Analysis of Expression of the ANG1, CaSR and FAK Proteins in Uterine Fibroids
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Immunohistochemical Protein Detection
4.3. Semi-Quantitative Evaluation of CaSR, ANG1, and FAK Protein Expression
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pavone, D.; Clemenza, S.; Sorbi, F.; Fambrini, M.; Petraglia, F. Epidemiology and Risk Factors of Uterine Fibroids. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 46, 3–11. [Google Scholar] [CrossRef]
- Islam, M.S.; Ciavattini, A.; Petraglia, F.; Castellucci, M.; Ciarmela, P. Extracellular Matrix in Uterine Leiomyoma Pathogenesis: A Potential Target for Future Therapeutics. Hum. Reprod. Update 2018, 24, 59–85. [Google Scholar] [CrossRef] [PubMed]
- Ciebiera, M.; Ali, M.; Prince, L.; Jackson-Bey, T.; Atabiekov, I.; Zgliczyński, S.; Al-Hendy, A. The Evolving Role of Natural Compounds in the Medical Treatment of Uterine Fibroids. J. Clin. Med. 2020, 9, 1479. [Google Scholar] [CrossRef] [PubMed]
- Moravek, M.B.; Yin, P.; Ono, M.; Coon, J.S.; Dyson, M.T.; Navarro, A.; Marsh, E.E.; Chakravarti, D.; Kim, J.J.; Wei, J.-J.; et al. Ovarian Steroids, Stem Cells and Uterine Leiomyoma: Therapeutic Implications. Hum. Reprod. Update 2015, 21, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Cardozo, E.R.; Foster, R.; Karmon, A.E.; Lee, A.E.; Gatune, L.W.; Rueda, B.R.; Styer, A.K. MicroRNA 21a-5p Overexpression Impacts Mediators of Extracellular Matrix Formation in Uterine Leiomyoma. Reprod. Biol. Endocrinol. 2018, 16, 46. [Google Scholar] [CrossRef] [PubMed]
- Firdaus, R.; Agrawal, P.; Anagani, M.; Vijayalakshmi, K.; Hasan, Q. Multiple Mutations in Exon-2 of Med-12 Identified in Uterine Leiomyomata. J. Reprod. Infertil. 2021, 22, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, X.; Mas, A.; Cervelló, I.; Taylor, H.; Simon, C. Uterine Stem Cells: From Basic Research to Advanced Cell Therapies. Hum. Reprod. Update 2018, 24, 673–693. [Google Scholar] [CrossRef] [PubMed]
- Ciebiera, M.; Włodarczyk, M.; Ciebiera, M.; Zaręba, K.; Łukaszuk, K.; Jakiel, G. Vitamin D and Uterine Fibroids-Review of the Literature and Novel Concepts. Int. J. Mol. Sci. 2018, 19, 2051. [Google Scholar] [CrossRef]
- Markowska, A.; Kurzawa, P.; Bednarek, W.; Gryboś, A.; Mardas, M.; Krzyżaniak, M.; Majewski, J.; Markowska, J.; Gryboś, M.; Żurawski, J. Immunohistochemical Expression of Vitamin D Receptor in Uterine Fibroids. Nutrients 2022, 14, 3371. [Google Scholar] [CrossRef]
- Lee, J.-W.; Choi, H.J.; Kim, E.-J.; Hwang, W.Y.; Jung, M.-H.; Kim, K.S. Fisetin Induces Apoptosis in Uterine Leiomyomas through Multiple Pathways. Sci. Rep. 2020, 10, 7993. [Google Scholar] [CrossRef]
