Research Progress of Pyroptosis in Diabetic Kidney Disease
Abstract
:1. Introduction
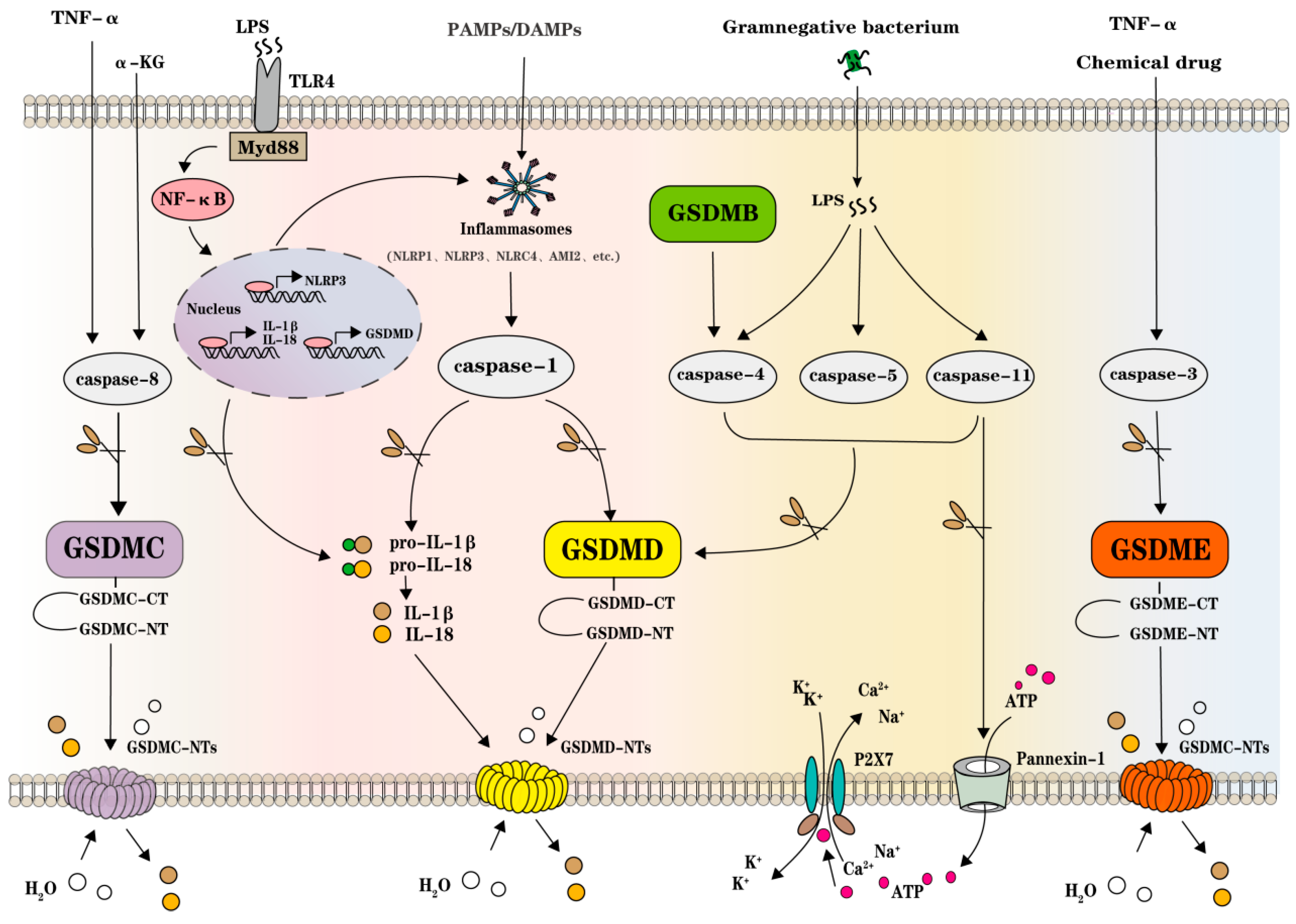
2. GSDMs, Inflammasomes, and Caspases in Pyroptosis
3. GSDMs Execute Pore-Forming Function in Pyroptosis
4. Inflammasomes and Other Pathogenic Sensor Signals Initiate Pyroptosis
5. Caspases Host Pyroptosis between Pathogenic Pathways and GSDMs
6. Multiple Pyroptosis Pathways Implicate DKD Pathology
7. TXNIP/NLRP3/Caspase-1/GSDMD Pathway
8. NF-κB/NLRP3/Caspase-1/GSDMD Signaling Pathway
9. Non-Coding RNA-RelatedNLRP3/Caspase-1/GSDMD Signaling Pathways
10. ATP/P2X4(7)/NLRP3/Caspase-1/GSDMD Signaling Pathway
11. Caspase-3/GSDME Signaling Pathway
12. Targeting Pyroptosis on DKD Treatment
13. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
Abbreviations
References
- Samsu, N. Diabetic Nephropathy: Challenges in Pathogenesis, Diagnosis, and Treatment. Biomed Res. Int. 2021, 2021, 1497449. [Google Scholar] [CrossRef]
- Kanaley, J.A.; Colberg, S.R.; Corcoran, M.H.; Malin, S.K.; Rodriguez, N.R.; Crespo, C.J.; Kirwan, J.P.; Zierath, J.R. Exercise/Physical Activity in Individuals with Type 2 Diabetes: A Consensus Statement from the American College of Sports Medicine. Med. Sci. Sports Exerc. 2022, 54, 353–368. [Google Scholar] [CrossRef]
- Gupta, S.; Dominguez, M.; Golestaneh, L. Diabetic Kidney Disease: An Update. Med. Clin. N. Am. 2023, 107, 689–705. [Google Scholar] [CrossRef]
- Tsai, J.L.; Chen, C.H.; Wu, M.J.; Tsai, S.F. New Approaches to Diabetic Nephropathy from Bed to Bench. Biomedicines 2022, 10, 876. [Google Scholar] [CrossRef] [PubMed]
- Elias, E.E.; Lyons, B.; Muruve, D.A. Gasdermins and pyroptosis in the kidney. Nat. Rev. Nephrol. 2023, 19, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Al, M.A.; Ara, M.A.; Wu, Y.; Zaeem, M.; Abdul, A.M.; Aktar, S.S.; Alyafeai, E.; Munir, F.; Xiao, J. Pyroptosis in diabetic nephropathy. Clin. Chim. Acta 2021, 523, 131–143. [Google Scholar] [CrossRef]
- Friedlander, A.M. Macrophages are sensitive to anthrax lethal toxin through an acid-dependent process. J. Biol. Chem. 1986, 261, 7123–7126. [Google Scholar] [CrossRef] [PubMed]
- Sansonetti, P.J.; Phalipon, A.; Arondel, J.; Thirumalai, K.; Banerjee, S.; Akira, S.; Takeda, K.; Zychlinsky, A. Caspase-1 activation of IL-1beta and IL-18 are essential for Shigella flexneri-induced inflammation. Immunity 2000, 12, 581–590. [Google Scholar] [CrossRef]
- Zychlinsky, A.; Prevost, M.C.; Sansonetti, P.J. Shigella flexneri induces apoptosis in infected macrophages. Nature 1992, 358, 167–169. [Google Scholar] [CrossRef]
- Zychlinsky, A.; Fitting, C.; Cavaillon, J.M.; Sansonetti, P.J. Interleukin 1 is released by murine macrophages during apoptosis induced by Shigella flexneri. J. Clin. Investig. 1994, 94, 1328–1332. [Google Scholar] [CrossRef]
- Cookson, B.T.; Brennan, M.A. Pro-inflammatory programmed cell death. Trends Microbiol. 2001, 9, 113–114. [Google Scholar] [CrossRef]
- Bergsbaken, T.; Fink, S.L.; Cookson, B.T. Pyroptosis: Host cell death and inflammation. Nat. Rev. Microbiol. 2009, 7, 99–109. [Google Scholar] [CrossRef]
- Dai, Z.; Liu, W.C.; Chen, X.Y.; Wang, X.; Li, J.L.; Zhang, X. Gasdermin D-mediated pyroptosis: Mechanisms, diseases, and inhibitors. Front. Immunol. 2023, 14, 1178662. [Google Scholar] [CrossRef]
- Devant, P.; Kagan, J.C. Molecular mechanisms of gasdermin D pore-forming activity. Nat. Immunol. 2023, 24, 1064–1075. [Google Scholar] [CrossRef]
- Li, R.; Xue, W.; Wei, H.; Fan, Q.; Li, X.; Qiu, Y.; Cui, D. Research Progress of Pyroptosis in Fatty Liver Disease. Int. J. Mol. Sci. 2023, 24, 13065. [Google Scholar] [CrossRef]
- Li, S.; Feng, L.; Li, G.; Liu, R.; Ma, C.; Wang, L.; Gao, A.; Liu, C.; Cui, Y.; Jiang, Z.; et al. GSDME-dependent pyroptosis signaling pathway in diabetic nephropathy. Cell Death Discov. 2023, 9, 156. [Google Scholar] [CrossRef]
- Wei, H.; Cui, D. Pyroptosis and Insulin Resistance in Metabolic Organs. Int. J. Mol. Sci. 2022, 23, 11638. [Google Scholar] [CrossRef]
- Xue, W.; Cui, D.; Qiu, Y. Research Progress of Pyroptosis in Alzheimer’s Disease. Front. Molec. Neurosci. 2022, 15, 872471. [Google Scholar] [CrossRef]
- Ding, J.; Wang, K.; Liu, W.; She, Y.; Sun, Q.; Shi, J.; Sun, H.; Wang, D.C.; Shao, F. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature 2016, 535, 111–116. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, Y.; Wang, K.; Shi, X.; Wang, Y.; Huang, H.; Zhuang, Y.; Cai, T.; Wang, F.; Shao, F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 2015, 526, 660–665. [Google Scholar] [CrossRef]
- Li, Z.; Ji, S.; Jiang, M.L.; Xu, Y.; Zhang, C.J. The Regulation and Modification of GSDMD Signaling in Diseases. Front. Immunol. 2022, 13, 893912. [Google Scholar] [CrossRef]
- Muhammad, J.S.; Jayakumar, M.N.; Elemam, N.M.; Venkatachalam, T.; Raju, T.K.; Hamoudi, R.A.; Maghazachi, A.A. Gasdermin D Hypermethylation Inhibits Pyroptosis And LPS-Induced IL-1beta Release from NK92 Cells. Immunotargets Ther. 2019, 8, 29–41. [Google Scholar] [CrossRef]
- Liu, J.; Jia, S.; Yang, Y.; Piao, L.; Wang, Z.; Jin, Z.; Bai, L. Exercise induced meteorin-like protects chondrocytes against inflammation and pyroptosis in osteoarthritis by inhibiting PI3K/Akt/NF-kappaB and NLRP3/caspase-1/GSDMD signaling. Biomed. Pharmacother. 2023, 158, 114118. [Google Scholar] [CrossRef]
- Kayagaki, N.; Lee, B.L.; Stowe, I.B.; Kornfeld, O.S.; O’Rourke, K.; Mirrashidi, K.M.; Haley, B.; Watanabe, C.; Roose-Girma, M.; Modrusan, Z.; et al. IRF2 transcriptionally induces GSDMD expression for pyroptosis. Sci. Signal. 2019, 12, eaax4917. [Google Scholar] [CrossRef]
