and risk for select congenital anomalies. Birth Defects Res. Part A Clin. Mol. Teratol. 2008, 82, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Shalen, E.F.; McGrath, L.B.; Bhamidipati, C.M.; Garcia, I.C.; Ramsey, K.; Broberg, C.S.; Khan, A.M. Substance Use Disorders Are Prevalent in Adults With Congenital Heart Disease and Are Associated With Increased Healthcare Use. Am. J. Cardiol. 2023, 192, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Wang, L.; Yang, T.; Chen, L.; Wang, T.; Chen, L.; Zhao, L.; Zhang, S.; Zheng, Z.; Luo, L.; et al. Maternal Viral Infection and Risk of Fetal Congenital Heart Diseases: A Meta-Analysis of Observational Studies. J. Am. Heart Assoc. 2019, 8, e011264. [Google Scholar] [CrossRef] [PubMed]
- Cipollone, D.; Amati, F.; Carsetti, R.; Placidi, S.; Biancolella, M.; D’Amati, G.; Novelli, G.; Siracusa, G.; Marino, B. A multiple retinoic acid antagonist induces conotruncal anomalies, including transposition of the great arteries, in mice. Cardiovasc. Pathol. 2006, 15, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Obler, D.; Juraszek, A.L.; Smoot, L.B.; Natowicz, M.R. Double outlet right ventricle: Aetiologies and associations. J. Med. Genet. 2008, 45, 481–497. [Google Scholar] [CrossRef]
- Taylor, I.M.; Wiley, M.J.; Agur, A. Retinoic acid-induced heart malformations in the hamster. Teratology 1980, 21, 193–197. [Google Scholar] [CrossRef]
- Kuehl, K.S.; Loffredo, C.A. Genetic and environmental influences on malformations of the cardiac outflow tract. Expert Rev. Cardiovasc. Ther. 2005, 3, 1125–1130. [Google Scholar] [CrossRef]
- Loffredo, C.A.; Silbergeld, E.K.; Ferencz, C.; Zhang, J. Association of transposition of the great arteries in infants with maternalexposures to herbicides and rodenticides. Am. J. Epidemiol. 2001, 153, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Suhl, J.; Conway, K.M.; Rhoads, A.; Langlois, P.H.; Feldkamp, M.L.; Michalski, A.M.; Oleson, J.; Sidhu, A.; Scholz, T.D.; Kancherla, V.; et al. Prepregnancy exposure to dietary arsenic and congenital heart defects. Birth Defects Res. 2023, 115, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Unolt, M.; Putotto, C.; Silvestri, L.M.; Marino, D.; Scarabotti, A.; Massaccesi, V.; Caiaro, A.; Versacci, P.; Marino, B. Transposition of great arteries: New insights into the pathogenesis. Front. Pediatr. 2013, 1, 11. [Google Scholar] [CrossRef]
- Pexieder, T.; Blanc, O.; Pelouch, V.; Ostàdalovà, I.; Milerovà, M.; Ostàdal, B. Late fetal development of retinoic acid-induced transposition of great arteries: Morphology, physiology, and biochemistry. In Developmental Mechanism of Heart Disease; Clark, E.B., Markwald, R.R., Takao, A., Eds.; Futura Publishing: Armonk, NY, USA, 1995; pp. 297–307. [Google Scholar]
- Yasui, H.; Nakazawa, M.; Morishima, M.; Miyagawa-Tomita, S.; Momma, K. Morphological observations on the pathogenetic process of transposition of the great arteries induced by retinoic acid in mice. Circulation 1995, 91, 2478–2486. [Google Scholar] [CrossRef] [PubMed]
- Mahler, G.J.; Butcher, J.T. Cardiac developmental toxicity. Birth Defects Res. C Embryo Today 2011, 93, 291–297. [Google Scholar] [CrossRef]