- Afrin, S.; Islam, M.S.; Patzkowsky, K.; Malik, M.; Catherino, W.H.; Segars, J.H.; Borahay, M.A. Simvastatin Ameliorates Altered Mechanotransduction in Uterine Leiomyoma Cells. Am. J. Obstet. Gynecol. 2020, 223, 733.e1–733.e14. [Google Scholar] [CrossRef] [PubMed]
- Russo, T.A.; Banuth, A.M.M.; Nader, H.B.; Dreyfuss, J.L. Altered Shear Stress on Endothelial Cells Leads to Remodeling of Extracellular Matrix and Induction of Angiogenesis. PLoS ONE 2020, 15, e0241040. [Google Scholar] [CrossRef]
- Chauhan, A.; Khan, T. Focal Adhesion Kinase-An Emerging Viable Target in Cancer and Development of Focal Adhesion Kinase Inhibitors. Chem. Biol. Drug Des. 2021, 97, 774–794. [Google Scholar] [CrossRef] [PubMed]
- Machado-Lopez, A.; Simón, C.; Mas, A. Molecular and Cellular Insights into the Development of Uterine Fibroids. Int. J. Mol. Sci. 2021, 22, 8483. [Google Scholar] [CrossRef]
- Vega, R.; Carretero, M.; Travasso, R.D.M.; Bonilla, L.L. Notch Signaling and Taxis Mechanisms Regulate Early Stage Angiogenesis: A Mathematical and Computational Model. PLoS Comput. Biol. 2020, 16, e1006919. [Google Scholar] [CrossRef]
- Moccia, F.; Negri, S.; Shekha, M.; Faris, P.; Guerra, G. Endothelial Ca2+ Signaling, Angiogenesis and Vasculogenesis: Just What It Takes to Make a Blood Vessel. Int. J. Mol. Sci. 2019, 20, 3962. [Google Scholar] [CrossRef] [PubMed]
- Hwang-Bo, J.; Park, J.-H.; Chung, I.S. 3-O-Acetyloleanolic Acid Inhibits Angiopoietin-1-Induced Angiogenesis and Lymphangiogenesis via Suppression of Angiopoietin-1/Tie-2 Signaling. Phytother. Res. 2020, 34, 359–367. [Google Scholar] [CrossRef]
- Harel, S.; Sanchez-Gonzalez, V.; Echavarria, R.; Mayaki, D.; Hussain, S.N. Roles of miR-640 and Zinc Finger Protein 91 (ZFP91) in Angiopoietin-1-Induced In Vitro Angiogenesis. Cells 2020, 9, 1602. [Google Scholar] [CrossRef]
- Pafumi, I.; Favia, A.; Gambara, G.; Papacci, F.; Ziparo, E.; Palombi, F.; Filippini, A. Regulation of Angiogenic Functions by Angiopoietins through Calcium-Dependent Signaling Pathways. Biomed. Res. Int. 2015, 2015, 965271. [Google Scholar] [CrossRef]
- Yang, W.; Cheng, Z.; Dai, H. Calcium Concentration Response to Uterine Ischemia: A Comparison of Uterine Fibroid Cells and Adjacent Normal Myometrial Cells. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 174, 123–127. [Google Scholar] [CrossRef]
- Wang, S.; Duan, H.; Li, B. Rapid Effects of Oestrogen on Intracellular Ca2+ in the Uterine Junctional Myometrium of Patients With and Without Adenomyosis in Different Phases of the Menstrual Cycle. Reprod. Sci. 2020, 27, 1992–2001. [Google Scholar] [CrossRef]
