Abstract
Gα13 and Gα12, encoded by the GNA13 and GNA12 genes, respectively, are members of the G12 family of Gα proteins that, along with their associated Gβγ subunits, mediate signaling from specific G protein-coupled receptors (GPCRs). Advanced prostate cancers have increased expression of GPCRs such as CXC Motif Chemokine Receptor 4 (CXCR4), lysophosphatidic acid receptor (LPAR), and protease activated receptor 1 (PAR-1). These GPCRs signal through either the G12 family, or through Gα13 exclusively, often in addition to other G proteins. The effect of Gα13 can be distinct from that of Gα12, and the role of Gα13 in prostate cancer initiation and progression is largely unexplored. The oncogenic effect of Gα13 on cell migration and invasion in prostate cancer has been characterized, but little is known about other biological processes such as mitochondrial function and oxidative stress. Current knowledge on the link between Gα13 and oxidative stress is based on animal studies in which GPCR-Gα13 signaling decreased superoxide levels, and the overexpression of constitutively active Gα13 promoted antioxidant gene activation. In human samples, mitochondrial superoxide dismutase 2 (SOD2) correlates with prostate cancer risk and prognostic Gleason grade. However, overexpression of SOD2 in prostate cancer cells yielded conflicting results on cell growth and survival under basal versus oxidative stress conditions. Hence, it is necessary to explore the effect of Gα13 on prostate cancer tumorigenesis, as well as the effect of Gα13 on SOD2 in prostate cancer cell growth under oxidative stress conditions.
1. GPCR-Gα13 Signaling
1.1. Overview
G protein-coupled receptors (GPCRs) are seven-transmembrane receptors that form the largest family of transmembrane receptors, with over 800 in the human genome. GPCRs are involved in processes as diverse as embryogenesis, normal physiological functions, including metabolic and neuroendocrine control, as well as in the pathological processes of tumorigenesis and tumor progression [1]. As such, GPCRs govern a wide range of functions and processes, including sensory, neurotransmission, endocrine, exocrine, cell growth, and cell migration [2,3], several of which are also involved in malignancy. GPCRs are largely located at the plasma membrane and respond to diverse extracellular stimuli from both small molecule hormones and neurotransmitters, as well as peptides and even large protein signaling molecules [2]. More recently, it was recognized that GPCRs are also located at the membranes of intracellular compartments such as endoplasmic reticulum, Golgi, endosomes, cell division compartments, and inner and outer nuclear membranes [3,4]. In regard to cancer, ligand activation of overexpressed wild-type GPCRs, or constitutively active mutant GPCRs, can induce oncogenic transformation in vitro and in vivo [1,5,6,7,8]. In human samples, nearly 20% of cancers harbor GPCR mutations [9,10]. Furthermore, GPCR signaling drives cancer cell stemness, treatment resistance, and progression [11]. Hence, aberrant GPCR signaling likely contributes to tumorigenesis in many cell types and can affect cellular processes in different cellular compartments.
GPCRs are coupled to heterotrimeric G proteins, which consist of α, β, and γ subunits. There are 21 Gα proteins, 6 Gβ proteins, and 14 Gγ proteins [12,13,14]. Gα proteins are bound to guanosine diphosphate (GDP) in the inactive state and exchange for guanosine triphosphate (GTP) when activated by GPCR. This triggers the dissociation of Gα-GTP from Gβγ dimers, both of which can activate distinct signaling pathways. Once activated, GPCRs are often quickly desensitized via phosphorylation by GPCR kinases and coupling to β-arrestin or ubiquitination, then internalized by endocytosis, dephosphorylated, or deubiquitinated, and recycled to the membrane to couple with Gα-GDP and Gβγ [15]. This forms the classic cycle of GPCR activation.
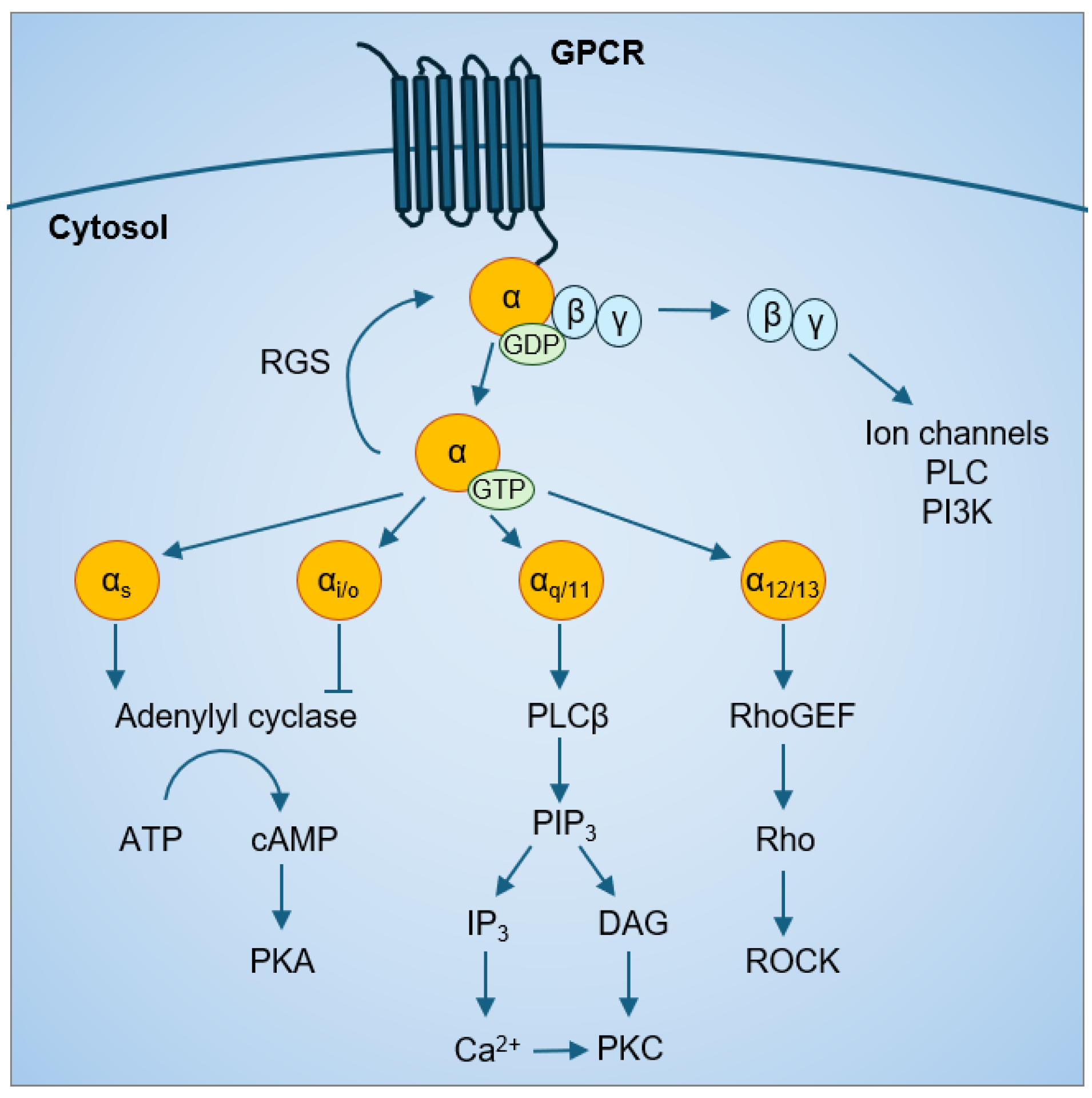
Based on sequence homology and functional similarities, Gα proteins are categorized into four major families: Gαs, Gαi/o, Gαq/11, and Gα12/13. Each Gα family has preferential downstream effectors but can also activate multiple signaling cascades (Figure 1) Both the Gαs and Gαi family affect the cyclic adenosine monophosphate (cAMP) signal pathway through their effects on adenylyl cyclase; Gαs is stimulatory, while Gαi is inhibitory [16,17,18]. Gαq activates phospholipase Cβ and the phosphatidylinositol pathway to increase intracellular calcium levels [19]. Gα12/13 mainly activate the Rho family of GTPases (Rho) and the Rho-associated protein kinases (ROCK) pathways. Following Gα protein activation by a GPCR, the intrinsic GTPase activity of Gα proteins converts the bound GTP to GDP, which is the inactive Gα-GDP state. In addition, regulator of G protein signaling (RGS) proteins have GTPase activating protein (GAP) activity that can accelerate the intrinsic GTPase activity of Gα proteins [20], prevent Gα-GTP binding to effectors [21,22], and enhance Gα affinity for Gβγ binding [23,24]. This forms the classic cycle of Gα protein activation.
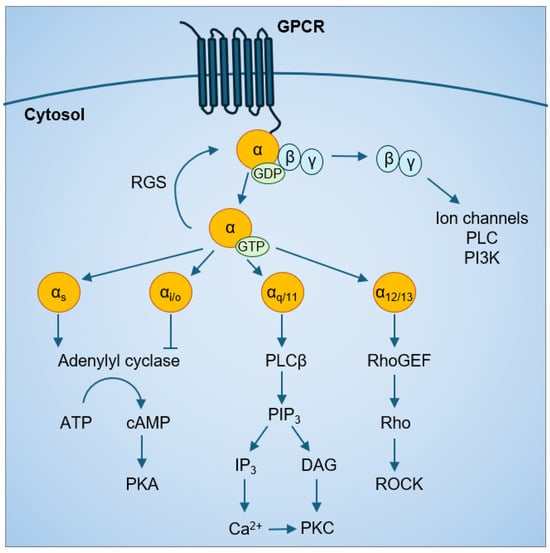
Figure 1.
GPCR-Gα protein signaling.
The Gα12/13 family consists of Gα12 and Gα13 proteins that are 67% homologous to each other [25]. Different from other families of Gα proteins, which share less than 45% identity with the Gα12/13 family, Gα12 and Gα13 are not targets of pertussis toxin or cholera toxin [26]. Activated Gα12/13 are capable of stimulating guanine nucleotide exchange factors (GEFs) for another class of GTPases, the Rho/Rac small GTPases [27]. For example, p115-RhoGEF [28,29], leukemia-related RhoGEF (LARG) [30,31,32], and PDZ, PSD-95/Dlg/ZO-1 homology-RhoGEF (PDZ-RhoGEF) [33,34] can activate the downstream Rho [35,36,37,38,39], cell division control protein 42 homolog (CDC42) [40], and Rac small GTPases [41]. Then, activated Rho-GTP binds to ROCK at its the carboxyl-terminus containing the Rho-binding domain. There are two ROCK isoforms, ROCK1 and ROCK2, and both bind to Gα12/13. Gα12/13 signaling via ROCK can increase phosphorylation of myosin phosphatase target subunit 1 (MYPT1), myosin light chain (MLC) [31,42] and the LIN-11, Isl-1, MEC-3 kinases (LIMK) [43] to regulate actin cytoskeletal remodeling, which, in turn, can activate the YAP/TAZ pathway [44,45].
Gα12/13 signaling can also regulate mitogen-activated protein kinase (MAPK) [40,46,47,48,49,50,51], phospholipases [37,51], protein kinases [52,53,54], Na+/H+ ion channels [40,48,54,55,56], and the Hippo signaling pathway [57]; whether all these require initial activation of Rho/Rac is not clear. These signaling pathways converge in the nucleus to regulate transcription factor activity and gene transcription. Gα12/13-regulated transcription factors include the nuclear factor kappa B (NF-κB) [53,58,59], activator protein 1 (AP-1) [41,60], serum response factor (SRF) [61,62], nuclear factor of activated T-cell c1 (NFATc1) [63,64], signal transducer and activator of transcription 3 (STAT3) [65], and yes-associated protein and the transcriptional coactivator with PDZ-binding motif (YAP/TAZ) [57,66].
Both in vitro models as well as results from knockout mice, both individually and in combination, have linked Gα12/13 signaling to numerous normal physiological processes, such as embryonic development and cell differentiation [63,67,68], cell metabolism, cell growth, actin cytoskeletal remodeling [35,69,70], cell migration [63], angiogenesis [71], platelet activation [72], osteogenesis [63,64,73], immune response [74,75], and neuronal responses [36,76,77]. Aberrant Gα12/13 signaling is also involved in oncogenic transformation [49,78,79], cancer cell growth [60], apoptosis [80,81,82], metastatic invasion [39,83,84], hypertension [31,85], inflammation, metabolic, fibrotic, and renal diseases [55].
Although Gα12 and Gα13 signaling appear to be very similar, their biochemistry and function are not completely overlapping [26]. Gα13 knockout is embryonic lethal in mice between embryonic day 9 and 10 due to angiogenic defects [86], whereas Gα12 knockout is viable without apparent abnormalities [87,88]. Moreover, Gα12 and Gα13 activate Na+/H+ exchanger via different signaling mechanisms [54] and have contrasting roles in osteogenesis [63,64,73]. In addition, Gα13, but not Gα12, enhances resistance against cytotoxic drugs and irradiation in primary head and neck squamous cell carcinomas [81]. It is purported that downstream effectors are able to discriminate Gα12 and Gα13, possibly due to the difference in abundance and cellular localization in addition to their sequence differences [26]. Gα12 is mainly located at the plasma membrane, while Gα13 appears to be located in the cytoplasm in multiple cell types [89]. Furthermore, some GPCRs selectively couple to either Gα12 or Gα13 [55,90,91]. In addition to the conventional model of GPCR-Gα13 signaling, Gα13 can also be activated independent of GPCR activation [92,93,94] or interact with non-GPCRs such as receptor tyrosine kinases (RTKs), smoothened [95], and integrins [96,97,98,99]. Hence, exploring the role of Gα13 protein will add to the current knowledge of the functions of Gα12 protein to increase the understanding of the biology of the Gα12/13 family.
1.2. Gα13 in Solid Cancers
Gα12 and Gα13 are also known as gep proto-oncogenes and are the only Gα proteins that induce oncogenic transformation when overexpressed, even without mutation [78,100,101]. Multiple studies have shown that Gα12/13 family can impact tumorigenesis, cancer cell migration and invasion, and drug resistance [102,103,104,105,106]. While the role of Gα13 in tumorigenesis and progression can be distinct from Gα12, it is relatively less studied, even though the oncogenic role of Gα13 was discovered several decades ago when overexpression of the GNA13 cDNA in NIH3T3 fibroblasts was found to confer cell transformation [101]. Subsequent in vitro studies have mainly focused on the constitutively active Gα13 (Q226L) mutant, which does not exist in nature [49,79,101,107]. The only naturally occurring activating Gα13 mutation is at the arginine-200 site in bladder cancer [66], as well as inactivating mutations found in certain B cell lymphomas (a field of its own, and not covered here) [108,109]. In addition to being tumor-suppressive in lymphomas, Gα13 has also been reported to be tumor-suppressive in vitro in melanoma [110,111], glioma [112], and in genetically engineered KRas/Tp53 pancreatic cancer mouse models [113]. However, Gα13 is thought to be largely oncogenic in humans, where the most common alteration in Gα13 is GNA13 gene amplification [114] or in enhanced activity of GPCRs coupled to Gα13, such as PAR-1 [115,116], CXCR4 [117], LPAR [118,119], thromboxane A2 receptor (TA2R) [9], and thyrotropin receptor (TSHR) [120]. This section will mainly focus on the role of Ga13 in solid cancers.
The oncogenic role of Gα13 is supported by patient data. The overall survival rate or progression-free survival rate is worse in those expressing higher levels Gα13 compared to those with lower levels of Gα13. Gα13 is a prognostic factor in gastric [121], hepatocellular [122], esophageal [123], and head and neck [81] cancers (Table 1). A meta-analysis of overall survival outcomes across 39 cancer types from ~18,000 human samples showed that GNA13 gene expression is associated with poor prognosis in breast, lung, kidney, and ovarian cancers [124].

Table 1.
Prognosis of patients with tumors expressing Gα13 protein at higher versus lower levels, as assessed by immunohistochemistry staining in tumor samples.
The activation of Gα13 promotes in vitro cell proliferation and in vivo tumor growth in several cancer cell types such as head and neck squamous cell carcinoma [81], lung squamous cell carcinoma [125], small cell lung cancer [126], gastric cancer [121], and ovarian cancer [57] cells. The oncogenic transformation and mitogenic effects of Gα13 is at least partially mediated by RhoA-dependent activation of MAPK pathways (c-Jun, c-Jun N-terminal kinase (JNK), serum response factor), NF-κB and YAP/TAZ transcription factors [57,66,81,115,116,127,128] (Figure 2). Interestingly, overexpression of Gα13 did not promote cell proliferation in hepatocellular cancer cells [122], and expression of the constitutively active Gα13(Q226L) mutant in breast [83,129] and prostate [84,130] cancer cells had no effect on cell growth. However, limited studies have assessed the effect of wild-type Gα13 on anchorage-independent proliferation or tumor growth in mouse xenografts [81,83,125,131]. Hence, the oncogenic effect of Gα13 can be context-specific and cell type-dependent, and it is necessary to study the effect of wild-type Gα13 at endogenous levels in anchorage-independent growth models.
In vitro studies have revealed the role of Gα13 in other hallmarks of cancer. Gα13 is involved in cancer cell migration and invasion in solid cancers, such as prostate [130,132], breast [83,129], ovarian [57,131,133], lung [125], hepatocellular [122], pancreatic [134,135], and colorectal [136,137] cancers. Gα13 also protects against cell death induced by cytotoxic drugs or γ-irradiation in head and neck cancers [81], hepatocellular [138], and non-small cell lung cancer [139]. This enhanced resistance against cytotoxicity seems to be a function of Gα13, but not Gα12 [81]. In support of this, ROCK signaling also protects against apoptosis in prostate cancer [140], bladder cancer [141], and leukemia [142]. However, there are other hallmarks of cancer that are yet to be explored for Gα13, such as the regulation of metabolism.
