Microcin C7 as a Potential Antibacterial-Immunomodulatory Agent in the Postantibiotic Era: Overview of Its Bioactivity Aspects and Applications
Abstract
:1. Introduction
2. Bacteriocins and Their Classifications
2.1. The Bacteriocins Produced by Gram-Positive Bacteria
2.2. Most Gram-Negative Bacteriocins Are from Enterobacteriaceae
3. Structure of McC7
4. Problems in the Application of AMPs and McC7 Biosynthesis
4.1. Problems in the Application of AMPs
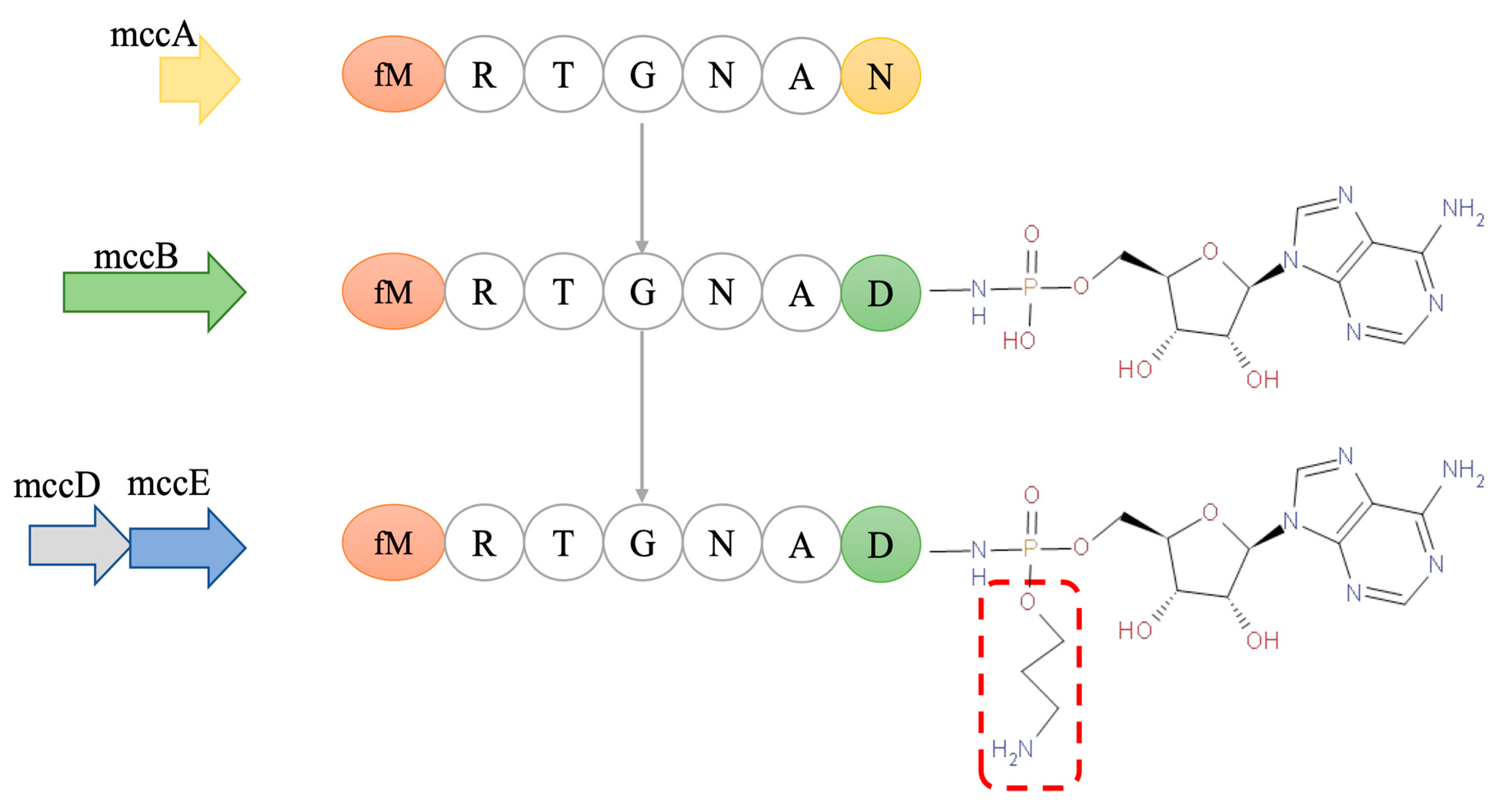
4.2. Biosynthesis of McC7
5. Research Progress on McC7 Mutations
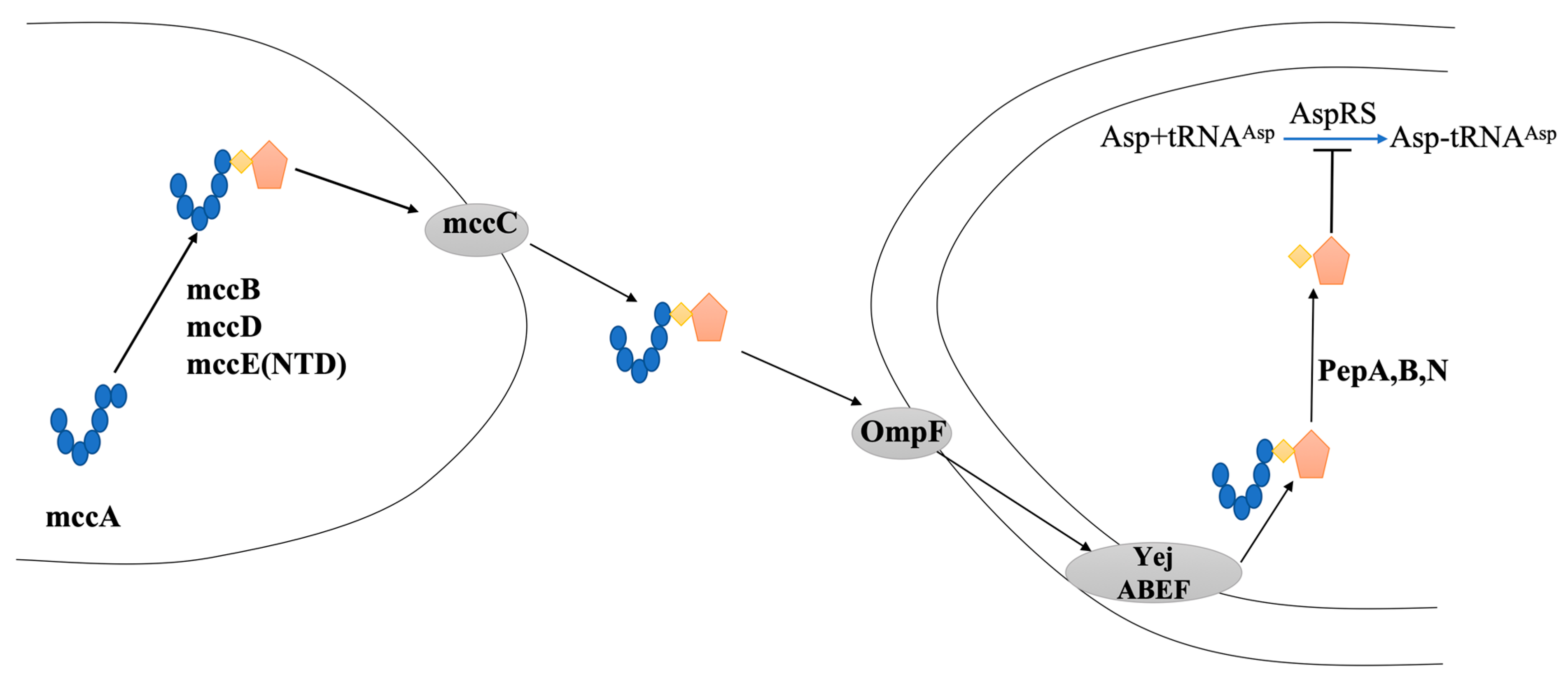
6. McC7′s Antibacterial Mechanism
6.1. The Immune Mechanism
6.2. The Immune Mechanism
7. In Vivo Function and McC7 Application
7.1. In Vivo Anti-Infection Studies: Direct Antibacterial and Anti-Inflammatory Activities and Intestinal Health Regulation
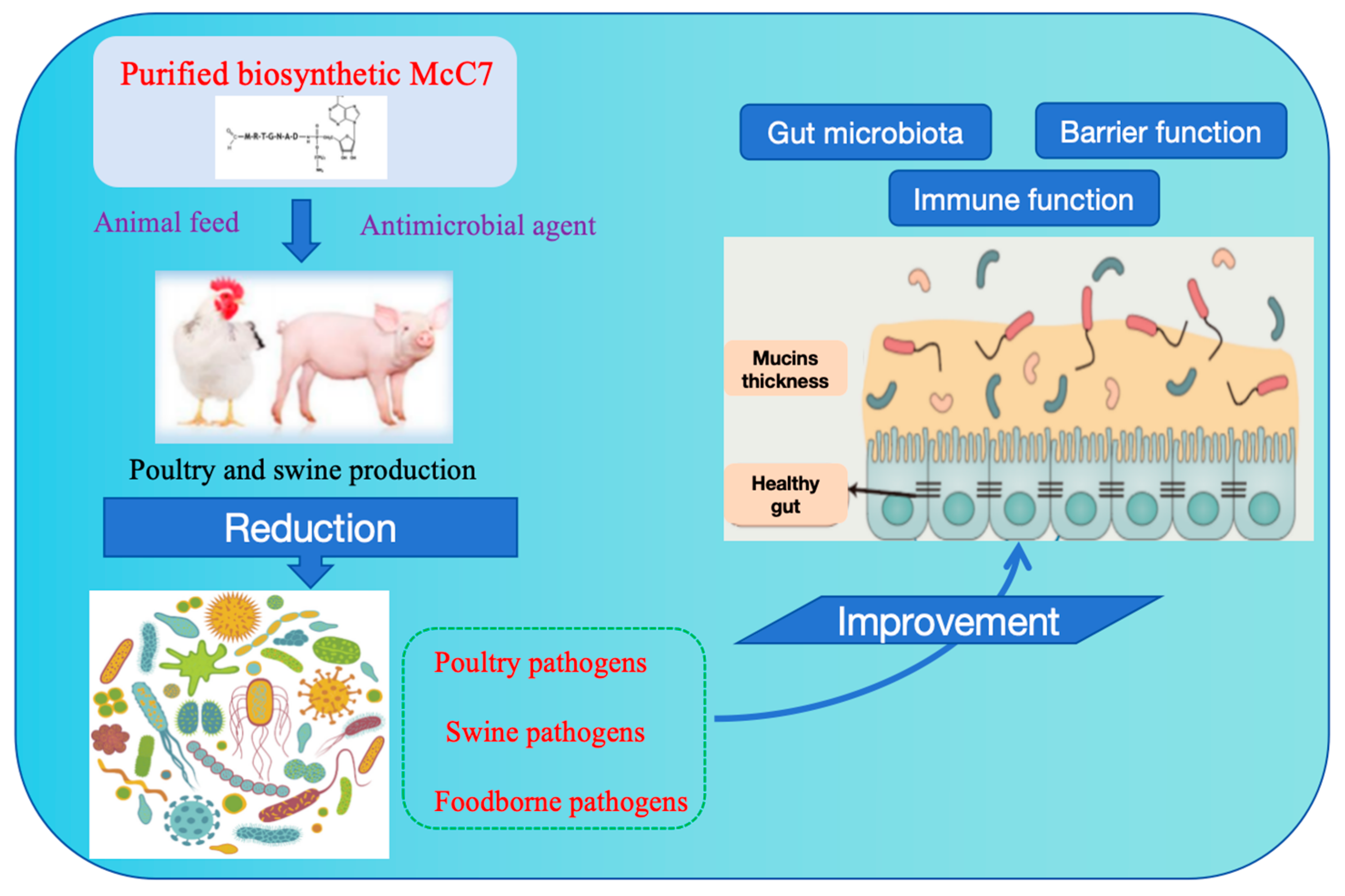
7.2. Applications in Poultry and Pigs Production
7.3. Applications in Immunomodulation and Infection Prevention
8. A Potential Method for Improving the Antibacterial Spectrum of McC7 via the Conjugation of Micro/Nanoparticles
9. Conclusions and Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Sethi, S.; Murphy, T.F. Bacterial infection in chronic obstructive pulmonary disease in 2000: A state-of-the-art review. Clin. Microbiol. Rev. 2001, 14, 336–363. [Google Scholar] [CrossRef] [PubMed]
- Andersson, D.I.; Hughes, D. Antibiotic resistance and its cost: Is it possible to reverse resistance? Nat. Rev. Microbiol. 2010, 8, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, N.R.; Nunn, P.; Dheda, K.; Schaaf, H.S.; Zignol, M.; van Soolingen, D.; Jensen, P.; Bayona, J. Multidrug-resistant and extensively drug-resistant tuberculosis: A threat to global control of tuberculosis. Lancet 2010, 375, 1830–1843. [Google Scholar] [CrossRef] [PubMed]
