Integrative Bioinformatics Analysis Reveals a Transcription Factor EB-Driven MicroRNA Regulatory Network in Endothelial Cells
Abstract
:1. Introduction
2. Results
2.1. Characterization of the TFEB Regulatory Landscape in HUVECs through ChIP-Seq
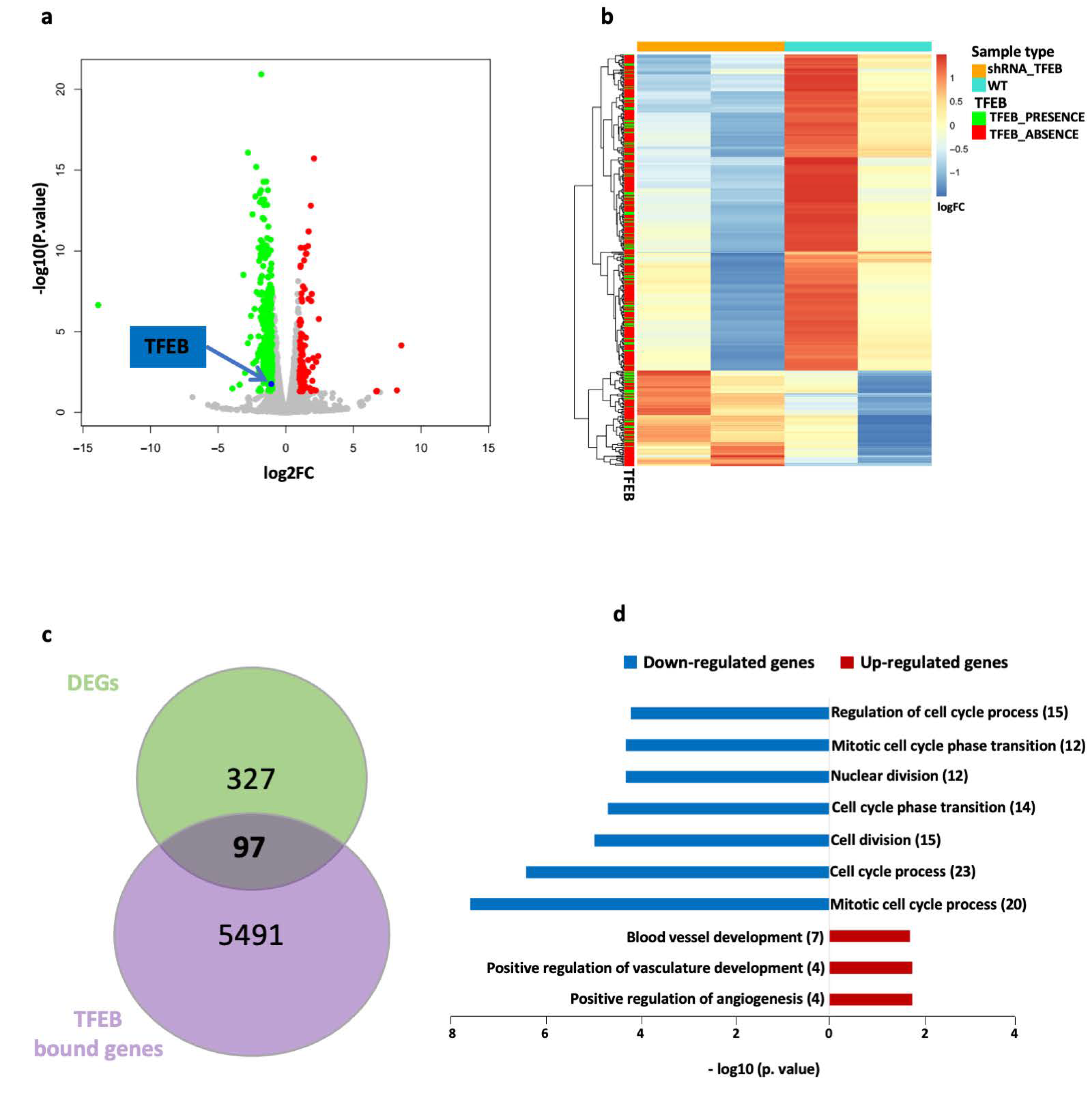
2.2. mRNA-Seq Reveals Differential Gene Expression Patterns Associated with TFEB in HUVECs
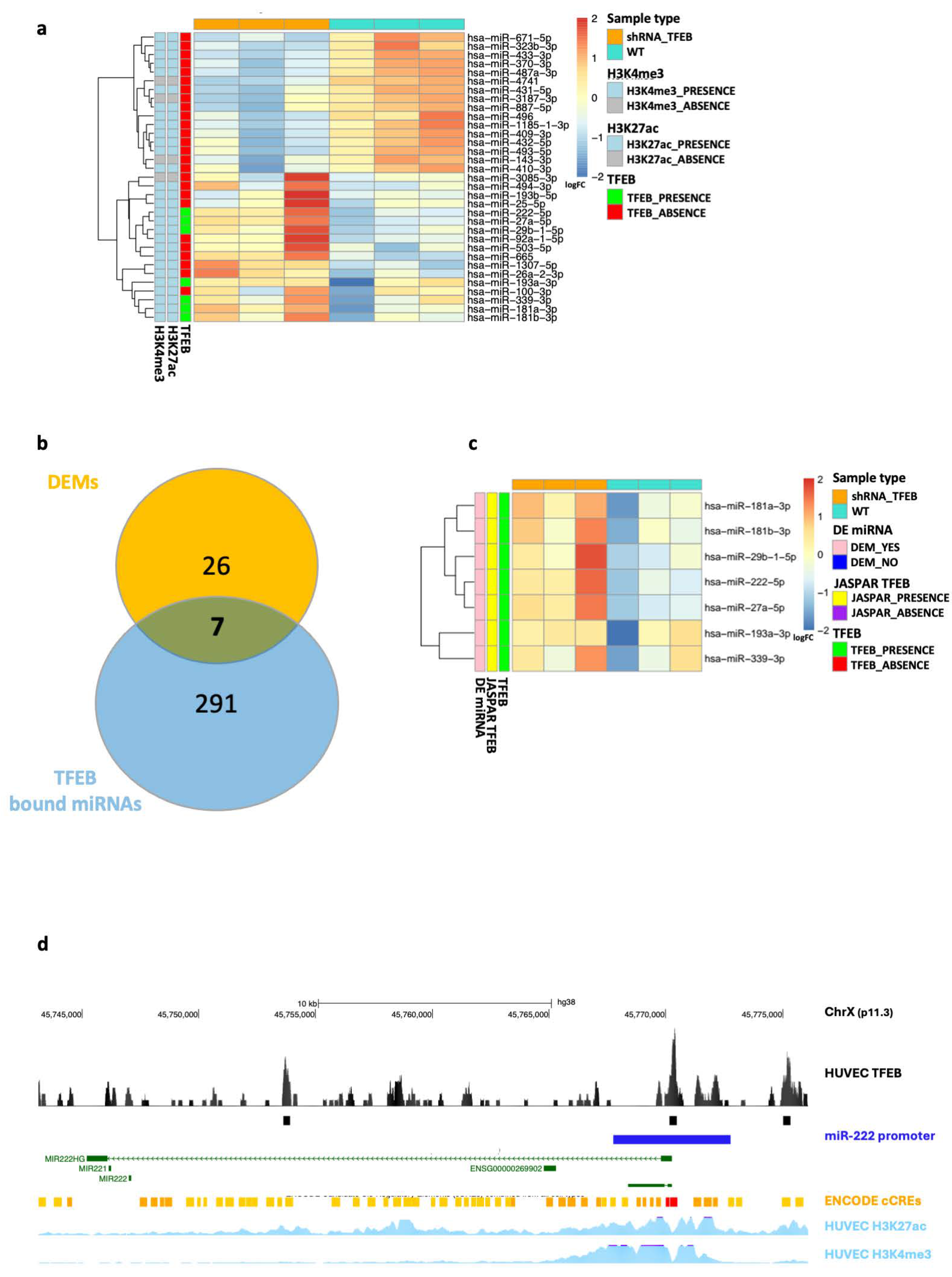
2.3. TFEB Transcriptional Regulation of miRNAs in HUVECs
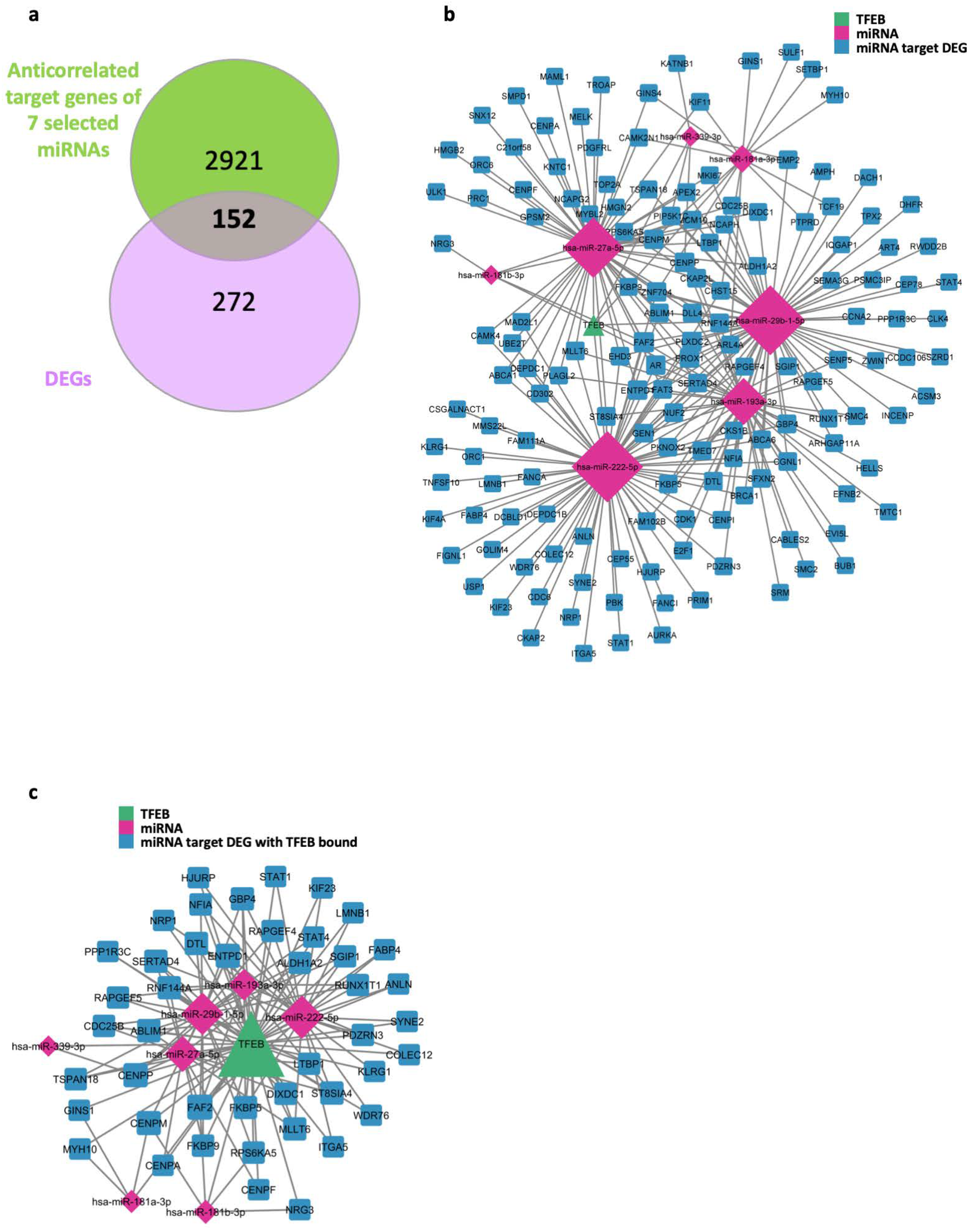
2.4. TFEB Mediates miRNA Regulatory Network in HUVECs
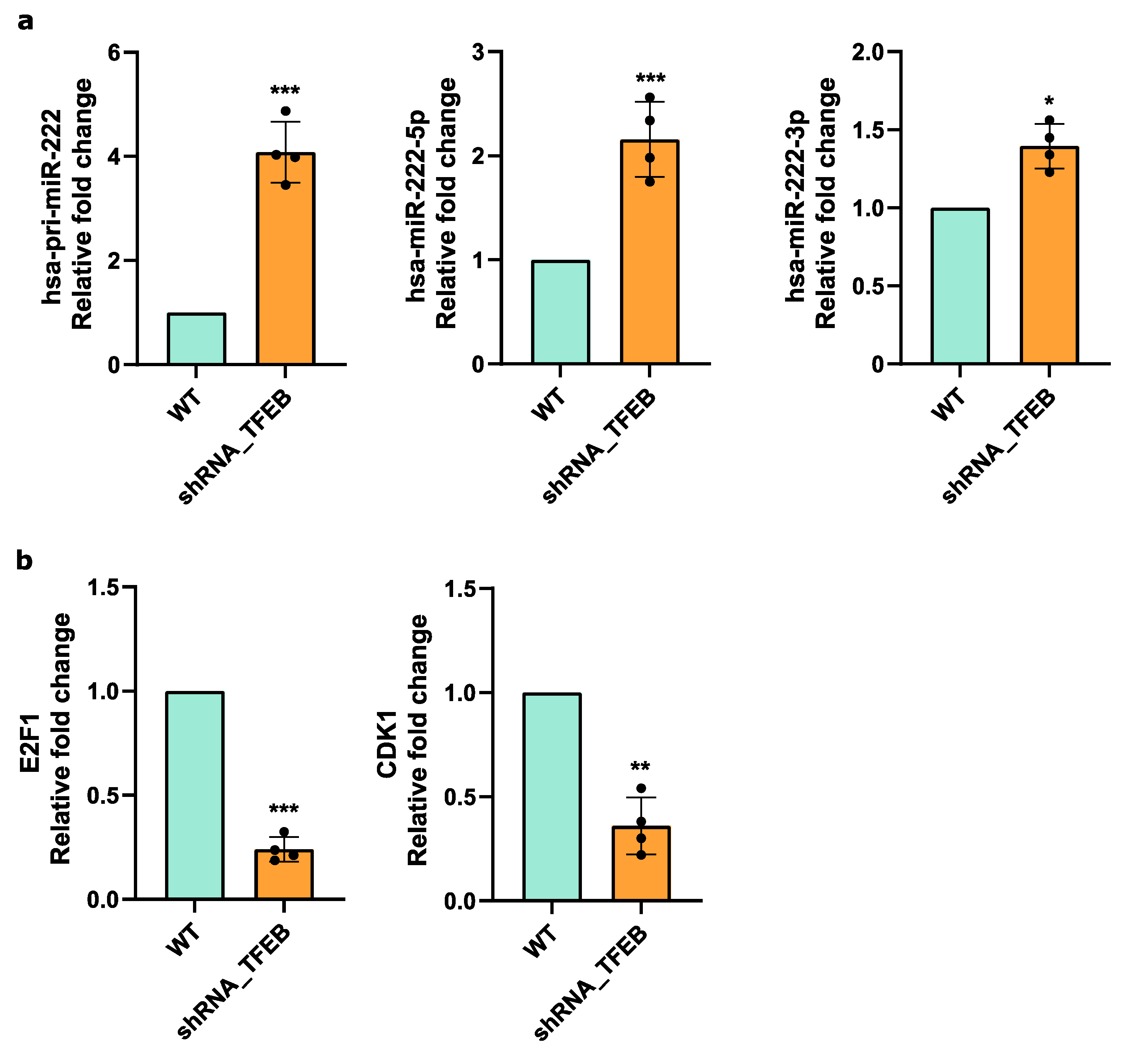
2.5. TFEB Modulates E2F1 and CDK1 via miR-222 in HUVECs
3. Discussion
- –
- miR-181a-3p is associated with slowed progression of atherosclerosis due to its role in inhibiting vascular inflammation. Specifically, it exerts an anti-inflammatory effect in ECs by reducing the expression of adhesion molecules, such as intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion protein 1 (VCAM-1), as well as NF-κB essential modulator (NEMO), which is involved in NF-κB signaling [40];
- –
- miR-339-3p is upregulated in vascular smooth muscle cells (VSMCs) in response to angiotensin II receptor-1 autoantibody (AT1-AA), contributing to vascular inflammation [41];
- –
- miR-181b-3b is overexpressed in chronic obstructive pulmonary disease (CODP), where it is associated with pulmonary endothelial dysfunction. Its overexpression in HUVECs in vitro leads to reduced endothelial sprouting and, consequently, diminished angiogenesis [42];
- –
- miR-29b-1-5p is downregulated in damaged endometrial stromal cells (ESCs) co-cultured with Wharton’s jelly mesenchymal stem cells (WJ-MSCs), which, through the upregulation of RAPB1, positively influences angiogenesis [43];
- –
- miR-222-5p is known for negatively regulating the angiogenic activity of the stem cell factor (SCF) by targeting c-Kit [30]. It is also upregulated in the vascular smooth muscle cells (VSMCs) treated with oxidized low-density lipoprotein (ox-LDL), contributing to the development of atherosclerosis [44];
- –