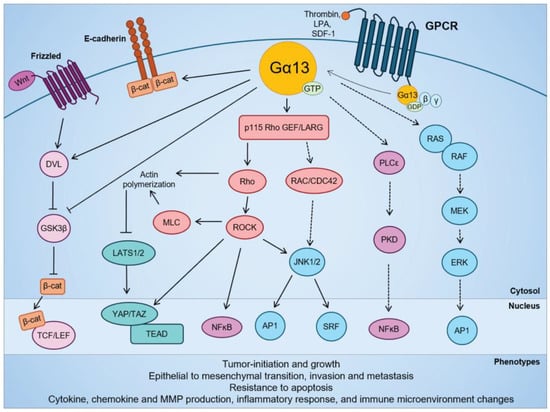
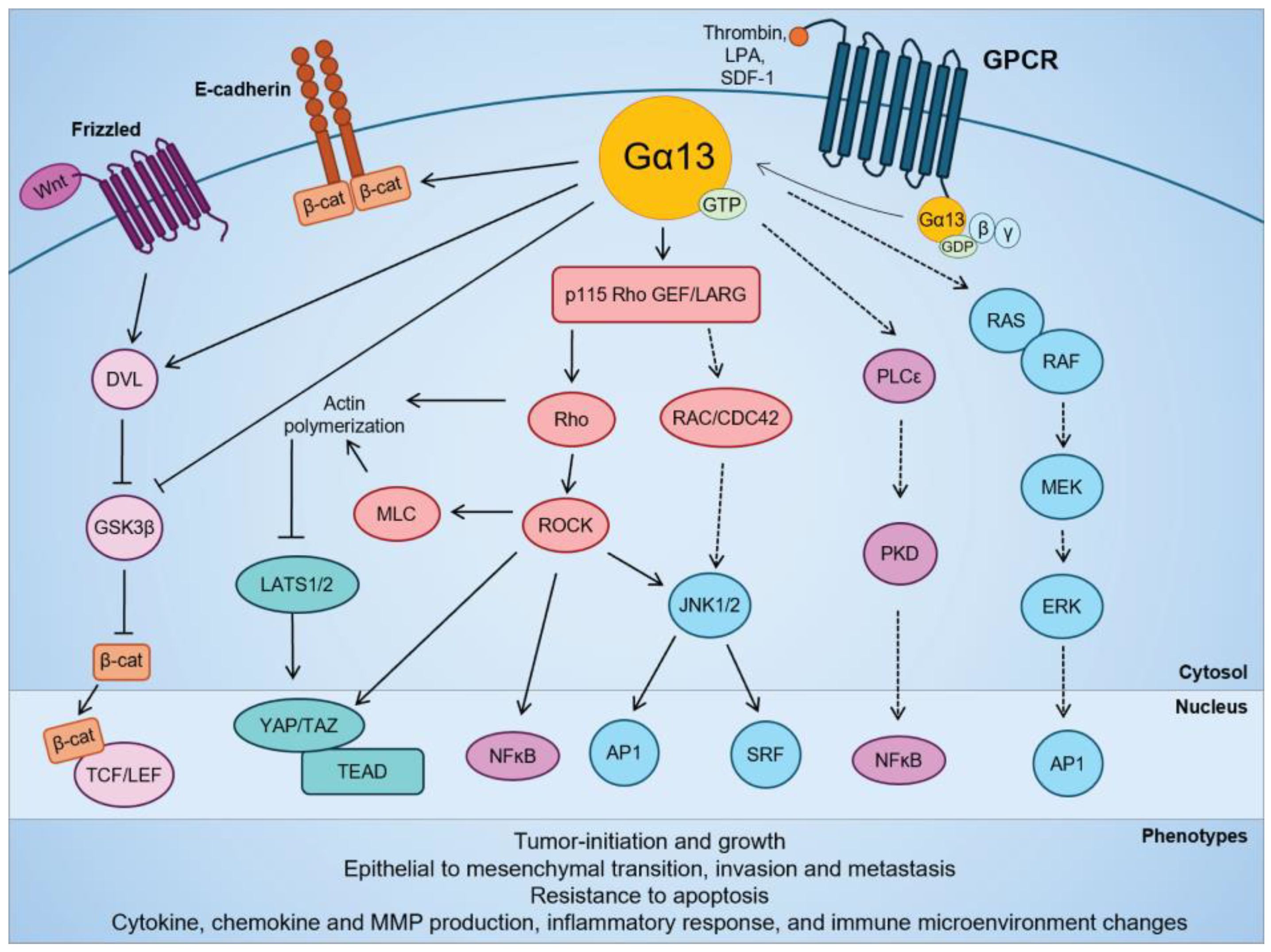
Figure 2.
Signaling pathways regulated by Gα13 in cellular transformation and in solid cancers. Modified from Rasheed et al. (2022) [143].
Figure 2.
Signaling pathways regulated by Gα13 in cellular transformation and in solid cancers. Modified from Rasheed et al. (2022) [143].
1.3. Gα13 in Mitochondrial Function
There is limited evidence on the role of Gα13 on mitochondrial processes. Although Gα13 does not appear to be present in the mitochondria of cells [144], it has been reported to alter the expression of genes involved in mitochondrial biogenesis, oxidative phosphorylation and insulin insensitivity in osteoclasts [73], muscle [145], and liver [146], as seen in mice with tissue-specific deletion of GNA13. Moreover, Gα13 potentially mediates mitochondrial trafficking in neurons in GNA12 and GNA13 double-knockout HEK293 cells [147]. Additionally, several studies have shown that GPCR signaling regulates mitochondria morphology and cellular metabolism in normal cells [148,149,150,151]. Even though these studies indicate that Gα13 negatively regulates mitochondrial functions in non-cancerous cells, the role of Gα13 on the mitochondria in cancer cells and its effect on tumorigenesis is largely unstudied. Furthermore, GPCRs are localized and function on the mitochondrion [3,152], but none of these mitochondrially localized GPCRs have been shown to stimulate Gα13 proteins. In studies on human cancers, the GPCR succinate receptor 1 (SUCNR1/GPR91) inhibited mitochondrial respiration in gastric, lung, and pancreatic cancer cell lines that were addicted to glutamine [153]. In another study, the GPCR ligand lysophosphatidic acid (LPA) stimulated a transient increase in mitochondrial calcium ion uptake in colorectal cancer cells [154]. Finally, the GPCR ligand stromal cell-derived factor 1 (SDF-1), also known as CXC motif chemokine ligand 12 (CXCL12), induced mitochondrial superoxide dismutase 2 expression in human hepatocellular carcinoma cells [155]. Again, none of these plasma membrane GPCRs, even though capable of activating Gα13, have been yet shown to impact mitochondrial function by such activation.
2. Oxidative Stress in Cancer
2.1. Overview
Reactive oxygen species (ROS) include superoxide anion (O2•−), hydrogen peroxide (H2O2), hydroxyl radicals (HO•), and singlet oxygen (1O2). They are mainly generated intracellularly by electron leakage during mitochondrial oxidative phosphorylation in the electron transport chain (ETC), at the endoplasmic reticulum (ER) during unfolded protein response, and at the plasma and nuclear membranes by nicotinamide adenine dinucleotide phosphate oxidase (NADPH oxidase, NOX) [156]. The oxidizing effect of ROS creates oxidative damage to DNA, proteins, and lipids, and its accumulation eventually promotes tumorigenesis [156,157]. The strongest evidence for ROS-induced tumorigenesis is from superoxide scavenging by superoxide dismutase (SOD), the enzyme that catalyzes the transformation of superoxide to H2O2. Mice deficient in cytoplasmic Sod1 developed liver cancer [158], and mice with heterozygous deletion of mitochondrial Sod2 formed lymphoma and pituitary adenoma [159]. Mice deficient in peroxiredoxin-1, which reduces H2O2 to H2O, developed several types of malignancies [160]. In addition, mice deficient in antioxidant gene transcription factor nuclear factor erythroid 2-related factor 2 (Nrf2) demonstrated increased sensitivity of chemical carcinogenesis [161].
While many studies have focused on nuclear H2O2 that induces oxidative damage on genomic DNA, H2O2 is a relatively weak oxidant compared to superoxide [156]. In addition, mitochondria oxidative stress can directly lead to intrinsic apoptosis or other forms of cell death [162,163,164]. The major source of mitochondrial ROS generation is by ETC complex I and III [165], which are components of oxidative phosphorylation in energy metabolism. Complex I and III produce superoxide anions, which are catalyzed to H2O2 by SOD2 in the mitochondrial matrix and SOD1 in the intermembrane space. Then, H2O2 is catalyzed to H2O by catalase, glutathione peroxidase, and peroxiredoxin. Therefore, targeting the initial superoxide accumulation and oxidizing effects might be more effective than targeting H2O2. Moreover, anchorage-independent growth leads to metabolic reprogramming and increase in mitochondrial superoxide production [166,167,168], which could enhance the tumor cell inherent sensitivity to superoxide levels.
2.2. Gα13 in Oxidative Stress
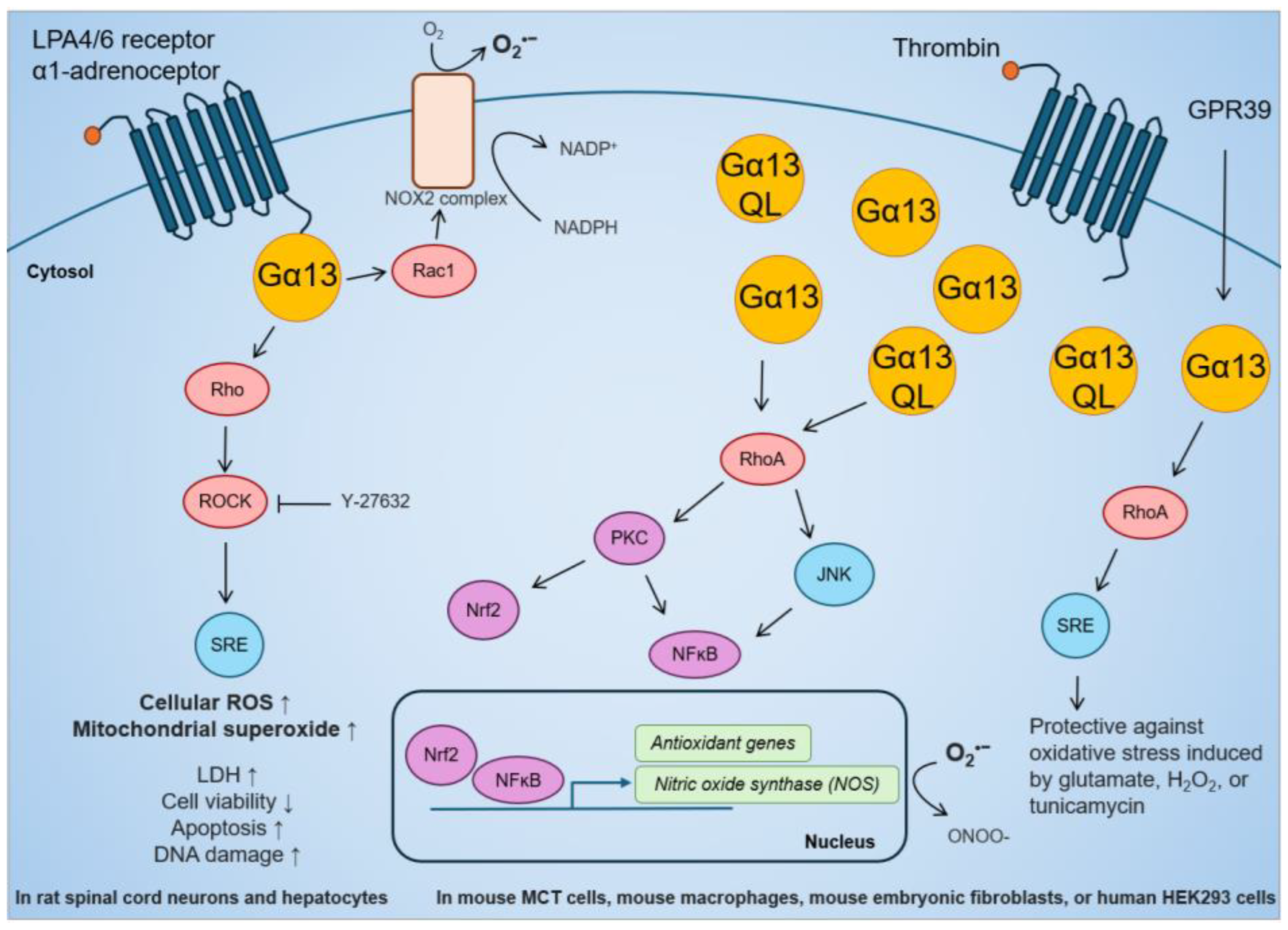
The role of Gα13 in regulating oxidative stress in human cancer is only now beginning to be studied. Current knowledge on the link between Gα13 and oxidative stress is mostly from mouse and rat studies (Figure 3). While it has been reported that GPCR activation of Gα13 can increase oxidative stress, most studies have reached the opposite conclusion. In regard to the first, LPA stimulation in rat spinal cord neurons increased ROS detected by 2′,7′–dichlorofluorescin diacetate (DCFDA) and MitoSOX Red Staining, which was suppressed by ROCK inhibition [169]. This is supported by a study in rat hepatocytes in which stimulation of the GPCR α1-adrenoceptor activated the NADPH oxidase 2 (NOX2) complex via Gα13-Rac1 [170]. NOX2 transports electrons from NADPH to O2 and generates superoxide, which is rapidly converted into H2O2 [170]. In terms of the second, stable overexpression of Gα13 or Gα13(Q226L) in the mouse renal proximal tubule MCT cell line increased inducible nitric oxide synthase (iNOS) activity through mRNA transcription [171]. In mouse macrophage Raw264.7 cells, iNOS activity was induced by thrombin stimulation and Gα13(Q226L) expression [172]. iNOS catalyzes the production of nitric oxide (NO) that can react with superoxide to form peroxynitrite (ONOO-), which is a strong reactive nitrogen species (RNS) that has been shown to promote immune evasion in cancer cells [173]. The scope of this review does not further cover the role of Gα13 in RNS or nitrosative stress. Moreover, in mouse hippocampal neuronal HT-22 cells and human HEK293 cells, the overexpression of the GPCR GPR39 confers protection via the Gα13-RhoA-serum response element (SRE) pathway against oxidative stress induced by glutamate, H2O2, or tunicamycin [174]. Finally, in mouse embryonic fibroblasts, the expression of constitutively active Gα13(Q226L) mutant promoted Nrf2 activity and antioxidant gene transcription via Rho-protein kinase C-delta (PKCδ)-Nrf2 pathway [175]. Overall, Gα13 is clearly capable of inducing expression of antioxidant genes to suppress oxidative stress. However, the effect of Gα13 on oxidative stress in cancer biology needs to be further explored (see sections below).
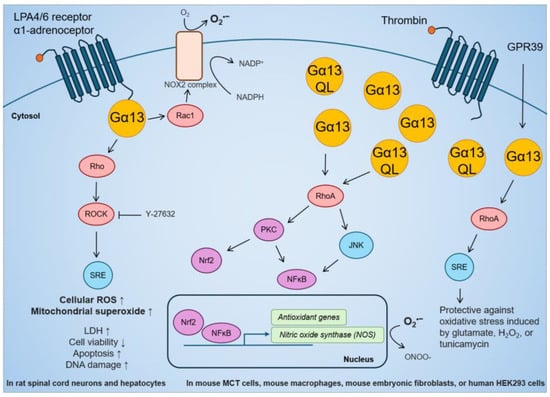
Figure 3.
Effect of GPCR-Gα13 stimulation, overexpression of Gα13, or constitutively active Gα13(Q226L) mutant on cellular ROS, antioxidant gene activation, and resistance against oxidative stress in rat and mouse studies.
3. Prostate Cancer
3.1. Overview
The prostate is an exocrine gland important for male reproduction that responds to the male hormones testosterone and dihydrotestosterone. Hence, androgen deprivation therapy (ADT) is the mainstay of prostate cancer treatment [176,177]. Despite being the second-most commonly diagnosed cancer in males [178,179], most prostate cancers are indolent and have low aggressiveness, with an overall five-year survival of 83–97% [180,181]. However, most ADT-responsive prostate cancers eventually become ADT-resistant, also known as castration-resistant prostate cancer (CRPC), which drastically increases morbidity and mortality with a median survival of 13–30 months [182]. Moreover, aggressive forms of prostate cancer and CRPC are more fatal in men under the age of 50 [183,184,185].
Although there is a strong genetic component to primary prostate cancer, no single driver mutation is solely responsible for tumorigenesis or aggressiveness [186]. In metastatic CRPC, the most frequently altered genes include the androgen receptor gene AR (62.7%), the erythroblast transformation specific (ETS) family (56.7%), tumor protein P53 gene TP53 (53.3%), and phosphatase and tensin homolog gene PTEN (40.7%) [186,187,188]. The AR pathway is activated in all metastatic, androgen-independent CRPC through AR gene amplification, constitutively active mutations or overexpression, as well as mutations in AR coactivators [186,188]. In addition, the protein kinase B (AKT), MAPK and retinoblastoma (Rb) signaling pathways are altered with higher frequencies in metastases compared to primary tumors [188,189]. Crosstalk between these pathways and AR enhances prostate cancer tumor growth [190,191]. AR stimulation activates the Src-rapidly accelerated fibrosarcoma (RAF)-MAPK kinase (MEK) pathway, resulting in MAPK activation [192,193,194,195], while ADT-resistance activates the phosphatidylinositol-3 kinase (PI3K)-AKT-mammalian target of rapamycin (mTOR) pathway, leading to mitogenic growth [196,197,198,199,200,201]. AR signaling also phosphorylates Rb protein controlling cell cycle G1 to S phase progression [202,203,204], and dysfunctional Rb protein increases AR target gene expression [205,206].