- Yelin, I.; Kishony, R. Antibiotic Resistance. Cell 2018, 172, 1136.e1. [Google Scholar] [CrossRef] [PubMed]
- Kumarasamy, K.K.; Toleman, M.A.; Walsh, T.R.; Bagaria, J.; Butt, F.; Balakrishnan, R.; Chaudhary, U.; Doumith, M.; Giske, C.G.; Irfan, S.; et al. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: A molecular, biological, and epidemiological study. Lancet Infect Dis. 2010, 10, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Caruso, R.; Lo, B.C.; Núñez, G. Host-microbiota interactions in inflammatory bowel disease. Nat. Rev. Immunol. 2020, 20, 411–426. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, S.; Toh, H.; Hase, K.; Oshima, K.; Nakanishi, Y.; Yoshimura, K.; Tobe, T.; Clarke, J.M.; Topping, D.L.; Suzuki, T.; et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 2011, 469, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Smith, F.; Clark, J.E.; Overman, B.L.; Tozel, C.C.; Huang, J.H.; Rivier, J.E.F.; Blikslager, A.T.; Moeser, J.A. Early weaning stress impairs development of mucosal barrier function in the porcine intestine. Am. J. Physiol.-Gastroint. Liver Physiol. 2010, 298, G352–G363. [Google Scholar] [CrossRef] [PubMed]
- Peterson, L.W.; Artis, D. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 2014, 14, 141–153. [Google Scholar] [CrossRef]
- Rooks, M.G.; Garrett, W.S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 2016, 16, 341–352. [Google Scholar] [CrossRef]
- Huang, C.; Ming, D.; Wang, W.; Wang, Z.; Hu, Y.; Ma, X.; Wang, F. Pyrroloquinoline quinone alleviates jejunal mucosal barrier function damage and regulates colonic microbiota in piglets challenged with enterotoxigenic Escherichia coli. Front. Microbiol. 2020, 24, 1754. