CAR-T Cells Therapy in Glioblastoma: A Systematic Review on Molecular Targets and Treatment Strategies
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Review
2.2. Data Extraction
2.3. Outcomes
2.4. Risk of Bias Assessment
2.5. Statistical Analysis
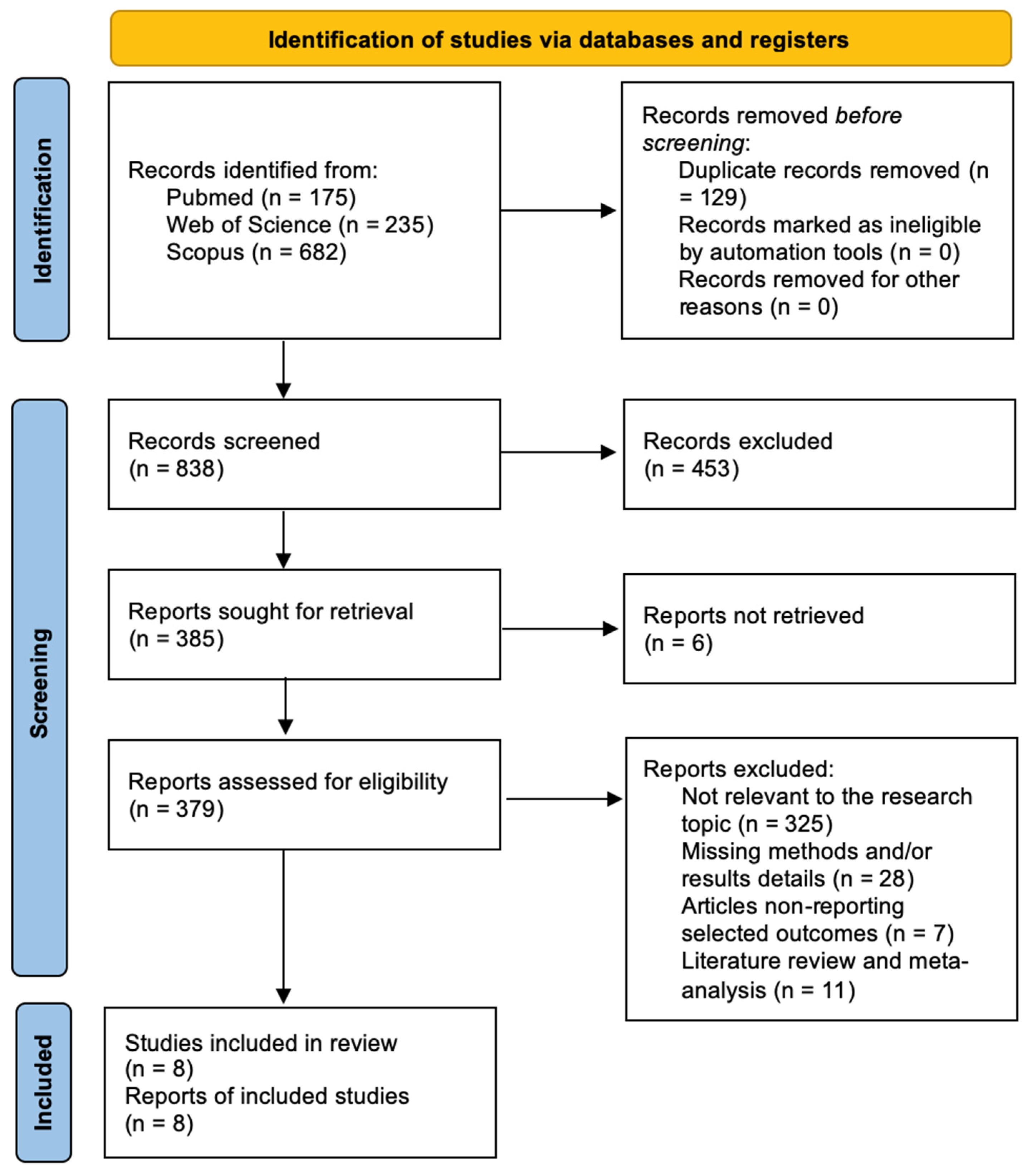
3. Results
3.1. Literature Review
3.2. Data Analysis
4. Discussion
4.1. Molecular Mechanisms of CAR-T Cell Immunotherapy
4.2. Targeted Therapies Proposed Using CAR-T Cells
4.3. Limitations and Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Yuan, B.; Wang, G.; Tang, X.; Tong, A.; Zhou, L. Immunotherapy of Glioblastoma: Recent Advances and Future Prospects. Hum. Vaccines Immunother. 2022, 18, 2055417. [Google Scholar] [CrossRef] [PubMed]
- Vik-Mo, E.O.; Nyakas, M.; Mikkelsen, B.V.; Moe, M.C.; Due-Tønnesen, P.; Suso, E.M.I.; Sæbøe-Larssen, S.; Sandberg, C.; Brinchmann, J.E.; Helseth, E.; et al. Therapeutic Vaccination against Autologous Cancer Stem Cells with mRNA-Transfected Dendritic Cells in Patients with Glioblastoma. Cancer Immunol. Immunother. 2013, 62, 1499–1509. [Google Scholar] [CrossRef] [PubMed]
- Markovic, D.S.; Glass, R.; Synowitz, M.; van Rooijen, N.; Kettenmann, H. Microglia Stimulate the Invasiveness of Glioma Cells by Increasing the Activity of Metalloprotease-2. J. Neuropathol. Exp. Neurol. 2005, 64, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Luksik, A.S.; Yazigi, E.; Shah, P.; Jackson, C.M. CAR T Cell Therapy in Glioblastoma: Overcoming Challenges Related to Antigen Expression. Cancers 2023, 15, 1414. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Stang, A. Critical Evaluation of the Newcastle-Ottawa Scale for the Assessment of the Quality of Nonrandomized Studies in Meta-Analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- Ahmed, N.; Brawley, V.; Hegde, M.; Bielamowicz, K.; Kalra, M.; Landi, D.; Robertson, C.; Gray, T.L.; Diouf, O.; Wakefield, A.; et al. HER2-Specific Chimeric Antigen Receptor—Modified Virus-Specific T Cells for Progressive Glioblastoma: A Phase 1 Dose-Escalation Trial. JAMA Oncol. 2017, 3, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.E.; Badie, B.; Barish, M.E.; Weng, L.; Ostberg, J.R.; Chang, W.-C.; Naranjo, A.; Starr, R.; Wagner, J.; Wright, C.; et al. Bioactivity and Safety of IL13Rα2-Redirected Chimeric Antigen Receptor CD8+ T Cells in Patients with Recurrent Glioblastoma. Clin. Cancer Res. 2015, 21, 4062–4072. [Google Scholar] [CrossRef]
