Overview and Evolution of Insect Fibroin Heavy Chain (FibH)
Abstract
1. Introduction
2. Results
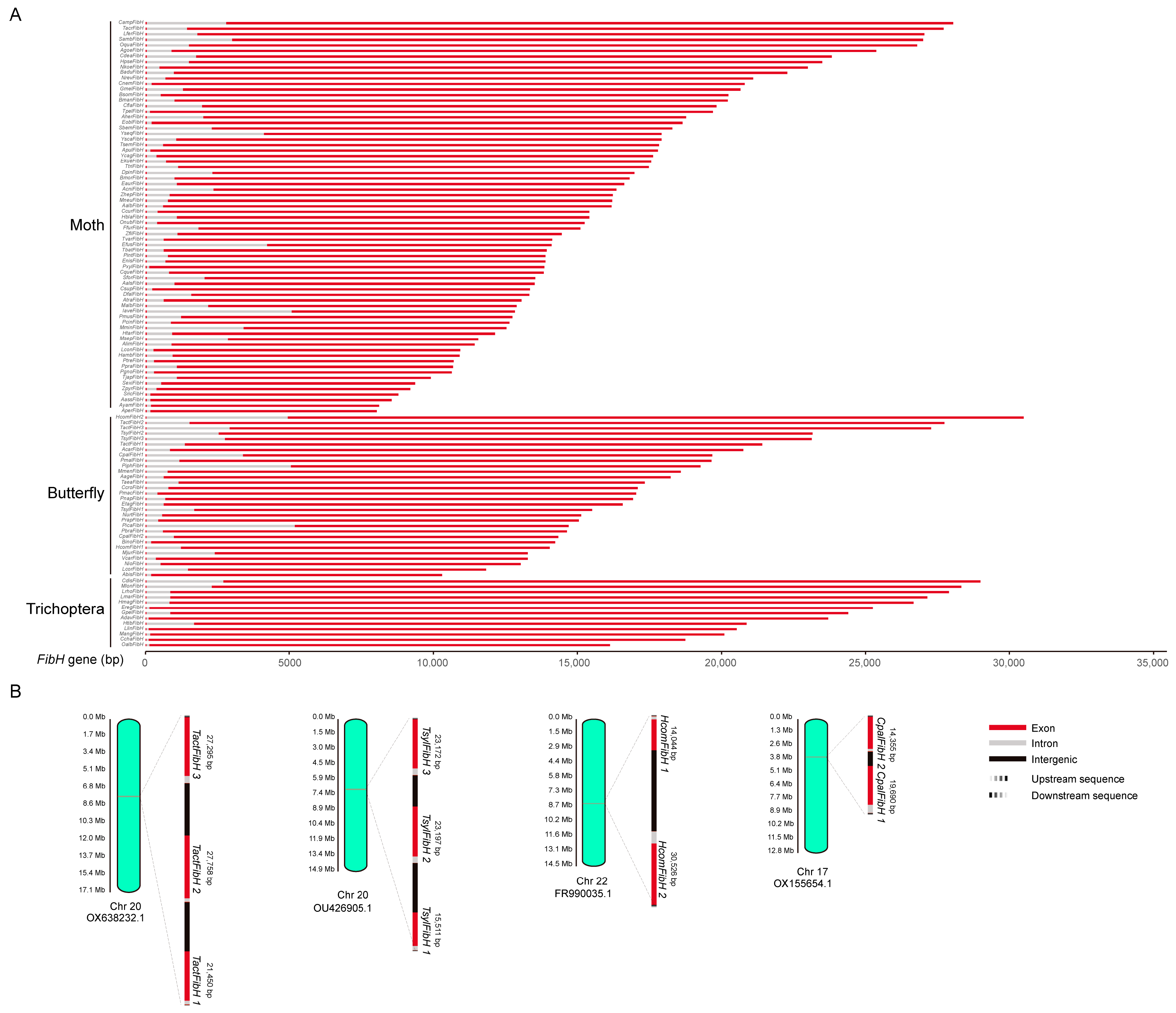
2.1. FibH Gene Copy Number Variation Occurs Only in Skipper (Hesperiidae)
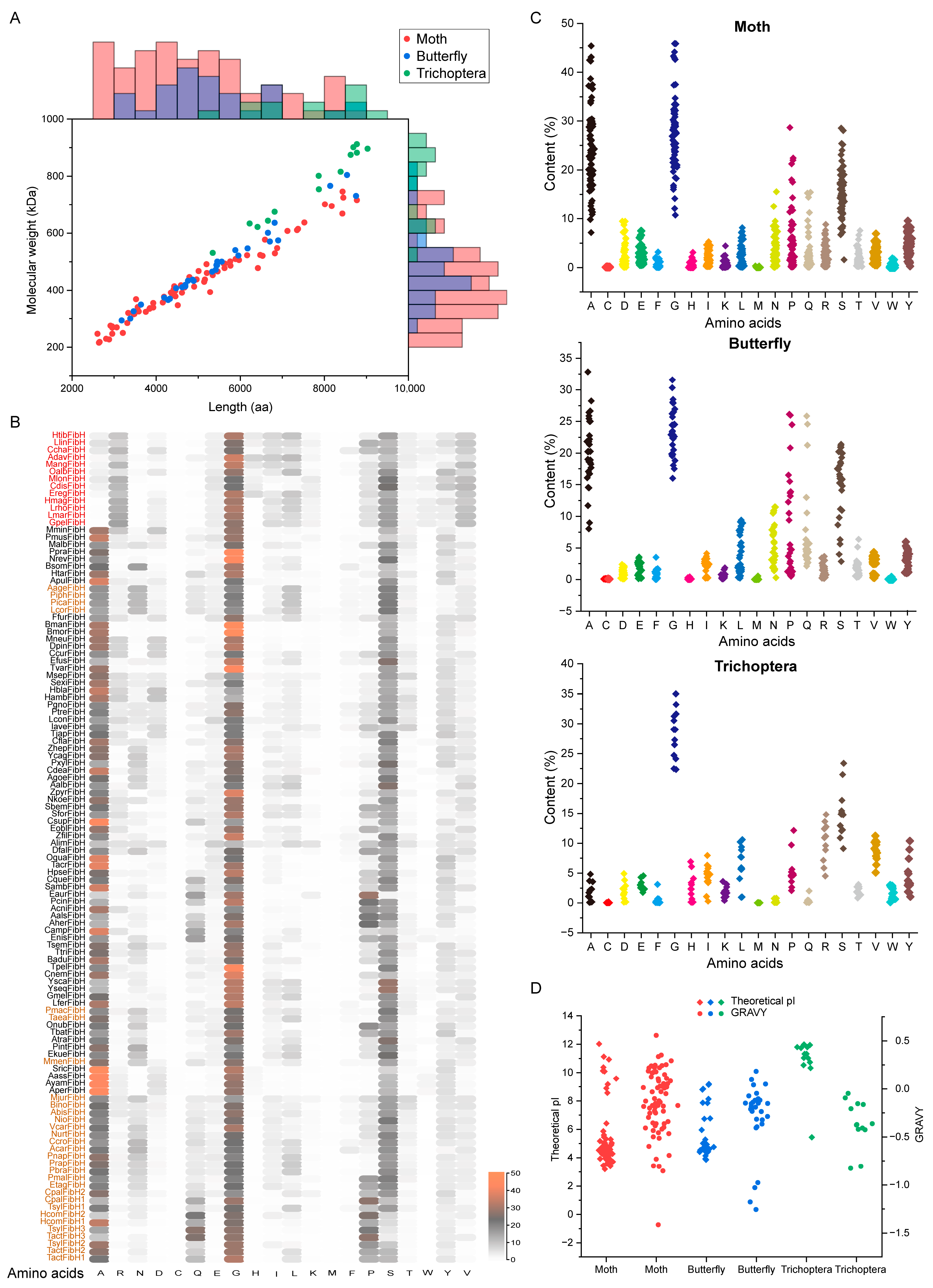
2.2. FibH Differs in Length, Amino Acid Composition, and Properties among Insects
2.3. Insect FibH Has Developed Unique Motifs during Evolution
3. Discussion
4. Materials and Methods
4.1. Extract the Complete FibH Gene Sequence from Database
4.2. Data Analysis and Graphing
4.3. Three-Dimensional Structure Prediction of FibH
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sutherland, T.D.; Young, J.H.; Weisman, S.; Hayashi, C.Y.; Merritt, D.J. Insect Silk: One Name, Many Materials. Annu. Rev. Entomol. 2010, 55, 171–188. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Li, S.; Feng, Q. Advances in Silkworm Studies Accelerated by the Genome Sequencing of Bombyx mori. Annu. Rev. Entomol. 2014, 59, 513–536. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, T.D.; Weisman, S.; Trueman, H.E.; Sriskantha, A.; Trueman, J.W.H.; Haritos, V.S. Conservation of Essential Design Features in Coiled Coil Silks. Mol. Biol. Evol. 2007, 24, 2424–2432. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Lua, S.; Du, N.; Liu, X.; Song, J. Identification, recombinant production and structural characterization of four silk proteins from the Asiatic honeybee Apis cerana. Biomaterials 2008, 29, 2820–2828. [Google Scholar] [CrossRef] [PubMed]
- Inoue, S.; Tanaka, K.; Arisaka, F.; Kimura, S.; Ohtomo, K.; Mizuno, S. Silk fibroin of Bombyx mori is secreted, assembling a high molecular mass elementary unit consisting of H-chain, L-chain, and P25, with a 6:6:1 molar ratio. J. Biol. Chem. 2000, 275, 40517–40528. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Yang, X.; Liu, C.; Dong, Z.; Wang, F.; Wang, X.; Hu, W.; Zhang, X.; Zhao, P.; Xia, Q. Structural and Mechanical Properties of Silk from Different Instars of Bombyx mori. Biomacromolecules 2019, 20, 1203–1216. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Li, J.; Guo, K.; Lu, M.; Zhang, Y.; Zhang, X.; Zeng, Y.; Wang, X.; Xia, Q.; Zhao, P.; et al. Insights into the structure and composition of mineralized hard cocoons constructed by the oriental moth, Monema (Cnidocampa) flavescens Walker. Insect Biochem. Mol. Biol. 2022, 151, 103878. [Google Scholar] [CrossRef] [PubMed]