- Nicholson, T.A.; Pelage, J.P.; Ettles, D.F. Fibroid Calcification after Uterine Artery Embolization: Ultrasonographic Appearance and Pathology. J. Vasc. Interv. Radiol. 2001, 12, 443–446. [Google Scholar] [CrossRef]
- Gorvin, C.M. Calcium-Sensing Receptor Signaling—How Human Disease Informs Biology. Curr. Opin. Endocr. Metab. Res. 2021, 16, 10–28. [Google Scholar] [CrossRef] [PubMed]
- Lechertier, T.; Reynolds, L.E.; Kim, H.; Pedrosa, A.R.; Gómez-Escudero, J.; Muñoz-Félix, J.M.; Batista, S.; Dukinfield, M.; Demircioglu, F.; Wong, P.P.; et al. Pericyte FAK Negatively Regulates Gas6/Axl Signalling to Suppress Tumour Angiogenesis and Tumour Growth. Nat. Commun. 2020, 11, 2810. [Google Scholar] [CrossRef]
- Pedrosa, A.-R.; Bodrug, N.; Gomez-Escudero, J.; Carter, E.P.; Reynolds, L.E.; Georgiou, P.N.; Fernandez, I.; Lees, D.M.; Kostourou, V.; Alexopoulou, A.N.; et al. Tumor Angiogenesis Is Differentially Regulated by Phosphorylation of Endothelial Cell Focal Adhesion Kinase Tyrosines-397 and -861. Cancer Res. 2019, 79, 4371–4386. [Google Scholar] [CrossRef]
- Islam, M.S.; Castellucci, C.; Fiorini, R.; Greco, S.; Gagliardi, R.; Zannotti, A.; Giannubilo, S.R.; Ciavattini, A.; Frega, N.G.; Pacetti, D.; et al. Omega-3 Fatty Acids Modulate the Lipid Profile, Membrane Architecture, and Gene Expression of Leiomyoma Cells. J. Cell Physiol. 2018, 233, 7143–7156. [Google Scholar] [CrossRef]
- Katoh, K. FAK-Dependent Cell Motility and Cell Elongation. Cells 2020, 9, 192. [Google Scholar] [CrossRef]
- Tavora, B.; Reynolds, L.E.; Batista, S.; Demircioglu, F.; Fernandez, I.; Lechertier, T.; Lees, D.M.; Wong, P.-P.; Alexopoulou, A.; Elia, G.; et al. Endothelial-Cell FAK Targeting Sensitizes Tumours to DNA-Damaging Therapy. Nature 2014, 514, 112–116. [Google Scholar] [CrossRef]
- Lopez-Mejia, I.C.; Pijuan, J.; Navaridas, R.; Santacana, M.; Gatius, S.; Velasco, A.; Castellà, G.; Panosa, A.; Cabiscol, E.; Pinyol, M.; et al. Oxidative Stress-Induced FAK Activation Contributes to Uterine Serous Carcinoma Aggressiveness. Mol. Oncol. 2023, 17, 98–118. [Google Scholar] [CrossRef] [PubMed]
- Munro, M.G.; Critchley, H.O.D.; Fraser, I.S. The FIGO Classification of Causes of Abnormal Uterine Bleeding in the Reproductive Years. Fertil. Steril. 2011, 95, 2204–2208.e3. [Google Scholar] [CrossRef] [PubMed]