Several clinical trials have targeted the aforementioned upregulated signaling pathways, either individually or synergistically with ADT. However, these new therapies have made only slight improvement in prostate-specific antigen (PSA) levels or progression free survival. For example, a phase III trial (IPATential150) evaluating the combination therapy of pan-AKT inhibitor ipatasertib and androgen biosynthesis inhibitor abiraterone found only slight improvement in median radiographic progression free survival (rPFS) of metastatic CRPC patients with PTEN deficiency (18.5 months versus 16.5 months; hazard ratio 0.77; p = 0.0335) [207,208]. In a phase II trial, PI3K/mTOR inhibitor samotolisib + AR inhibitor enzalutamide improved median rPFS than placebo + enzalutamide (10.2 vs. 5.5 months; p = 0.03) [209]. Another phase II trial on PI3K inhibitor buparlisib + enzalutamide improved median PFS compared to buparlisib alone (3.5 vs. 1.9 months) [210]. The phase III READY trial evaluating Src inhibitor dasatinib + anti-mitotic docetaxel versus docetaxel alone had no significant improvement in PSA levels or PFS [211]. Overall, these pathway inhibitors did not demonstrate significant activity in metastatic CRPC, suggesting that they are insufficient to reverse CRPC progression. Hence, exploring new biological mechanisms in prostate cancer is needed.
3.2. Gα13 in Prostate Cancers
Advanced prostate cancers exhibit increased expression of GPCRs such as CXCR4 [117], LPAR1-3 [118,119,212], and PAR-1 [213]. These GPCRs signal through either the Gα12/13 family, or through Gα13 exclusively [55,91]. In addition, stimulation with GPCR ligands such as LPA [214,215,216,217] and thrombin [213] can promote cell growth in both androgen-responsive and androgen-independent prostate cancer cell lines [218,219,220,221,222,223,224,225,226,227,228,229,230]. Moreover, exome sequencing data sets show that the GNA13 gene is amplified in prostate cancer samples at a level of 4% (21/492) in primary adenocarcinoma in The Cancer Genome Atlas (TCGA) and at 28% (123/444) in metastatic CRPC in the SU2C/PCF data set [231,232,233]. GNA13 mRNA levels also positively and significantly correlated to prognostic Gleason scores in the TCGA and SU2C/PCF data sets. Although Gα13 has not been directly linked to AR signaling, the stimulation of RhoA leads to transcriptional activation of AR [234]. Activation of AR is linked to androgen-independence and aggressive phenotypes. Although there is no available data on the immunohistochemistry staining of Gα13 in prostate cancer samples, the protein expression of Gα13 in prostate cancer cell lines correlates with androgen-independence and aggressiveness. Gα13 protein expression in the more tumorigenic, invasive, androgen-independent PC3 and DU145 cells is significantly higher than in the less tumorigenic, non-invasive, androgen-responsive LNCaP cell line [84,130,132]. Hence, Gα13 may play a significant role in prostate cancer biology.
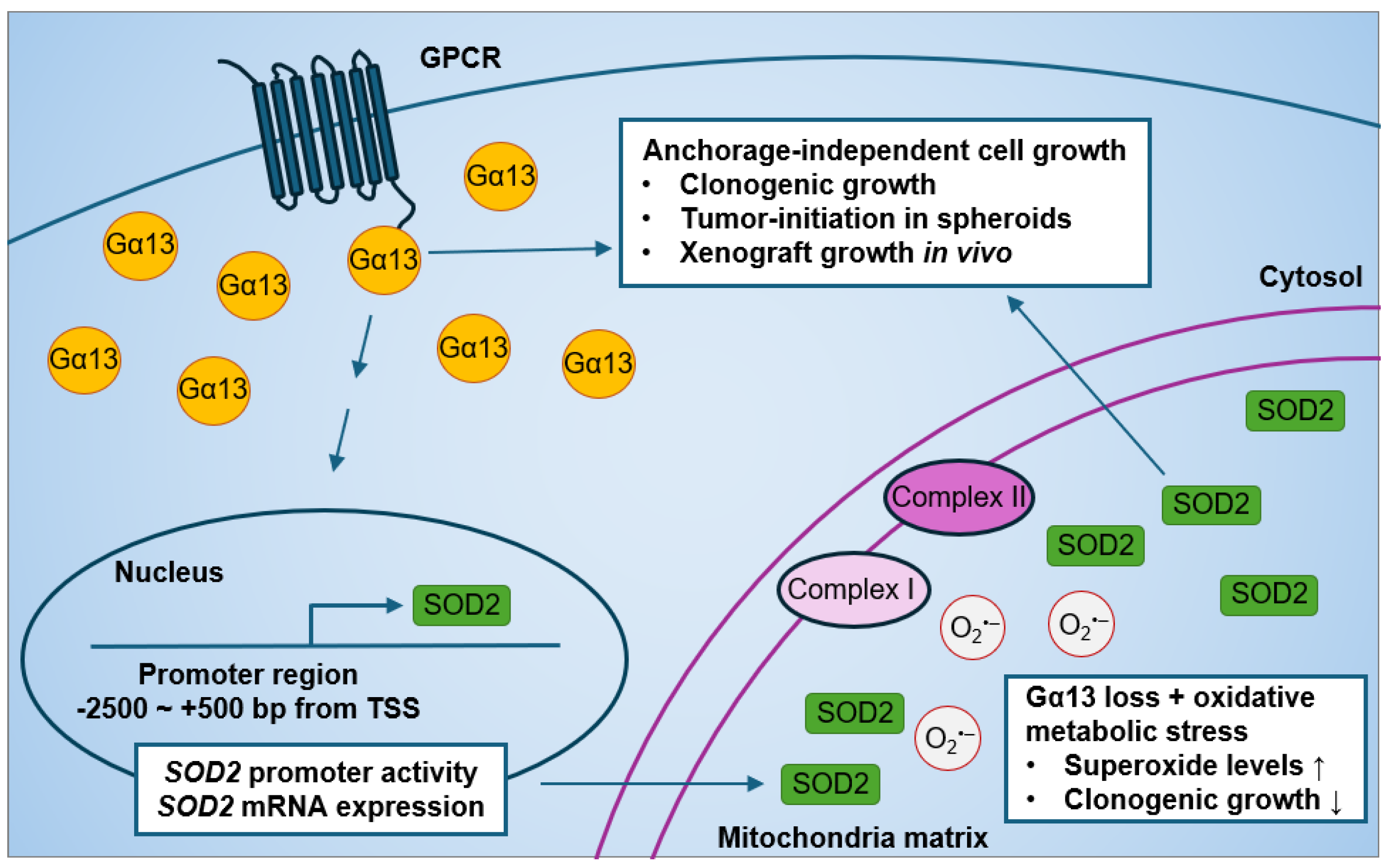
Current evidence on the effect of Gα13 on prostate cancer cell growth is limited to monolayer proliferation. Forced expression of an activated Gα13(Q226L) mutant has no effect on monolayer cell growth as assessed by thymidine incorporation assays after 12 h serum stimulation in both PC3 and Du145 cells [84]. Although the expression of p115-RGS (a dominant-negative inhibitor of both Gα12 and Gα13) in PC3 and Du145 cells suppressed RhoA activation by thrombin stimulation [84], its expression had no effect on short-term monolayer cell growth in PC3 and Du145 cells [84]. Stable expression of p115-RGS in PC3 cells also had no significant change in soft agar colony formation in soft agar or in xenograft tumors [84]. However, it was not known until recently whether silencing endogenous Gα13 rather than overexpressing the constitutively active Gα13(Q226L) mutant (that is not naturally occurring) affects prostate cancer anchorage-independent cell growth. In our recent study, we showed that the knockdown of GNA13 in the high-Gα13-expressing PC3 cells reduced anchorage-independent cell growth in in vitro and in vivo models (Figure 4) [233]. Gα13 loss in PC3 cells suppressed soft agar colony formation, spheroid formation upon serial re-plating, and xenograft tumor growth. Likewise, the overexpression of Gα13 in the low-Gα13-expressing LNCaP cells promoted anchorage-independent cell growth. Hence, modulating wild-type Gα13 expression at endogenous levels in prostate cancer cells affects anchorage-independent cell growth in vitro and tumor growth in mouse xenografts.
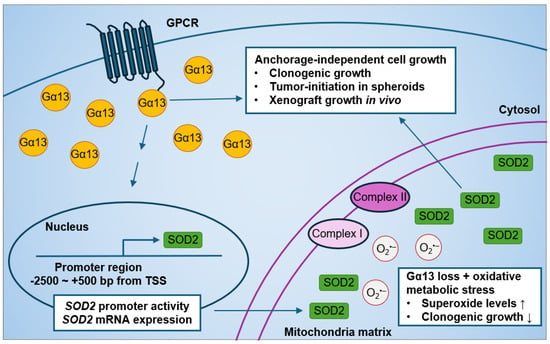
Figure 4.
Effect of modulating Gα13 on mitochondrial SOD2 expression and anchorage-independent cell growth in human prostate cancer cells.
The most well-characterized oncogenic effect of Gα12/13 in prostate cancer is cell migration and invasion. Modulation of Gα13 positively regulated RhoA activation in the prostate cancer cell lines PC3, Du145, and LNCaP [59,84,130,132]. The activated Gα13(Q226L) mutant promoted cell invasion in both PC3 and Du145 cells [84], whereas transient knockdown of GNA13 in PC3 cells suppressed cell migration and invasion [130,132]. Overexpression of Gα13 in LNCaP cells increased invasion to CXCR4 ligand SDF-1/CXCL12 as chemoattractant [130]. In addition, Gα13 promoted Rho-dependent expression of chemokine CXCL5 via the NFκB complex [59]. Hence, it is worthwhile to explore other oncogenic biological processes in which Gα13 may impact.
Interestingly, we recently found a novel role of Gα13 in mitochondrial oxidative metabolic stress response (Figure 4) [233]. The effect of Gα13 loss on oxidative stress was more evident when cells were cultured in glucose-free media supplemented with non-glycolytic metabolites, such as galactose, succinate, glutamate, and malate, which, in turn, increased oxidative metabolic stress in the mitochondria. This effect of Gα13 on superoxide levels was specific to the mitochondria, as little to no change in cytosolic superoxide levels were detected in PC3 cells. Phenotypically, increased oxidative metabolic stress induced by non-glycolytic metabolites had no significant effect on colony formation in PC3 control cells. However, GNA13 knockdown resulted in decreased soft agar colony formation when cells were cultured in galactose, succinate, glutamate, and malate as compared to glucose culture. These results suggest that Gα13 loss increases sensitivity to oxidative metabolic stress-induced non-glycolytic metabolites in PC3 cells.
3.3. Oxidative Stress in Prostate Cancer
Oxidative stress plays an important role in prostate cancer initiation and progression. The expression of antioxidant enzymes SOD1, SOD2, and catalase are lower in prostate cancer than in normal tissue [235]. Oxidative stress also crosstalks with AR signaling and is involved in the development of androgen-independent CRPC [236,237,238]. Androgen deprivation and silencing of AR increases intracellular ROS, which, in turn, promotes AR expression and signaling resulting in androgen-independence [238]. In patients, prostate cancer tissue specimens from patients who have received ADT had higher lipid peroxidation oxidative damage compared to those from patients who have not received ADT [239]. On the other hand, it seems that after prostate cancer cells become androgen-independent, the increased expression of antioxidant enzymes lowers intracellular ROS. Proteomics analysis of androgen-independent versus androgen-dependent LNCaP cells showed a higher expression of antioxidant enzymes such as antioxidant protein 2, SOD1, thioredoxin peroxidase, and peroxiredoxin-2 [240,241]. Moreover, androgen-resistant 22RV1 cells had lower intracellular H2O2 levels compared to control cells both before and after irradiation [242]. In addition, polymorphisms in the antioxidant SOD2, glutathione S-transferase mu 1 and 3 (GSTM1 and GSTM3), glutathione S-transferase pi 1 (GSTP1), catalase (CAT), and paraoxonase 1 (PON1) genes have been associated with increased susceptibility to prostate cancer or increased risk of progression to metastatic CRPC [243,244]. Although a link between ROS in the form of H2O2 is linked to prostate cancer progression, less is known about the effect of ROS in the form of superoxide and of SOD enzymes on prostate cancer.
Mitochondrial SOD2 is associated with prostate cancer risk, whereas no significant association was detected for cytoplasmic/nuclear SOD1 or extracellular SOD3 [245]. In patient samples, SOD2 protein levels were significantly increased in tumor (Gleason 3–9) compared to hyperplasic or control tissues [246] and correlated with prostate cancer patient Gleason scores [247]. Control sample and low-grade tumor (Gleason 5) had much lower SOD2 levels than medium-grade tumors (Gleason 7), and high-grade tumors (Gleason 8) had even higher SOD2 protein levels. When androgen-responsive LNCaP cells were stimulated to neuroendocrine differentiation, the process also increased SOD2 protein levels [247]. Meta-analyses and other studies have concluded that SOD2 (rs4880) polymorphism increases prostate cancer risk and aggressiveness [244,248,249,250]. Moreover, men homozygous for SOD2 (rs4880) have increased odds for high-grade tumors [251,252]. However, homozygous SOD2 (rs4880) was not predictive of prostate cancer recurrence [253] or overall survival [248,254] after radical prostatectomy. In addition, intratumoral SOD2 levels should be distinguished from serum SOD2 levels [255]. Given the relevance of SOD2 in prostate cancer patients, the role of mitochondrial SOD2 in prostate cancer tumorigenesis needs to be explored.
In addition to the roles of Gα13 in anchorage-independent cell growth and mitochondrial oxidative metabolic stress response in prostate cancer cells, we recently also found that modulating Gα13 levels impacted mitochondria SOD2 expression (Figure 4) [233]. Of note, both PC3 and LNCaP harbor the SOD2 (rs4880) polymorphism [256]. First, we found strong positive correlations between GNA13 and SOD2 mRNA expression in The Cancer Genome Atlas database. This led to the finding that Gα13 lower-expressing LNCaP cells have lower SOD2 protein levels compared to Gα13 higher-expressing PC3 cells, which is consistent with previous studies [256,257]. Most importantly, we showed that knockdown of GNA13 in PC3 cells reduced SOD2 protein expression in the mitochondria, as well as SOD2 mRNA expression and promoter activity. Likewise, the overexpression of Gα13 in LNCaP cells increased mitochondrial SOD2 expression. In contrast, modulating Gα13 in prostate cancer cells had no impact on SOD1 expression. Hence, it seems clear that Gα13 regulates SOD2 expression in prostate cancer cells.
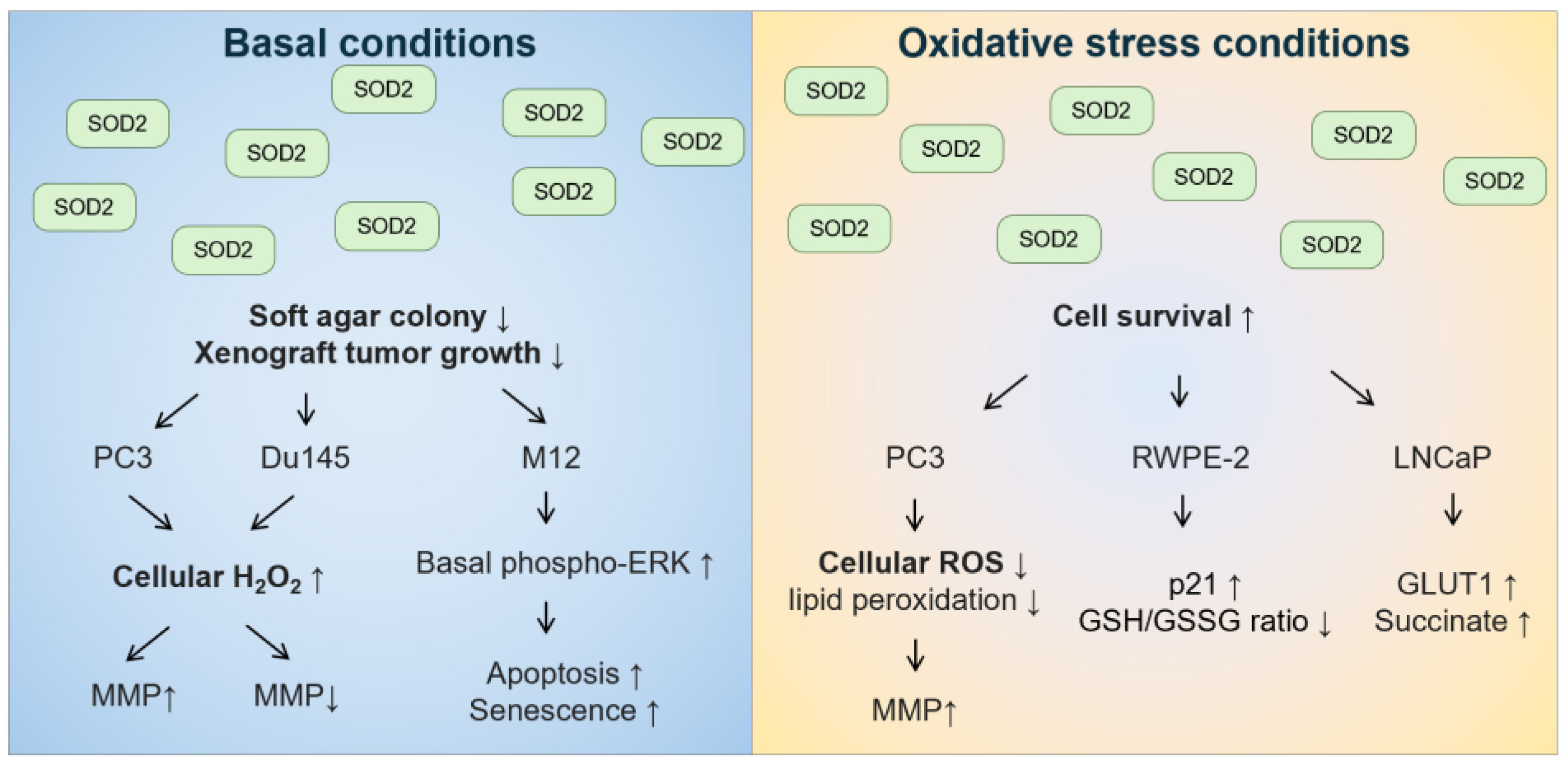
With regard to role of the mitochondrial SOD2 in prostate cancer tumorigenesis, the in vitro data are conflicting (Figure 5). Under basal conditions, the overexpression of wild-type SOD2 suppressed soft agar colony formation or xenograft tumor growth in androgen-independent PC3 [258] and Du145 cells [259], as well as in androgen-responsive M12 cells [260]. In these cell lines, overexpression of SOD2 or treatment with SOD2-mimetic resulted in changes in cell cycle with a delay in the G1 phase or a decrease in the G2+M phase [258,260,261]. Although the decreased growth rate was not due to necrosis, apoptosis, or senescence in PC3 cells [258], increased apoptosis and senescence were observed in M12 cells overexpressing SOD2 [260]. M12 cells overexpressing SOD2 also had increased basal levels of phosphorylated extracellular signal-regulated kinase (ERK) [260]. In both PC3 and Du145 cells, overexpression of wild-type SOD2 increased H2O2 levels [258,259]. Differences between PC3 and Du145 cells included that overexpression of SOD2 in the mitochondria increased mitochondrial membrane potential in PC3 cells [258,262] but decreased mitochondrial membrane potential in Du145 cells [259]. In contrast, under oxidative stress conditions, such as hyperthermia, PC3 cells with overexpression of SOD2 had better survival than control cells [262]. In addition, androgen-responsive RWPE-2 cells overexpressing wild-type SOD2 were less sensitive to high levels of sodium selenite that generate superoxide, but not to glutathione-depleting agent buthionine sulfoximine [263]. Moreover, androgen-responsive LNCaP cells overexpressing wild-type SOD2 were more resistant to glucose deprivation-induced cell death, whereby glucose deprivation can increase mitochondrial superoxide levels [264]. Hence, these data suggest that the effect of SOD2 on prostate cancer tumorigenesis should be distinguished between basal conditions and oxidative stress conditions, especially for conditions that increase superoxide stress.
