Intramuscular Pulsed Radiofrequency Upregulates BNDF-TrKB Expression in the Spinal Cord in Rats as an Alternative Treatment for Complicated Pain
Abstract
:1. Introduction
2. Results
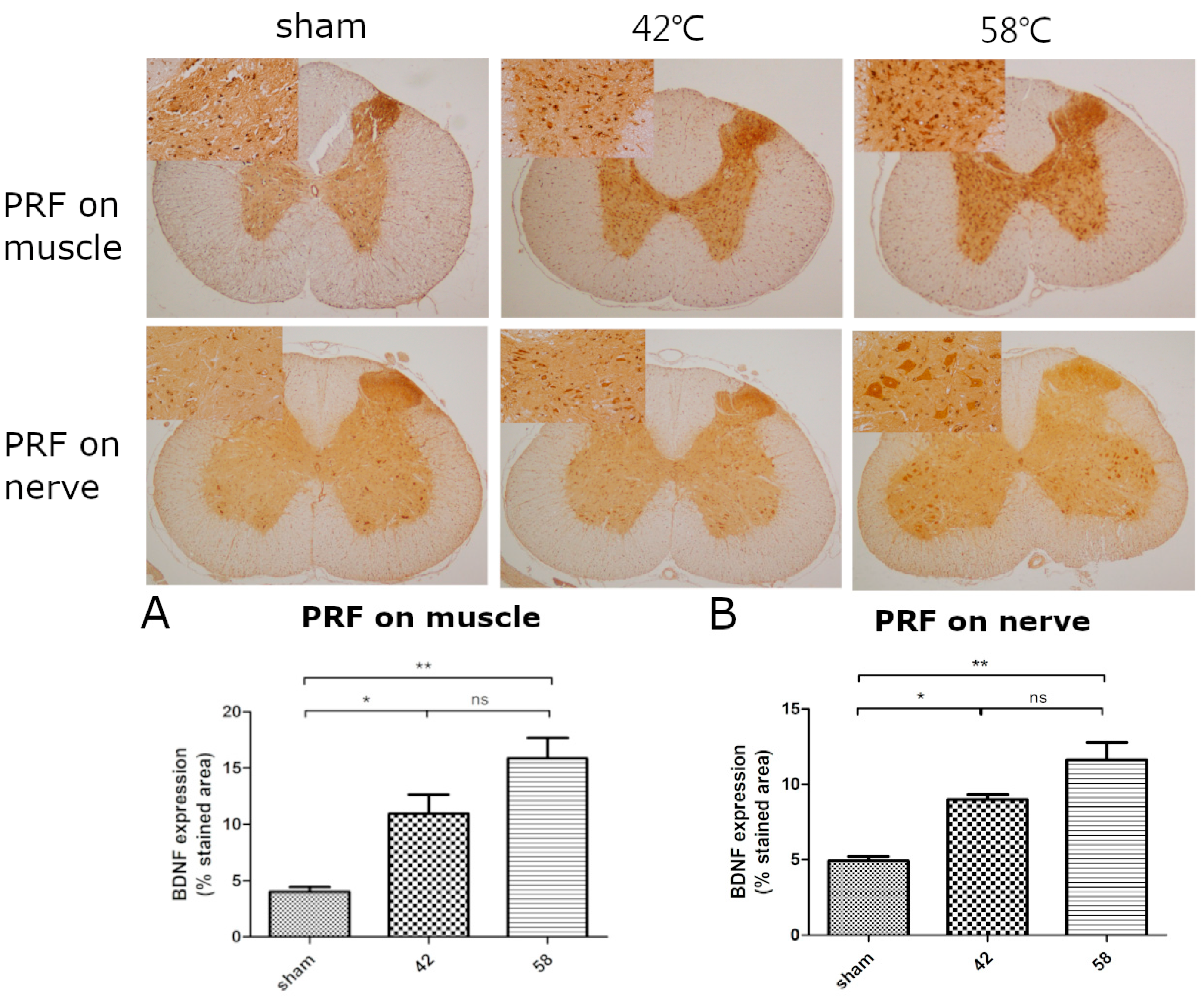
2.1. The First Stage
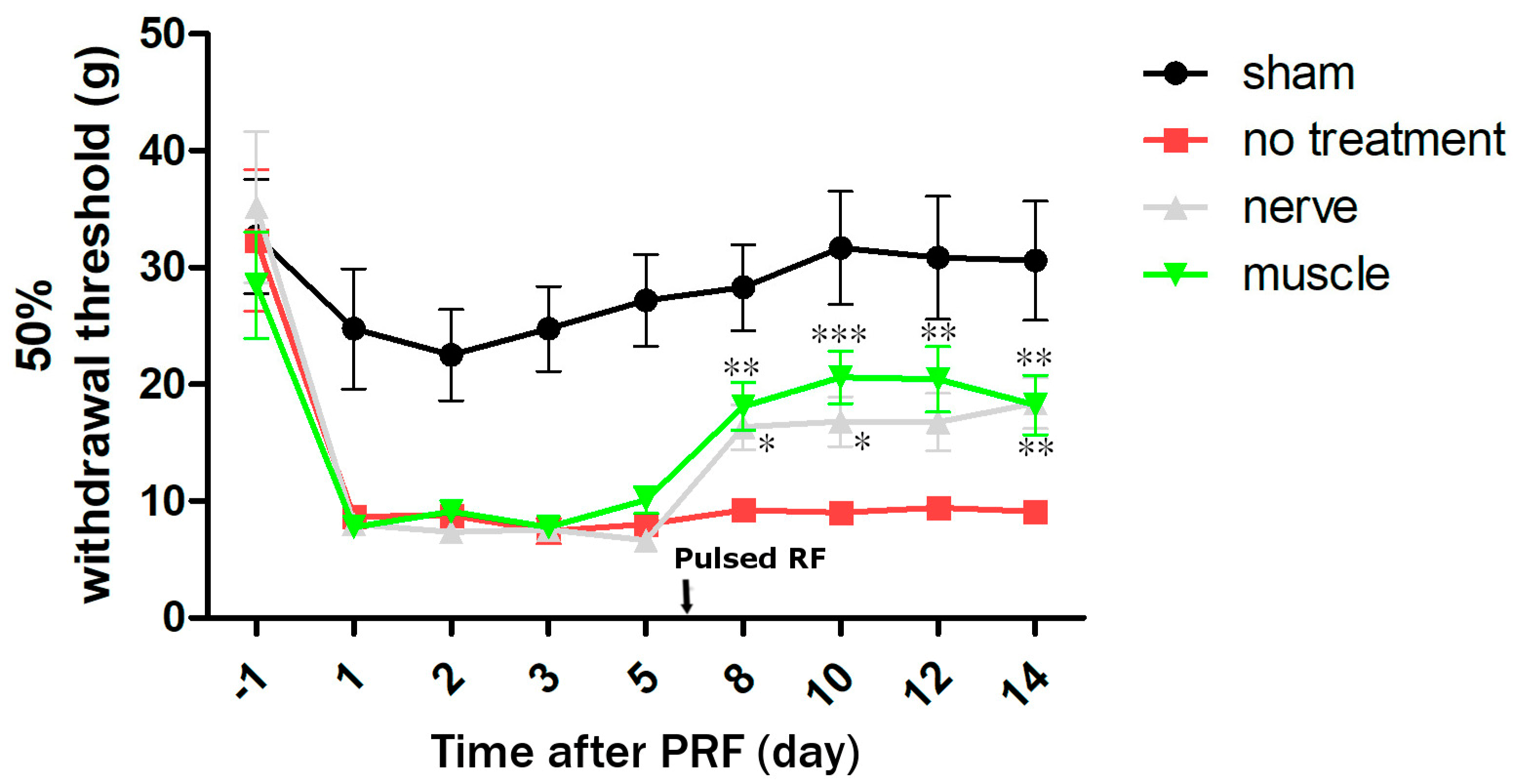
2.2. The Second Stage
3. Discussion
4. Materials and Methods
4.1. First Stage: PRF on the Muscle and Sciatic Nerve without Injury
4.1.1. PRF Treatment
4.1.2. Muscle Groups
4.1.3. Sciatic Nerve Group
4.1.4. Neurological Function
4.1.5. Determination of Intercellular Adhesion Molecule 1 (ICAM-1), Vascular Endothelial Growth Factor (VEGF), and BDNF Levels
4.1.6. Histology and Immunohistochemistry
4.2. Second Stage: PRF on Muscle and Sciatic Nerve after Nerve Injury
4.2.1. Experimental Protocol (The Second Stage)
4.2.2. Neurological Function for Pain
4.2.3. BDNF Determination
4.2.4. Histology and Immunohistochemistry
4.3. Statistical Analysis
4.3.1. The First Stage
4.3.2. The Second Stage
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Zundert, J.; Raj, P.; Erdine, S.; van Kleef, M. Application of radiofrequency treatment in practical pain management: State of the art. Pain Pract. 2002, 2, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Bogduk, N. Pulsed radiofrequency. Pain Med. 2006, 7, 396–407. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, B.D.; Wang, J.; Arnold, P.M.; Hermsmeyer, J.; Norvell, D.C.; Brodke, D.S. Predicting the risk of adjacent segment pathology after lumbar fusion: A systematic review. Spine 2012, 37, S123–S132. [Google Scholar] [CrossRef] [PubMed]
- Klessinger, S. Zygapophysial joint pain in post lumbar surgery syndrome. The efficacy of medial branch blocks and radiofrequency neurotomy. Pain Med. 2013, 14, 374–377. [Google Scholar] [CrossRef] [PubMed]
- Herzberg, D.E.; Hedie, A. Bustamante animal models of chronic pain. Are naturally occurring diseases a potential model for translational research. Austral J. Vet. Sci. 2021, 53, 47–54. [Google Scholar] [CrossRef]