- Rouhová, L.; Sehadová, H.; Pauchová, L.; Hradilová, M.; Žurovcová, M.; Šerý, M.; Rindoš, M.; Žurovec, M. Using the multi-omics approach to reveal the silk composition in Plectrocnemia conspersa. Front. Mol. Biosci. 2022, 9, 945239. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.Z.; Confalonieri, F.; Jacquet, M.; Perasso, R.; Li, Z.G.; Janin, J. Silk fibroin: Structural implications of a remarkable amino acid sequence. Proteins 2001, 44, 119–122. [Google Scholar] [CrossRef]
- He, Y.-X.; Zhang, N.-N.; Li, W.-F.; Jia, N.; Chen, B.-Y.; Zhou, K.; Zhang, J.; Chen, Y.; Zhou, C.-Z. N-Terminal Domain of Bombyx mori Fibroin Mediates the Assembly of Silk in Response to pH Decrease. J. Mol. Biol. 2012, 418, 197–207. [Google Scholar] [CrossRef]
- Hu, W.; Lu, W.; Wei, L.; Zhang, Y.; Xia, Q. Molecular nature of dominant naked pupa mutation reveals novel insights into silk production in Bombyx mori. Insect Biochem. Mol. Biol. 2019, 109, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; Long, D.; Zhang, Y.; Umuhoza, D.; Dai, J.; Xu, Z.; Zhang, G.; Meng, W.; Xiang, Z.; Zhao, A. New insight into the mechanism of in vivo fibroin self-assembly and secretion in the silkworm, Bombyx mori. Int. J. Biol. Macromol. 2021, 169, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Ma, S.; Sun, L.; Zhang, T.; Wang, X.; Feng, M.; Wang, A.; Shi, R.; Jia, L.; Xia, Q. Combined CRISPR toolkits reveal the domestication landscape and function of the ultra-long and highly repetitive silk genes. Acta Biomater. 2023, 158, 190–202. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Tortolero, R.O.; Luo, Y.; Parmeggiani, F.; Skaer, N.; Walker, R.; Serpell, L.; Holland, C.; Davis, S.A. Silk Road Revealed: Mechanism of silk fibre formation in Bombyx mori. bioRxiv 2023. [Google Scholar] [CrossRef]