- Süleyman, H.; Demircan, B.; Karagöz, Y. Anti-inflammatory and side effects of cyclooxygenase inhibitors. Pharmacol. Rep. 2007, 59, 247–258. [Google Scholar] [PubMed]
- Weber, M.; Schweitzer, M.; Mur, J.M.; Andre, J.M.; Tridon, P.; Ver, T.P. Epilepsy, anticonvulsants and pregnancy. Arch. Fr. Pediatr. 1977, 34, 374–383. [Google Scholar] [PubMed]
- Redline, R.W.; Abramowsky, C.R. Transposition of the great vessels in an infant exposed to massive doses of oral contraceptives. Am. J. Obstet. Gynecol. 1981, 141, 468–469. [Google Scholar] [CrossRef]
- Ferencz, C.; Loffredo, C.A.; Corea-Villasenor, A.; Wilson, P.D. Genetic and environmental risk factors of major cardiovascular malformations: The Baltimore-Washington Infant Study, 1981–1989. In Perspectives in Pediatric Cardiology, 1st ed.; Ferencz, C., Loffredo, C.A., Corea-Villasenor, A., Wilson, P.D., Eds.; Futura Publishing Co. Inc.: Armonk, NY, USA, 1997; Volume 5, pp. 867–868. [Google Scholar]
- Tararbit, K.; Houyel, L.; Bonnet, D.; De Vigan, C.; Lelong, N.; Goffinet, F.; Khoshnood, B. Risk of congenital heart defects associated with assisted reproductive technologies: A population-based evaluation. Eur. Heart J. 2011, 32, 500–508. [Google Scholar] [CrossRef]
- Giorgione, V.; Parazzini, F.; Fesslova, V. Congenital heart defects in IVF/ICSI pregnancy: Systemic review and meta-analysis. Ultrasound Obstet. Gynecol. 2018, 51, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Chen, L.; Yu, H.; Wang, H.; Li, Q.; Yu, R.; Qin, J. Which type of congenital malformations is significantly increased in singleton pregnancies following after in vitro fertilization/intracytoplasmic sperm injection: A systemic review and meta-analysis. Oncotarget 2017, 9, 4267–4278. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Abbas, A.K.; Aster, J.C. (Eds.) Robbins: Basic Book Company Pathology, 9th ed.; Elsevier Saunders: Amsterdam, The Netherlands, 2013; pp. 399–440. [Google Scholar]
- Van Weerd, J.H.; Koshiba-Takeuchi, K.; Kwon, C.; Takeuchi, J.K. Epigenetic Factors and Cardiac Development. Cardiovasc. Res. 2011, 91, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Meier, K.; Brehm, A. Chromatin Regulation: How Complex Does It Get? Epigenetics 2014, 9, 1485–1495. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Wang, Z.; Bassel-Duby, R.; Olson, E.N. Genetic and epigenetic regulation of cardiomyocytes in development, regeneration and disease. Development 2018, 145, dev171983. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Li, W.; Yang, J.; Shang, C.; Lin, C.H.; Cheng, W.; Hang, C.T.; Cheng, H.L.; Chen, C.H.; Wong, J.; et al. Epigenetic response to environmental stress: Assembly of BRG1-G9a/GLP-DNMT3 repressive chromatin complex on Myh6 promoter in pathologically stressed hearts. Biochim. Biophys. Acta 2016, 1863, 1772–1781. [Google Scholar] [CrossRef] [PubMed]