- Brown, C.E.; Alizadeh, D.; Starr, R.; Weng, L.; Wagner, J.R.; Naranjo, A.; Ostberg, J.R.; Blanchard, M.S.; Kilpatrick, J.; Simpson, J.; et al. Regression of Glioblastoma after Chimeric Antigen Receptor T-Cell Therapy. N. Engl. J. Med. 2016, 375, 2561–2569. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, D.M.; Nasrallah, M.P.; Desai, A.; Melenhorst, J.J.; Mansfield, K.; Morrissette, J.J.D.; Martinez-Lage, M.; Brem, S.; Maloney, E.; Shen, A.; et al. A Single Dose of Peripherally Infused EGFRvIII-Directed CAR-T cells Mediates Antigen Loss and Induces Adaptive Resistance in Patients with Recurrent Glioblastoma. Sci. Transl. Med. 2017, 9, eaaa0984. [Google Scholar] [CrossRef]
- Lin, Q.; Ba, T.; Ho, J.; Chen, D.; Cheng, Y.; Wang, L.; Xu, G.; Xu, L.; Zhou, Y.; Wei, Y.; et al. First-in-Human Trial of EphA2-Redirected CAR T-Cells in Patients with Recurrent Glioblastoma: A Preliminary Report of Three Cases at the Starting Dose. Front. Oncol. 2021, 11, 694941. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; O’Rourke, D.M.; Chawla, S.; Verma, G.; Nasrallah, M.P.; Morrissette, J.J.D.; Plesa, G.; June, C.H.; Brem, S.; Maloney, E.; et al. Multiparametric Magnetic Resonance Imaging in the Assessment of Anti-EGFRvIII Chimeric Antigen Receptor T Cell Therapy in Patients with Recurrent Glioblastoma. Br. J. Cancer 2019, 120, 54–56. [Google Scholar] [CrossRef] [PubMed]
- Goff, S.L.; Morgan, R.A.; Yang, J.C.; Sherry, R.M.; Robbins, P.F.; Restifo, N.P.; Feldman, S.A.; Lu, Y.-C.; Lu, L.; Zheng, Z.; et al. Pilot Trial of Adoptive Transfer of Chimeric Antigen Receptor–Transduced T Cells Targeting EGFRvIII in Patients with Glioblastoma. J. Immunother. 2019, 42, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhou, J.; Yang, X.; Liu, Y.; Zou, C.; Lv, W.; Chen, C.; Cheng, K.K.; Chen, T.; Chang, L.-J.; et al. Safety and Antitumor Activity of GD2-Specific 4SCAR-T Cells in Patients with Glioblastoma. Mol. Cancer 2023, 22, 3. [Google Scholar] [CrossRef] [PubMed]
- Agosti, E.; Zeppieri, M.; De Maria, L.; Tedeschi, C.; Fontanella, M.M.; Panciani, P.P.; Ius, T. Glioblastoma Immunotherapy: A Systematic Review of the Present Strategies and Prospects for Advancements. Int. J. Mol. Sci. 2023, 24, 15037. [Google Scholar] [CrossRef] [PubMed]
- Agosti, E.; Panciani, P.P.; Zeppieri, M.; De Maria, L.; Pasqualetti, F.; Tel, A.; Zanin, L.; Fontanella, M.M.; Ius, T. Tumor Microenvironment and Glioblastoma Cell Interplay as Promoters of Therapeutic Resistance. Biology 2023, 12, 736. [Google Scholar] [CrossRef] [PubMed]
- June, C.H.; O’Connor, R.S.; Kawalekar, O.U.; Ghassemi, S.; Milone, M.C. CAR T Cell Immunotherapy for Human Cancer. Science 2018, 359, 1361–1365. [Google Scholar] [CrossRef] [PubMed]