- Fernández-de-Las-Peñas, C.; Nijs, J.; Cagnie, B.; Gerwin, R.D.; Plaza-Manzano, G.; Valera-Calero, J.A.; Arendt-Nielsen, L. Myofascial pain syndrome: A nociceptive condition comorbid with neuropathic or nociplastic pain. Life 2023, 13, 694. [Google Scholar] [CrossRef] [PubMed]
- Constandil, L.; Aguilera, R.; Goich, M.; Hernández, A.; Alvarez, P.; Infantec, C.; Pelissier, T. Involvement of spinal cord BDNF in the generation and maintenance of chronic neuropathic pain in rats. Brain Res. Bull. 2011, 86, 454–459. [Google Scholar] [CrossRef]
- Thompson, S.W.N.; Bennett, D.L.H.; Kerr, B.J.; Bradbury, E.J.; McMahon, S.B. Brain-derived neurotrophic factor is an endogenous modulator of nociceptive responses in the spinal cord. Proc. Natl. Acad. Sci. USA 1999, 96, 7714–7718. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.Y.; Huang, K.F.; Chen, J.C.; Lai, M.F.; Ma, K.H.; Yeh, C.C. The clinical application of pulsed radiofrequency induces inflammatory pain via MAPKs activation: A novel hint for pulsed radiofrequency treatment. Int. J. Mol. Sci. 2021, 22, 11865. [Google Scholar] [CrossRef] [PubMed]
- Sluijter, M.; Racz, G. Technical aspects of radiofrequency. Pain Pract. 2002, 2, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Liliang, P.C.; Lu, K.; Hsieh, C.H.; Kao, C.Y.; Wang, K.W.; Chen, H.J. Pulsed radiofrequency of cervical medial branches for treatment of whiplash-related cervical zygapophysial joint pain. Surg. Neurol. 2008, 70 (Suppl. S1), 50–55. [Google Scholar] [CrossRef] [PubMed]
- Skootsky, S.A.; Jaeger, B.; Oye, R. Prevalence of myofascial pain in general internal medicine practice. West J. Med. 1989, 151, 157–160. [Google Scholar] [PubMed]
- Mazin, A.; Michael, H.M.; Jason, K. A case series of pulsed radiofrequency treatment of myofascial trigger points and scar neuromas. Pain Med. 2009, 10, 1140–1143. [Google Scholar] [CrossRef]
- Robert, B. Myofascial pain syndromes and their evaluation. Best Pract. Res. Clin. Rheumatol. 2007, 21, 427–445. [Google Scholar] [CrossRef]