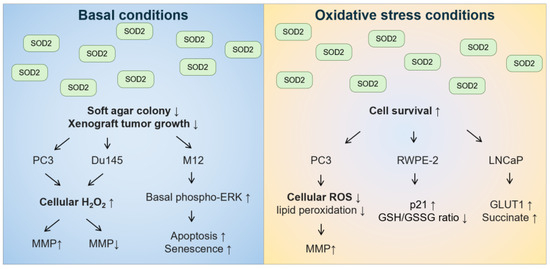
Figure 5.
The effect of overexpressing SOD2 on anchorage-independent cell growth and cell survival under basal and oxidative stress conditions in human prostate cancer cell lines.
As mentioned above, the knockdown of GNA13 in PC3 cells reduced soft agar colony formation, suppressed mitochondrial SOD2 expression, and increased mitochondrial superoxide levels (Figure 4) [233]. The phenotypes displayed by SOD2 loss induced by GNA13 knockdown were more evident under oxidative stress conditions. For example, the increase in mitochondrial superoxide levels induced by GNA13 knockdown was more pronounced when cells were cultured in oxidative metabolic stress conditions (i.e., non-glycolytic metabolites). Moreover, mitochondrial oxidative metabolic stress further suppressed in colony formation that was already suppressed by Gα13 loss, and, in turn, SOD2 loss, in PC3 cells. Our data further validate that Gα13 and mitochondrial SOD2 promote cell survival under oxidative stress conditions in prostate cancer cells.
Many studies on antioxidants and prostate cancer have focused on suppressing ROS to, in turn, suppress AR expression and signaling. Natural antioxidants such as carotenoids can suppress prostate-specific antigen levels [265,266], and tocopherols improved overall prostate cancer survival in randomized, controlled clinical trials [267]. In in vitro studies, the antioxidant N-acetyl cysteine reduced AR expression, and along with ADT, it decreased xenograft tumor growth in androgen-responsive LNCaP and 22Rv1 cells [239]. SOD mimetics such as 4-hydroxy-2,2,6,6-tetramethylpiperidin-1-oxyl (TEMPOL), manganese(III) tetrakis(4-benzoic acid)porphyrin (MnTBAP) and manganese(III) tetrakis(1-methyl-4-pyridyl)porphyrin (MnTMPyP) reduced AR expression and decreased cell viability in androgen-responsive LNCaP, CWR22Rv1, and LAPC-4AD cells [268]. However, TEMPOL had little effect on androgen-independent PC3 cells [268]. Hence, it is necessary to further explore the effect of SOD2 in androgen-independent prostate cancer cells and to describe the effect of silencing SOD2, in contrast to overexpression, on prostate cancer growth.
It now seems clear that loss of Gα13 suppresses anchorage-independent cell growth in multiple prostate cancer cell lines. In this regard, in PC3 cells in which GNA13 was silenced, the effect of Gα13 loss on suppression of anchorage-independent cell growth was rescued by overexpression of SOD2 in a fashion dependent on the catalytic activity of SOD2, since colony formation was not rescued when a catalytically inactive SOD2(Q167A) mutant was expressed. In support of this, anchorage-independent cell growth was attenuated upon SOD2 knockdown in Gα13-overexpressed LNCaP cells. Furthermore, at the time of tumor injection in the xenograft mouse model, PC3 cells in which GNA13 was knocked down had approximately 40% lower SOD2 protein levels compared to control cells; this corresponded to a 40% reduction in xenograft tumor growth in GNA13 knockdown cells compared to control cells. Overall, these studies highlight a novel biological route of Gα13-mediated anchorage-independent growth and response to oxidative metabolic stress through regulation of SOD2 expression in prostate cancer cells.
4. Conclusions
Prostate cancers harbor aberrant GPCR-Gα13 signaling, which has been linked to oncogenic transformation, cancer cell growth, migration, and invasion. Modulating wild-type Gα13 expression at endogenous levels in prostate cancer cells affects anchorage-independent cell growth in vitro and tumor growth in vivo. Furthermore, a novel role of Gα13 was recently found in mitochondrial oxidative metabolic stress response. Although a link between Gα13 and oxidative stress has been shown in animal studies, the data in human biology was lacking until now. While ROS in the form of H2O2 has been linked to prostate cancer progression, much less is known about the effect of ROS in the form of superoxide and of SOD enzymes on prostate cancer. Now that Gα13 has been implicated in mitochondrial SOD2 expression in prostate cancer cells, which has been shown to correlate with prostate cancer risk and prognostic Gleason score, it is clear that the role of Gα13 and mitochondrial SOD2 in prostate cancer tumorigenesis needs to be further explored.
Author Contributions
Conceptualization, D.W. and P.J.C.; writing—original draft, D.W.; writing—review and editing, P.J.C.; supervision, P.J.C.; funding acquisition, P.J.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by Ministry of Education, Singapore, MOE2018-T2-1-147.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Arang, N.; Gutkind, J.S. G Protein-Coupled receptors and heterotrimeric G proteins as cancer drivers. FEBS Lett. 2020, 594, 4201–4232. [Google Scholar] [CrossRef]
- Majumdar, S.; Chiu, Y.T.; Pickett, J.E.; Roth, B.L. Illuminating the understudied GPCR-ome. Drug Discov. Today 2023, 29, 103848. [Google Scholar] [CrossRef] [PubMed]
- Fasciani, I.; Carli, M.; Petragnano, F.; Colaianni, F.; Aloisi, G.; Maggio, R.; Scarselli, M.; Rossi, M. GPCRs in Intracellular Compartments: New Targets for Drug Discovery. Biomolecules 2022, 12, 1343. [Google Scholar] [CrossRef]
- Liccardo, F.; Luini, A.; Di Martino, R. Endomembrane-Based Signaling by GPCRs and G-Proteins. Cells 2022, 11, 528. [Google Scholar] [CrossRef]
- Young, D.; Waitches, G.; Birchmeier, C.; Fasano, O.; Wigler, M. Isolation and characterization of a new cellular oncogene encoding a protein with multiple potential transmembrane domains. Cell 1986, 45, 711–719. [Google Scholar] [CrossRef]
- Julius, D.; Livelli, T.J.; Jessell, T.M.; Axel, R. Ectopic expression of the serotonin 1c receptor and the triggering of malignant transformation. Science 1989, 244, 1057–1062. [Google Scholar] [CrossRef] [PubMed]
- Gutkind, J.S.; Novotny, E.A.; Brann, M.R.; Robbins, K.C. Muscarinic acetylcholine receptor subtypes as agonist-dependent oncogenes. Proc. Natl. Acad. Sci. USA 1991, 88, 4703–4707. [Google Scholar] [CrossRef] [PubMed]
- Allen, L.F.; Lefkowitz, R.J.; Caron, M.G.; Cotecchia, S. G-protein-coupled receptor genes as protooncogenes: Constitutively activating mutation of the alpha 1B-adrenergic receptor enhances mitogenesis and tumorigenicity. Proc. Natl. Acad. Sci. USA 1991, 88, 11354–11358. [Google Scholar] [CrossRef]
- O’Hayre, M.; Vázquez-Prado, J.; Kufareva, I.; Stawiski, E.W.; Handel, T.M.; Seshagiri, S.; Gutkind, J.S. The emerging mutational landscape of G proteins and G-protein-coupled receptors in cancer. Nat. Rev. Cancer 2013, 13, 412–424. [Google Scholar] [CrossRef]
- Parma, J.; Duprez, L.; Van Sande, J.; Cochaux, P.; Gervy, C.; Mockel, J.; Dumont, J.; Vassart, G. Somatic mutations in the thyrotropin receptor gene cause hyperfunctioning thyroid adenomas. Nature 1993, 365, 649–651. [Google Scholar] [CrossRef]
- Lynch, J.R.; Wang, J.Y. G Protein-Coupled Receptor Signaling in Stem Cells and Cancer. Int. J. Mol. Sci. 2016, 17, 707. [Google Scholar] [CrossRef] [PubMed]
- Syrovatkina, V.; Alegre, K.O.; Dey, R.; Huang, X.Y. Regulation, Signaling, and Physiological Functions of G-Proteins. J. Mol. Biol. 2016, 428, 3850–3868. [Google Scholar] [CrossRef] [PubMed]
- Milligan, G.; Kostenis, E. Heterotrimeric G-proteins: A short history. Br. J. Pharmacol. 2006, 147 (Suppl. 1), S46–S55. [Google Scholar] [CrossRef] [PubMed]
- Oldham, W.M.; Hamm, H.E. Heterotrimeric G protein activation by G-protein-coupled receptors. Nat. Rev. Mol. Cell Biol. 2008, 9, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Patwardhan, A.; Cheng, N.; Trejo, J. Post-Translational Modifications of G Protein-Coupled Receptors Control Cellular Signaling Dynamics in Space and Time. Pharmacol. Rev. 2021, 73, 120–151. [Google Scholar] [CrossRef] [PubMed]
- Taussig, R.; Iñiguez-Lluhi, J.A.; Gilman, A.G. Inhibition of adenylyl cyclase by Gi alpha. Science 1993, 261, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Graziano, M.P.; Casey, P.J.; Gilman, A.G. Expression of cDNAs for G proteins in Escherichia coli. Two forms of Gs alpha stimulate adenylate cyclase. J. Biol. Chem. 1987, 262, 11375–11381. [Google Scholar] [CrossRef]
- Duhe, R.J.; Dittman, A.H.; Wu, Z.; Storm, D.R. Regulation of the mammalian adenylyl cyclases. In Biomembranes: A Multi-Volume Treatise; Lee, A.G., Ed.; JAI Press Inc.: Greenwich, CT, USA, 1997; Volume 6, pp. 107–128. [Google Scholar]
- Smrcka, A.V.; Hepler, J.R.; Brown, K.O.; Sternweis, P.C. Regulation of polyphosphoinositide-specific phospholipase C activity by purified Gq. Science 1991, 251, 804–807. [Google Scholar] [CrossRef]
- Posner, B.A.; Mukhopadhyay, S.; Tesmer, J.J.; Gilman, A.G.; Ross, E.M. Modulation of the affinity and selectivity of RGS protein interaction with G alpha subunits by a conserved asparagine/serine residue. Biochemistry 1999, 38, 7773–7779. [Google Scholar] [CrossRef]
- Roy, A.A.; Baragli, A.; Bernstein, L.S.; Hepler, J.R.; Hébert, T.E.; Chidiac, P. RGS2 interacts with Gs and adenylyl cyclase in living cells. Cell. Signal. 2006, 18, 336–348. [Google Scholar] [CrossRef]
- Schoeber, J.P.; Topala, C.N.; Wang, X.; Diepens, R.J.; Lambers, T.T.; Hoenderop, J.G.; Bindels, R.J. RGS2 inhibits the epithelial Ca2+ channel TRPV6. J. Biol. Chem. 2006, 281, 29669–29674. [Google Scholar] [CrossRef] [PubMed]
- De Vries, L.; Zheng, B.; Fischer, T.; Elenko, E.; Farquhar, M.G. The regulator of G protein signaling family. Annu. Rev. Pharmacol. Toxicol. 2000, 40, 235–271. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Wade, S.M.; Woolf, P.J.; Linderman, J.J.; Traynor, J.R.; Neubig, R.R. A spatial focusing model for G protein signals. Regulator of G protein signaling (RGS) protien-mediated kinetic scaffolding. J. Biol. Chem. 2003, 278, 7278–7284. [Google Scholar] [CrossRef]
- Strathmann, M.P.; Simon, M.I. G alpha 12 and G alpha 13 subunits define a fourth class of G protein alpha subunits. Proc. Natl. Acad. Sci. USA 1991, 88, 5582–5586. [Google Scholar] [CrossRef] [PubMed]
- Harhammer, R.; Nürnberg, B.; Harteneck, C.; Leopoldt, D.; Exner, T.; Schultz, G. Distinct biochemical properties of the native members of the G12 G-protein subfamily. Characterization of G alpha 12 purified from rat brain. Biochem. J. 1996, 319, 165–171. [Google Scholar] [CrossRef]
- Dutt, P.; Nguyen, N.; Toksoz, D. Role of Lbc RhoGEF in Galpha12/13-induced signals to Rho GTPase. Cell. Signal. 2004, 16, 201–209. [Google Scholar] [CrossRef]
- Kozasa, T.; Jiang, X.; Hart, M.J.; Sternweis, P.M.; Singer, W.D.; Gilman, A.G.; Bollag, G.; Sternweis, P.C. p115 RhoGEF, a GTPase activating protein for Galpha12 and Galpha13. Science 1998, 280, 2109–2111. [Google Scholar] [CrossRef]
- Hart, M.J.; Jiang, X.; Kozasa, T.; Roscoe, W.; Singer, W.D.; Gilman, A.G.; Sternweis, P.C.; Bollag, G. Direct stimulation of the guanine nucleotide exchange activity of p115 RhoGEF by Galpha13. Science 1998, 280, 2112–2114. [Google Scholar] [CrossRef] [PubMed]
- Fukuhara, S.; Chikumi, H.; Gutkind, J.S. Leukemia-associated Rho guanine nucleotide exchange factor (LARG) links heterotrimeric G proteins of the G(12) family to Rho. FEBS Lett. 2000, 485, 183–188. [Google Scholar] [CrossRef]
- Wirth, A.; Benyó, Z.; Lukasova, M.; Leutgeb, B.; Wettschureck, N.; Gorbey, S.; Orsy, P.; Horváth, B.; Maser-Gluth, C.; Greiner, E.; et al. G12-G13-LARG-mediated signaling in vascular smooth muscle is required for salt-induced hypertension. Nat. Med. 2008, 14, 64–68. [Google Scholar] [CrossRef]
- Suzuki, N.; Nakamura, S.; Mano, H.; Kozasa, T. Galpha 12 activates Rho GTPase through tyrosine-phosphorylated leukemia-associated RhoGEF. Proc. Natl. Acad. Sci. USA 2003, 100, 733–738. [Google Scholar] [CrossRef]
- Fukuhara, S.; Murga, C.; Zohar, M.; Igishi, T.; Gutkind, J.S. A novel PDZ domain containing guanine nucleotide exchange factor links heterotrimeric G proteins to Rho. J. Biol. Chem. 1999, 274, 5868–5879. [Google Scholar] [CrossRef]
- Mikelis, C.M.; Palmby, T.R.; Simaan, M.; Li, W.; Szabo, R.; Lyons, R.; Martin, D.; Yagi, H.; Fukuhara, S.; Chikumi, H.; et al. PDZ-RhoGEF and LARG are essential for embryonic development and provide a link between thrombin and LPA receptors and Rho activation. J. Biol. Chem. 2013, 288, 12232–12243. [Google Scholar] [CrossRef]
- Buhl, A.M.; Johnson, N.L.; Dhanasekaran, N.; Johnson, G.L. G alpha 12 and G alpha 13 stimulate Rho-dependent stress fiber formation and focal adhesion assembly. J. Biol. Chem. 1995, 270, 24631–24634. [Google Scholar] [CrossRef]
- Katoh, H.; Aoki, J.; Yamaguchi, Y.; Kitano, Y.; Ichikawa, A.; Negishi, M. Constitutively active Galpha12, Galpha13, and Galphaq induce Rho-dependent neurite retraction through different signaling pathways. J. Biol. Chem. 1998, 273, 28700–28707. [Google Scholar] [CrossRef]
- Yuan, J.; Slice, L.W.; Rozengurt, E. Activation of protein kinase D by signaling through Rho and the alpha subunit of the heterotrimeric G protein G13. J. Biol. Chem. 2001, 276, 38619–38627. [Google Scholar] [CrossRef]
- Slice, L.W.; Walsh, J.H.; Rozengurt, E. Galpha(13) stimulates Rho-dependent activation of the cyclooxygenase-2 promoter. J. Biol. Chem. 1999, 274, 27562–27566. [Google Scholar] [CrossRef]
- Yuan, B.; Cui, J.; Wang, W.; Deng, K. Gα12/13 signaling promotes cervical cancer invasion through the RhoA/ROCK-JNK signaling axis. Biochem. Biophys. Res. Commun. 2016, 473, 1240–1246. [Google Scholar] [CrossRef]
- Hooley, R.; Yu, C.Y.; Symons, M.; Barber, D.L. G alpha 13 stimulates Na+-H+ exchange through distinct Cdc42-dependent and RhoA-dependent pathways. J. Biol. Chem. 1996, 271, 6152–6158. [Google Scholar] [CrossRef]
- Lee, S.J.; Yang, J.W.; Cho, I.J.; Kim, W.D.; Cho, M.K.; Lee, C.H.; Kim, S.G. The gep oncogenes, Galpha(12) and Galpha(13), upregulate the transforming growth factor-beta1 gene. Oncogene 2009, 28, 1230–1240. [Google Scholar] [CrossRef]
- Hersch, E.; Huang, J.; Grider, J.R.; Murthy, K.S. Gq/G13 signaling by ET-1 in smooth muscle: MYPT1 phosphorylation via ETA and CPI-17 dephosphorylation via ETB. Am. J. Physiol. Cell Physiol. 2004, 287, C1209–C1218. [Google Scholar] [CrossRef]
- Fiedler, S.E.; Bajpai, M.; Carr, D.W. Identification and characterization of RHOA-interacting proteins in bovine spermatozoa. Biol. Reprod. 2008, 78, 184–192. [Google Scholar] [CrossRef]
- Yu, F.X.; Zhao, B.; Panupinthu, N.; Jewell, J.L.; Lian, I.; Wang, L.H.; Zhao, J.; Yuan, H.; Tumaneng, K.; Li, H.; et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell 2012, 150, 780–791. [Google Scholar] [CrossRef]