- miR-27a-5p is downregulated in exosomes derived from Tsp-1-expressing microglia, leading to increased Smad3 expression in ECs, which reduces retinal neovascularization, suggesting a protective role in maintaining vascular homeostasis and suppressing pathological angiogenesis [45];
- –
4. Materials and Methods
4.1. Cells and Genetic Manipulation
4.2. mRNA-Seq Sample Preparation
4.3. miRNA-Seq Sample Preparation
4.4. mRNA-Seq Analysis
4.5. miRNA-Seq Analysis
4.6. ChIP-Seq Analysis
4.7. Bioinformatic Data Integration
4.8. Functional Annotation of Differentially Expressed Genes
4.9. Real-Time PCR
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Doronzo, G.; Astanina, E.; Bussolino, F. The Oncogene Transcription Factor EB Regulates Vascular Functions. Front. Physiol. 2021, 12, 640061. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, G.; Ballabio, A. TFEB at a Glance. J. Cell Sci. 2016, 129, 2475–2481. [Google Scholar] [CrossRef] [PubMed]
- Takla, M.; Keshri, S.; Rubinsztein, D.C. The Post-translational Regulation of Transcription Factor EB (TFEB) in Health and Disease. EMBO Rep. 2023, 24, e57574. [Google Scholar] [CrossRef] [PubMed]
- Sardiello, M.; Palmieri, M.; Di Ronza, A.; Medina, D.L.; Valenza, M.; Gennarino, V.A.; Di Malta, C.; Donaudy, F.; Embrione, V.; Polishchuk, R.S.; et al. A Gene Network Regulating Lysosomal Biogenesis and Function. Science 2009, 325, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Medina, D.L.; Fraldi, A.; Bouche, V.; Annunziata, F.; Mansueto, G.; Spampanato, C.; Puri, C.; Pignata, A.; Martina, J.A.; Sardiello, M.; et al. Transcriptional Activation of Lysosomal Exocytosis Promotes Cellular Clearance. Dev. Cell 2011, 21, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, M.; Impey, S.; Kang, H.; Di Ronza, A.; Pelz, C.; Sardiello, M.; Ballabio, A. Characterization of the CLEAR Network Reveals an Integrated Control of Cellular Clearance Pathways. Hum. Mol. Genet. 2011, 20, 3852–3866. [Google Scholar] [CrossRef] [PubMed]
- Settembre, C.; Di Malta, C.; Polito, V.A.; Arencibia, M.G.; Vetrini, F.; Erdin, S.; Erdin, S.U.; Huynh, T.; Medina, D.; Colella, P.; et al. TFEB Links Autophagy to Lysosomal Biogenesis. Science 2011, 332, 1429–1433. [Google Scholar] [CrossRef]