- Op, D.B.K.; Van Camp, G.; Thys, S.; Cools, N.; Callebaut, I.; Vrijens, K.; Van Nassauw, L.; Van Tendeloo, V.F.; Timmermans, J.P.; Van Laer, L. The DFNA5 gene, responsible for hearing loss and involved in cancer, encodes a novel apoptosis-inducing protein. Eur. J. Hum. Genet. 2011, 19, 965–973. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, W.; Shi, X.; Ding, J.; Liu, W.; He, H.; Wang, K.; Shao, F. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature 2017, 547, 99–103. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Xia, S.; Kong, Q.; Li, S.; Liu, X.; Junqueira, C.; Meza-Sosa, K.F.; Mok, T.; Ansara, J.; et al. Gasdermin E suppresses tumour growth by activating anti-tumour immunity. Nature 2020, 579, 415–420. [Google Scholar] [CrossRef]
- Yang, C.; Wang, Z.Q.; Zhang, Z.C.; Lou, G.; Jin, W.L. CBL0137 activates ROS/BAX signaling to promote caspase-3/GSDME-dependent pyroptosis in ovarian cancer cells. Biomed. Pharmacother. 2023, 161, 114529. [Google Scholar] [CrossRef]
- Li, X.; Zhang, T.; Kang, L.; Xin, R.; Sun, M.; Chen, Q.; Pei, J.; Chen, Q.; Gao, X.; Lin, Z. Apoptotic caspase-7 activation inhibits non-canonical pyroptosis by GSDMB cleavage. Cell Death Differ. 2023, 30, 2120–2134. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Zhou, B.; Sun, R.Y.; Ai, Y.L.; Cheng, K.; Li, F.N.; Wang, B.R.; Liu, F.J.; Jiang, Z.H.; Wang, W.J.; et al. The metabolite alpha-KG induces GSDMC-dependent pyroptosis through death receptor 6-activated caspase-8. Cell Res. 2021, 31, 980–997. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Zhao, R.; Xia, W.; Chang, C.W.; You, Y.; Hsu, J.M.; Nie, L.; Chen, Y.; Wang, Y.C.; Liu, C.; et al. PD-L1-mediated gasdermin C expression switches apoptosis to pyroptosis in cancer cells and facilitates tumour necrosis. Nat. Cell Biol. 2020, 22, 1264–1275. [Google Scholar] [CrossRef]
- Kovacs, S.B.; Miao, E.A. Gasdermins: Effectors of Pyroptosis. Trends Cell Biol. 2017, 27, 673–684. [Google Scholar] [CrossRef]
- Barnett, K.C.; Li, S.; Liang, K.; Ting, J.P. A 360 degrees view of the inflammasome: Mechanisms of activation, cell death, and diseases. Cell 2023, 186, 2288–2312. [Google Scholar] [CrossRef]
- Oh, S.; Lee, J.; Oh, J.; Yu, G.; Ryu, H.; Kim, D.; Lee, S. Integrated NLRP3, AIM2, NLRC4, Pyrin inflammasome activation and assembly drive PANoptosis. Cell. Mol. Immunol. 2023, 20, 1513–1526. [Google Scholar] [CrossRef]
- Wawrocki, S.; Druszczynska, M. Inflammasomes in Mycobacterium tuberculosis-Driven Immunity. Can. J. Infect. Dis. Med. Microbiol. 2017, 2017, 2309478. [Google Scholar] [CrossRef]
- Huang, M.; Zhang, X.; Toh, G.A.; Gong, Q.; Wang, J.; Han, Z.; Wu, B.; Zhong, F.; Chai, J. Structural and biochemical mechanisms of NLRP1 inhibition by DPP9. Nature 2021, 592, 773–777. [Google Scholar] [CrossRef]
- Xu, Q.; Zhao, B.; Ye, Y.; Li, Y.; Zhang, Y.; Xiong, X.; Gu, L. Relevant mediators involved in and therapies targeting the inflammatory response induced by activation of the NLRP3 inflammasome in ischemic stroke. J. Neuroinflamm. 2021, 18, 123. [Google Scholar] [CrossRef]
- Liu, Z.; Gan, L.; Xu, Y.; Luo, D.; Ren, Q.; Wu, S.; Sun, C. Melatonin alleviates inflammasome-induced pyroptosis through inhibiting NF-kappaB/GSDMD signal in mice adipose tissue. J. Pineal Res. 2017, 63, e12414. [Google Scholar] [CrossRef]
- Zahid, A.; Li, B.; Kombe, A.; Jin, T.; Tao, J. Pharmacological Inhibitors of the NLRP3 Inflammasome. Front. Immunol. 2019, 10, 2538. [Google Scholar] [CrossRef]
- Song, M.Y.; Wang, J.X.; Sun, Y.L.; Han, Z.F.; Zhou, Y.T.; Liu, Y.; Fan, T.H.; Li, Z.G.; Qi, X.M.; Luo, Y.; et al. Tetrandrine alleviates silicosis by inhibiting canonical and non-canonical NLRP3 inflammasome activation in lung macrophages. Acta Pharmacol. Sin. 2022, 43, 1274–1284. [Google Scholar] [CrossRef]
- Baatarjav, C.; Komada, T.; Karasawa, T.; Yamada, N.; Sampilvanjil, A.; Matsumura, T.; Takahashi, M. dsDNA-induced AIM2 pyroptosis halts aberrant inflammation during rhabdomyolysis-induced acute kidney injury. Cell Death Differ. 2022, 29, 2487–2502. [Google Scholar] [CrossRef] [PubMed]
- Kayagaki, N.; Stowe, I.B.; Lee, B.L.; O’Rourke, K.; Anderson, K.; Warming, S.; Cuellar, T.; Haley, B.; Roose-Girma, M.; Phung, Q.T.; et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 2015, 526, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Gao, W.; Shao, F. Pyroptosis: Gasdermin-Mediated Programmed Necrotic Cell Death. Trends Biochem. Sci. 2017, 42, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Sun, J.; Meng, X. Pyroptosis by caspase-11 inflammasome-Gasdermin D pathway in autoimmune diseases. Pharmacol. Res. 2021, 165, 105408. [Google Scholar] [CrossRef]
- Yin, F.; Zheng, P.Q.; Zhao, L.Q.; Wang, Y.Z.; Miao, N.J.; Zhou, Z.L.; Cheng, Q.; Chen, P.P.; Xie, H.Y.; Li, J.Y.; et al. Caspase-11 promotes NLRP3 inflammasome activation via the cleavage of pannexin1 in acute kidney disease. Acta Pharmacol. Sin. 2022, 43, 86–95. [Google Scholar] [CrossRef]
- Zuo, Y.; Chen, L.; Gu, H.; He, X.; Ye, Z.; Wang, Z.; Shao, Q.; Xue, C. GSDMD-mediated pyroptosis: A critical mechanism of diabetic nephropathy. Expert. Rev. Mol. Med. 2021, 23, e23. [Google Scholar] [CrossRef]
- Zhang, K.J.; Wu, Q.; Jiang, S.M.; Ding, L.; Liu, C.X.; Xu, M.; Wang, Y.; Zhou, Y.; Li, L. Pyroptosis: A New Frontier in Kidney Diseases. Oxidative Med. Cell. Longev. 2021, 2021, 6686617. [Google Scholar] [CrossRef]
- Wang, Z.; Gu, Z.; Hou, Q.; Chen, W.; Yang, D. Zebrafish GSDMEb Cleavage-Gated Pyroptosis Drives Septic-Acute Kidney Injury In Vivo. J. Immunol. 2020, 204, i1901456. [Google Scholar] [CrossRef]
- Munshi, R.; Johnson, A.; Siew, E.D.; Ikizler, T.A.; Ware, L.B.; Wurfel, M.M.; Himmelfarb, J.; Zager, R.A.; Munshi, R.; Johnson, A. MCP-1 gene activation marks acute kidney injury. J. Am. Soc. Nephrol. JASN 2011, 22, 165–175. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, Q. The Role of Pyroptosis in the Pathogenesis of Kidney Diseases. Kidney Dis. 2023, 9, 443–458. [Google Scholar] [CrossRef]
- Kang, Q.; Yang, C. Oxidative stress and diabetic retinopathy: Molecular mechanisms, pathogenetic role and therapeutic implications. Redox Biol. 2020, 37, 101799. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Ma, G.; Wang, Z.; Liang, W.; Gao, W. Mechanisms of Kidney Cell Pyroptosis in Chronic Kidney Disease and the Effects of Traditional Chinese Medicine. Evid.-Based Complement. Altern. Med. 2021, 2021, 1173324. [Google Scholar] [CrossRef]