- Kim, M.; Kim, M.; Lee, S.; Kuninaka, S.; Saya, H.; Lee, H.; Lee, S.; Lim, D.S. cAMP/PKA signalling reinforces the LATS-YAP pathway to fully suppress YAP in response to actin cytoskeletal changes. EMBO J. 2013, 32, 1543–1555. [Google Scholar] [CrossRef]
- Prasad, M.V.; Dermott, J.M.; Heasley, L.E.; Johnson, G.L.; Dhanasekaran, N. Activation of Jun kinase/stress-activated protein kinase by GTPase-deficient mutants of G alpha 12 and G alpha 13. J. Biol. Chem. 1995, 270, 18655–18659. [Google Scholar] [CrossRef]
- Collins, L.R.; Minden, A.; Karin, M.; Brown, J.H. Galpha12 stimulates c-Jun NH2-terminal kinase through the small G proteins Ras and Rac. J. Biol. Chem. 1996, 271, 17349–17353. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Voyno-Yasenetskaya, T.A.; Hooley, R.; Lin, C.Y.; Orlowski, J.; Barber, D.L. Galpha12 differentially regulates Na+-H+ exchanger isoforms. J. Biol. Chem. 1996, 271, 22604–22610. [Google Scholar] [CrossRef] [PubMed]
- Voyno-Yasenetskaya, T.A.; Pace, A.M.; Bourne, H.R. Mutant alpha subunits of G12 and G13 proteins induce neoplastic transformation of Rat-1 fibroblasts. Oncogene 1994, 9, 2559–2565. [Google Scholar] [PubMed]
- Jho, E.H.; Davis, R.J.; Malbon, C.C. c-Jun amino-terminal kinase is regulated by Galpha12/Galpha13 and obligate for differentiation of P19 embryonal carcinoma cells by retinoic acid. J. Biol. Chem. 1997, 272, 24468–24474. [Google Scholar] [CrossRef]
- Lopez, I.; Mak, E.C.; Ding, J.; Hamm, H.E.; Lomasney, J.W. A novel bifunctional phospholipase c that is regulated by Galpha 12 and stimulates the Ras/mitogen-activated protein kinase pathway. J. Biol. Chem. 2001, 276, 2758–2765. [Google Scholar] [CrossRef]
- Yuan, J.; Slice, L.W.; Gu, J.; Rozengurt, E. Cooperation of Gq, Gi, and G12/13 in protein kinase D activation and phosphorylation induced by lysophosphatidic acid. J. Biol. Chem. 2003, 278, 4882–4891. [Google Scholar] [CrossRef] [PubMed]
- Dusaban, S.S.; Purcell, N.H.; Rockenstein, E.; Masliah, E.; Cho, M.K.; Smrcka, A.V.; Brown, J.H. Phospholipase C epsilon links G protein-coupled receptor activation to inflammatory astrocytic responses. Proc. Natl. Acad. Sci. USA 2013, 110, 3609–3614. [Google Scholar] [CrossRef] [PubMed]
- Dhanasekaran, N.; Prasad, M.V.; Wadsworth, S.J.; Dermott, J.M.; van Rossum, G. Protein kinase C-dependent and -independent activation of Na+/H+ exchanger by G alpha 12 class of G proteins. J. Biol. Chem. 1994, 269, 11802–11806. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Tai, Y.; Wang, M.; Sun, H.; Zhang, L.; Wei, W.; Xiang, Y.K.; Wang, Q. Gα(12) and Gα(13): Versatility in Physiology and Pathology. Front. Cell Dev. Biol. 2022, 10, 809425. [Google Scholar] [CrossRef] [PubMed]
- Voyno-Yasenetskaya, T.; Conklin, B.R.; Gilbert, R.L.; Hooley, R.; Bourne, H.R.; Barber, D.L. G alpha 13 stimulates Na-H exchange. J. Biol. Chem. 1994, 269, 4721–4724. [Google Scholar] [CrossRef]
- Yagi, H.; Onoyama, I.; Asanoma, K.; Hori, E.; Yasunaga, M.; Kodama, K.; Kijima, M.; Ohgami, T.; Kaneki, E.; Okugawa, K.; et al. Gα(13)-mediated LATS1 down-regulation contributes to epithelial-mesenchymal transition in ovarian cancer. FASEB J. 2019, 33, 13683–13694. [Google Scholar] [CrossRef]
- Ki, S.H.; Choi, M.J.; Lee, C.H.; Kim, S.G. Galpha12 specifically regulates COX-2 induction by sphingosine 1-phosphate. Role for JNK-dependent ubiquitination and degradation of IkappaBalpha. J. Biol. Chem. 2007, 282, 1938–1947. [Google Scholar] [CrossRef]
- Lim, W.K.; Chai, X.; Ghosh, S.; Ray, D.; Wang, M.; Rasheed, S.A.K.; Casey, P.J. Gα-13 induces CXC motif chemokine ligand 5 expression in prostate cancer cells by transactivating NF-κB. J. Biol. Chem. 2019, 294, 18192–18206. [Google Scholar] [CrossRef]
- Aragay, A.M.; Collins, L.R.; Post, G.R.; Watson, A.J.; Feramisco, J.R.; Brown, J.H.; Simon, M.I. G12 requirement for thrombin-stimulated gene expression and DNA synthesis in 1321N1 astrocytoma cells. J. Biol. Chem. 1995, 270, 20073–20077. [Google Scholar] [CrossRef]
- Mao, J.; Yuan, H.; Xie, W.; Simon, M.I.; Wu, D. Specific involvement of G proteins in regulation of serum response factor-mediated gene transcription by different receptors. J. Biol. Chem. 1998, 273, 27118–27123. [Google Scholar] [CrossRef]
- Fromm, C.; Coso, O.A.; Montaner, S.; Xu, N.; Gutkind, J.S. The small GTP-binding protein Rho links G protein-coupled receptors and Galpha12 to the serum response element and to cellular transformation. Proc. Natl. Acad. Sci. USA 1997, 94, 10098–10103. [Google Scholar] [CrossRef] [PubMed]
- Song, M.K.; Park, C.; Lee, Y.D.; Kim, H.; Kim, M.K.; Kwon, J.O.; Koo, J.H.; Joo, M.S.; Kim, S.G.; Kim, H.H. Gα12 regulates osteoclastogenesis by modulating NFATc1 expression. J. Cell. Mol. Med. 2018, 22, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Chen, W.; Lu, Y.; Zhu, G.; Hao, L.; Li, Y.P. Gα13 negatively controls osteoclastogenesis through inhibition of the Akt-GSK3β-NFATc1 signalling pathway. Nat. Commun. 2017, 8, 13700. [Google Scholar] [CrossRef]
- Kumar, R.N.; Shore, S.K.; Dhanasekaran, N. Neoplastic transformation by the gep oncogene, Galpha12, involves signaling by STAT3. Oncogene 2006, 25, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Maziarz, M.; Federico, A.; Zhao, J.; Dujmusic, L.; Zhao, Z.; Monti, S.; Varelas, X.; Garcia-Marcos, M. Naturally occurring hotspot cancer mutations in Gα(13) promote oncogenic signaling. J. Biol. Chem. 2020, 295, 16897–16904. [Google Scholar] [CrossRef]
- Dettlaff-Swiercz, D.A.; Wettschureck, N.; Moers, A.; Huber, K.; Offermanns, S. Characteristic defects in neural crest cell-specific Galphaq/Galpha11- and Galpha12/Galpha13-deficient mice. Dev. Biol. 2005, 282, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Jho, E.H.; Malbon, C.C. Galpha12 and Galpha13 mediate differentiation of P19 mouse embryonal carcinoma cells in response to retinoic acid. J. Biol. Chem. 1997, 272, 24461–24467. [Google Scholar] [CrossRef]
- Xie, S.; Mason, F.M.; Martin, A.C. Loss of Gα12/13 exacerbates apical area dependence of actomyosin contractility. Mol. Biol. Cell 2016, 27, 3526–3536. [Google Scholar] [CrossRef]
- Xu, J.; Wang, F.; Van Keymeulen, A.; Herzmark, P.; Straight, A.; Kelly, K.; Takuwa, Y.; Sugimoto, N.; Mitchison, T.; Bourne, H.R. Divergent signals and cytoskeletal assemblies regulate self-organizing polarity in neutrophils. Cell 2003, 114, 201–214. [Google Scholar] [CrossRef]
- Sivaraj, K.K.; Takefuji, M.; Schmidt, I.; Adams, R.H.; Offermanns, S.; Wettschureck, N. G13 controls angiogenesis through regulation of VEGFR-2 expression. Dev. Cell 2013, 25, 427–434. [Google Scholar] [CrossRef]
- Offermanns, S.; Laugwitz, K.L.; Spicher, K.; Schultz, G. G proteins of the G12 family are activated via thromboxane A2 and thrombin receptors in human platelets. Proc. Natl. Acad. Sci. USA 1994, 91, 504–508. [Google Scholar] [CrossRef] [PubMed]
- Nakano, S.; Inoue, K.; Xu, C.; Deng, Z.; Syrovatkina, V.; Vitone, G.; Zhao, L.; Huang, X.Y.; Zhao, B. G-protein Gα(13) functions as a cytoskeletal and mitochondrial regulator to restrain osteoclast function. Sci. Rep. 2019, 9, 4236. [Google Scholar] [CrossRef] [PubMed]
- Healy, J.A.; Nugent, A.; Rempel, R.E.; Moffitt, A.B.; Davis, N.S.; Jiang, X.; Shingleton, J.R.; Zhang, J.; Love, C.; Datta, J.; et al. GNA13 loss in germinal center B cells leads to impaired apoptosis and promotes lymphoma in vivo. Blood 2016, 127, 2723–2731. [Google Scholar] [CrossRef]
- Rieken, S.; Sassmann, A.; Herroeder, S.; Wallenwein, B.; Moers, A.; Offermanns, S.; Wettschureck, N. G12/G13 family G proteins regulate marginal zone B cell maturation, migration, and polarization. J. Immunol. 2006, 177, 2985–2993. [Google Scholar] [CrossRef] [PubMed]
- Kranenburg, O.; Poland, M.; van Horck, F.P.; Drechsel, D.; Hall, A.; Moolenaar, W.H. Activation of RhoA by lysophosphatidic acid and Galpha12/13 subunits in neuronal cells: Induction of neurite retraction. Mol. Biol. Cell 1999, 10, 1851–1857. [Google Scholar] [CrossRef] [PubMed]
- Moers, A.; Nürnberg, A.; Goebbels, S.; Wettschureck, N.; Offermanns, S. Galpha12/Galpha13 deficiency causes localized overmigration of neurons in the developing cerebral and cerebellar cortices. Mol. Cell. Biol. 2008, 28, 1480–1488. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Wu, D.; Simon, M.I. The transforming activity of activated G alpha 12. FEBS Lett. 1993, 330, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Bradley, L.; Ambdukar, I.; Gutkind, J.S. A mutant alpha subunit of G12 potentiates the eicosanoid pathway and is highly oncogenic in NIH 3T3 cells. Proc. Natl. Acad. Sci. USA 1993, 90, 6741–6745. [Google Scholar] [CrossRef]
- Berestetskaya, Y.V.; Faure, M.P.; Ichijo, H.; Voyno-Yasenetskaya, T.A. Regulation of apoptosis by alpha-subunits of G12 and G13 proteins via apoptosis signal-regulating kinase-1. J. Biol. Chem. 1998, 273, 27816–27823. [Google Scholar] [CrossRef]
- Rasheed, S.A.K.; Leong, H.S.; Lakshmanan, M.; Raju, A.; Dadlani, D.; Chong, F.T.; Shannon, N.B.; Rajarethinam, R.; Skanthakumar, T.; Tan, E.Y.; et al. GNA13 expression promotes drug resistance and tumor-initiating phenotypes in squamous cell cancers. Oncogene 2018, 37, 1340–1353. [Google Scholar] [CrossRef]
- Althoefer, H.; Eversole-Cire, P.; Simon, M.I. Constitutively active Galphaq and Galpha13 trigger apoptosis through different pathways. J. Biol. Chem. 1997, 272, 24380–24386. [Google Scholar] [CrossRef] [PubMed]
- Kelly, P.; Moeller, B.J.; Juneja, J.; Booden, M.A.; Der, C.J.; Daaka, Y.; Dewhirst, M.W.; Fields, T.A.; Casey, P.J. The G12 family of heterotrimeric G proteins promotes breast cancer invasion and metastasis. Proc. Natl. Acad. Sci. USA 2006, 103, 8173–8178. [Google Scholar] [CrossRef] [PubMed]
- Kelly, P.; Stemmle, L.N.; Madden, J.F.; Fields, T.A.; Daaka, Y.; Casey, P.J. A role for the G12 family of heterotrimeric G proteins in prostate cancer invasion. J. Biol. Chem. 2006, 281, 26483–26490. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, N.; Ogata, T.; Naito, D.; Miyagawa, K.; Taniguchi, T.; Hamaoka, T.; Maruyama, N.; Kasahara, T.; Nishi, M.; Matoba, S.; et al. MURC deficiency in smooth muscle attenuates pulmonary hypertension. Nat. Commun. 2016, 7, 12417. [Google Scholar] [CrossRef] [PubMed]
- Offermanns, S.; Mancino, V.; Revel, J.P.; Simon, M.I. Vascular system defects and impaired cell chemokinesis as a result of Galpha13 deficiency. Science 1997, 275, 533–536. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.L.; Müller, S.; Mancino, V.; Offermanns, S.; Simon, M.I. Interaction of G alpha(12) with G alpha(13) and G alpha(q) signaling pathways. Proc. Natl. Acad. Sci. USA 2002, 99, 9352–9357. [Google Scholar] [CrossRef]
- Offermanns, S. In vivo functions of heterotrimeric G-proteins: Studies in Galpha-deficient mice. Oncogene 2001, 20, 1635–1642. [Google Scholar] [CrossRef]
- Yamazaki, J.; Katoh, H.; Yamaguchi, Y.; Negishi, M. Two G12 family G proteins, G alpha12 and G alpha13, show different subcellular localization. Biochem. Biophys. Res. Commun. 2005, 332, 782–786. [Google Scholar] [CrossRef] [PubMed]
- Riobo, N.A.; Manning, D.R. Receptors coupled to heterotrimeric G proteins of the G12 family. Trends Pharmacol. Sci. 2005, 26, 146–154. [Google Scholar] [CrossRef]
- Mackenzie, A.E.; Quon, T.; Lin, L.C.; Hauser, A.S.; Jenkins, L.; Inoue, A.; Tobin, A.B.; Gloriam, D.E.; Hudson, B.D.; Milligan, G. Receptor selectivity between the G proteins Gα(12) and Gα(13) is defined by a single leucine-to-isoleucine variation. FASEB J. 2019, 33, 5005–5017. [Google Scholar] [CrossRef]
- Tall, G.G.; Krumins, A.M.; Gilman, A.G. Mammalian Ric-8A (synembryn) is a heterotrimeric Galpha protein guanine nucleotide exchange factor. J. Biol. Chem. 2003, 278, 8356–8362. [Google Scholar] [CrossRef] [PubMed]
- Takesono, A.; Cismowski, M.J.; Ribas, C.; Bernard, M.; Chung, P.; Hazard, S., 3rd; Duzic, E.; Lanier, S.M. Receptor-independent activators of heterotrimeric G-protein signaling pathways. J. Biol. Chem. 1999, 274, 33202–33205. [Google Scholar] [CrossRef] [PubMed]
- Shan, D.; Chen, L.; Wang, D.; Tan, Y.C.; Gu, J.L.; Huang, X.Y. The G protein G alpha(13) is required for growth factor-induced cell migration. Dev. Cell 2006, 10, 707–718. [Google Scholar] [CrossRef]
- Qu, C.; Liu, Y.; Kunkalla, K.; Singh, R.R.; Blonska, M.; Lin, X.; Agarwal, N.K.; Vega, F. Trimeric G protein-CARMA1 axis links smoothened, the hedgehog receptor transducer, to NF-κB activation in diffuse large B-cell lymphoma. Blood 2013, 121, 4718–4728. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Zhao, X.; O’Brien, K.A.; Stojanovic-Terpo, A.; Delaney, M.K.; Kim, K.; Cho, J.; Lam, S.C.; Du, X. A directional switch of integrin signalling and a new anti-thrombotic strategy. Nature 2013, 503, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Estevez, B.; Xu, Z.; Kreutz, B.; Karginov, A.; Bai, Y.; Qian, F.; Norifumi, U.; Mosher, D.; Du, X. The interaction of Gα13 with integrin β1 mediates cell migration by dynamic regulation of RhoA. Mol. Biol. Cell 2015, 26, 3658–3670. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Delaney, M.K.; Du, X. Inside-out, outside-in, and inside-outside-in: G protein signaling in integrin-mediated cell adhesion, spreading, and retraction. Curr. Opin. Cell Biol. 2012, 24, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Shen, B.; Flevaris, P.; Chow, C.; Lam, S.C.; Voyno-Yasenetskaya, T.A.; Kozasa, T.; Du, X. G protein subunit Galpha13 binds to integrin alphaIIbbeta3 and mediates integrin "outside-in" signaling. Science 2010, 327, 340–343. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.M.; Fleming, T.P.; McGovern, E.S.; Chedid, M.; Miki, T.; Aaronson, S.A. Expression cDNA cloning of a transforming gene encoding the wild-type G alpha 12 gene product. Mol. Cell Biol. 1993, 13, 762–768. [Google Scholar] [CrossRef]
- Xu, N.; Voyno-Yasenetskaya, T.; Silvio Gutkind, J. Potent transforming activity of the G13 α subunit defines a novel family of oncogenes. 1994, 201, 603–609. [CrossRef]
- Kelly, P.; Casey, P.J.; Meigs, T.E. Biologic functions of the G12 subfamily of heterotrimeric G proteins: Growth, migration, and metastasis. Biochemistry 2007, 46, 6677–6687. [Google Scholar] [CrossRef]
- Dorsam, R.T.; Gutkind, J.S. G-protein-coupled receptors and cancer. Nat. Rev. Cancer 2007, 7, 79–94. [Google Scholar] [CrossRef] [PubMed]