- Abokyi, S.; Ghartey-Kwansah, G.; Tse, D.Y. TFEB Is a Central Regulator of the Aging Process and Age-Related Diseases. Ageing Res. Rev. 2023, 89, 101985. [Google Scholar] [CrossRef]
- Ariano, C.; Riganti, C.; Corà, D.; Valdembri, D.; Mana, G.; Astanina, E.; Serini, G.; Bussolino, F.; Doronzo, G. TFEB Controls Integrin-Mediated Endothelial Cell Adhesion by the Regulation of Cholesterol Metabolism. Angiogenesis 2022, 25, 471–492. [Google Scholar] [CrossRef]
- Astanina, E.; Bussolino, F.; Doronzo, G. Transcription Factor EB Controls Both Motogenic and Mitogenic Cell Activities. FEBS Lett. 2022, 596, 1973–1980. [Google Scholar] [CrossRef]
- Doronzo, G.; Astanina, E.; Corà, D.; Chiabotto, G.; Comunanza, V.; Noghero, A.; Neri, F.; Puliafito, A.; Primo, L.; Spampanato, C.; et al. TFEB Controls Vascular Development by Regulating the Proliferation of Endothelial Cells. EMBO J. 2019, 38, e98250. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, Z.; Zhang, L.; Qin, Y.; Yu, D. Dissecting the Multifaced Function of Transcription Factor EB (TFEB) in Human Diseases: From Molecular Mechanism to Pharmacological Modulation. Biochem. Pharmacol. 2023, 215, 115698. [Google Scholar] [CrossRef] [PubMed]
- Agostini, F.; Agostinis, R.; Medina, D.L.; Bisaglia, M.; Greggio, E.; Plotegher, N. The Regulation of MiTF/TFE Transcription Factors Across Model Organisms: From Brain Physiology to Implication for Neurodegeneration. Mol. Neurobiol. 2022, 59, 5000–5023. [Google Scholar] [CrossRef] [PubMed]
- Jiao, F.; Zhou, B.; Meng, L. The Regulatory Mechanism and Therapeutic Potential of Transcription Factor EB in Neurodegenerative Diseases. CNS Neurosci. Ther. 2023, 29, 37–59. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.; Prasad, R.; Lee, C.; Jho, E. Past, Present, and Future Perspectives of Transcription Factor EB (TFEB): Mechanisms of Regulation and Association with Disease. Cell Death Differ. 2022, 29, 1433–1449. [Google Scholar] [CrossRef] [PubMed]
- Ariano, C.; Costanza, F.; Akman, M.; Riganti, C.; Corà, D.; Casanova, E.; Astanina, E.; Comunanza, V.; Bussolino, F.; Doronzo, G. TFEB Inhibition Induces Melanoma Shut-down by Blocking the Cell Cycle and Rewiring Metabolism. Cell Death Dis. 2023, 14, 314. [Google Scholar] [CrossRef] [PubMed]