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.T.; Shang, L.J.; Yang, G.Y.; Dai, Z.Q.; Zeng, X.F.; Qiao, S. Biosynthetic microcin J25 exerts strong antibacterial, antiinflammatory activities, low cytotoxicity without increasing drugresistance to bacteria target. Front. Immunol. 2022, 18, 811378. [Google Scholar]
- Johnson, A.M.; Kaushik, R.S.; Hardwidge, P.R. Disruption of transepithelial resistance by enterotoxigenic Escherichia coli. Vet. Microbiol. 2010, 141, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Sun, K.J.; Wu, Y.J.; Yang, Y.; Tso, P.; Wu, Z.L. Interactions between intestinal microbiota and host immune response in inflammatory bowl disease. Front. Immunol. 2017, 14, 942. [Google Scholar]
- Pluske, J.R.; Turpin, D.L.; Kim, J.C. Gastrointestinal tract (gut) health in the young pig. Anim. Nutr. 2018, 4, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.H.; Xu, K.J.; Wang, H.Y.; Tan, P.K.J.; Fan, W.M.; Venkatraman, S.S.; Li, L.; Yang, Y.Y. Self-assembled cationic peptide nanoparticles as an efficient antimicrobial agent. Nat. Nanotechnol. 2009, 4, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.J.; O’Brien-Simpson, N.M.; Pantarat, N.; Sulistio, A.; Wong, E.H.H.; Chen, Y.Y.; Lenzo, J.C.; Holden, J.A.; Blencowe, A.; Reynolds, E.C.; et al. Combating multidrug-resistant Gram-negative bacteria with structurally nanoengineered antimicrobial peptide polymers. Nat. Microbiol. 2016, 1, 16162. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.T.; Ding, X.L.; Li, N.; Zhang, X.Y.; Zeng, X.F.; Wang, S.; Liu, H.B.; Wang, Y.M.; Jia, H.M.; Qiao, S.Y. Dietary supplemented antimicrobial peptide microcin J25 improves the growth performance, apparent total tract digestibility, fecal microbiota, and intestinal barrier function of weaned pigs. J. Anim. Sci. 2017, 95, 5064–5076. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Garrido-Maestu, A.; Jeong, K.C. Application, mode of action, and in vivo activity of chitosan and its micro-and nanoparticles as antimicrobial agents: A review. Carbohyd. Polym. 2017, 176, 257–265. [Google Scholar] [CrossRef]
- Ma, Z.X.; Kang, M.Y.; Meng, S.Y.; Tong, Z.H.; Yoon, S.D.; Jang, Y.S.; Jeong, K.C. Selective Killing of Shiga Toxin-Producing Escherichia coli with Antibody-Conjugated Chitosan Nanoparticles in the Gastrointestinal Tract. ACS Appl. Mater. Interfaces 2020, 12, 18332–18341. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.J.; Yang, Y.Z.; Bao, C.L.; Cao, Y.H. High-level expression of an acidic thermostable xylanase in Pichia pastoris and its application in weaned piglets. J Anim. Sci. 2020, 98, skz364. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.X.; Bao, C.L.; Wang, J.; Zang, J.J.; Cao, Y.H. Administration of Saccharomyces boulardii mafic-1701 improves feed conversion ratio, promotes antioxidant capacity, alleviates intestinal inflammation and modulates gut microbiota in weaned piglets. J. Anim. Sci. Biotechnol. 2020, 11, 112. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.T.; Ma, Z.X.; Meng, S.Y.; Qiao, S.Y.; Zeng, X.F.; Tong, Z.H.; Jeong, K.C. A novel nanohybrid antimicrobial based on chitosan nanoparticles and antimicrobial peptide microcin J25 with low toxicity. Carbohydr. Polym. 2021, 253, 117309. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, X.Y.; Ma, Y.H.; Cai, S.; Yang, L.J.; Fan, Y.X.; Zeng, X.F.; Qiao, S. Lactobacillus reuteri improves the development and maturation of fecal microbiota in piglets through mother-to-infant microbe and metabolite vertical transmission. Microbiome 2022, 10, 211. [Google Scholar] [CrossRef] [PubMed]