- Shahzad, K.; Fatima, S.; Khawaja, H.; Elwakiel, A.; Gadi, I.; Ambreen, S.; Zimmermann, S.; Mertens, P.R.; Biemann, R.; Isermann, B. Podocyte-specific Nlrp3 inflammasome activation promotes diabetic kidney disease. Kidney Int. 2022, 102, 766–779. [Google Scholar] [CrossRef]
- Wu, M.; Han, W.; Song, S.; Du, Y.; Liu, C.; Chen, N.; Wu, H.; Shi, Y.; Duan, H. NLRP3 deficiency ameliorates renal inflammation and fibrosis in diabetic mice. Mol. Cell. Endocrinol. 2018, 478, 115–125. [Google Scholar] [CrossRef]
- Wu, M.; Yang, Z.; Zhang, C.; Shi, Y.; Han, W.; Song, S.; Mu, L.; Du, C.; Shi, Y. Inhibition of NLRP3 inflammasome ameliorates podocyte damage by suppressing lipid accumulation in diabetic nephropathy. Metab.-Clin. Exp. 2021, 118, 154748. [Google Scholar] [CrossRef]
- Zhou, R.; Tardivel, A.; Thorens, B.; Choi, I.; Tschopp, J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 2010, 11, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.M. LncRNA-antisense non-coding RNA in the INK4 locus promotes pyroptosis via miR-497/thioredoxin-interacting protein axis in diabetic nephropathy. Life Sci. 2021, 264, 118728. [Google Scholar] [CrossRef]
- Song, Y.; Guo, F.; Zhao, Y.Y.; Ma, X.J.; Wu, L.N.; Yu, J.F.; Ji, H.F.; Shao, M.W.; Huang, F.J.; Zhao, L.; et al. Novel lncRNA-prader willi/angelman region RNA, SNRPN neighbour (PWARSN) aggravates tubular epithelial cell pyroptosis by regulating TXNIP via dual way in diabetic kidney disease. Cell Prolif. 2023, 56, e13349. [Google Scholar] [CrossRef]
- Ke, R.; Wang, Y.; Hong, S.; Xiao, L. Endoplasmic reticulum stress related factor IRE1alpha regulates TXNIP/NLRP3-mediated pyroptosis in diabetic nephropathy. Exp. Cell Res. 2020, 396, 112293. [Google Scholar] [CrossRef]
- Wan, L.; Bai, X.; Zhou, Q.; Chen, C.; Wang, H.; Liu, T.; Xue, J.; Wei, C.; Xie, L. The advanced glycation end-products (AGEs)/ROS/NLRP3 inflammasome axis contributes to delayed diabetic corneal wound healing and nerve regeneration. Int. J. Biol. Sci. 2022, 18, 809–825. [Google Scholar] [CrossRef]
- An, X.; Zhang, Y.; Cao, Y.; Chen, J.; Qin, H.; Yang, L. Punicalagin Protects Diabetic Nephropathy by Inhibiting Pyroptosis Based on TXNIP/NLRP3 Pathway. Nutrients 2020, 12, 1516. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, S.; Li, H.; Chen, F.; Shi, J. Naringin ameliorates diabetic nephropathy by inhibiting NADPH oxidase 4. Eur. J. Pharmacol. 2017, 804, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Guan, Y.B.; Zhang, K.J.; Li, L.; Zhou, Y. Tanshinone IIA mediates protection from diabetes kidney disease by inhibiting oxidative stress induced pyroptosis. J. Ethnopharmacol. 2023, 316, 116667. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Wang, J.; Chen, Y.; Li, X.; Wang, L.; Li, Y.; Jin, X.; Gu, X.; Hao, M.; Zhu, X.; et al. Novel biphenyl diester derivative AB-38b inhibits NLRP3 inflammasome through Nrf2 activation in diabetic nephropathy. Cell Biol. Toxicol. 2020, 36, 243–260. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Zhao, Q.; Datan, E.; Lin, G.Q.; Minn, I.; Pomper, M.G.; Yu, B.; Romo, D.; He, Q.L.; Liu, J.O. Triptolide: Reflections on two decades of research and prospects for the future. Nat. Prod. Rep. 2021, 38, 843–860. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.; Cheng, T.; Zhang, B.; Sun, K.; Lu, K. Triptolide protects against podocyte injury in diabetic nephropathy by activating the Nrf2/HO-1 pathway and inhibiting the NLRP3 inflammasome pathway. Ren. Fail. 2023, 45, 2165103. [Google Scholar] [CrossRef] [PubMed]
- Hinz, M.; Scheidereit, C. The IkappaB kinase complex in NF-kappaB regulation and beyond. EMBO Rep. 2014, 15, 46–61. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Qin, Z.; Zhang, C.; Mi, X.; Zhang, C.; Zhou, F.; Wang, J.; Zhang, L.; Hua, F. TRIM29 promotes podocyte pyroptosis in diabetic nephropathy through the NF-kB/NLRP3 inflammasome pathway. Cell Biol. Int. 2023, 47, 1126–1135. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Chadban, S.J.; Zhao, C.Y.; Chen, X.; Kwan, T.; Panchapakesan, U.; Pollock, C.A.; Wu, H. TLR4 activation promotes podocyte injury and interstitial fibrosis in diabetic nephropathy. PLoS ONE 2014, 9, e97985. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, K.; Hou, L.; Liao, J.; Zhang, H.; Han, Q.; Guo, J.; Li, Y.; Hu, L.; Pan, J.; et al. Endoplasmic reticulum stress contributes to pyroptosis through NF-kappaB/NLRP3 pathway in diabetic nephropathy. Life Sci. 2023, 322, 121656. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, X.; Yuan, S.; Wen, S.; Liu, X.; Wang, C.; Qu, Z.; Li, J.; Liu, H.; Sun, L.; et al. TLR4/NF-kappaB Signaling Induces GSDMD-Related Pyroptosis in Tubular Cells in Diabetic Kidney Disease. Front. Endocrinol. 2019, 10, 603. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Chen, G.; Wyburn, K.R.; Yin, J.; Bertolino, P.; Eris, J.M.; Alexander, S.I.; Sharland, A.F.; Chadban, S.J. TLR4 activation mediates kidney ischemia/reperfusion injury. J. Clin. Investig. 2007, 117, 2847–2859. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.Y.; Xu, S.J.; Qian, J.C.; Yang, L.B.; Chen, P.Q.; Wang, Y.; Hu, X.; Zhang, Y.L.; Luo, W.; Liang, G. Pharmacological inhibition of MyD88 suppresses inflammation in tubular epithelial cells and prevents diabetic nephropathy in experimental mice. Acta Pharmacol. Sin. 2022, 43, 354–366. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Han, J.; Yuan, R.; Xue, L.; Pang, W. Berberine ameliorates diabetic nephropathy by inhibiting TLR4/NF-kappaB pathway. Biol. Res. 2018, 51, 9. [Google Scholar] [CrossRef]