- Zhou, C.Z.; Confalonieri, F.; Medina, N.; Zivanovic, Y.; Esnault, C.; Yang, T.; Jacquet, M.; Janin, J.; Duguet, M.; Perasso, R.; et al. Fine organization of Bombyx mori fibroin heavy chain gene. Nucleic Acids Res. 2000, 28, 2413–2419. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Zhang, T.; Zhang, Q.; Zhang, N.; Jia, L.; Ma, S.; Xia, Q. FibH Gene Complete Sequences (FibHome) Revealed Silkworm Pedigree. Insects 2023, 14, 244. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Chen, X.; Shao, Z.; Huang, Y.; Knight, D.P. Effect of Metallic Ions on Silk Formation in the Mulberry Silkworm, Bombyx mori. J. Phys. Chem. B 2005, 109, 16937–16945. [Google Scholar] [CrossRef] [PubMed]
- Greving, I.; Cai, M.; Vollrath, F.; Schniepp, H.C. Shear-Induced Self-Assembly of Native Silk Proteins into Fibrils Studied by Atomic Force Microscopy. Biomacromolecules 2012, 13, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Han, Y.; Ling, S.; Huang, Y.; Yao, J.; Shao, Z.; Chen, X. Direct Observation of Native Silk Fibroin Conformation in Silk Gland of Bombyx mori Silkworm. ACS Biomater. Sci. Eng. 2020, 6, 1874–1879. [Google Scholar] [CrossRef]
- Suzuki, Y.; Morie, S.; Okamura, H.; Asakura, T.; Naito, A. Real-Time Monitoring of the Structural Transition of Bombyx mori Liquid Silk under Pressure by Solid-State NMR. J. Am. Chem. Soc. 2023, 145, 22925–22933. [Google Scholar] [CrossRef]
- Ma, S.; Shi, R.; Wang, X.; Liu, Y.; Chang, J.; Gao, J.; Lu, W.; Zhang, J.; Zhao, P.; Xia, Q. Genome editing of BmFib-H gene provides an empty Bombyx mori silk gland for a highly efficient bioreactor. Sci. Rep. 2014, 4, 6867. [Google Scholar] [CrossRef]
- Malay, A.D.; Sato, R.; Yazawa, K.; Watanabe, H.; Ifuku, N.; Masunaga, H.; Hikima, T.; Guan, J.; Mandal, B.B.; Damrongsakkul, S.; et al. Relationships between physical properties and sequence in silkworm silks. Sci. Rep. 2016, 6, 27573. [Google Scholar] [CrossRef] [PubMed]
- Frandsen, P.B.; Hotaling, S.; Powell, A.; Heckenhauer, J.; Kawahara, A.Y.; Baker, R.H.; Hayashi, C.Y.; Ríos-Touma, B.; Holzenthal, R.; Pauls, S.U.; et al. Allelic resolution of insect and spider silk genes reveals hidden genetic diversity. Proc. Natl. Acad. Sci. USA 2023, 120, e2221528120. [Google Scholar] [CrossRef] [PubMed]