- Scott, M.G.; Dullaghan, E.; Mookherjee, N.; Glavas, N.; Waldbrook, M.; Thompson, A.; Wang, A.; Lee, K.; Doria, S.; Hamill, P.; et al. An anti-infective peptide that selectively modulates the innate immune response. Nat. Biotechnol. 2007, 25, 465–472. [Google Scholar] [CrossRef]
- Lai, Y.P.; Gallo, R.L. AMPed up immunity: How antimicrobial peptides have multiple roles in immune defense. Trends Immunol. 2009, 30, 131–141. [Google Scholar] [CrossRef]
- Wang, S.; Thacker, P.A.; Watford, M.; Qiao, S.Y. Functions of antimicrobial peptides in gut homeostasis. Curr. Protein Pept. Sci. 2015, 16, 582–591. [Google Scholar] [CrossRef]
- Wang, S.; Zeng, X.F.; Yang, Q.; Qiao, S.Y. Antimicrobial peptides as potential alternatives to antibiotics in food animal industry. Int. J. Mol. Sci. 2016, 17, 603. [Google Scholar] [CrossRef]
- Li, S.Q.; Wang, Y.J.; Xue, Z.H.; Jia, Y.N.; Li, R.L.; He, C.W.; Chen, H.X. The structure-mechanism relationship and mode of actions of antimicrobial peptides: A review. Trends Food Sci. Tech. 2021, 109, 103–115. [Google Scholar] [CrossRef]
- Fu, J.; Zong, X.; Jin, M.L.; Min, J.X.; Wang, F.D.; Wang, Y.Z. Mechanisms and regulation of defensins in host defense. Signal Transduct. Target. Ther. 2023, 8, 300. [Google Scholar] [CrossRef]
- Gao, N.; Wang, J.; Fang, C.; Bai, P.; Sun, Y.; Wu, W.; Shan, A. Combating bacterial infections with host defense peptides: Shifting focus from bacteria to host immunity. Drug. Resist. Updates 2023, 72, 101030. [Google Scholar] [CrossRef]
- Li, G.Y.; Lai, Z.H.; Shan, A.S. Advances of Antimicrobial Peptide-Based Biomaterials for the Treatment of Bacterial Infections. Adv. Sci. 2023, 10, e2206602. [Google Scholar] [CrossRef]
- Duquesne, S.; Destoumieux-Garzón, D.; Peduzzi, J.; Rebuffat, S. Microcins, gene-encoded antibacterial peptides from enterobacteria. Nat. Prod. Rep. 2007, 24, 708–734. [Google Scholar] [CrossRef] [PubMed]