- Lu, S.; Zhang, H.; Wei, X.; Huang, X.; Chen, L.; Jiang, L.; Wu, X.; Zhou, X.; Qin, L.; Li, Y.; et al. 2-dodecyl-6-methoxycyclohexa-2,5-diene-1,4-dione isolated from Averrhoa carambola L. root ameliorates diabetic nephropathy by inhibiting the TLR4/MyD88/NF-kappaB pathway. Diabetes Metab. Syndr. Obes. 2019, 12, 1355–1363. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Li, F.; Xu, X.; Li, S.; Dong, X.; Chen, L.; Bai, B.; Wang, Y.; Qiu, M.; Dong, Y. 1,25(OH)(2)D(3) provides protection against diabetic kidney disease by downregulating the TLR4-MyD88-NF-kappaB pathway. Exp. Mol. Pathol. 2020, 114, 104434. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Chen, J.; Li, Y. Dioscin protects against diabetic nephropathy by inhibiting renal inflammation through TLR4/NF-kappaB pathway in mice. Immunobiology 2020, 225, 151941. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.Y.; He, Y.H.; Cheng, Y.; Fang, Q.; Ma, R.Y.; Zhou, S.J.; Hao, J.Q. Icariin ameliorates streptozocin-induced diabetic nephropathy through suppressing the TLR4/NF-kappaB signal pathway. Food Funct. 2021, 12, 1241–1251. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Shen, S.; Wang, L.; Zhao, M.; Li, Y.; Huang, S. Grifola frondosa Polysaccharide Ameliorates Early Diabetic Nephropathy by Suppressing the TLR4/NF-kappaB Pathway. Appl. Biochem. Biotechnol. 2022, 194, 4093–4104. [Google Scholar] [CrossRef]
- Qiu, D.; Song, S.; Chen, N.; Bian, Y.; Yuan, C.; Zhang, W.; Duan, H.; Shi, Y. NQO1 alleviates renal fibrosis by inhibiting the TLR4/NF-kappaB and TGF-beta/Smad signaling pathways in diabetic nephropathy. Cell. Signal. 2023, 108, 110712. [Google Scholar] [CrossRef]
- Guo, M.; Gao, J.; Jiang, L.; Dai, Y. Astragalus Polysaccharide Ameliorates Renal Inflammatory Responses in a Diabetic Nephropathy by Suppressing the TLR4/NF-kappaB Pathway. Drug Des. Devel Ther. 2023, 17, 2107–2118. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Ma, Q.; Liu, Y.; Wu, W.; Tu, Y.; Huang, L.; Long, Y.; Wang, W.; Yee, H.; Wan, Z.; et al. Huangkui capsule alleviates renal tubular epithelial-mesenchymal transition in diabetic nephropathy via inhibiting NLRP3 inflammasome activation and TLR4/NF-kappaB signaling. Phytomedicine 2019, 57, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Ma, Y.; Chen, F.; Wang, S.; Chen, B.; Shi, J. Artesunate ameliorates high glucose-induced rat glomerular mesangial cell injury by suppressing the TLR4/NF-kappaB/NLRP3 inflammasome pathway. Chem.-Biol. Interact. 2018, 293, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Li, Y.; Yuan, S.; Huang, Y.; Chen, X.; Han, Y.; Liu, Z.; Li, Z.; Xiao, Y.; Wang, Y.; et al. Alpha-kinase1 promotes tubular injury and interstitial inflammation in diabetic nephropathy by canonical pyroptosis pathway. Biol. Res. 2023, 56, 5. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; He, C.; Jiang, J.; Zhao, Z.; Yuan, H.; Wang, F.; Shen, B. The role of discoid domain receptor 1 on renal tubular epithelial pyroptosis in diabetic nephropathy. Korean J. Physiol. Pharmacol. 2022, 26, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, L.; Hua, F.; Zhang, C.; Zhang, C.; Mi, X.; Qin, N.; Wang, J.; Zhu, A.; Qin, Z.; et al. FOXM1-activated SIRT4 inhibits NF-kappaB signaling and NLRP3 inflammasome to alleviate kidney injury and podocyte pyroptosis in diabetic nephropathy. Exp. Cell Res. 2021, 408, 112863. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yang, Y.; Lv, Z.; Shu, A.; Du, Q.; Wang, W.; Chen, Y.; Xu, H. Study on the inhibitive effect of Catalpol on diabetic nephropathy. Life Sci. 2020, 257, 118120. [Google Scholar] [CrossRef]
- Li, Y.; Hou, J.G.; Liu, Z.; Gong, X.J.; Hu, J.N.; Wang, Y.P.; Liu, W.C.; Lin, X.H.; Wang, Z.; Li, W. Alleviative effects of 20(R)-Rg3 on HFD/STZ-induced diabetic nephropathy via MAPK/NF-kappaB signaling pathways in C57BL/6 mice. J. Ethnopharmacol. 2021, 267, 113500. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Chen, Y.; Yang, M.J.; Fan, X.R.; Xie, H.; Zhang, L.; Nie, Y.S.; Yan, M. Celastrol attenuates renal injury in diabetic rats via MAPK/NF-kappaB pathway. Phytother. Res. 2019, 33, 1191–1198. [Google Scholar] [CrossRef]
- Han, J.; Pang, X.; Zhang, Y.; Peng, Z.; Shi, X.; Xing, Y. Hirudin Protects Against Kidney Damage in Streptozotocin-Induced Diabetic Nephropathy Rats by Inhibiting Inflammation via P38 MAPK/NF-kappaB Pathway. Drug Des. Devel Ther. 2020, 14, 3223–3234. [Google Scholar] [CrossRef]
- Ge, Y.; Wang, J.; Wu, D.; Zhou, Y.; Qiu, S.; Chen, J.; Zhu, X.; Xiang, X.; Li, H.; Zhang, D. lncRNA NR_038323 Suppresses Renal Fibrosis in Diabetic Nephropathy by Targeting the miR-324–3p/DUSP1 Axis. Mol. Ther.-Nucl. Acids 2019, 17, 741–753. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.; Peng, R.; Zhang, L.Y.; Sun, Y.; Peng, H.M.; Liu, H.D.; Yu, L.J.; Li, A.L.; Zhang, Y.J.; Jiang, W.H.; et al. LincRNA-Gm4419 knockdown ameliorates NF-kappaB/NLRP3 inflammasome-mediated inflammation in diabetic nephropathy. Cell Death Dis. 2017, 8, e2583. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Cui, J.; Shi, Y.; Wang, T.; Xin, J.; Li, Y.; Shan, X.; Zhu, Z.; Gao, Y. Astragaloside IV Attenuates High-Glucose-Induced Impairment in Diabetic Nephropathy by Increasing Klotho Expression via the NF-kappaB/NLRP3 Axis. J. Diabetes Res. 2023, 2023, 7423661. [Google Scholar] [CrossRef]
- Ram, C.; Gairola, S.; Verma, S.; Mugale, M.N.; Bonam, S.R.; Murty, U.S.; Sahu, B.D. Biochanin A Ameliorates Nephropathy in High-Fat Diet/Streptozotocin-Induced Diabetic Rats: Effects on NF-kB/NLRP3 Axis, Pyroptosis, and Fibrosis. Antioxidants 2023, 12, 1052. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Zhai, B.; Liu, Y.; Chen, Y.; Xie, Z.; Wang, Q.; Wu, Y.; Liu, Z.; Chen, J.; Mei, S.; et al. Pyrroloquinoline quinone ameliorates renal fibrosis in diabetic nephropathy by inhibiting the pyroptosis pathway in C57BL/6 mice and human kidney 2 cells. Biomed. Pharmacother. 2022, 150, 112998. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; He, L.; Deng, W.; Lu, M.; Zhai, Y.; Pei, F.; Liu, S.; Zhang, C. Natural swietenine attenuates diabetic nephropathy by regulating the NF-kappaB/NLRP3/Caspase-1 signaling pathways: In vivo and in vitro study. Environ. Toxicol. 2022, 37, 2977–2989. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Huang, W.; Zhang, W.; Zhao, T.; Gao, C.; Gan, W.; Rao, M.; Chen, Q.; Guo, M.; Xu, Y.; et al. Sodium butyrate alleviates high-glucose-induced renal glomerular endothelial cells damage via inhibiting pyroptosis. Int. Immunopharmacol. 2019, 75, 105832. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Gao, Y.; Yue, R.; Wang, X.; Shi, Y.; Xu, J.; Wu, B.; Li, Y. Ginsenoside Rg1 Alleviates Podocyte Injury Induced by Hyperlipidemia via Targeting the mTOR/NF-kappaB/NLRP3 Axis. Evid.-Based Complement. Altern. Med. 2020, 2020, 2735714. [Google Scholar] [CrossRef]