- Astanina, E.; Bussolino, F.; Doronzo, G. Multifaceted Activities of Transcription Factor EB in Cancer Onset and Progression. Mol. Oncol. 2021, 15, 327–346. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Yao, R.; Li, Y.; Zhao, P.; Ren, C.; Du, X.; Yao, Y. The Role and Regulatory Mechanism of Transcription Factor EB in Health and Diseases. Front. Cell Dev. Biol. 2021, 9, 667750. [Google Scholar] [CrossRef] [PubMed]
- Zoncu, R.; Perera, R.M. Emerging Roles of the MiT/TFE Factors in Cancer. Trends Cancer 2023, 9, 817–827. [Google Scholar] [CrossRef]
- Shang, R.; Lee, S.; Senavirathne, G.; Lai, E.C. microRNAs in action: Biogenesis, function and regulation. Nat. Rev. Genet. 2023, 24, 816–833. [Google Scholar] [CrossRef]
- Corà, D.; Re, A.; Caselle, M.; Bussolino, F. MicroRNA-Mediated Regulatory Circuits: Outlook and Perspectives. Phys. Biol. 2017, 14, 045001. [Google Scholar] [CrossRef]
- Bracken, C.P.; Scott, H.S.; Goodall, G.J. A network-biology perspective of microRNA function and dysfunction in cancer. Nat. Rev. Genet. 2016, 17, 719–732. [Google Scholar] [CrossRef] [PubMed]
- Corà, D.; Bussolino, F.; Doronzo, G. TFEB Signalling-Related MicroRNAs and Autophagy. Biomolecules 2021, 11, 985. [Google Scholar] [CrossRef] [PubMed]
- Martin, F.J.; Amode, M.R.; Aneja, A.; Austine-Orimoloye, O.; Azov, A.G.; Barnes, I.; Becker, A.; Bennett, R.; Berry, A.; Bhai, J.; et al. Ensembl 2023. Nucleic Acids Res. 2023, 51, D933–D941. [Google Scholar] [CrossRef]
- De Rie, D.; Abugessaisa, I.; Alam, T.; Arner, E.; Arner, P.; Ashoor, H.; Åström, G.; Babina, M.; Bertin, N.; Burroughs, A.M.; et al. An Integrated Expression Atlas of miRNAs and Their Promoters in Human and Mouse. Nat. Biotechnol. 2017, 35, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Bardes, E.E.; Aronow, B.J.; Jegga, A.G. ToppGene Suite for Gene List Enrichment Analysis and Candidate Gene Prioritization. Nucleic Acids Res. 2009, 37, W305–W311. [Google Scholar] [CrossRef]
- The ENCODE Project Consortium. An Integrated Encyclopedia of DNA Elements in the Human Genome. Nature 2012, 489, 57–74. [Google Scholar] [CrossRef]