- Juneja, J.; Casey, P.J. Role of G12 proteins in oncogenesis and metastasis. Br. J. Pharmacol. 2009, 158, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Radhika, V.; Dhanasekaran, N. Transforming G proteins. Oncogene 2001, 20, 1607–1614. [Google Scholar] [CrossRef] [PubMed]
- Syrovatkina, V.; Huang, X.Y. Signaling mechanisms and physiological functions of G-protein Gα(13) in blood vessel formation, bone homeostasis, and cancer. Protein Sci. 2019, 28, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Vara Prasad, M.V.; Shore, S.K.; Dhanasekaran, N. Activated mutant of G alpha 13 induces Egr-1, c-fos, and transformation in NIH 3T3 cells. Oncogene 1994, 9, 2425–2429. [Google Scholar] [PubMed]
- O’Hayre, M.; Inoue, A.; Kufareva, I.; Wang, Z.; Mikelis, C.M.; Drummond, R.A.; Avino, S.; Finkel, K.; Kalim, K.W.; DiPasquale, G.; et al. Inactivating mutations in GNA13 and RHOA in Burkitt’s lymphoma and diffuse large B-cell lymphoma: A tumor suppressor function for the Gα13/RhoA axis in B cells. Oncogene 2016, 35, 3771–3780. [Google Scholar] [CrossRef] [PubMed]
- Muppidi, J.R.; Schmitz, R.; Green, J.A.; Xiao, W.; Larsen, A.B.; Braun, S.E.; An, J.; Xu, Y.; Rosenwald, A.; Ott, G.; et al. Loss of signalling via Gα13 in germinal centre B-cell-derived lymphoma. Nature 2014, 516, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Wels, C.; Beham-Schmid, C.; Fukunaga-Kalabis, M.; Holmen, S.L.; Otte, M.; Herlyn, M.; Waldhoer, M.; Schaider, H. Gα13 mediates human cytomegalovirus-encoded chemokine receptor US28-induced cell death in melanoma. Int. J. Cancer 2015, 137, 1503–1508. [Google Scholar] [CrossRef] [PubMed]
- Bartolomé, R.A.; Wright, N.; Molina-Ortiz, I.; Sánchez-Luque, F.J.; Teixidó, J. Activated G(alpha)13 impairs cell invasiveness through p190RhoGAP-mediated inhibition of RhoA activity. Cancer Res. 2008, 68, 8221–8230. [Google Scholar] [CrossRef]
- Liu, Y.; Du, Z.; Xu, Z.; Jin, T.; Xu, K.; Huang, M.; Wang, S.; Zheng, Y.; Liu, M.; Xu, H. Overexpressed GNA13 induces temozolomide sensitization via down-regulating MGMT and p-RELA in glioma. Am. J. Transl. Res. 2021, 13, 11413–11426. [Google Scholar]
- Shields, M.A.; Spaulding, C.; Metropulos, A.E.; Khalafalla, M.G.; Pham, T.N.D.; Munshi, H.G. Gα13 loss in Kras/Tp53 mouse model of pancreatic tumorigenesis promotes tumors susceptible to rapamycin. Cell Rep. 2022, 38, 110441. [Google Scholar] [CrossRef] [PubMed]
- AACR Project GENIE Consortium. AACR Project GENIE: Powering Precision Medicine through an International Consortium. Cancer Discov. 2017, 7, 818–831. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.B.; Mahon, G.M.; Klinger, M.B.; Kay, R.J.; Symons, M.; Der, C.J.; Whitehead, I.P. The thrombin receptor, PAR-1, causes transformation by activation of Rho-mediated signaling pathways. Oncogene 2001, 20, 1953–1963. [Google Scholar] [CrossRef] [PubMed]
- Marinissen, M.J.; Servitja, J.M.; Offermanns, S.; Simon, M.I.; Gutkind, J.S. Thrombin protease-activated receptor-1 signals through Gq- and G13-initiated MAPK cascades regulating c-Jun expression to induce cell transformation. J. Biol. Chem. 2003, 278, 46814–46825. [Google Scholar] [CrossRef]
- Sun, Y.X.; Wang, J.; Shelburne, C.E.; Lopatin, D.E.; Chinnaiyan, A.M.; Rubin, M.A.; Pienta, K.J.; Taichman, R.S. Expression of CXCR4 and CXCL12 (SDF-1) in human prostate cancers (PCa) in vivo. J. Cell. Biochem. 2003, 89, 462–473. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Gibbs, T.C.; Mukhin, Y.V.; Meier, K.E. Role for 18:1 lysophosphatidic acid as an autocrine mediator in prostate cancer cells. J. Biol. Chem. 2002, 277, 32516–32526. [Google Scholar] [CrossRef] [PubMed]
- Ward, Y.; Lake, R.; Yin, J.J.; Heger, C.D.; Raffeld, M.; Goldsmith, P.K.; Merino, M.; Kelly, K. LPA receptor heterodimerizes with CD97 to amplify LPA-initiated RHO-dependent signaling and invasion in prostate cancer cells. Cancer Res. 2011, 71, 7301–7311. [Google Scholar] [CrossRef] [PubMed]
- Feng, F.; Han, H.; Wu, S.; Wang, H. Crosstalk Between Abnormal TSHR Signaling Activation and PTEN/PI3K in the Dedifferentiation of Thyroid Cancer Cells. Front. Oncol. 2021, 11, 718578. [Google Scholar] [CrossRef]
- Zhang, J.X.; Yun, M.; Xu, Y.; Chen, J.W.; Weng, H.W.; Zheng, Z.S.; Chen, C.; Xie, D.; Ye, S. GNA13 as a prognostic factor and mediator of gastric cancer progression. Oncotarget 2016, 7, 4414–4427. [Google Scholar] [CrossRef]
- Xu, Y.; Rong, J.; Duan, S.; Chen, C.; Li, Y.; Peng, B.; Yi, B.; Zheng, Z.; Gao, Y.; Wang, K.; et al. High expression of GNA13 is associated with poor prognosis in hepatocellular carcinoma. Sci. Rep. 2016, 6, 35948. [Google Scholar] [CrossRef]
- Pan, Z.; Zheng, Z.; Ye, W.; Chen, C.; Ye, S. Overexpression of GNA13 correlates with poor prognosis in esophageal squamous cell carcinoma after esophagectomy. Int. J. Biol. Markers 2022, 37, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Gentles, A.J.; Newman, A.M.; Liu, C.L.; Bratman, S.V.; Feng, W.; Kim, D.; Nair, V.S.; Xu, Y.; Khuong, A.; Hoang, C.D.; et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat. Med. 2015, 21, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Na, J.; Zhou, W.; Yin, M.; Hu, Y.; Ma, X. GNA13 promotes the proliferation and migration of lung squamous cell carcinoma cells through regulating the PI3K/AKT signaling pathway. Tissue Cell 2022, 76, 101795. [Google Scholar] [CrossRef] [PubMed]
- Grzelinski, M.; Pinkenburg, O.; Büch, T.; Gold, M.; Stohr, S.; Kalwa, H.; Gudermann, T.; Aigner, A. Critical role of G(alpha)12 and G(alpha)13 for human small cell lung cancer cell proliferation in vitro and tumor growth in vivo. Clin. Cancer Res. 2010, 16, 1402–1415. [Google Scholar] [CrossRef]
- Voyno-Yasenetskaya, T.A.; Faure, M.P.; Ahn, N.G.; Bourne, H.R. Galpha12 and Galpha13 regulate extracellular signal-regulated kinase and c-Jun kinase pathways by different mechanisms in COS-7 cells. J. Biol. Chem. 1996, 271, 21081–21087. [Google Scholar] [CrossRef]
- Castellone, M.D.; Laukkanen, M.O.; Teramoto, H.; Bellelli, R.; Alì, G.; Fontanini, G.; Santoro, M.; Gutkind, J.S. Cross talk between the bombesin neuropeptide receptor and Sonic hedgehog pathways in small cell lung carcinoma. Oncogene 2015, 34, 1679–1687. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, S.A.; Teo, C.R.; Beillard, E.J.; Voorhoeve, P.M.; Zhou, W.; Ghosh, S.; Casey, P.J. MicroRNA-31 controls G protein alpha-13 (GNA13) expression and cell invasion in breast cancer cells. Mol. Cancer 2015, 14, 67. [Google Scholar] [CrossRef]
- Rasheed, S.A.K.; Teo, C.R.; Beillard, E.J.; Voorhoeve, P.M.; Casey, P.J. MicroRNA-182 and microRNA-200a control G-protein subunit α-13 (GNA13) expression and cell invasion synergistically in prostate cancer cells. J. Biol. Chem. 2013, 288, 7986–7995. [Google Scholar] [CrossRef]
- Ha, J.H.; Gomathinayagam, R.; Yan, M.; Jayaraman, M.; Ramesh, R.; Dhanasekaran, D.N. Determinant role for the gep oncogenes, Gα12/13, in ovarian cancer cell proliferation and xenograft tumor growth. Genes. Cancer 2015, 6, 356–364. [Google Scholar] [CrossRef][Green Version]
- El-Haibi, C.P.; Sharma, P.; Singh, R.; Gupta, P.; Taub, D.D.; Singh, S.; Lillard, J.W., Jr. Differential G protein subunit expression by prostate cancer cells and their interaction with CXCR5. Mol. Cancer 2013, 12, 64. [Google Scholar] [CrossRef]
- Bian, D.; Mahanivong, C.; Yu, J.; Frisch, S.M.; Pan, Z.K.; Ye, R.D.; Huang, S. The G12/13-RhoA signaling pathway contributes to efficient lysophosphatidic acid-stimulated cell migration. Oncogene 2006, 25, 2234–2244. [Google Scholar] [CrossRef] [PubMed]
- Gardner, J.A.; Ha, J.H.; Jayaraman, M.; Dhanasekaran, D.N. The gep proto-oncogene Gα13 mediates lysophosphatidic acid-mediated migration of pancreatic cancer cells. Pancreas 2013, 42, 819–828. [Google Scholar] [CrossRef]
- Chow, C.R.; Ebine, K.; Knab, L.M.; Bentrem, D.J.; Kumar, K.; Munshi, H.G. Cancer Cell Invasion in Three-dimensional Collagen Is Regulated Differentially by Gα13 Protein and Discoidin Domain Receptor 1-Par3 Protein Signaling. J. Biol. Chem. 2016, 291, 1605–1618. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, S.; Tang, Q.; Wei, L.; Zhang, Q.; Wang, G.; Muhammad, B.U.; Kaur, K.; Kamchedalova, T.; Gang, Z.; Jiang, Z.; et al. miRNA-30d serves a critical function in colorectal cancer initiation, progression and invasion via directly targeting the GNA13 gene. Exp. Ther. Med. 2019, 17, 260–272. [Google Scholar] [CrossRef]
- Patel, M.; Kawano, T.; Suzuki, N.; Hamakubo, T.; Karginov, A.V.; Kozasa, T. Gα13/PDZ-RhoGEF/RhoA signaling is essential for gastrin-releasing peptide receptor-mediated colon cancer cell migration. Mol. Pharmacol. 2014, 86, 252–262. [Google Scholar] [CrossRef]
- Yang, Y.M.; Lee, S.; Nam, C.W.; Ha, J.H.; Jayaraman, M.; Dhanasekaran, D.N.; Lee, C.H.; Kwak, M.K.; Kim, S.G. G(alpha)12/13 inhibition enhances the anticancer effect of bortezomib through PSMB5 downregulation. Carcinogenesis 2010, 31, 1230–1237. [Google Scholar] [CrossRef]
- Zhang, H.H.; Zhang, Z.Y.; Che, C.L.; Mei, Y.F.; Shi, Y.Z. Array analysis for potential biomarker of gemcitabine identification in non-small cell lung cancer cell lines. Int. J. Clin. Exp. Pathol. 2013, 6, 1734–1746. [Google Scholar] [PubMed]
- Xu, B.; Huang, Y.; Niu, X.; Tao, T.; Jiang, L.; Tong, N.; Chen, S.; Liu, N.; Zhu, W.; Chen, M. Hsa-miR-146a-5p modulates androgen-independent prostate cancer cells apoptosis by targeting ROCK1. Prostate 2015, 75, 1896–1903. [Google Scholar] [CrossRef]
- Abe, H.; Kamai, T.; Hayashi, K.; Anzai, N.; Shirataki, H.; Mizuno, T.; Yamaguchi, Y.; Masuda, A.; Yuki, H.; Betsunoh, H.; et al. The Rho-kinase inhibitor HA-1077 suppresses proliferation/migration and induces apoptosis of urothelial cancer cells. BMC Cancer 2014, 14, 412. [Google Scholar] [CrossRef]
- Li, F.; Jiang, Q.; Shi, K.J.; Luo, H.; Yang, Y.; Xu, C.M. RhoA modulates functional and physical interaction between ROCK1 and Erk1/2 in selenite-induced apoptosis of leukaemia cells. Cell Death Dis. 2013, 4, e708. [Google Scholar] [CrossRef]
- Rasheed, S.A.K.; Subramanyan, L.V.; Lim, W.K.; Udayappan, U.K.; Wang, M.; Casey, P.J. The emerging roles of Gα12/13 proteins on the hallmarks of cancer in solid tumors. Oncogene 2022, 41, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Andreeva, A.V.; Kutuzov, M.A.; Voyno-Yasenetskaya, T.A. G alpha12 is targeted to the mitochondria and affects mitochondrial morphology and motility. FASEB J. 2008, 22, 2821–2831. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.H.; Kim, T.H.; Park, S.Y.; Joo, M.S.; Han, C.Y.; Choi, C.S.; Kim, S.G. Gα13 ablation reprograms myofibers to oxidative phenotype and enhances whole-body metabolism. J. Clin. Investig. 2017, 127, 3845–3860. [Google Scholar] [CrossRef]
- Kim, T.H.; Koo, J.H.; Heo, M.J.; Han, C.Y.; Kim, Y.I.; Park, S.Y.; Cho, I.J.; Lee, C.H.; Choi, C.S.; Lee, J.W.; et al. Overproduction of inter-α-trypsin inhibitor heavy chain 1 after loss of Gα(13) in liver exacerbates systemic insulin resistance in mice. Sci. Transl. Med. 2019, 11, eaan4735. [Google Scholar] [CrossRef] [PubMed]
- Sabbir, M.G.; Calcutt, N.A.; Fernyhough, P. Muscarinic Acetylcholine Type 1 Receptor Activity Constrains Neurite Outgrowth by Inhibiting Microtubule Polymerization and Mitochondrial Trafficking in Adult Sensory Neurons. Front. Neurosci. 2018, 12, 402. [Google Scholar] [CrossRef]
- Pedro, M.P.; Lund, K.; Kang, S.W.S.; Chen, T.; Stuelten, C.H.; Porat-Shliom, N.; Iglesias-Bartolome, R. GPCR screening reveals that the metabolite receptor HCAR3 regulates epithelial proliferation, migration, and cellular respiration. J. Investig. Dermatol. 2024, 144, 1311–1321.e7. [Google Scholar] [CrossRef]
- Kim, S.; Sieburth, D. FSHR-1/GPCR Regulates the Mitochondrial Unfolded Protein Response in Caenorhabditis elegans. Genetics 2020, 214, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Sun, W.; Fu, C.; Nazmi, K.; Veerman, E.C.I.; Jaspers, R.T.; Bolscher, J.G.M.; Bikker, F.J.; Wu, G. GPCR/endocytosis/ERK signaling/S2R is involved in the regulation of the internalization, mitochondria-targeting and -activating properties of human salivary histatin 1. Int. J. Oral. Sci. 2022, 14, 42. [Google Scholar] [CrossRef]
- Ishii, M.; Beeson, G.; Beeson, C.; Rohrer, B. Mitochondrial C3a Receptor Activation in Oxidatively Stressed Epithelial Cells Reduces Mitochondrial Respiration and Metabolism. Front. Immunol. 2021, 12, 628062. [Google Scholar] [CrossRef]
- Picard, M.; Shirihai, O.S. Mitochondrial signal transduction. Cell Metab. 2022, 34, 1620–1653. [Google Scholar] [CrossRef]
- Rabe, P.; Liebing, A.D.; Krumbholz, P.; Kraft, R.; Stäubert, C. Succinate receptor 1 inhibits mitochondrial respiration in cancer cells addicted to glutamine. Cancer Lett. 2022, 526, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Robert, A.; Crottès, D.; Bourgeais, J.; Gueguen, N.; Chevrollier, A.; Dumas, J.-F.; Servais, S.; Domingo, I.; Chadet, S.; Sobilo, J.; et al. Encoding of the colorectal cancer metabolic program through MICU2. bioRxiv 2023. [Google Scholar] [CrossRef]
- Kim, N.Y.; Ha, I.J.; Um, J.Y.; Kumar, A.P.; Sethi, G.; Ahn, K.S. Loganic acid regulates the transition between epithelial and mesenchymal-like phenotypes by alleviating MnSOD expression in hepatocellular carcinoma cells. Life Sci. 2023, 317, 121458. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef]
- Gill, J.G.; Piskounova, E.; Morrison, S.J. Cancer, Oxidative Stress, and Metastasis. Cold Spring Harb. Symp. Quant. Biol. 2016, 81, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Elchuri, S.; Oberley, T.D.; Qi, W.; Eisenstein, R.S.; Jackson Roberts, L.; Van Remmen, H.; Epstein, C.J.; Huang, T.T. CuZnSOD deficiency leads to persistent and widespread oxidative damage and hepatocarcinogenesis later in life. Oncogene 2005, 24, 367–380. [Google Scholar] [CrossRef]
- Van Remmen, H.; Ikeno, Y.; Hamilton, M.; Pahlavani, M.; Wolf, N.; Thorpe, S.R.; Alderson, N.L.; Baynes, J.W.; Epstein, C.J.; Huang, T.T.; et al. Life-long reduction in MnSOD activity results in increased DNA damage and higher incidence of cancer but does not accelerate aging. Physiol. Genomics 2003, 16, 29–37. [Google Scholar] [CrossRef]
- Neumann, C.A.; Krause, D.S.; Carman, C.V.; Das, S.; Dubey, D.P.; Abraham, J.L.; Bronson, R.T.; Fujiwara, Y.; Orkin, S.H.; Van Etten, R.A. Essential role for the peroxiredoxin Prdx1 in erythrocyte antioxidant defence and tumour suppression. Nature 2003, 424, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Gomez, M.; Kwak, M.K.; Dolan, P.M.; Itoh, K.; Yamamoto, M.; Talalay, P.; Kensler, T.W. Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in nrf2 transcription factor-deficient mice. Proc. Natl. Acad. Sci. USA 2001, 98, 3410–3415. [Google Scholar] [CrossRef]