- Takasu, Y.; Yamada, N.; Kojima, K.; Iga, M.; Yukuhiro, F.; Iizuka, T.; Yoshioka, T. Fibroin heavy chain gene replacement with a highly ordered synthetic repeat sequence in Bombyx mori. Insect Biochem. Mol. Biol. 2023, 161, 104002. [Google Scholar] [CrossRef] [PubMed]
- Malay, A.D.; Craig, H.C.; Chen, J.; Oktaviani, N.A.; Numata, K. Complexity of Spider Dragline Silk. Biomacromolecules 2022, 23, 1827–1840. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Kono, N.; Mori, M.; Masunaga, H.; Numata, K.; Arakawa, K. Composition of Minor Ampullate Silk Makes Its Properties Different from Those of Major Ampullate Silk. Biomacromolecules 2023, 24, 2042–2051. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Shi, R.; Li, X.; Ma, S.; Yang, D.; Shang, D.; Xia, Q. A review on complete silk gene sequencing and de novo assembly of artificial silk. Int. J. Biol. Macromol. 2024, 264, 130444. [Google Scholar] [CrossRef] [PubMed]
- Kono, N.; Ohtoshi, R.; Malay, A.D.; Mori, M.; Masunaga, H.; Yoshida, Y.; Nakamura, H.; Numata, K.; Arakawa, K. Darwin’s bark spider shares a spidroin repertoire with Caerostris extrusa but achieves extraordinary silk toughness through gene expression. Open Biol. 2021, 11, 210242. [Google Scholar] [CrossRef] [PubMed]
- Kono, N.; Nakamura, H.; Mori, M.; Yoshida, Y.; Ohtoshi, R.; Malay, A.D.; Moran, D.A.P.; Tomita, M.; Numata, K.; Arakawa, K. Multicomponent nature underlies the extraordinary mechanical properties of spider dragline silk. Proc. Natl. Acad. Sci. USA 2021, 118, e2107065118. [Google Scholar] [CrossRef]
- Song, K.; Wang, Y.; Dong, W.; Li, Z.; Xia, Q.; Zhu, P.; He, H. Decoding silkworm spinning programmed by pH and metal ions. Sci. Bull. 2024, 69, 792–802. [Google Scholar] [CrossRef]