- Margalef, R.; Sisquella, M.; Bosque, M.; Romeu, C.; Mayoral, O.; Monterde, S.; Priego, M.; Guerra-Perez, R.; Ortiz, N.; Tomàs, J.; et al. Experimental myofascial trigger point creation in rodents. Appl. Physiol. 2019, 126, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, L.; Garrie, K.; Turner, M.D. Role of S100 proteins in health and disease. Biochimica et Biophysica Acta Acta Mol. Cell Res. 2020, 1867, 118677. [Google Scholar] [CrossRef] [PubMed]
- Van Acker, N.; Ragé, M.; Sluydts, E.; Knaapen, M.W.; De Bie, M.; Timmers, M.; Fransen, E.; Duymelinck, C.; De Schepper, S.; Anand, P.; et al. Automated PGP9.5 immunofluorescence staining: A valuable tool in the assessment of small fiber neuropathy? BMC Res. Notes 2016, 9, 280. [Google Scholar] [CrossRef] [PubMed]
- Reinders, M.E.; Sho, M.; Izawa, A.; Wang, P.; Mukhopadhyay, D.; Koss, K.E.; Geehan, C.S.; Luster, A.D.; Sayegh, M.H.; Briscoe, D.M. Proinflammatory functions of vascular endothelial growth factor in alloimmunity. J. Clin. Investig. 2003, 112, 1655–1665. [Google Scholar] [CrossRef] [PubMed]
- Merighi, A.; Salio, C.; Ghirri, A.; Lossi, L.; Ferrini, F.; Betelli, C.; Bardoni, R. BDNF as a pain modulator. Prog. Neurobiol. 2008, 85, 297–317. [Google Scholar] [CrossRef] [PubMed]
- Kerr, B.J.; Bradbury, E.J.; Bennett, D.L.H.; Trivedi, P.M.; Dassan, P.; French, J.; Shelton, D.B.; McMahon, S.B.; Thompson, S.W.N. Brain-derived neurotrophic factor modulates nociceptive sensory inputs and NMDA-evoked responses in the rat spinal cord. J. Neurosci. 1999, 19, 5138–5148. [Google Scholar] [CrossRef] [PubMed]
- Mannion, R.J.; Costigan, M.; Decosterd, I.; Amaya, F.; Ma, Q.P.; Holstege, J.C.; Ji, R.R.; Acheson, A.; Lindsay, R.M.; Wilkinson, G.A.; et al. Neurotrophins: Peripherally and centrally acting modulators of tactile stimulus-induced inflammatory pain hypersensitivity. Proc. Natl. Acad. Sci. USA 1999, 96, 9385–9390. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.L.; Wang, S.W.; Chen, H.J.; Tsai, Y.D.; Chen, J.S.; Wang, H.K.; Wang, K.W. Optimal cut-off points of sagittal spinopelvic parameters as a morphological parameter to predict efficiency in nerve block and pulsed radiofrequency for lumbar facet joint pain: A retrospective study. J. Pain Res. 2021, 14, 1949–1957. [Google Scholar] [CrossRef] [PubMed]