- Li, Y.; Deng, X.; Zhuang, W.; Li, Y.; Xue, H.; Lv, X.; Zhu, S. Tanshinone IIA down-regulates -transforming growth factor beta 1 to relieve renal tubular epithelial cell inflammation and pyroptosis caused by high glucose. Bioengineered 2022, 13, 12224–12236. [Google Scholar] [CrossRef]
- Li, C.; Cai, F.; Yang, Y.; Zhao, X.; Wang, C.; Li, J.; Jia, Y.; Tang, J.; Liu, Q. Tetrahydroxystilbene glucoside ameliorates diabetic nephropathy in rats: Involvement of SIRT1 and TGF-beta1 pathway. Eur. J. Pharmacol. 2010, 649, 382–389. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, Z.; Ying, K.; Wang, H.; Liu, P.; Ji, X.; Chi, T.; Zou, L.; Wang, S.; He, Z. WJ-39, an Aldose Reductase Inhibitor, Ameliorates Renal Lesions in Diabetic Nephropathy by Activating Nrf2 Signaling. Oxidative Med. Cell. Longev. 2020, 2020, 7950457. [Google Scholar] [CrossRef]
- Zhu, X.; Shi, J.; Li, H. Liquiritigenin attenuates high glucose-induced mesangial matrix accumulation, oxidative stress, and inflammation by suppression of the NF-kappaB and NLRP3 inflammasome pathways. Biomed. Pharmacother. 2018, 106, 976–982. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, X.; Zhang, X.; Lu, D.; Guo, R. Electro-Acupuncture Protects Diabetic Nephropathy-Induced Inflammation Through Suppression of NLRP3 Inflammasome in Renal Macrophage Isolation. Endocr. Metab. Immune Disord.-Drug Targets 2021, 21, 2075–2083. [Google Scholar] [CrossRef]
- Qiao, Y.; Tian, X.; Men, L.; Li, S.; Chen, Y.; Xue, M.; Hu, Y.; Zhou, P.; Long, G.; Shi, Y.; et al. Spleen tyrosine kinase promotes NLR family pyrin domain containing 3 inflammasome-mediated IL-1beta secretion via c-Jun N-terminal kinase activation and cell apoptosis during diabetic nephropathy. Mol. Med. Rep. 2018, 18, 1995–2008. [Google Scholar] [CrossRef]
- Shi, Y.; Huang, C.; Zhao, Y.; Cao, Q.; Yi, H.; Chen, X.; Pollock, C. RIPK3 blockade attenuates tubulointerstitial fibrosis in a mouse model of diabetic nephropathy. Sci. Rep. 2020, 10, 10458. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Jiang, S.; Shang, J.; Jiang, Y.; Dai, Y.; Xu, B.; Yu, Y.; Liang, Z.; Yang, Y. LncRNA: Shedding light on mechanisms and opportunities in fibrosis and aging. Ageing Res. Rev. 2019, 52, 17–31. [Google Scholar] [CrossRef]
- Bridges, M.C.; Daulagala, A.C.; Kourtidis, A. LNCcation: lncRNA localization and function. J. Cell Biol. 2021, 220, e202009045. [Google Scholar] [CrossRef] [PubMed]
- Panni, S.; Lovering, R.C.; Porras, P.; Orchard, S. Non-coding RNA regulatory networks. Biochim. Biophys. Acta-Gene Regul. Mech. 2020, 1863, 194417. [Google Scholar] [CrossRef]
- Li, C.; Su, F.; Liang, Z.; Zhang, L.; Liu, F.; Fan, W.; Li, Z. Macrophage M1 regulatory diabetic nephropathy is mediated by m6A methylation modification of lncRNA expression. Mol. Immunol. 2022, 144, 16–25. [Google Scholar] [CrossRef]
- Xie, C.; Wu, W.; Tang, A.; Luo, N.; Tan, Y. lncRNA GAS5/miR-452–5p Reduces Oxidative Stress and Pyroptosis of High-Glucose-Stimulated Renal Tubular Cells. Diabetes Metab. Syndr. Obes. 2019, 12, 2609–2617. [Google Scholar] [CrossRef]
- Zhan, J.F.; Huang, H.W.; Huang, C.; Hu, L.L.; Xu, W.W. Long Non-Coding RNA NEAT1 Regulates Pyroptosis in Diabetic Nephropathy via Mediating the miR-34c/NLRP3 Axis. Kidney Blood Pressure Res. 2020, 45, 589–602. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.Y.; Zhou, X.; Liu, Z.W.; Xu, X.Q.; Liu, C. LncRNA NEAT1 accelerates renal tubular epithelial cell damage by modulating mitophagy via miR-150–5p-DRP1 axis in diabetic nephropathy. Exp. Physiol. 2021, 106, 1631–1642. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.T.; Wang, Q.L.; Yu, C.; Gao, M. lncRNA PVT1 modulates NLRP3-mediated pyroptosis in septic acute kidney injury by targeting miR-20a-5p. Mol. Med. Rep. 2021, 23, 271. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Fan, J.; He, J.; Zhao, L.; Tang, H. Knockdown of LncRNA DLX6-AS1 inhibits HK-2 cell pyroptosis via regulating miR-223–3p/NLRP3 pathway in lipopolysaccharide-induced acute kidney injury. J. Bioenerg. Biomembr. 2020, 52, 367–376. [Google Scholar] [CrossRef]
- Lv, P.; Liu, H.; Ye, T.; Yang, X.; Duan, C.; Yao, X.; Li, B.; Tang, K.; Chen, Z.; Liu, J.; et al. XIST Inhibition Attenuates Calcium Oxalate Nephrocalcinosis-Induced Renal Inflammation and Oxidative Injury via the miR-223/NLRP3 Pathway. Oxidative Med. Cell. Longev. 2021, 2021, 1676152. [Google Scholar] [CrossRef] [PubMed]
- Chi, K.; Geng, X.; Liu, C.; Zhang, Y.; Cui, J.; Cai, G.; Chen, X.; Wang, F.; Hong, Q. LncRNA-HOTAIR promotes endothelial cell pyroptosis by regulating the miR-22/NLRP3 axis in hyperuricaemia. J. Cell. Mol. Med. 2021, 25, 8504–8521. [Google Scholar] [CrossRef]
- Song, Z.; Zhang, Y.; Gong, B.; Xu, H.; Hao, Z.; Liang, C. Long noncoding RNA LINC00339 promotes renal tubular epithelial pyroptosis by regulating the miR-22–3p/NLRP3 axis in calcium oxalate-induced kidney stone. J. Cell. Biochem. 2019, 120, 10452–10462. [Google Scholar] [CrossRef]
- Hu, J.; Wu, H.; Wang, D.; Yang, Z.; Dong, J. LncRNA ANRIL promotes NLRP3 inflammasome activation in uric acid nephropathy through miR-122–5p/BRCC3 axis. Biochimie 2019, 157, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Song, W.; Zhang, X.; Long, F.; Yin, J.; He, X.; Lv, L. Downregulating LncRNA XIST attenuated contrast-induced nephropathy injury via regulating miR-133a-3p/NLRP3 axis. J. Thromb. Thrombolysis 2021, 52, 440–453. [Google Scholar] [CrossRef]