- Yoshioka, T.; Tsubota, T.; Tashiro, K.; Jouraku, A.; Kameda, T. A study of the extraordinarily strong and tough silk produced by bagworms. Nat. Commun. 2019, 10, 1469. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Peng, D.; Zheng, L.; Liu, D.; Han, C.; Wang, X.; Yang, Y.; Song, L.; Zhao, M.; Wei, Y.; Li, J.; et al. Large-language models facilitate discovery of the molecular signatures regulating sleep and activity. Nat. Commun. 2024, 15, 3685. [Google Scholar] [CrossRef]
- Lin, Z.; Akin, H.; Rao, R.; Hie, B.; Zhu, Z.; Lu, W.; Smetanin, N.; Verkuil, R.; Kabeli, O.; Shmueli, Y.; et al. Evolutionary-scale prediction of atomic-level protein structure with a language model. Science 2023, 379, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Ruffolo, J.A.; Nayfach, S.; Gallagher, J.; Bhatnagar, A.; Beazer, J.; Hussain, R.; Russ, J.; Yip, J.; Hill, E.; Pacesa, M.; et al. Design of highly functional genome editors by modeling the universe of CRISPR-Cas sequences. bioRxiv 2024. [Google Scholar] [CrossRef]
- Jiang, M.; Shu, T.; Ye, C.; Ren, J.; Ling, S. Predicting the conformations of the silk protein through deep learning. Analyst 2021, 146, 2490–2498. [Google Scholar] [CrossRef]
- Kim, Y.; Yoon, T.; Park, W.B.; Na, S. Predicting mechanical properties of silk from its amino acid sequences via machine learning. J. Mech. Behav. Biomed. Mater. 2023, 140, 105739. [Google Scholar] [CrossRef]
- Fazio, V.; Pugno, N.M.; Giustolisi, O.; Puglisi, G. Hierarchical physically based machine learning in material science: The case study of spider silk. arXiv 2023. [Google Scholar] [CrossRef]
- Almagro Armenteros, J.J.; Tsirigos, K.D.; Sønderby, C.K.; Petersen, T.N.; Winther, O.; Brunak, S.; Von Heijne, G.; Nielsen, H. SignalP 5.0 improves signal peptide predictions using deep neural networks. Nat. Biotechnol. 2019, 37, 420–423. [Google Scholar] [CrossRef]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; Von Haeseler, A.; Lanfear, R. IQ-TREE 2: New Models and Efficient Methods for Phylogenetic Inference in the Genomic Era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef]
- Mirdita, M.; Schütze, K.; Moriwaki, Y.; Heo, L.; Ovchinnikov, S.; Steinegger, M. ColabFold: Making protein folding accessible to all. Nat. Methods 2022, 19, 679–682. [Google Scholar] [CrossRef] [PubMed]