- Shannon, M.L.; Noelle, F.; Shaw, P.A.; Richard, A.; Barrett, D.M.; Bunin, N.J.; Chew, A.; Gonzalez, V.E.; Zheng, Z.; Lacey, S.F.; et al. Chimeric Antigen Receptor T Cells for Sustained Remissions in Leukemia. N. Engl. J. Med. 2014, 371, 1507–1517. [Google Scholar] [CrossRef]
- Guedan, S.; Posey, A.D.; Shaw, C.; Wing, A.; Da, T.; Patel, P.R.; McGettigan, S.E.; Casado-Medrano, V.; Kawalekar, O.U.; Uribe-Herranz, M.; et al. Enhancing CAR T Cell Persistence through ICOS and 4-1BB Costimulation. JCI Insight 2018, 3, e96976. [Google Scholar] [CrossRef]
- Roybal, K.T.; Rupp, L.J.; Morsut, L.; Walker, W.J.; McNally, K.A.; Park, J.S.; Lim, W.A. Precision Tumor Recognition by T Cells with Combinatorial Antigen-Sensing Circuits. Cell 2016, 164, 770–779. [Google Scholar] [CrossRef]
- Neelapu, S.S.; Tummala, S.; Kebriaei, P.; Wierda, W.; Gutierrez, C.; Locke, F.L.; Komanduri, K.V.; Lin, Y.; Jain, N.; Daver, N.; et al. Chimeric Antigen Receptor T-Cell Therapy—Assessment and Management of Toxicities. Nat. Rev. Clin. Oncol. 2018, 15, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Chang, M.; Wang, Y.; Xing, B.; Ma, W. B7-H3 in Brain Malignancies: Immunology and Immunotherapy. Int. J. Biol. Sci. 2023, 19, 3762–3780. [Google Scholar] [CrossRef] [PubMed]
- Majzner, R.G.; Theruvath, J.L.; Nellan, A.; Heitzeneder, S.; Cui, Y.; Mount, C.W.; Rietberg, S.P.; Linde, M.H.; Xu, P.; Rota, C.; et al. CAR-T cells Targeting B7-H3, A Pan-Cancer Antigen, Demonstrate Potent Preclinical Activity against Pediatric Solid Tumors and Brain Tumors. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019, 25, 2560–2574. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Hirabayashi, K.; Ahn, S.; Kren, N.P.; Montgomery, S.A.; Wang, X.; Tiruthani, K.; Mirlekar, B.; Michaud, D.; Greene, K.; et al. Antitumor Responses in the Absence of Toxicity in Solid Tumors by Targeting B7-H3 via Chimeric Antigen Receptor T Cells. Cancer Cell 2019, 35, 221. [Google Scholar] [CrossRef] [PubMed]
- An, Z.; Aksoy, O.; Zheng, T.; Fan, Q.W.; Weiss, W.A. Epidermal Growth Factor Receptor and EGFRvIII in Glioblastoma: Signaling Pathways and Targeted Therapies. Oncogene 2018, 37, 1561–1575. [Google Scholar] [CrossRef] [PubMed]
- Chuntova, P.; Hou, Y.; Naka, R.; Yamamichi, A.; Chen, T.; Goretsky, Y.; Hatae, R.; Nejo, T.; Kohanbash, G.; Mende, A.L.; et al. Novel EGFRvIII-CAR Transgenic Mice for Rigorous Preclinical Studies in Syngeneic Mice. Neuro-Oncol. 2022, 24, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Zhang, J.; Yang, Y.-Z.; Wang, F.; Jiang, H.; Chen, H.-D.; Wu, H.-Y.; Sai, K.; Hu, W.-M. IL13RA2 Is Overexpressed in Malignant Gliomas and Related to Clinical Outcome of Patients. Am. J. Transl. Res. 2020, 12, 4702–4714. [Google Scholar] [PubMed]