- Ott, M.; Gogvadze, V.; Orrenius, S.; Zhivotovsky, B. Mitochondria, oxidative stress and cell death. Apoptosis 2007, 12, 913–922. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, X.; Zeng, Y.; Mo, X.; Hong, S.; He, H.; Li, J.; Fatima, S.; Liu, Q. Oxidative stress induces mitochondrial iron overload and ferroptotic cell death. Sci. Rep. 2023, 13, 15515. [Google Scholar] [CrossRef]
- Lee, C.; Nam, J.S.; Lee, C.G.; Park, M.; Yoo, C.M.; Rhee, H.W.; Seo, J.K.; Kwon, T.H. Analysing the mechanism of mitochondrial oxidation-induced cell death using a multifunctional iridium(III) photosensitiser. Nat. Commun. 2021, 12, 26. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, V.; Ciccarese, F.; Ciminale, V. Oncogenic pathways and the electron transport chain: A dangeROS liaison. Br. J. Cancer 2020, 122, 168–181. [Google Scholar] [CrossRef]
- Weinberg, F.; Hamanaka, R.; Wheaton, W.W.; Weinberg, S.; Joseph, J.; Lopez, M.; Kalyanaraman, B.; Mutlu, G.M.; Budinger, G.R.; Chandel, N.S. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc. Natl. Acad. Sci. USA 2010, 107, 8788–8793. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Shestov, A.A.; Swain, P.; Yang, C.; Parker, S.J.; Wang, Q.A.; Terada, L.S.; Adams, N.D.; McCabe, M.T.; Pietrak, B.; et al. Reductive carboxylation supports redox homeostasis during anchorage-independent growth. Nature 2016, 532, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Schafer, Z.T.; Grassian, A.R.; Song, L.; Jiang, Z.; Gerhart-Hines, Z.; Irie, H.Y.; Gao, S.; Puigserver, P.; Brugge, J.S. Antioxidant and oncogene rescue of metabolic defects caused by loss of matrix attachment. Nature 2009, 461, 109–113. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, J.; Su, Q.; Wu, Y.; Li, Q.; Ma, Z.; Ding, T. Lysophosphatidic Acid Induced Apoptosis, DNA Damage, and Oxidative Stress in Spinal Cord Neurons by Upregulating LPA4/LPA6 Receptors. Mediators Inflamm. 2022, 2022, 1818758. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Cruz, A.; Vilchis-Landeros, M.M.; Guinzberg, R.; Villalobos-Molina, R.; Piña, E. NOX2 activated by α1-adrenoceptors modulates hepatic metabolic routes stimulated by β-adrenoceptors. Free Radic. Res. 2011, 45, 1366–1378. [Google Scholar] [CrossRef]
- Kitamura, K.; Singer, W.D.; Star, R.A.; Muallem, S.; Miller, R.T. Induction of inducible nitric-oxide synthase by the heterotrimeric G protein Galpha13. J. Biol. Chem. 1996, 271, 7412–7415. [Google Scholar] [CrossRef]
- Kang, K.W.; Choi, S.Y.; Cho, M.K.; Lee, C.H.; Kim, S.G. Thrombin induces nitric-oxide synthase via Galpha12/13-coupled protein kinase C-dependent I-kappaBalpha phosphorylation and JNK-mediated I-kappaBalpha degradation. J. Biol. Chem. 2003, 278, 17368–17378. [Google Scholar] [CrossRef]
- Tcyganov, E.N.; Sanseviero, E.; Marvel, D.; Beer, T.; Tang, H.Y.; Hembach, P.; Speicher, D.W.; Zhang, Q.; Donthireddy, L.R.; Mostafa, A.; et al. Peroxynitrite in the tumor microenvironment changes the profile of antigens allowing escape from cancer immunotherapy. Cancer Cell 2022, 40, 1173–1189.e6. [Google Scholar] [CrossRef]
- Dittmer, S.; Sahin, M.; Pantlen, A.; Saxena, A.; Toutzaris, D.; Pina, A.L.; Geerts, A.; Golz, S.; Methner, A. The constitutively active orphan G-protein-coupled receptor GPR39 protects from cell death by increasing secretion of pigment epithelium-derived growth factor. J. Biol. Chem. 2008, 283, 7074–7081. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.K.; Kim, W.D.; Ki, S.H.; Hwang, J.I.; Choi, S.; Lee, C.H.; Kim, S.G. Role of Galpha12 and Galpha13 as novel switches for the activity of Nrf2, a key antioxidative transcription factor. Mol. Cell Biol. 2007, 27, 6195–6208. [Google Scholar] [CrossRef]
- Schaeffer, E.M.; Srinivas, S.; Adra, N.; An, Y.; Barocas, D.; Bitting, R.; Bryce, A.; Chapin, B.; Cheng, H.H.; D’Amico, A.V.; et al. NCCN Guidelines® Insights: Prostate Cancer, Version 1.2023. J. Natl. Compr. Cancer Netw. 2022, 20, 1288–1298. [Google Scholar] [PubMed]
- Parker, C.; Castro, E.; Fizazi, K.; Heidenreich, A.; Ost, P.; Procopio, G.; Tombal, B.; Gillessen, S. Prostate cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 1119–1134. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Ferlay, J.E.M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today. Available online: https://gco.iarc.who.int/today (accessed on 15 February 2024).
- Allemani, C.; Weir, H.K.; Carreira, H.; Harewood, R.; Spika, D.; Wang, X.S.; Bannon, F.; Ahn, J.V.; Johnson, C.J.; Bonaventure, A.; et al. Global surveillance of cancer survival 1995-2009: Analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet 2015, 385, 977–1010. [Google Scholar] [CrossRef]
- Surveillance Research Program National Cancer Institute. SEER*Explorer: An Interactive Website for SEER Cancer Statistics. Available online: https://seer.cancer.gov/statistics-network/explorer/ (accessed on 15 February 2024).
- Aly, M.; Leval, A.; Schain, F.; Liwing, J.; Lawson, J.; Vágó, E.; Nordström, T.; Andersson, T.M.; Sjöland, E.; Wang, C.; et al. Survival in patients diagnosed with castration-resistant prostate cancer: A population-based observational study in Sweden. Scand. J. Urol. 2020, 54, 115–121. [Google Scholar] [CrossRef]
- Merrill, R.M.; Bird, J.S. Effect of young age on prostate cancer survival: A population-based assessment (United States). Cancer Causes Control. 2002, 13, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.W.; Porter, M.; Montgomery, B. Treatment and survival outcomes in young men diagnosed with prostate cancer: A Population-based Cohort Study. Cancer 2009, 115, 2863–2871. [Google Scholar] [CrossRef]
- Salinas, C.A.; Tsodikov, A.; Ishak-Howard, M.; Cooney, K.A. Prostate cancer in young men: An important clinical entity. Nat. Rev. Urol. 2014, 11, 317–323. [Google Scholar] [CrossRef] [PubMed]
- The Cancer Genome Atlas Research Network. The Molecular Taxonomy of Primary Prostate Cancer. Cell 2015, 163, 1011–1025. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.; Van Allen, E.M.; Wu, Y.M.; Schultz, N.; Lonigro, R.J.; Mosquera, J.M.; Montgomery, B.; Taplin, M.E.; Pritchard, C.C.; Attard, G.; et al. Integrative clinical genomics of advanced prostate cancer. Cell 2015, 161, 1215–1228. [Google Scholar] [CrossRef] [PubMed]
- Taylor, B.S.; Schultz, N.; Hieronymus, H.; Gopalan, A.; Xiao, Y.; Carver, B.S.; Arora, V.K.; Kaushik, P.; Cerami, E.; Reva, B.; et al. Integrative genomic profiling of human prostate cancer. Cancer Cell 2010, 18, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.P.; Amstad, P.; He, P.; Robles, A.; Lupold, S.; Kaneko, I.; Ichimiya, M.; Sengupta, S.; Mechanic, L.; Okamura, S.; et al. p53-induced up-regulation of MnSOD and GPx but not catalase increases oxidative stress and apoptosis. Cancer Res. 2004, 64, 2350–2356. [Google Scholar] [CrossRef] [PubMed]
- Michmerhuizen, A.R.; Spratt, D.E.; Pierce, L.J.; Speers, C.W. ARe we there yet? Understanding androgen receptor signaling in breast cancer. npj Breast Cancer 2020, 6, 47. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.L.; Kyprianou, N. Androgen receptor and growth factor signaling cross-talk in prostate cancer cells. Endocr. Relat. Cancer 2008, 15, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Peterziel, H.; Mink, S.; Schonert, A.; Becker, M.; Klocker, H.; Cato, A.C. Rapid signalling by androgen receptor in prostate cancer cells. Oncogene 1999, 18, 6322–6329. [Google Scholar] [CrossRef]
- Migliaccio, A.; Castoria, G.; Di Domenico, M.; de Falco, A.; Bilancio, A.; Lombardi, M.; Barone, M.V.; Ametrano, D.; Zannini, M.S.; Abbondanza, C.; et al. Steroid-induced androgen receptor-oestradiol receptor beta-Src complex triggers prostate cancer cell proliferation. EMBO J. 2000, 19, 5406–5417. [Google Scholar] [CrossRef]
- Pandini, G.; Mineo, R.; Frasca, F.; Roberts, C.T., Jr.; Marcelli, M.; Vigneri, R.; Belfiore, A. Androgens up-regulate the insulin-like growth factor-I receptor in prostate cancer cells. Cancer Res. 2005, 65, 1849–1857. [Google Scholar] [CrossRef]
- Ware, K.E.; Gupta, S.; Eng, J.; Kemeny, G.; Puviindran, B.J.; Foo, W.-C.; Crawford, L.A.; Almquist, R.G.; Runyambo, D.; Thomas, B.C.; et al. Convergent evolution of p38/MAPK activation in hormone resistant prostate cancer mediates pro-survival, immune evasive, and metastatic phenotypes. bioRxiv 2020. [Google Scholar] [CrossRef]
- Cinar, B.; Mukhopadhyay, N.K.; Meng, G.; Freeman, M.R. Phosphoinositide 3-kinase-independent non-genomic signals transit from the androgen receptor to Akt1 in membrane raft microdomains. J. Biol. Chem. 2007, 282, 29584–29593. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Morales, C.; Cooke, L.S.; Johnson, B.; Somer, B.; Mahadevan, D. Reciprocal feedback inhibition of the androgen receptor and PI3K as a novel therapy for castrate-sensitive and -resistant prostate cancer. Oncotarget 2015, 6, 41976–41987. [Google Scholar] [CrossRef] [PubMed]
- Carver, B.S.; Chapinski, C.; Wongvipat, J.; Hieronymus, H.; Chen, Y.; Chandarlapaty, S.; Arora, V.K.; Le, C.; Koutcher, J.; Scher, H.; et al. Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer Cell 2011, 19, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Lamoureux, F.; Crafter, C.; Davies, B.R.; Beraldi, E.; Fazli, L.; Kim, S.; Thaper, D.; Gleave, M.E.; Zoubeidi, A. Synergistic targeting of PI3K/AKT pathway and androgen receptor axis significantly delays castration-resistant prostate cancer progression in vivo. Mol. Cancer Ther. 2013, 12, 2342–2355. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, S.Y.; Ross, K.N.; Balk, S.P. Androgens induce prostate cancer cell proliferation through mammalian target of rapamycin activation and post-transcriptional increases in cyclin D proteins. Cancer Res. 2006, 66, 7783–7792. [Google Scholar] [CrossRef] [PubMed]
- Mulholland, D.J.; Tran, L.M.; Li, Y.; Cai, H.; Morim, A.; Wang, S.; Plaisier, S.; Garraway, I.P.; Huang, J.; Graeber, T.G.; et al. Cell autonomous role of PTEN in regulating castration-resistant prostate cancer growth. Cancer Cell 2011, 19, 792–804. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, K.E.; Arden, K.C.; Cavenee, W.K. Multiple G1 regulatory elements control the androgen-dependent proliferation of prostatic carcinoma cells. J. Biol. Chem. 1998, 273, 20213–20222. [Google Scholar] [CrossRef]
- Taneja, S.S.; Ha, S.; Garabedian, M.J. Androgen stimulated cellular proliferation in the human prostate cancer cell line LNCaP is associated with reduced retinoblastoma protein expression. J. Cell Biochem. 2001, 84, 188–199. [Google Scholar] [CrossRef]
- Fribourg, A.F.; Knudsen, K.E.; Strobeck, M.W.; Lindhorst, C.M.; Knudsen, E.S. Differential requirements for ras and the retinoblastoma tumor suppressor protein in the androgen dependence of prostatic adenocarcinoma cells. Cell Growth Differ. 2000, 11, 361–372. [Google Scholar]
- Sharma, A.; Yeow, W.S.; Ertel, A.; Coleman, I.; Clegg, N.; Thangavel, C.; Morrissey, C.; Zhang, X.; Comstock, C.E.; Witkiewicz, A.K.; et al. The retinoblastoma tumor suppressor controls androgen signaling and human prostate cancer progression. J. Clin. Investig. 2010, 120, 4478–4492. [Google Scholar] [CrossRef]
- Ku, S.Y.; Rosario, S.; Wang, Y.; Mu, P.; Seshadri, M.; Goodrich, Z.W.; Goodrich, M.M.; Labbé, D.P.; Gomez, E.C.; Wang, J.; et al. Rb1 and Trp53 cooperate to suppress prostate cancer lineage plasticity, metastasis, and antiandrogen resistance. Science 2017, 355, 78–83. [Google Scholar] [CrossRef]
- Sweeney, C.; Bracarda, S.; Sternberg, C.N.; Chi, K.N.; Olmos, D.; Sandhu, S.; Massard, C.; Matsubara, N.; Alekseev, B.; Parnis, F.; et al. Ipatasertib plus abiraterone and prednisolone in metastatic castration-resistant prostate cancer (IPATential150): A multicentre, randomised, double-blind, phase 3 trial. Lancet 2021, 398, 131–142. [Google Scholar] [CrossRef] [PubMed]
- de Bono, J.S.; De Giorgi, U.; Rodrigues, D.N.; Massard, C.; Bracarda, S.; Font, A.; Arranz Arija, J.A.; Shih, K.C.; Radavoi, G.D.; Xu, N.; et al. Randomized Phase II Study Evaluating Akt Blockade with Ipatasertib, in Combination with Abiraterone, in Patients with Metastatic Prostate Cancer with and without PTEN Loss. Clin. Cancer Res. 2019, 25, 928–936. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, C.J.; Percent, I.J.; Babu, S.; Cultrera, J.L.; Mehlhaff, B.A.; Goodman, O.B.; Morris, D.S.; Schnadig, I.D.; Albany, C.; Shore, N.D.; et al. Phase Ib/II Study of Enzalutamide with Samotolisib (LY3023414) or Placebo in Patients with Metastatic Castration-Resistant Prostate Cancer. Clin. Cancer Res. 2022, 28, 2237–2247. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.J.; Halabi, S.; Healy, P.; Alumkal, J.J.; Winters, C.; Kephart, J.; Bitting, R.L.; Hobbs, C.; Soleau, C.F.; Beer, T.M.; et al. Phase II trial of the PI3 kinase inhibitor buparlisib (BKM-120) with or without enzalutamide in men with metastatic castration resistant prostate cancer. Eur. J. Cancer 2017, 81, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Araujo, J.C.; Trudel, G.C.; Saad, F.; Armstrong, A.J.; Yu, E.Y.; Bellmunt, J.; Wilding, G.; McCaffrey, J.; Serrano, S.V.; Matveev, V.B.; et al. Docetaxel and dasatinib or placebo in men with metastatic castration-resistant prostate cancer (READY): A randomised, double-blind phase 3 trial. Lancet Oncol. 2013, 14, 1307–1316. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Kasbohm, E.A.; Arora, P.; Sample, C.J.; Baban, B.; Sud, N.; Sivashanmugam, P.; Moniri, N.H.; Daaka, Y. Expression and function of lysophosphatidic acid LPA1 receptor in prostate cancer cells. Endocrinology 2006, 147, 4883–4892. [Google Scholar] [CrossRef] [PubMed]
- Chay, C.H.; Cooper, C.R.; Gendernalik, J.D.; Dhanasekaran, S.M.; Chinnaiyan, A.M.; Rubin, M.A.; Schmaier, A.H.; Pienta, K.J. A functional thrombin receptor (PAR1) is expressed on bone-derived prostate cancer cell lines. Urology 2002, 60, 760–765. [Google Scholar] [CrossRef]
- Guo, C.; Luttrell, L.M.; Price, D.T. Mitogenic signaling in androgen sensitive and insensitive prostate cancer cell lines. J. Urol. 2000, 163, 1027–1032. [Google Scholar] [CrossRef]
- Kue, P.F.; Daaka, Y. Essential role for G proteins in prostate cancer cell growth and signaling. J. Urol. 2000, 164, 2162–2167. [Google Scholar] [CrossRef]
- Kue, P.F.; Taub, J.S.; Harrington, L.B.; Polakiewicz, R.D.; Ullrich, A.; Daaka, Y. Lysophosphatidic acid-regulated mitogenic ERK signaling in androgen-insensitive prostate cancer PC-3 cells. Int. J. Cancer 2002, 102, 572–579. [Google Scholar] [CrossRef]