- Huie, J.R.; Garraway, S.M.; Baumbauer, K.M.; Hoy, K.C., Jr.; Beas, B.S.; Montgomery, K.S.; Bizon, J.L.; Grau, J.W. Brain-derived neurotrophic factor promotes adaptive plasticity within the spinal cord and mediates the beneficial effects of controllable stimulation. Neuroscience 2012, 200, 74–90. [Google Scholar] [CrossRef] [PubMed]
- Cappoli, N.; Tabolacci, E.; Aceto, P.; Russo, C.D. The emerging role of the BDNF-TrkB signaling pathway in the modulation of pain perception. J. Neuroimmunol. 2020, 349, 577406. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Wang, F.; Wang, L.; Xu, Y.; Lv, L.; Duan, W.; Bai, R.; Meng, Z.; Shao, X. Involvement of the BDNF-TrkB-KCC2 pathway in neuropathic pain after brachial plexus avulsion. Brain Behav. 2022, 12, e2464. [Google Scholar] [CrossRef] [PubMed]
- Siddall, P.J.; Loeser, J.D. Pain following spinal cord injury. Spinal Cord 2001, 39, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Ge, H.Y.; Fernández-de-Las-Peñas, C.; Yue, S.W. Myofascial trigger points: Spontaneous electrical activity and its consequences for pain induction and propagation. Chin. Med. 2011, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, B.; Acevedo, E.O. BDNF as a biomarker for neuropathic pain: Consideration of mechanisms of action and associated measurement challenges. Brain Behav. 2023, 13, e2903. [Google Scholar] [CrossRef] [PubMed]
- Ge, H.Y.; Fernandez-de-Las-Penas, C.; Arendt-Nielsen, L. Sympathetic facilitation of hyperalgesia evoked from myofascial tender and trigger points in patients with unilateral shoulder pain. Clin. Neurophysiol. 2006, 117, 1545–1550. [Google Scholar] [CrossRef] [PubMed]
- Yap, E.C. Myofascial Pain—An Overview. Ann. Acad. Med. Singap. 2007, 36, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Mu, C.; Xin, Y. Role of BDNF-TrkB Signaling in Regulating Anxiety and Depression-Like Behavior in Diverse Brain Regions. Clin. Neurol. Neurosci. 2023, 7, 18–30. [Google Scholar] [CrossRef]