- Chalcarz, M.; Żurawski, J. Injection of Aquafilling® for Breast Augmentation Causes Inflammatory Responses Independent of Visible Symptoms. Aesthetic Plast. Surg. 2021, 45, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Ciarmela, P.; Delli Carpini, G.; Greco, S.; Zannotti, A.; Montik, N.; Giannella, L.; Giuliani, L.; Grelloni, C.; Panfoli, F.; Paolucci, M.; et al. Uterine Fibroid Vascularization: From Morphological Evidence to Clinical Implications. Reprod. BioMedicine Online 2022, 44, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Don, E.E.; Middelkoop, M.-A.; Hehenkamp, W.J.K.; Mijatovic, V.; Griffioen, A.W.; Huirne, J.A.F. Endometrial Angiogenesis of Abnormal Uterine Bleeding and Infertility in Patients with Uterine Fibroids-A Systematic Review. Int. J. Mol. Sci. 2023, 24, 7011. [Google Scholar] [CrossRef] [PubMed]
- Grube, M.; Neis, F.; Brucker, S.Y.; Kommoss, S.; Andress, J.; Weiss, M.; Hoffmann, S.; Taran, F.-A.; Krämer, B. Uterine Fibroids—Current Trends and Strategies. Surg. Technol. Int. 2019, 34, 257–263. [Google Scholar] [PubMed]
- Bowler, E.; Oltean, S. Alternative Splicing in Angiogenesis. Int. J. Mol. Sci. 2019, 20, 2067. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, T.; Inaba, M.; Naito, S.; Mihara, Y.; Miura, S.; Taba, M.; Yoshizaki, A.; Wen, C.-Y.; Sekine, I. Expression of Angiopoietin-1, 2 and 4 and Tie-1 and 2 in Gastrointestinal Stromal Tumor, Leiomyoma and Schwannoma. World J. Gastroenterol. 2007, 13, 4473–4479. [Google Scholar] [CrossRef] [PubMed]
- Middelkoop, M.-A.; Don, E.E.; Hehenkamp, W.J.K.; Polman, N.J.; Griffioen, A.W.; Huirne, J.A.F. Angiogenesis in Abnormal Uterine Bleeding: A Narrative Review. Hum. Reprod. Update 2023, 29, 457–485. [Google Scholar] [CrossRef] [PubMed]
- Cai, E.; Yang, D.; Zhang, Y.; Cai, J.; Sun, S.; Yang, P.; Huang, Y.; Han, Q.; Xiong, Z.; Wang, S. Angiopoietin-1 Associated with a Decreased Risk of Lymph Node Metastasis in Early Stage Cervical Cancer. Histol. Histopathol. 2020, 35, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Adeboje-Jimoh, F.; Okunade, K.S.; Olorunfemi, G.; Olamijulo, J.A. Serum Calcium and Magnesium Levels in Women with Uterine Fibroids at a University Teaching Hospital in Southwest Nigeria: A Comparative Cross-Sectional Study. Res. Sq. 2023, rs.3.rs-2877359. [Google Scholar] [CrossRef]
- Li, S.; Chen, B.; Sheng, B.; Wang, J.; Zhu, X. The Associations between Serum Vitamin D, Calcium and Uterine Fibroids in Chinese Women: A Case-Controlled Study. J. Int. Med. Res. 2020, 48, 300060520923492. [Google Scholar] [CrossRef]
- Kropf, J.; Vo, M.; Cheyney, S.; Kayaleh, O.; McWhorter, J.; Carlan, S.J. Hypercalcemia Resulting from Necrotizing Leiomyoma in a Pregnant Female. Am. J. Case Rep. 2020, 21, e923412. [Google Scholar] [CrossRef] [PubMed]
- Brullo, C.; Tasso, B. New Insights on Fak and Fak Inhibitors. Curr. Med. Chem. 2021, 28, 3318–3338. [Google Scholar] [CrossRef] [PubMed]