- Luo, Y.; Hitz, B.C.; Gabdank, I.; Hilton, J.A.; Kagda, M.S.; Lam, B.; Myers, Z.; Sud, P.; Jou, J.; Lin, K.; et al. New Developments on the Encyclopedia of DNA Elements (ENCODE) Data Portal. Nucleic Acids Res. 2020, 48, D882–D889. [Google Scholar] [CrossRef]
- Castro-Mondragon, J.A.; Riudavets-Puig, R.; Rauluseviciute, I.; Berhanu Lemma, R.; Turchi, L.; Blanc-Mathieu, R.; Lucas, J.; Boddie, P.; Khan, A.; Manosalva Pérez, N.; et al. JASPAR 2022: The 9th Release of the Open-Access Database of Transcription Factor Binding Profiles. Nucleic Acids Res. 2022, 50, D165–D173. [Google Scholar] [CrossRef]
- Poliseno, L.; Tuccoli, A.; Mariani, L.; Evangelista, M.; Citti, L.; Woods, K.; Mercatanti, A.; Hammond, S.; Rainaldi, G. MicroRNAs modulate the angiogenic properties of HUVECs. Blood 2006, 108, 3068–3071. [Google Scholar] [CrossRef]
- Kent, W.J.; Sugnet, C.W.; Furey, T.S.; Roskin, K.M.; Pringle, T.H.; Zahler, A.M.; Haussler, A.D. The Human Genome Browser at UCSC. Genome Res. 2002, 12, 996–1006. [Google Scholar] [CrossRef] [PubMed]
- McGeary, S.E.; Lin, K.S.; Shi, C.Y.; Pham, T.M.; Bisaria, N.; Kelley, G.M.; Bartel, D.P. The Biochemical Basis of microRNA Targeting Efficacy. Science 2019, 366, eaav1741. [Google Scholar] [CrossRef] [PubMed]
- Malumbres, M.; Barbacid, M. Cell Cycle, CDKs and Cancer: A Changing Paradigm. Nat. Rev. Cancer 2009, 9, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Sliwinska, P.; Alitalo, K.; Allen, E.; Anisimov, A.; Aplin, A.C.; Auerbach, R.; Augustin, H.G.; Bates, D.O.; Van Beijnum, J.R.; Bender, R.H.F.; et al. Consensus guidelines for the use and interpretation of angiogenesis assays. Angiogenesis 2018, 21, 425–532. [Google Scholar] [CrossRef] [PubMed]
- Rosano, S.; Corà, D.; Parab, S.; Zaffuto, S.; Isella, C.; Porporato, R.; Hoza, R.M.; Calogero, R.A.; Riganti, C.; Bussolino, F.; et al. A regulatory microRNA network controls endothelial cell phenotypic switch during sprouting angiogenesis. Elife 2020, 9, e48095. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Hernando, C.; Suárez, Y. MicroRNAs in endothelial cell homeostasis and vascular disease. Curr. Opin. Hematol. 2018, 25, 227–236. [Google Scholar] [CrossRef]