- Kim, K.; Gwak, H.-S.; Han, N.; Hong, E.K.; Choi, B.K.; Lee, S.; Choi, S.; Park, J.-H.; Seok, J.-H.; Jeon, Y.; et al. Chimeric Antigen Receptor T Cells with Modified Interleukin-13 Preferentially Recognize IL13Rα2 and Suppress Malignant Glioma: A Preclinical Study. Front. Immunol. 2021, 12, 715000. [Google Scholar] [CrossRef] [PubMed]
- Agosti, E.; Zeppieri, M.; Ghidoni, M.; Ius, T.; Tel, A.; Fontanella, M.M.; Panciani, P.P. Role of Glioma Stem Cells in Promoting Tumor Chemo- and Radioresistance: A Systematic Review of Potential Targeted Treatments. World J. Stem Cells 2024, 16, 604–614. [Google Scholar] [CrossRef]
- De Maria, L.; Panciani, P.P.; Zeppieri, M.; Ius, T.; Serioli, S.; Piazza, A.; Di Giovanni, E.; Fontanella, M.M.; Agosti, E. A Systematic Review of the Metabolism of High-Grade Gliomas: Current Targeted Therapies and Future Perspectives. Int. J. Mol. Sci. 2024, 25, 724. [Google Scholar] [CrossRef]

| Author | Year | Trial Name | Phase | Patients (N) | Diagnosis (Target Glioma) | Follow-Up (Months, Median Value) | Treatment | Drug Delivery | Endpoints (OS, PFS) |
|---|---|---|---|---|---|---|---|---|---|
| Ahmed et al. [7] | 2015 | NCT01109095 | I | 17 | Recurrent GBM | N/A | HER2 CMV-specific CAR-T cells | Intravenous | OS: 11.1 mo from the first T-cell infusion and 24.5 mo from diagnosis. Three patients had no progression between 24 to 29 mo |
| Brown et al. [8] | 2016 | NCT00730613 | I | 3 | Recurrent GBM | N/A | IL13(E13Y)-zetakine+ CD8+ CTL clones | Intracranial | mOS: 10.3 mo |
| Brown et al. [9] | 2016 | NCT02208362 | I | 82 | Recurrent GBM | N/A | IL13 Rα2-specific CAR-T cells | Intracranial | PFS: 7.5 mo |
| O’Rourke et al. [10] | 2017 | NCT02209376 | I | 10 | Recurrent GBM | N/A | CAR T-EGFRvIII+ | Intravenous | mOS: 251 d |
| Lin et al. [11] | 2018 | NCT03423992 | I | 3 | Recurrent GBM | N/A | EphA2-41BBζ T cells | Intravenous | mOS: 5.5 mo |
| Wang et al. [12] | 2019 | N/A | N/A | 10 | Recurrent GBM | N/A | EGFRvIII CAR-T cells | N/A | mOS: 247 d |
| Goff et al. [13] | 2019 | NCT01454569 | I | 18 | Recurrent GBM | N/A | Autologous EGFRvIII-specific CAR-T cells | Intravenous | mOS: 6.9 mo; mPFS: 1.3 mo |
| Liu et al. [14] | 2023 | NCT03170141 | I | 8 | Recurrent GBM | 24 | Autologous GD2-specific 4SCAR-T cells | Intravenous or directly to the tumor location | mOS: 10 mo |
| Principal Investigator | Year | Trial Name | Phase | Status | Patients (N) | Diagnosis (Target Glioma) | Treatment | Drug Delivery | Primary Objectives |