- May, D.; Blow, M.J.; Kaplan, T.; McCulley, D.J.; Jensen, B.C.; Akiyama, J.A.; Holt, A.; Plajzer-Frick, I.; Shoukry, M.; Wright, C.; et al. Large-scale discovery of enhancers from human heart tissue. Nat. Genet. 2011, 44, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ransom, J.F.; Li, A.; Vedantham, V.; von Drehle, M.; Muth, A.N.; Tsuchihashi, T.; McManus, M.T.; Schwartz, R.J.; Srivastava, D. Dysregulation of cardiogenesis, cardiac conduction, and cell cycle in mice lacking miRNA-1-2. Cell 2007, 129, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.F.; Mandel, E.M.; Thomson, J.M.; Wu, Q.; Callis, T.E.; Hammond, S.M.; Conlon, F.L.; Wang, D.Z. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat. Genet. 2006, 38, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Potthoff, M.J.; Olson, E.N. MEF2: A central regulator of diverse developmental programs. Development 2007, 134, 4131–4140. [Google Scholar] [CrossRef]
- Anderson, R.H.; Becker, A.E.; Freedom, R.M.; Macartney, F.J.; Quero-Jimenez, M.; Shinebourne, E.A.; Wilkinson, J.L.; Tynan, M. Sequential segmental analysis of congenital heart disease. Pediatr. Cardiol. 1984, 5, 281–287. [Google Scholar] [CrossRef]
- Ho, S.Y.; Anderson, R.H. How constant anatomically is the tendon of Todaro as a marker for the triangle of Koch? J. Cardiovasc. Electrophysiol. 2000, 11, 83–89. [Google Scholar]
- Crucean, A.C.; Spicer, D.E.; Anderson, R.H. The Significance of Ventricular Topology in the Analysis of Congenitally Malformed Hearts. J. Cardiovasc. Dev. Dis. 2022, 9, 155. [Google Scholar] [CrossRef]
- Van Praagh, R. The Segmental Approach to Diagnosis in Congenital Heart Disease. Birth Defects. In Original Article Series 8; Williams and Wilkins: Baltimore, MD, USA, 1972; pp. 4–23. [Google Scholar]
- Van Praagh, R. Segmental approach to diagnosis. In Nadas’ Pediatric Cardiology, 2nd ed.; Keane, J.F., Lock, J.F., Flyer, D.C., Eds.; Elsevier: Philadelphia, PA, USA, 2006; pp. 39–47. [Google Scholar]
- Anderson, R.H. Heart Defects Congenital. In Pediatric Cardiology; Anderson, R.H., Becker, E.J., Redington, A., Rigby, M.L., Wernovsky, G., Eds.; Churchill Livingstone: Philadelphia, PA, USA, 2009; pp. 3–16. [Google Scholar]
- Shinebourne, E.A.; Macartney, F.J.; Andreson, R.H. Sequential chamber localization: Logical approach to diagnosis in congenital heart disease. Br. Heart J. 1976, 38, 327–340. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zubrzycki, M.; Schramm, R.; Costard-Jäckle, A.; Grohmann, J.; Gummert, J.F.; Zubrzycka, M. Cardiac Development and Factors Influencing the Development of Congenital Heart Defects (CHDs): Part I. Int. J. Mol. Sci. 2024, 25, 7117. https://doi.org/10.3390/ijms25137117
Zubrzycki M, Schramm R, Costard-Jäckle A, Grohmann J, Gummert JF, Zubrzycka M. Cardiac Development and Factors Influencing the Development of Congenital Heart Defects (CHDs): Part I. International Journal of Molecular Sciences. 2024; 25(13):7117. https://doi.org/10.3390/ijms25137117
Chicago/Turabian StyleZubrzycki, Marek, Rene Schramm, Angelika Costard-Jäckle, Jochen Grohmann, Jan F. Gummert, and Maria Zubrzycka. 2024. "Cardiac Development and Factors Influencing the Development of Congenital Heart Defects (CHDs): Part I" International Journal of Molecular Sciences 25, no. 13: 7117. https://doi.org/10.3390/ijms25137117
APA StyleZubrzycki, M., Schramm, R., Costard-Jäckle, A., Grohmann, J., Gummert, J. F., & Zubrzycka, M. (2024). Cardiac Development and Factors Influencing the Development of Congenital Heart Defects (CHDs): Part I. International Journal of Molecular Sciences, 25(13), 7117. https://doi.org/10.3390/ijms25137117