- Liu, C.; Zhuo, H.; Ye, M.Y.; Huang, G.X.; Fan, M.; Huang, X.Z. LncRNA MALAT1 promoted high glucose-induced pyroptosis of renal tubular epithelial cell by sponging miR-30c targeting for NLRP3. Kaohsiung J. Med. Sci. 2020, 36, 682–691. [Google Scholar] [CrossRef]
- Huang, J.; Xu, C. LncRNA MALAT1-deficiency restrains lipopolysaccharide (LPS)-induced pyroptotic cell death and inflammation in HK-2 cells by releasing microRNA-135b-5p. Ren. Fail. 2021, 43, 1288–1297. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Zhang, G.; Ge, Z. lncRNA MALAT1 Promotes Renal Fibrosis in Diabetic Nephropathy by Targeting the miR-2355–3p/IL6ST Axis. Front. Pharmacol. 2021, 12, 647650. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jiao, P.; Wei, X.; Zhou, Y. Silencing Long Non-coding RNA Kcnq1ot1 Limits Acute Kidney Injury by Promoting miR-204–5p and Blocking the Activation of NLRP3 Inflammasome. Front. Physiol. 2021, 12, 721524. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Gong, Y.; Li, N.; Liu, X.; Zhang, Y.; Ye, F.; Guo, Q.; Zheng, J. Long noncoding RNA Kcnq1ot1 promotes sC5b-9-induced podocyte pyroptosis by inhibiting miR-486a-3p and upregulating NLRP3. Am. J. Physiol.-Cell Physiol. 2021, 320, C355–C364. [Google Scholar] [CrossRef]
- Ding, H.; Li, J.; Li, Y.; Yang, M.; Nie, S.; Zhou, M.; Zhou, Z.; Yang, X.; Liu, Y.; Hou, F.F. MicroRNA-10 negatively regulates inflammation in diabetic kidney via targeting activation of the NLRP3 inflammasome. Mol. Ther. 2021, 29, 2308–2320. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, L.; Wang, R.; Guo, Y.; Yang, Z.; Yu, L.; Wang, L.; Liang, Y.; Tang, L. Circ_0004951 Promotes Pyroptosis of Renal Tubular Cells via the NLRP3 Inflammasome in Diabetic Kidney Disease. Front. Med. 2022, 9, 828240. [Google Scholar] [CrossRef]
- Fu, H.; Gu, Y.H.; Tan, J.; Yang, Y.N.; Wang, G.H. CircACTR2 in macrophages promotes renal fibrosis by activating macrophage inflammation and epithelial-mesenchymal transition of renal tubular epithelial cells. Cell. Mol. Life Sci. 2022, 79, 253. [Google Scholar] [CrossRef]
- Wen, S.; Li, S.; Li, L.; Fan, Q. circACTR2: A Novel Mechanism Regulating High Glucose-Induced Fibrosis in Renal Tubular Cells via Pyroptosis. Biol. Pharm. Bull. 2020, 43, 558–564. [Google Scholar] [CrossRef]
- Fu, H.; Chu, L.; Yuan, Y.S.; Liao, S.; Wang, G.H. Circular RNA ACTR2 activates M2 polarization of macrophages through activating Yes-associated protein signalling and contributes to renal fibrosis. Immunology 2022, 167, 606–621. [Google Scholar] [CrossRef]
- Sluyter, R. The P2X7 Receptor. Adv. Exp. Med. Biol. 2017, 1051, 17–53. [Google Scholar] [CrossRef]
- Kong, H.; Zhao, H.; Chen, T.; Song, Y.; Cui, Y. Targeted P2X7/NLRP3 signaling pathway against inflammation, apoptosis, and pyroptosis of retinal endothelial cells in diabetic retinopathy. Cell Death Dis. 2022, 13, 336. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wang, M.; Li, Y.; Shi, M.; Wang, Z.; Cao, C.; Hong, Y.; Hu, B.; Zhu, H.; Zhao, Z.; et al. Blocking connexin 43 and its promotion of ATP release from renal tubular epithelial cells ameliorates renal fibrosis. Cell Death Dis. 2022, 13, 511. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, K.; Jin, Y.; Liu, Y.; Chen, Y.; Zhang, X.; Yu, S.; Song, E.; Chen, S.; Zhang, J.; et al. H3 relaxin protects against calcium oxalate crystal-induced renal inflammatory pyroptosis. Cell Prolif. 2020, 53, e12902. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Zhang, J.; Zhang, W.; Zhang, J.; Yang, J.; Li, K.; He, Y. ATP-P2X4 signaling mediates NLRP3 inflammasome activation: A novel pathway of diabetic nephropathy. Int. J. Biochem. Cell Biol. 2013, 45, 932–943. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, X.N.; Fang, J.N.; Hua, F.F.; Han, J.Y.; Yuan, Z.Q.; Xie, A.M. The mechanism behind activation of the Nod-like receptor family protein 3 inflammasome in Parkinson’s disease. Neural Regen. Res. 2022, 17, 898–904. [Google Scholar] [CrossRef] [PubMed]
- Palygin, O.; Klemens, C.A.; Isaeva, E.; Levchenko, V.; Spires, D.R.; Dissanayake, L.V.; Nikolaienko, O.; Ilatovskaya, D.V.; Staruschenko, A. Characterization of purinergic receptor 2 signaling in podocytes from diabetic kidneys. iScience 2021, 24, 102528. [Google Scholar] [CrossRef] [PubMed]
- Menzies, R.I.; Booth, J.; Mullins, J.J.; Bailey, M.A.; Tam, F.; Norman, J.T.; Unwin, R.J. Hyperglycemia-induced Renal P2X7 Receptor Activation Enhances Diabetes-related Injury. EBioMedicine 2017, 19, 73–83. [Google Scholar] [CrossRef]
- Oda, K.; Miyamoto, S.; Kodera, R.; Wada, J.; Shikata, K. Suramin prevents the development of diabetic kidney disease by inhibiting NLRP3 inflammasome activation in KK-Ay mice. J. Diabetes Investig. 2023, 14, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Serralha, R.S.; Rodrigues, I.F.; Bertolini, A.; Lima, D.Y.; Nascimento, M.; Mouro, M.G.; Punaro, G.R.; Visona, I.; Rodrigues, A.M.; Higa, E. Esculin reduces P2X7 and reverses mitochondrial dysfunction in the renal cortex of diabetic rats. Life Sci. 2020, 254, 117787. [Google Scholar] [CrossRef]
- Wang, C.; Hou, X.X.; Rui, H.L.; Li, L.J.; Zhao, J.; Yang, M.; Sun, L.J.; Dong, H.R.; Cheng, H.; Chen, Y.P. Artificially Cultivated Ophiocordyceps sinensis Alleviates Diabetic Nephropathy and Its Podocyte Injury via Inhibiting P2X7R Expression and NLRP3 Inflammasome Activation. J. Diabetes Res. 2018, 2018, 1390418. [Google Scholar] [CrossRef]