|---|---|---|---|---|---|---|---|---|---|
| Badie | 2010 | NCT01082926 | I | Completed | 6 | Recurrent malignant glioma | GRm13Z40-2 (Allogeneic CD8+ Cytolitic T-Cell Line Genetically Modified to Express the IL 13-Zetakine and HyTK and to be Resistant to Glucocorticoids) in Combination With Interleukin-2 | Intratumoral | Safety |
| Badie | 2019 | NCT04003649 | I | Recruiting | 60 | Recurrent GBM | IL13 Rα2-targeted CAR-T cells with or without nivolumab and ipilimumab | Intravenous | AEs; DLT; feasibility; OS |
| Chen | 2019 | NCT04045847 | I | Unknown | 31 | Recurrent GBM | CD147-CART | Intracavity | Safety; Tolerance; Efficacy |
| O’Rourke | 2019 | NCT03726515 | I | Completed | 7 | GBM (unmethylated MGMT, EGFRvIII+) | CART-EGFRvIII + Pembrolizumab | NA | Safety; tolerability |
| Mackall | 2020 | NCT04196413 | I | Recruiting | 54 | H3K27M-mutated DIPG of the brainstem, or H3K27M-mutated DMG of the spinal cord | GD2 CAR-T cells; Fludarabine; Cyclophosphamide | Intravenous | mOS: 7.8 mo |
| Omer | 2020 | NCT04099797 | I | Recruiting | 34 | GD2-expressing newly diagnosed DMG (DIPG or HGG) | C7R-GD2.CART cells | Intracerebroventricularly | Efficacy |
| Badie | 2020 | NCT04214392 | I | Recruiting | 36 | Recurrent GBM | Chlorotoxin (EQ)-CD28-CD3zeta-CD19t-expressing CAR T-lymphocytes | Intracerebroventricularly | Feasibility; safety |
| Reinikainen | 2020 | NCT05063682 | I | Unknown | 10 | Leptomeningeal disease from glioblastoma | EGFRvIII-CAR-T cells | Intracerebroventricular | Feasibility; safety |
| Kuo | 2020 | NCT04717999 | NA | Unknown | 20 | Recurrent GBM | NKG2D CAR-T cell | Intracerebroventricular injection through an Ommaya catheter | DLT |
| Feldman | 2021 | NCT04661384 | I | Recruiting | 30 | leptomeningeal disease from glioblastoma, ependymoma, or medulloblastoma | IL13 Rα2-targeted CAR-T cells | Intracerebroventricular | AEs; OS |
| Xu | 2021 | NCT05131763 | I | Unknown | 3 | Hepatocellular carcinoma, GBM, medulloblastoma and colon cancer | NKG2D CAR-T cells | NA | AEs |
| Zhang | 2022 | NCT04385173 | I | Recruiting | 12 | Recurrent GBM | B7-H3-targeted CAR-T cells with temozolomide | Intratumoral/intracerebroventricular | AEs; MTD; OS; PFS |
| Cheng | 2022 | NCT05366179 | I | Recruiting | 36 | Relapsed/refractory GBM | B7-H3 CAR-T cells | Intraventricular infusion | AEs |
| Zhang | 2022 | NCT05241392 | I | Recruiting | 30 | Recurrent GBM | B7-H3 CAR-T cells | Intracranial tumor resection cavity or ventricular system using an Ommaya device | Incidence of AEs; DLT |