- Farì, G.; de Sire, A.; Fallea, C.; Albano, M.; Grossi, G.; Bettoni, E.; Di Paolo, S.; Agostini, F.; Bernetti, A.; Puntillo, F.; et al. Efficacy of Radiofrequency as Therapy and Diagnostic Support in the Management of Musculoskeletal Pain: A Systematic Review and Meta-Analysis. Diagnostics 2022, 12, 600. [Google Scholar] [CrossRef] [PubMed]
- Li, D.Y.; Meng, L.; Ji, N.; Luo, F. Effect of pulsed radiofrequency on rat sciatic nerve chronic constriction injury: A preliminary study. Chin. Med. J. 2015, 128, 540–544. [Google Scholar] [CrossRef] [PubMed]
- Deuis, J.R.; Dvorakova, L.S.; Vetter, I. Methods Used to Evaluate Pain Behaviors in Rodents. Front. Mol. Neurosci. 2017, 10, 284. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, C.-L.; Yen, C.-Y.; Wang, H.-K.; Tsai, Y.-D.; Chye, C.-L.; Wang, K.-W. Intramuscular Pulsed Radiofrequency Upregulates BNDF-TrKB Expression in the Spinal Cord in Rats as an Alternative Treatment for Complicated Pain. Int. J. Mol. Sci. 2024, 25, 7199. https://doi.org/10.3390/ijms25137199
Liang C-L, Yen C-Y, Wang H-K, Tsai Y-D, Chye C-L, Wang K-W. Intramuscular Pulsed Radiofrequency Upregulates BNDF-TrKB Expression in the Spinal Cord in Rats as an Alternative Treatment for Complicated Pain. International Journal of Molecular Sciences. 2024; 25(13):7199. https://doi.org/10.3390/ijms25137199
Chicago/Turabian StyleLiang, Cheng-Loong, Cheng-Yo Yen, Hao-Kuang Wang, Yu-Duan Tsai, Cien-Leong Chye, and Kuo-Wei Wang. 2024. "Intramuscular Pulsed Radiofrequency Upregulates BNDF-TrKB Expression in the Spinal Cord in Rats as an Alternative Treatment for Complicated Pain" International Journal of Molecular Sciences 25, no. 13: 7199. https://doi.org/10.3390/ijms25137199
APA StyleLiang, C.-L., Yen, C.-Y., Wang, H.-K., Tsai, Y.-D., Chye, C.-L., & Wang, K.-W. (2024). Intramuscular Pulsed Radiofrequency Upregulates BNDF-TrKB Expression in the Spinal Cord in Rats as an Alternative Treatment for Complicated Pain. International Journal of Molecular Sciences, 25(13), 7199. https://doi.org/10.3390/ijms25137199