- Annese, T.; Tamma, R.; De Giorgis, M.; Ribatti, D. microRNAs Biogenesis, Functions and Role in Tumor Angiogenesis. Front. Oncol. 2020, 10, 581007. [Google Scholar] [CrossRef] [PubMed]
- Moreau, P.; Tomas Bosch, V.; Bouvy-Liivrand, M.; Õunap, K.; Örd, T.; Pulkkinen, H.; Pölönen, P.; Heinäniemi, M.; Ylä-Herttuala, S.; Laakkonen, J.; et al. Profiling of Primary and Mature miRNA Expression in Atherosclerosis-Associated Cell Types. Arter. Thromb. Vasc. Biol. 2021, 41, 2149–2167. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.; Cui, Q.; Wang, J.; Zhou, Y. TransmiR v2.0: An updated transcription factor-microRNA regulation database. Nucleic Acids Res. 2019, 47, D253–D258. [Google Scholar] [CrossRef]
- Su, Y.; Yuan, J.; Zhang, F.; Lei, Q.; Zhang, T.; Li, K.; Guo, J.; Hong, Y.; Bu, G.; Lv, X.; et al. MicroRNA-181a-5p and microRNA-181a-3p cooperatively restrict vascular inflammation and atherosclerosis. Cell Death Dis. 2019, 10, 365. [Google Scholar] [CrossRef]
- Li, Y.; Long, Y.; Zhi, X.; Hao, H.; Wang, X.; Liu, H.; Wang, L. miR-339-3p promotes AT1-AA-induced vascular inflammation by upregulating NFATc3 protein expression in vascular smooth muscle cells. Acta Biochim. Biophys. Sin. 2023, 55, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Green, C.E.; Clarke, J.; Bicknell, R.; Turner, A.M. Pulmonary MicroRNA Changes Alter Angiogenesis in Chronic Obstructive Pulmonary Disease and Lung Cancer. Biomedicines 2021, 9, 830. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Sun, B.; Wang, D.; Zhu, Y.; Zhao, X.; Yang, X.; Zhang, Y. Circ6401, a novel circular RNA, is implicated in repair of the damaged endometrium by Wharton’s jelly-derived mesenchymal stem cells through regulation of the miR-29b-1-5p/RAP1B axis. Stem Cell Res. Ther. 2020, 11, 520. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jiang, G.; Lv, C.; Yang, C. miR-222-5p promotes dysfunction of human vascular smooth muscle cells by targeting RB1. Env. Toxicol. 2022, 4, 683–694. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Jiang, Z.; Jiang, J.; Wan, L.; Li, Y.; Huang, Y.; Qiu, J.; Yu, K.; Zhuang, J. Tsp-1+ microglia attenuate retinal neovascularization by maintaining the expression of Smad3 in endothelial cells through exosomes with decreased miR-27a-5p. Theranostics 2023, 11, 3689–3706. [Google Scholar] [CrossRef]