- Cheng, Q.; Pan, J.; Zhou, Z.L.; Yin, F.; Xie, H.Y.; Chen, P.P.; Li, J.Y.; Zheng, P.Q.; Zhou, L.; Zhang, W.; et al. Caspase-11/4 and gasdermin D-mediated pyroptosis contributes to podocyte injury in mouse diabetic nephropathy. Acta Pharmacol. Sin. 2021, 42, 954–963. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Xu, Y. Pyroptosis in Kidney Disease. J. Mol. Biol. 2022, 434, 167290. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yuan, Y.; Huang, Z.X.; Chen, H.; Lan, R.; Wang, Z.; Lai, K.; Chen, H.; Chen, Z.; Zou, Z.; et al. GSDME-mediated pyroptosis promotes inflammation and fibrosis in obstructive nephropathy. Cell Death Differ. 2021, 28, 2333–2350. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Wang, H.; Weng, C.; Jiang, H.; Chen, J. Caspase 3/GSDME-dependent pyroptosis contributes to chemotherapy drug-induced nephrotoxicity. Cell Death Dis. 2021, 12, 186. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.F.; Zhang, Q.; Ding, C.J.; Sun, H.Y.; Che, Y.; Huang, H.; Wang, Y.; Wu, J.W.; Hao, H.P.; Cao, L.J. Gasdermin E-derived caspase-3 inhibitors effectively protect mice from acute hepatic failure. Acta Pharmacol. Sin. 2021, 42, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Li, Y.; Wu, M.; Jin, Q.; Wang, Q.; Li, S.; Huang, S.; Zhang, A.; Zhang, Y.; Jia, Z. Gasdermin E deficiency attenuates acute kidney injury by inhibiting pyroptosis and inflammation. Cell Death Dis. 2021, 12, 139. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Yin, N.; Liu, W.; Cui, X.; Chen, S.; Wang, E. Curcumin Ameliorates Diabetic Nephropathy by Suppressing NLRP3 Inflammasome Signaling. Biomed. Res. Int. 2017, 2017, 1516985. [Google Scholar] [CrossRef]
- Ding, T.; Wang, S.; Zhang, X.; Zai, W.; Fan, J.; Chen, W.; Bian, Q.; Luan, J.; Shen, Y.; Zhang, Y.; et al. Kidney protection effects of dihydroquercetin on diabetic nephropathy through suppressing ROS and NLRP3 inflammasome. Phytomedicine 2018, 41, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhu, C.; Yang, H.; Deng, J.; Fan, D. Protective effect of ginsenoside Rg5 against kidney injury via inhibition of NLRP3 inflammasome activation and the MAPK signaling pathway in high-fat diet/streptozotocin-induced diabetic mice. Pharmacol. Res. 2020, 155, 104746. [Google Scholar] [CrossRef]
- Liu, Y.W.; Hao, Y.C.; Chen, Y.J.; Yin, S.Y.; Zhang, M.Y.; Kong, L.; Wang, T.Y. Protective effects of sarsasapogenin against early stage of diabetic nephropathy in rats. Phytother. Res. 2019, 33, 2470. [Google Scholar] [CrossRef]
- Yang, R.; Li, Y.; Mehmood, S.; Yan, C.; Huang, Y.; Cai, J.; Ji, J.; Pan, W.; Zhang, W.; Chen, Y. Polysaccharides from Armillariella tabescens mycelia ameliorate renal damage in type 2 diabetic mice. Int. J. Biol. Macromol. 2020, 162, 1682–1691. [Google Scholar] [CrossRef] [PubMed]
- Pourshabanan, P.; Momeni, A.; Mahmoudnia, L.; Kheiri, S. Effect of pioglitazone on decreasing of proteinuria in type 2 diabetic patients with nephropathy. Diabetes Metab. Syndr.-Clin. Res. Rev. 2019, 13, 132–136. [Google Scholar] [CrossRef]
- Tang, S.; Gao, C.; Long, Y.; Huang, W.; Chen, J.; Fan, F.; Jiang, C.; Xu, Y. Maresin 1 Mitigates High Glucose-Induced Mouse Glomerular Mesangial Cell Injury by Inhibiting Inflammation and Fibrosis. Mediat. Inflamm. 2017, 2017, 2438247. [Google Scholar] [CrossRef] [PubMed]
- Iacobini, C.; Menini, S.; Blasetti Fantauzzi, C.; Pesce, C.M.; Giaccari, A.; Salomone, E.; Lapolla, A.; Orioli, M.; Aldini, G.; Pugliese, G. FL-926–16, a novel bioavailable carnosinase-resistant carnosine derivative, prevents onset and stops progression of diabetic nephropathy in db/db mice. Br. J. Pharmacol. 2017, 175, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Xiao, M.; Tan, Q.; Lyu, J.; Lu, F. The effect of aerobic exercise on oxidative stress in patients with chronic kidney disease: A systematic review and meta-analysis with trial sequential analysis. Ren. Fail. 2023, 45, 2252093. [Google Scholar] [CrossRef]
- Ghosh, S.; Khazaei, M.; Moien-Afshari, F.; Ang, L.S.; Granville, D.J.; Verchere, C.B.; Dunn, S.R.; McCue, P.; Mizisin, A.; Sharma, K.; et al. Moderate exercise attenuates caspase-3 activity, oxidative stress, and inhibits progression of diabetic renal disease in db/db mice. Am. J. Physiol.-Renal Physiol. 2009, 296, F700–F708. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Ying, C.; Zhou, X.; Shi, Y.; Xu, J.; Zhu, Y.; Wang, M.; Li, Y.; Li, X.; Xiang, J. Aerobic exercise training alleviates renal injury in db/db mice through inhibiting Nox4-mediated NLRP3 inflammasome activation. Exp. Gerontol. 2022, 168, 111934. [Google Scholar] [CrossRef] [PubMed]
- Monno, I.; Ogura, Y.; Xu, J.; Koya, D.; Kitada, M. Exercise Ameliorates Diabetic Kidney Disease in Type 2 Diabetic Fatty Rats. Antioxidants 2021, 10, 1754. [Google Scholar] [CrossRef] [PubMed]
- de Alcantara, S.R.; Guzzoni, V.; Silva, K.; Aragao, D.S.; de Paula, V.R.; Bertoncello, N.; Schor, N.; Aimbire, F.; Casarini, D.E.; Cunha, T.S. Resistance exercise shifts the balance of renin-angiotensin system toward ACE2/Ang 1–7 axis and reduces inflammation in the kidney of diabetic rats. Life Sci. 2021, 287, 120058. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Jafar, T.H.; Nitsch, D.; Neuen, B.L.; Perkovic, V. Chronic kidney disease. Lancet 2021, 398, 786–802. [Google Scholar] [CrossRef]
- Li, Y.J.; Chen, X.; Kwan, T.K.; Loh, Y.W.; Singer, J.; Liu, Y.; Ma, J.; Tan, J.; Macia, L.; Mackay, C.R.; et al. Dietary Fiber Protects against Diabetic Nephropathy through Short-Chain Fatty Acid-Mediated Activation of G Protein-Coupled Receptors GPR43 and GPR109A. J. Am. Soc. Nephrol. 2020, 31, 1267–1281. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Y.; Shi, D.; He, C.; Xia, G. Vitamin D Alleviates Type 2 Diabetes Mellitus by Mitigating Oxidative Stress-Induced Pancreatic beta-Cell Impairment. Exp. Clin. Endocrinol. Diabet. 2023, 131, 656–666. [Google Scholar] [CrossRef]

| Upstream | Non-Coding RNA | Downstream |
|---|---|---|
| lncRNA-038323/miR-324-3p | DUSP1/p38MAPK/ERK1/2 | |
| lncRNA-Gm4919 | NF-κB | |
| Nucleus |
| TXNIP |
| Nucleus |
| NLRP3 inflammasomes |
| Pyroptosis Pathway or Component | Treatment Agents | Results (Compared with Controls) | Potential Clinical Translation and Efficacy | Reference |
|---|---|---|---|---|
| TXNIP/NLRP3/ caspase-1/GSDMD pathway | Punicalagin | ↓BUN, ↓CREA, and ↓UACR | alleviating DKD by reducing inflammation and pyroptosis | [61] |
| Naringin | ↓Scr, and ↓BUN | improving renal function in DKD by reducing oxidative stress and inflammation | [62] | |