- Dawson, J.C.; Serrels, A.; Stupack, D.G.; Schlaepfer, D.D.; Frame, M.C. Targeting FAK in Anticancer Combination Therapies. Nat. Rev. Cancer 2021, 21, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Quispe, P.A.; Lavecchia, M.J.; León, I.E. Focal Adhesion Kinase Inhibitors in the Treatment of Solid Tumors: Preclinical and Clinical Evidence. Drug Discov. Today 2022, 27, 664–674. [Google Scholar] [CrossRef]

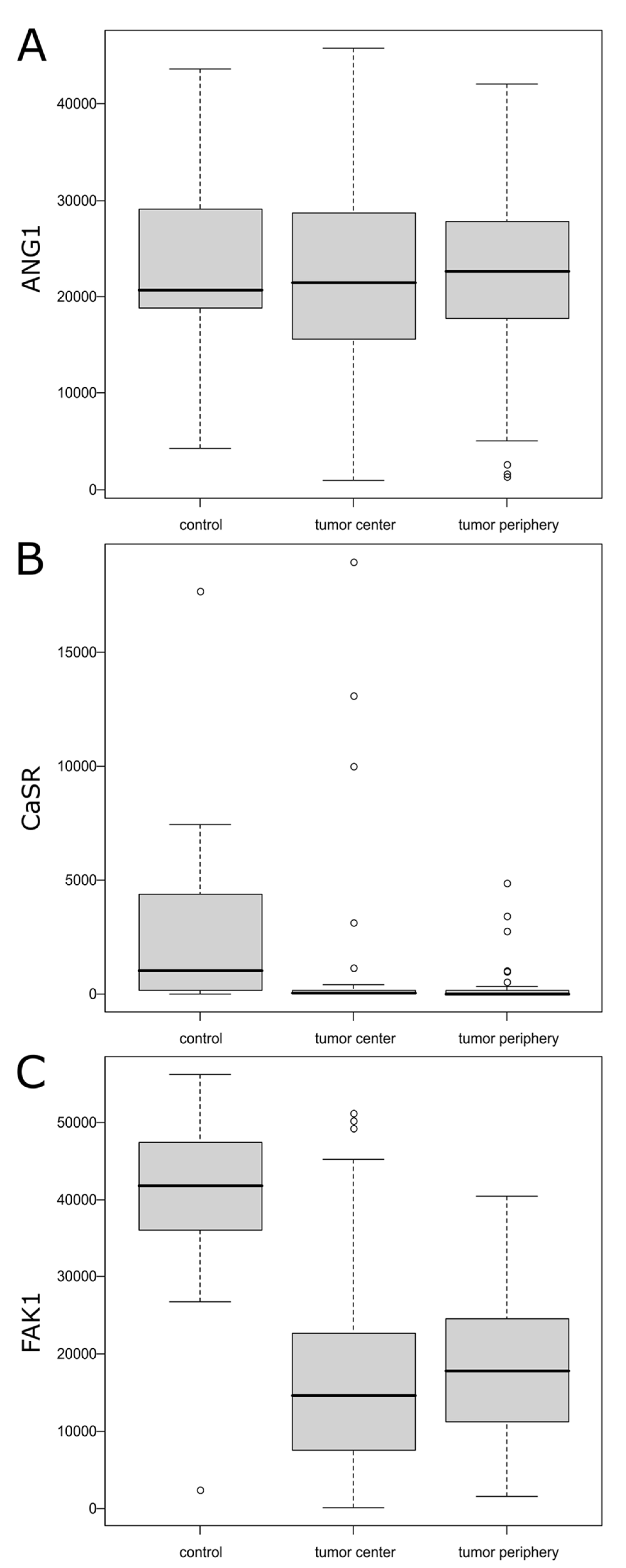
| N | Mean ± SD/ Median (Q1; Q3) | Range | p | Post hoc | |
|---|---|---|---|---|---|
| ANG1 | |||||
| Tumor center | 70 | 22,355.37 ± 9961.68 | (940.08; 45,829.46) | 0.983 | |
| Tumor periphery | 70 | 22,370.11 ± 8540.59 | (1313.09; 42,077.36) | ||
| Control | 12 | 22,900.08 ± 10,762.30 | (4256.68; 43,689.11) | ||
| CaSR | |||||
| Tumor center | 70 | 34.31 (7.64; 179.37) | (0.00; 18,997.74) | 0.001 | Center < control Periphery < control |
| Tumor periphery | 70 | 14.00 (2.08; 145.02) | (0.00; 4906.41) | ||
| Control | 12 | 1056.15 (171.81; 4343.91) | (1.38; 17,712.65) | ||
| FAK | |||||
| Tumor center | 70 | 16,037.69 ± 11,342.30 | (66.05; 51,188.30) | <0.001 | Center < control Periphery < control |
| Tumor periphery | 70 | 18,338.54 ± 8824.04 | (1601.30; 40,503.59) | ||
| Control | 12 | 39,665.24 ± 14,231.92 | (2358.86; 56,274.69) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Markowska, A.; de Mezer, M.; Kurzawa, P.; Bednarek, W.; Gryboś, A.; Krzyżaniak, M.; Markowska, J.; Gryboś, M.; Żurawski, J. Analysis of Expression of the ANG1, CaSR and FAK Proteins in Uterine Fibroids. Int. J. Mol. Sci. 2024, 25, 7164. https://doi.org/10.3390/ijms25137164
Markowska A, de Mezer M, Kurzawa P, Bednarek W, Gryboś A, Krzyżaniak M, Markowska J, Gryboś M, Żurawski J. Analysis of Expression of the ANG1, CaSR and FAK Proteins in Uterine Fibroids. International Journal of Molecular Sciences. 2024; 25(13):7164. https://doi.org/10.3390/ijms25137164
Chicago/Turabian StyleMarkowska, Anna, Mateusz de Mezer, Paweł Kurzawa, Wiesława Bednarek, Anna Gryboś, Monika Krzyżaniak, Janina Markowska, Marian Gryboś, and Jakub Żurawski. 2024. "Analysis of Expression of the ANG1, CaSR and FAK Proteins in Uterine Fibroids" International Journal of Molecular Sciences 25, no. 13: 7164. https://doi.org/10.3390/ijms25137164
APA StyleMarkowska, A., de Mezer, M., Kurzawa, P., Bednarek, W., Gryboś, A., Krzyżaniak, M., Markowska, J., Gryboś, M., & Żurawski, J. (2024). Analysis of Expression of the ANG1, CaSR and FAK Proteins in Uterine Fibroids. International Journal of Molecular Sciences, 25(13), 7164. https://doi.org/10.3390/ijms25137164