- Chen, Q.; Lin, G.; Huang, J.; Chen, L.; Liu, Y.; Huang, J.; Zhang, S.; Lin, Q. Inhibition of miR-193a-3p protects human umbilical vein endothelial cells against intermittent hypoxia-induced endothelial injury by targeting FAIM2. Aging 2020, 12, 1899–1909. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, Y.; Piao, H.; Li, B.; Zhu, Z.; Li, D.; Wang Tv Liu, K. Bioinformatics Analysis Reveals MicroRNA-193a-3p Regulates ACTG2 to Control Phenotype Switch in Human Vascular Smooth Muscle Cells. Front. Genet. 2021, 11, 572707. [Google Scholar] [CrossRef] [PubMed]
- Khoo, C.P.; Roubelakis, M.G.; Schrader, J.B.; Tsaknakis, G.; Konietzny, R.; Kessler, B.; Harris, A.L.; Watt, S.M. miR-193a-3p interaction with HMGB1 downregulates human endothelial cell proliferation and migration. Sci. Rep. 2017, 7, 44137. [Google Scholar] [CrossRef] [PubMed]
- Pasquier, A.; Pastore, N.; D’Orsi, L.; Colonna, R.; Esposito, A.; Maffia, V.; De Cegli, R.; Mutarelli, M.; Ambrosio, S.; Tufano, G.; et al. TFEB and TFE3 control glucose homeostasis by regulating insulin gene expression. EMBO J. 2023, 42, e113928. [Google Scholar] [CrossRef]
- Gambardella, G.; Staiano, L.; Moretti, M.N.; De Cegli, R.; Fagnocchi, L.; Di Tullio, G.; Polletti, S.; Braccia, C.; Armirotti, A.; Zippo, A.; et al. GADD34 is a modulator of autophagy during starvation. Sci. Adv. 2020, 6, eabb0205. [Google Scholar] [CrossRef]
- Follenzi, A.; Ailles, L.E.; Bakovic, S.; Geuna, M.; Naldini, L. Gene Transfer by Lentiviral Vectors Is Limited by Nuclear Translocation and Rescued by HIV-1 Pol Sequences. Nat. Genet. 2000, 25, 217–222. [Google Scholar] [CrossRef]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data, (Version 1.9) 2010 [Online]. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 1 May 2024).