- Raj, G.V.; Barki-Harrington, L.; Kue, P.F.; Daaka, Y. Guanosine phosphate binding protein coupled receptors in prostate cancer: A review. J. Urol. 2002, 167, 1458–1463. [Google Scholar] [CrossRef] [PubMed]
- Deeble, P.D.; Murphy, D.J.; Parsons, S.J.; Cox, M.E. Interleukin-6- and cyclic AMP-mediated signaling potentiates neuroendocrine differentiation of LNCaP prostate tumor cells. Mol. Cell Biol. 2001, 21, 8471–8482. [Google Scholar] [CrossRef] [PubMed]
- Aprikian, A.G.; Tremblay, L.; Han, K.; Chevalier, S. Bombesin stimulates the motility of human prostate-carcinoma cells through tyrosine phosphorylation of focal adhesion kinase and of integrin-associated proteins. Int. J. Cancer 1997, 72, 498–504. [Google Scholar] [CrossRef]
- Lin, J.; Freeman, M.R. Transactivation of ErbB1 and ErbB2 receptors by angiotensin II in normal human prostate stromal cells. Prostate 2003, 54, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.F.; Guan, J.; Qiu, Y.; Kung, H.J. Neuropeptide-induced androgen independence in prostate cancer cells: Roles of nonreceptor tyrosine kinases Etk/Bmx, Src, and focal adhesion kinase. Mol. Cell Biol. 2001, 21, 8385–8397. [Google Scholar] [CrossRef] [PubMed]
- Taub, J.S.; Guo, R.; Leeb-Lundberg, L.M.; Madden, J.F.; Daaka, Y. Bradykinin receptor subtype 1 expression and function in prostate cancer. Cancer Res. 2003, 63, 2037–2041. [Google Scholar]
- Barki-Harrington, L.; Daaka, Y. Bradykinin induced mitogenesis of androgen independent prostate cancer cells. J. Urol. 2001, 165, 2121–2125. [Google Scholar] [CrossRef]
- Barki-Harrington, L.; Bookout, A.L.; Wang, G.; Lamb, M.E.; Leeb-Lundberg, L.M.; Daaka, Y. Requirement for direct cross-talk between B1 and B2 kinin receptors for the proliferation of androgen-insensitive prostate cancer PC3 cells. Biochem. J. 2003, 371, 581–587. [Google Scholar] [CrossRef]
- Nelson, J.B.; Chan-Tack, K.; Hedican, S.P.; Magnuson, S.R.; Opgenorth, T.J.; Bova, G.S.; Simons, J.W. Endothelin-1 production and decreased endothelin B receptor expression in advanced prostate cancer. Cancer Res. 1996, 56, 663–668. [Google Scholar]
- Nelson, J.; Bagnato, A.; Battistini, B.; Nisen, P. The endothelin axis: Emerging role in cancer. Nat. Rev. Cancer 2003, 3, 110–116. [Google Scholar] [CrossRef]
- Porter, A.T.; Ben-Josef, E. Humoral mechanisms in prostate cancer: A role for FSH. Urol. Oncol. 2001, 6, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Cho, R.W.; Stork, P.J.; Weber, M.J. Elevation of cyclic adenosine 3’,5’-monophosphate potentiates activation of mitogen-activated protein kinase by growth factors in LNCaP prostate cancer cells. Cancer Res. 1999, 59, 213–218. [Google Scholar] [PubMed]
- Seethalakshmi, L.; Mitra, S.P.; Dobner, P.R.; Menon, M.; Carraway, R.E. Neurotensin receptor expression in prostate cancer cell line and growth effect of NT at physiological concentrations. Prostate 1997, 31, 183–192. [Google Scholar] [CrossRef]
- Liu, X.H.; Kirschenbaum, A.; Lu, M.; Yao, S.; Dosoretz, A.; Holland, J.F.; Levine, A.C. Prostaglandin E2 induces hypoxia-inducible factor-1alpha stabilization and nuclear localization in a human prostate cancer cell line. J. Biol. Chem. 2002, 277, 50081–50086. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal 2013, 6, pl1. [Google Scholar] [CrossRef]
- Abida, W.; Cyrta, J.; Heller, G.; Prandi, D.; Armenia, J.; Coleman, I.; Cieslik, M.; Benelli, M.; Robinson, D.; Van Allen, E.M.; et al. Genomic correlates of clinical outcome in advanced prostate cancer. Proc. Natl. Acad. Sci. USA 2019, 116, 11428–11436. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Lim, W.K.; Chai, X.; Seshachalam, V.P.; Wang, M.; Rasheed, S.A.K.; Ghosh, S.; Casey, P.J. Gα13 promotes clonogenic growth by increasing tolerance to oxidative metabolic stress in prostate cancer cells. (under review).
- Metzger, E.; Müller, J.M.; Ferrari, S.; Buettner, R.; Schüle, R. A novel inducible transactivation domain in the androgen receptor: Implications for PRK in prostate cancer. EMBO J. 2003, 22, 270–280. [Google Scholar] [CrossRef]
- Bostwick, D.G.; Alexander, E.E.; Singh, R.; Shan, A.; Qian, J.; Santella, R.M.; Oberley, L.W.; Yan, T.; Zhong, W.; Jiang, X.; et al. Antioxidant enzyme expression and reactive oxygen species damage in prostatic intraepithelial neoplasia and cancer. Cancer 2000, 89, 123–134. [Google Scholar] [CrossRef]
- Shiota, M.; Yokomizo, A.; Naito, S. Oxidative stress and androgen receptor signaling in the development and progression of castration-resistant prostate cancer. Free Radic. Biol. Med. 2011, 51, 1320–1328. [Google Scholar] [CrossRef]
- Sharifi, N.; Hurt, E.M.; Thomas, S.B.; Farrar, W.L. Effects of manganese superoxide dismutase silencing on androgen receptor function and gene regulation: Implications for castration-resistant prostate cancer. Clin. Cancer Res. 2008, 14, 6073–6080. [Google Scholar] [CrossRef] [PubMed]
- Shiota, M.; Yokomizo, A.; Tada, Y.; Inokuchi, J.; Kashiwagi, E.; Masubuchi, D.; Eto, M.; Uchiumi, T.; Naito, S. Castration resistance of prostate cancer cells caused by castration-induced oxidative stress through Twist1 and androgen receptor overexpression. Oncogene 2010, 29, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Shiota, M.; Song, Y.; Takeuchi, A.; Yokomizo, A.; Kashiwagi, E.; Kuroiwa, K.; Tatsugami, K.; Uchiumi, T.; Oda, Y.; Naito, S. Antioxidant therapy alleviates oxidative stress by androgen deprivation and prevents conversion from androgen dependent to castration resistant prostate cancer. J. Urol. 2012, 187, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Kuruma, H.; Egawa, S.; Oh-Ishi, M.; Kodera, Y.; Satoh, M.; Chen, W.; Okusa, H.; Matsumoto, K.; Maeda, T.; Baba, S. High molecular mass proteome of androgen-independent prostate cancer. Proteomics 2005, 5, 1097–1112. [Google Scholar] [CrossRef] [PubMed]
- Shiota, M.; Yokomizo, A.; Kashiwagi, E.; Takeuchi, A.; Fujimoto, N.; Uchiumi, T.; Naito, S. Peroxiredoxin 2 in the nucleus and cytoplasm distinctly regulates androgen receptor activity in prostate cancer cells. Free Radic. Biol. Med. 2011, 51, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.T.; Chen, W.C.; Liao, S.K.; Hsu, C.L.; Lee, K.D.; Chen, M.F. The radiation response of hormone-resistant prostate cancer induced by long-term hormone therapy. Endocr. Relat. Cancer 2007, 14, 633–643. [Google Scholar] [CrossRef]
- Shiota, M.; Fujimoto, N.; Itsumi, M.; Takeuchi, A.; Inokuchi, J.; Tatsugami, K.; Yokomizo, A.; Kajioka, S.; Uchiumi, T.; Eto, M. Gene polymorphisms in antioxidant enzymes correlate with the efficacy of androgen-deprivation therapy for prostate cancer with implications of oxidative stress. Ann. Oncol. 2017, 28, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-González, B.; Porras-Quesada, P.; Arenas-Rodríguez, V.; Tamayo-Gómez, A.; Vázquez-Alonso, F.; Martínez-González, L.J.; Hernández, A.F.; Álvarez-Cubero, M.J. Genetic variants of antioxidant and xenobiotic metabolizing enzymes and their association with prostate cancer: A meta-analysis and functional in silico analysis. Sci. Total Environ. 2023, 898, 165530. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Lee, K.M.; Park, S.K.; Berndt, S.I.; Peters, U.; Reding, D.; Chatterjee, N.; Welch, R.; Chanock, S.; Huang, W.Y.; et al. Functional variant of manganese superoxide dismutase (SOD2 V16A) polymorphism is associated with prostate cancer risk in the prostate, lung, colorectal, and ovarian cancer study. Cancer Epidemiol. Biomarkers Prev. 2007, 16, 1581–1586. [Google Scholar] [CrossRef]
- Miar, A.; Hevia, D.; Muñoz-Cimadevilla, H.; Astudillo, A.; Velasco, J.; Sainz, R.M.; Mayo, J.C. Manganese superoxide dismutase (SOD2/MnSOD)/catalase and SOD2/GPx1 ratios as biomarkers for tumor progression and metastasis in prostate, colon, and lung cancer. Free Radic. Biol. Med. 2015, 85, 45–55. [Google Scholar] [CrossRef]
- Quirós, I.; Sáinz, R.M.; Hevia, D.; García-Suárez, O.; Astudillo, A.; Rivas, M.; Mayo, J.C. Upregulation of manganese superoxide dismutase (SOD2) is a common pathway for neuroendocrine differentiation in prostate cancer cells. Int. J. Cancer 2009, 125, 1497–1504. [Google Scholar] [CrossRef] [PubMed]
- Djokic, M.; Radic, T.; Santric, V.; Dragicevic, D.; Suvakov, S.; Mihailovic, S.; Stankovic, V.; Cekerevac, M.; Simic, T.; Nikitovic, M.; et al. The Association of Polymorphisms in Genes Encoding Antioxidant Enzymes GPX1 (rs1050450), SOD2 (rs4880) and Transcriptional Factor Nrf2 (rs6721961) with the Risk and Development of Prostate Cancer. Medicina 2022, 58, 1414. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.F.; Xu, K.; Tang, B.W.; Zhang, W.; Yuan, W.; Yue, C.; Shi, L.; Mi, Y.Y.; Zuo, L.; Zhu, L.J. Association between SOD2 V16A variant and urological cancer risk. Aging 2020, 12, 825–843. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Shen, M.; Cai, H.; Liu, K.; Liu, Y.; Huang, Z.; Liang, C.; Deng, X.; Ye, J.; Zou, Q.; et al. Association between manganese superoxide dismutase (MnSOD) polymorphism and prostate cancer susceptibility: A meta-analysis. Int. J. Biol. Markers 2016, 31, e422–e430. [Google Scholar] [CrossRef] [PubMed]
- Woodson, K.; Tangrea, J.A.; Lehman, T.A.; Modali, R.; Taylor, K.M.; Snyder, K.; Taylor, P.R.; Virtamo, J.; Albanes, D. Manganese superoxide dismutase (MnSOD) polymorphism, alpha-tocopherol supplementation and prostate cancer risk in the alpha-tocopherol, beta-carotene cancer prevention study (Finland). Cancer Causes Control 2003, 14, 513–518. [Google Scholar] [CrossRef]
- Ergen, H.A.; Narter, F.; Timirci, O.; Isbir, T. Effects of manganase superoxide dismutase Ala-9Val polymorphism on prostate cancer: A case-control study. Anticancer. Res. 2007, 27, 1227–1230. [Google Scholar] [PubMed]
- Bauer, S.R.; Richman, E.L.; Sosa, E.; Weinberg, V.; Song, X.; Witte, J.S.; Carroll, P.R.; Chan, J.M. Antioxidant and vitamin E transport genes and risk of high-grade prostate cancer and prostate cancer recurrence. Prostate 2013, 73, 1786–1795. [Google Scholar] [CrossRef]
- Margalit, D.N.; Jordahl, K.M.; Werner, L.; Wang, X.; Gwo-Shu Lee, M.; Penney, K.L.; Batista, J.L.; Martin, N.E.; Chan, J.M.; Kantoff, P.W.; et al. GermLine Variation in Superoxide Dismutase-2 (SOD2) and Survival Outcomes After Radiation Therapy for Prostate Cancer: Results of a Test and Validation Set Analysis. Clin. Genitourin. Cancer 2015, 13, 370–377.e1. [Google Scholar] [CrossRef] [PubMed]
- Drozdz-Afelt, J.M.; Koim-Puchowska, B.B.; Kaminski, P. Analysis of oxidative stress indicators in Polish patients with prostate cancer. Environ. Sci. Pollut. Res. Int. 2022, 29, 4632–4640. [Google Scholar] [CrossRef]
- Sweeney, J.D.; Debeljak, M.; Riel, S.; Millena, A.C.; Eshleman, J.R.; Paller, C.J.; Odero-Marah, V. Val16A SOD2 Polymorphism Promotes Epithelial-Mesenchymal Transition Antagonized by Muscadine Grape Skin Extract in Prostate Cancer Cells. Antioxidants 2021, 10, 213. [Google Scholar] [CrossRef]
- Xu, Y.; Fang, F.; Dhar, S.K.; Bosch, A.; St Clair, W.H.; Kasarskis, E.J.; St Clair, D.K. Mutations in the SOD2 promoter reveal a molecular basis for an activating protein 2-dependent dysregulation of manganese superoxide dismutase expression in cancer cells. Mol. Cancer Res. 2008, 6, 1881–1893. [Google Scholar] [CrossRef] [PubMed]
- Venkataraman, S.; Jiang, X.; Weydert, C.; Zhang, Y.; Zhang, H.J.; Goswami, P.C.; Ritchie, J.M.; Oberley, L.W.; Buettner, G.R. Manganese superoxide dismutase overexpression inhibits the growth of androgen-independent prostate cancer cells. Oncogene 2005, 24, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Oberley, T.D.; Oberley, L.W.; Zhong, W. Overexpression of manganese superoxide dismutase in DU145 human prostate carcinoma cells has multiple effects on cell phenotype. Prostate 1998, 35, 221–233. [Google Scholar] [CrossRef]
- Plymate, S.R.; Haugk, K.H.; Sprenger, C.C.; Nelson, P.S.; Tennant, M.K.; Zhang, Y.; Oberley, L.W.; Zhong, W.; Drivdahl, R.; Oberley, T.D. Increased manganese superoxide dismutase (SOD-2) is part of the mechanism for prostate tumor suppression by Mac25/insulin-like growth factor binding-protein-related protein-1. Oncogene 2003, 22, 1024–1034. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Berto, M.D.; Bica, C.G.; de Sá, G.P.; Barbisan, F.; Azzolin, V.F.; Rogalski, F.; Duarte, M.M.; da Cruz, I.B. The effect of superoxide anion and hydrogen peroxide imbalance on prostate cancer: An integrative in vivo and in vitro analysis. Med. Oncol. 2015, 32, 251. [Google Scholar] [CrossRef] [PubMed]
- Venkataraman, S.; Wagner, B.A.; Jiang, X.; Wang, H.P.; Schafer, F.Q.; Ritchie, J.M.; Patrick, B.C.; Oberley, L.W.; Buettner, G.R. Overexpression of manganese superoxide dismutase promotes the survival of prostate cancer cells exposed to hyperthermia. Free Radic. Res. 2004, 38, 1119–1132. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Yan, T.; Webber, M.M.; Oberley, T.D. Alteration of cellular phenotype and responses to oxidative stress by manganese superoxide dismutase and a superoxide dismutase mimic in RWPE-2 human prostate adenocarcinoma cells. Antioxid. Redox Signal 2004, 6, 513–522. [Google Scholar] [CrossRef]
- Quiros-Gonzalez, I.; Gonzalez-Menendez, P.; Mayo, J.C.; Hevia, D.; Artime-Naveda, F.; Fernandez-Vega, S.; Fernandez-Fernandez, M.; Rodriguez-Gonzalez, P.; Garcia-Alonso, J.I.; Sainz, R.M. Androgen-Dependent Prostate Cancer Cells Reprogram Their Metabolic Signature upon GLUT1 Upregulation by Manganese Superoxide Dismutase. Antioxidants 2022, 11, 313. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Q.; Neil, B.; Chen, X. Effect of lycopene on androgen receptor and prostate-specific antigen velocity. Chin. Med. J. 2010, 123, 2231–2236. [Google Scholar]
- Ansari, M.S.; Gupta, N.P. A comparison of lycopene and orchidectomy vs orchidectomy alone in the management of advanced prostate cancer. BJU Int. 2003, 92, 375–378; discussion 378. [Google Scholar] [CrossRef]
- Watters, J.L.; Gail, M.H.; Weinstein, S.J.; Virtamo, J.; Albanes, D. Associations between alpha-tocopherol, beta-carotene, and retinol and prostate cancer survival. Cancer Res. 2009, 69, 3833–3841. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.; Sharifi, N. SOD mimetics: A novel class of androgen receptor inhibitors that suppresses castration-resistant growth of prostate cancer. Mol. Cancer Ther. 2012, 11, 87–97. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).