| Gene | Exon 1 (bp) | Intron (bp) | CDS (bp) | Protein (aa) | Signal Peptide (aa) | PI | Grand Average of Hydropathicity (GRAVY) | Top 5 Amino Acids |
|---|---|---|---|---|---|---|---|---|
| BmorFibH | 42 | 971 | 15,792 | 5263 | 18 | 4.39 | 0.216 | Gly, Ala, Ser, Tyr, Val |
| HcomFibH 1 | 42 | 1189 | 12,855 | 4284 | 18 | 9.19 | −0.288 | Ala, Gly, Pro, Gln, Ser |
| HcomFibH 2 | 42 | 4906 | 25,620 | 8539 | 18 | 8.85 | −1.029 | Gly, Pro, Gln, Ala, Ser |
| TactFibH 1 | 42 | 1338 | 20,112 | 6703 | 18 | 4.89 | −0.323 | Gly, Ala, Pro, Ser, Gln |
| TactFibH 2 | 42 | 1493 | 26,265 | 8754 | 18 | 7.87 | −0.153 | Gly, Ala, Pro, Ser, Gln |
| TactFibH 3 | 42 | 2878 | 24,417 | 8138 | 18 | 7.85 | −1.256 | Gly, Pro, Gln, Ala, Ser |
| TsylFibH 1 | 42 | 1648 | 13,863 | 4620 | 18 | 5.29 | −0.104 | Gly, Ala, Ser, Pro, Gln |
| TsylFibH 2 | 42 | 2491 | 20,706 | 6901 | 18 | 9.14 | −0.155 | Ala, Gly, Pro, Ser, Gln |
| TsylFibH 3 | 42 | 2718 | 20,454 | 6817 | 18 | 8.14 | −1.178 | Gly, Gln, Pro, Ala, Ser |
| CpalFibH 1 | 42 | 3349 | 16,341 | 5446 | 18 | 6.74 | −0.977 | Gly, Pro, Gln, Ala, Ser |
| CpalFibH 2 | 42 | 933 | 13,422 | 4473 | 18 | 5.02 | −0.395 | Ala, Gly, Ser, Pro, Tyr |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.; Ma, S.; Zhang, Z.; Guo, Y.; Yang, D.; Lu, W. Overview and Evolution of Insect Fibroin Heavy Chain (FibH). Int. J. Mol. Sci. 2024, 25, 7179. https://doi.org/10.3390/ijms25137179
Zhang T, Ma S, Zhang Z, Guo Y, Yang D, Lu W. Overview and Evolution of Insect Fibroin Heavy Chain (FibH). International Journal of Molecular Sciences. 2024; 25(13):7179. https://doi.org/10.3390/ijms25137179
Chicago/Turabian StyleZhang, Tong, Sanyuan Ma, Ziyang Zhang, Yongkang Guo, Daiying Yang, and Wei Lu. 2024. "Overview and Evolution of Insect Fibroin Heavy Chain (FibH)" International Journal of Molecular Sciences 25, no. 13: 7179. https://doi.org/10.3390/ijms25137179
APA StyleZhang, T., Ma, S., Zhang, Z., Guo, Y., Yang, D., & Lu, W. (2024). Overview and Evolution of Insect Fibroin Heavy Chain (FibH). International Journal of Molecular Sciences, 25(13), 7179. https://doi.org/10.3390/ijms25137179