- Martin, M. Cutadapt Removes Adapter Sequences from High-Throughput Sequencing Reads. EMBnet J. 2011, 17, 10. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate Transcript Quantification from RNA-Seq Data with or without a Reference Genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast Universal RNA-Seq Aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Friedländer, M.R.; Mackowiak, S.D.; Li, N.; Chen, W.; Rajewsky, N. miRDeep2 Accurately Identifies Known and Hundreds of Novel microRNA Genes in Seven Animal Clades. Nucleic Acids Res. 2012, 40, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Kozomara, A.; Birgaoanu, M.; Griffiths-Jones, S. miRBase: From microRNA Sequences to Function. Nucleic Acids Res. 2019, 47, D155–D162. [Google Scholar] [CrossRef] [PubMed]
- Langmead, B.; Salzberg, S.L. Fast Gapped-Read Alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, T.; Meyer, C.A.; Eeckhoute, J.; Johnson, D.S.; Bernstein, B.E.; Nusbaum, C.; Myers, R.M.; Brown, M.; Li, W.; et al. Model-Based Analysis of ChIP-Seq (MACS). Genome Biol. 2008, 9, R137. [Google Scholar] [CrossRef]
- Quinlan, A.R.; Hall, I.M. BEDTools: A Flexible Suite of Utilities for Comparing Genomic Features. Bioinformatics 2010, 26, 841–842. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gravina, T.; Favero, F.; Rosano, S.; Parab, S.; Diaz Alcalde, A.; Bussolino, F.; Doronzo, G.; Corà, D. Integrative Bioinformatics Analysis Reveals a Transcription Factor EB-Driven MicroRNA Regulatory Network in Endothelial Cells. Int. J. Mol. Sci. 2024, 25, 7123. https://doi.org/10.3390/ijms25137123
Gravina T, Favero F, Rosano S, Parab S, Diaz Alcalde A, Bussolino F, Doronzo G, Corà D. Integrative Bioinformatics Analysis Reveals a Transcription Factor EB-Driven MicroRNA Regulatory Network in Endothelial Cells. International Journal of Molecular Sciences. 2024; 25(13):7123. https://doi.org/10.3390/ijms25137123
Chicago/Turabian StyleGravina, Teresa, Francesco Favero, Stefania Rosano, Sushant Parab, Alejandra Diaz Alcalde, Federico Bussolino, Gabriella Doronzo, and Davide Corà. 2024. "Integrative Bioinformatics Analysis Reveals a Transcription Factor EB-Driven MicroRNA Regulatory Network in Endothelial Cells" International Journal of Molecular Sciences 25, no. 13: 7123. https://doi.org/10.3390/ijms25137123