| Mackall | 2022 | NCT05474378 | I | Recruiting | 39 | Recurrent GBM | B7-H3 CAR-T cells | ICV or both ICV and intratumorally (IT) | Number of successful manufacturing product (B7-H3CART) that met minimum assigned dose level range; MTD or Recommended phase 2 dose |
| Zhang | 2023 | NCT04077866 | I/II | Recruiting | 40 | Recurrent GBM | B7-H3-targeted CAR-T cells with or without temozolomide | Intratumoral/intracerebroventricular | OS; PFS |
| Xuejun | 2023 | NCT05802693 | I | Not yet recruiting | 22 | Recurrent GBM | EGFRvIII CAR-T cells | Infusion with Omaya capsule? | Incidence of AEs; DLT |
| Bagley | 2023 | NCT05168423 | I | Recruiting | 6 | Recurrent GBM | EGFR-IL13Rα2 CAR-T cells | Intrathecal | Incidence of AEs; DLT |
| Doyle | 2023 | NCT05835687 | I | Recruiting | 36 | Relapsed/refractory non-brainstem primary CNS tumors and brainstem high-grade neoplasms | B7-H3 CAR-T cells | CNS reservoir catheter | MTD |
| Litten | 2023 | NCT05627323 | I | Recruiting | 42 | Recurrent GBM | CHM-1101 CAR-T cells | Intracavitary/intratumoral and intraventricular | Incidence of AEs; DLT |
| Zhang | 2023 | NCT05577091 | I | Recruiting | 10 | Recurrent GBM | IL7Rα modified CAR T lymphocytes | Intratumoral or intraventricular administration via Ommaya reservoir | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agosti, E.; Garaba, A.; Antonietti, S.; Ius, T.; Fontanella, M.M.; Zeppieri, M.; Panciani, P.P. CAR-T Cells Therapy in Glioblastoma: A Systematic Review on Molecular Targets and Treatment Strategies. Int. J. Mol. Sci. 2024, 25, 7174. https://doi.org/10.3390/ijms25137174
Agosti E, Garaba A, Antonietti S, Ius T, Fontanella MM, Zeppieri M, Panciani PP. CAR-T Cells Therapy in Glioblastoma: A Systematic Review on Molecular Targets and Treatment Strategies. International Journal of Molecular Sciences. 2024; 25(13):7174. https://doi.org/10.3390/ijms25137174
Chicago/Turabian StyleAgosti, Edoardo, Alexandru Garaba, Sara Antonietti, Tamara Ius, Marco Maria Fontanella, Marco Zeppieri, and Pier Paolo Panciani. 2024. "CAR-T Cells Therapy in Glioblastoma: A Systematic Review on Molecular Targets and Treatment Strategies" International Journal of Molecular Sciences 25, no. 13: 7174. https://doi.org/10.3390/ijms25137174
APA StyleAgosti, E., Garaba, A., Antonietti, S., Ius, T., Fontanella, M. M., Zeppieri, M., & Panciani, P. P. (2024). CAR-T Cells Therapy in Glioblastoma: A Systematic Review on Molecular Targets and Treatment Strategies. International Journal of Molecular Sciences, 25(13), 7174. https://doi.org/10.3390/ijms25137174