| Tan IIA | ↓Scr, ↓BUN, and ↓albuminuria | reducing renal tubular epithelial cell inflammation and pyroptosis in DKD | [63] | |
| AB-38b | ↓Scr, and ↓BUN | improving renal function and reducing fibrosis in DKD by targeting oxidative stress and NLRP3 inflammasome | [64] | |
| TP | ↓Scr, and ↓BUN | alleviating podocyte injury and improving renal function in DKD by reducing oxidative stress and pyroptosis | [65] | |
| NF-κB/NLRP3/ caspase-1/GSDMD pathway | 4-PBA | ↓ER stress, and ↓pyroptosis | alleviating ER stress-induced pyroptosis in diabetic nephropathy | [70] |
| BYA11–7082 | ↓tubular injury score | reducing NLRP3-mediated pyroptosis in DKD | ||
| TAK-242 | ↓caspase-1, ↓GSDMD-NT, ↓IL-18, and ↓IL-1β | alleviating TLR4-mediated GSDMD-induced pyroptosis in DKD | [71] | |
| LM8 | ↓Scr, ↓BUN, and ↓ACR | therapeutic inhibition of MyD88-mediated renal inflammation in diabetes | [77] | |
| HKC | ↓Scr, ↓BUN, ↓UAlb, and ↓Alb | therapeutic intervention in renal tubular and inflammation in DKD | [82] | |
| PGF | ↓Scr, and ↓BUN | can be developed as a complementary treatment for diabetic nephropathy, potentially improving glucose control and renal protection alongside existing therapies | [79] | |
| 1,25(OH)2D3 | ↓Scr, and ↓BUN | consideration for anti-inflammatory and antifibrotic therapies in renal diseases | [76] | |
| DMDD | ↓Scr, and ↓BUN | treating diabetic nephropathy by targeting inflammation and improving kidney function and metabolic parameters | [75] | |
| ICA | ↓Scr, ↓BUN, and ↓UAlb | mitigating renal inflammation and oxidative stress | [68] | |
| BBR | ↓24h-urinaryproteinlevel, ↓Scr, and ↓BUN | reducing renal injury and inflammation and benefitting podocyte survival in DKD | [74] | |
| ART | ↓Scr, ↓BUN, and ↓UAlb | reducing inflammation, oxidative stress, and extracellular matrix accumulation in DKD | [83] | |
| GE | ↓Scr and, ↓BUN | reducing kidney inflammation and dysfunction in DKD | [87] | |
| Cat | ↓Scr and, ↓BUN | reducing inflammation, oxidative stress, and preventing kidney damage in DKD | [87] | |
| 20(R)-Rg3 | ↓Scr and, ↓BUN | alleviating symptoms of diabetic nephropathy by improving renal function and metabolic profiles. | [88] | |
| celastrol | ↓Scr, ↓BUN, and ↓urinary protein | protecting kidneys in diabetic nephropathy by reducing inflammation and delaying renal damage | [89] | |
| hirudin | ↓Scr, and ↓BUN | protecting against kidney damage in diabetic nephropathy by inhibiting inflammation and preventing podocyte apoptosis | [90] | |
| AS-IV | ↓Scr, ↓BUN, and ↓UACR | improving renal function and reducing podocyte damage in DKD models | [93] | |
| BCA | ↓Scr, and ↓BUN | protecting against renal injury and fibrosis | [94] | |
| PQQ | ↓Scr, and ↓BUN | reducing inflammatory cytokine levels associated with DKD and protecting against oxidative stress-induced renal damage | [95] | |
| SWI | ↓Scr, ↓BUN, and ↓UACR | reducing inflammatory markers associated with DKD progression and protecting renal function | [96] | |
| Ginsenoside Rg1 | ↓Scr, ↓BUN, and ↓UACR | alleviating renal damage induced by hyperlipidemia in DKD | [98] | |
| TSG | ↓Scr, and ↓BUN | improving renal function, reducing proteinuria in diabetic patients, and enhancing antioxidant defenses in diabetic conditions | [100] | |
| WJ-39 | ↓ACR, and ↓Scr | improving renal function and reducing fibrosis in DKD patients | [101] | |
| ATP/P2X4 (7)/ NLRP3/caspase-1/ GSDMD pathway | AZ11657312 | ↓Scr, and ↓AER | reducing renal macrophage accumulation and fibrosis and mitigating glomerular mesangial expansion and preserving renal function | [137] |
| Suramin | ↓UACR, and ↓AER | preventing or slowing the progression of early-stage DKD | [138] | |
| ACOS | ↓Scr | preventing or attenuating kidney damage in diabetes through anti-inflammatory and podocyte-protective effects | [140] | |
| Caspase-3/GSDME pathway | Ac-DMPD-CMK | ↓ALT/AST, and ↓LDH | advancement as specific caspase-3 inhibitors for clinical use and alleviating liver injury | [145] |
| Ac-DMLD-CMK | ||||
| NLRP3 | Curcumin | ↓IL-1β, ↓caspase-1, and ↓NLRP3 level | a potent antifibrotic agent that inhibits NLRP3 activity to protect kidney | [147] |
| DHQ | ↓ECM, and ↓mesangial matrix expansion | reducing urine microalbumin excretion, hyperglycemia, and lipid metabolism disorders in DKD | [148] | |
| Rg5 | ↓insulin levels, ↓serum creatinine, ↓serum urea, ↓and serum UA | improving renal function and histopathology in diabetic mice and reducing kidney inflammation | [149] | |
| SAR | ↓albuminuria, ↓kidney weight index, ↓serum uric acid, and ↓ECM | improving renal function and morphology in diabetic rats, and reducing renal inflammation and fibrosis | [150] | |
| AT | ↓renal function-related indices, ↓LPS, ↓IL-1β, and ↓IL-18 | improving intestinal health, which could translate to broader metabolic benefits in diabetic patients | [151] | |
| MAR 1 | ↓ROS, ↓NLRP3, ↓caspase-1, ↓IL-1β, and ↓TGF-β1 level | mitigating inflammation and fibrosis in DKD, potentially preventing disease progression | [153] | |
| FL-926–16 | ↓creatinine; ↓albuminuria, and ↓proteinuria | preventing onset and halting progression of DKD | [154] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Q.; Li, R.; Wei, H.; Xue, W.; Li, X.; Xia, Z.; Zhao, L.; Qiu, Y.; Cui, D. Research Progress of Pyroptosis in Diabetic Kidney Disease. Int. J. Mol. Sci. 2024, 25, 7130. https://doi.org/10.3390/ijms25137130
Fan Q, Li R, Wei H, Xue W, Li X, Xia Z, Zhao L, Qiu Y, Cui D. Research Progress of Pyroptosis in Diabetic Kidney Disease. International Journal of Molecular Sciences. 2024; 25(13):7130. https://doi.org/10.3390/ijms25137130
Chicago/Turabian StyleFan, Qingqing, Rongxuan Li, Huiting Wei, Weiyue Xue, Xiang Li, Ziyao Xia, Le Zhao, Ye Qiu, and Di Cui. 2024. "Research Progress of Pyroptosis in Diabetic Kidney Disease" International Journal of Molecular Sciences 25, no. 13: 7130. https://doi.org/10.3390/ijms25137130