- Sassone-Corsi, M.; Nuccio, S.; Liu, H.; Hernandez, D.; Vu, C.T.; Takahashi, A.A.; Edwards, A.R.; Raffatellu, M. Microcins mediate competition among Enterobacteriaceae in the inflamed gut. Nature 2016, 540, 280–283. [Google Scholar] [CrossRef] [PubMed]
- Gabant, P.; Borrero, J. PARAGEN 1.0: A standardized synthetic gene library for fast cell-free bacteriocin synthesis. Front. Bioeng. Biotech. 2019, 7, 213–218. [Google Scholar] [CrossRef]
- Soltani, S.; Hammami, R.; Cotter, P.D.; Rebuffat, S.; Said, L.B.; Gaudreau, H.; Bédard, F.; Biron, E.; Drider, D.; Fliss, I. Bacteriocins as a new generation of antimicrobials: Toxicity aspects and regulations. FEMS Microbiol. Rev. 2021, 45, fuaa039. [Google Scholar] [CrossRef] [PubMed]
- Duquesne, S.; Petit, V.; Peduzzi, J.; Rebuffat, S. Structural and functional diversity of microcins, gene-encoded antibacterial peptides from enterobacteria. J. Mol. Microbiol. Biotech. 2007, 13, 200–209. [Google Scholar] [CrossRef]
- Crunkhorn, S. Antibacterials: Microcins limit intestinal infection. Nat. Rev. Drug Discov. 2016, 16, 18. [Google Scholar]
- Yu, H.T.; Wang, Y.M.; Zeng, X.F.; Cai, S.; Wang, G.; Liu, L.; Huang, S.; Li, N.; Liu, H.; Ding, X.; et al. Therapeutic administration of the recombinant antimicrobial peptide microcin J25 effectively enhances host defenses against gut inflammation and epithelial barrier injury induced by enterotoxigenic Escherichia coli infection. FASEB J. 2020, 34, 1018–1037. [Google Scholar] [CrossRef]
- Guijarro, J.I.; González-Pastor, J.E.; Baleux, F.; San Millán, J.L.; Castilla, M.A.; Rico, M.; Moreno, F.; Delepierre, M. Chemical structure and translation inhibition studies of the antibiotic microcin C7. J. Biol. Chem. 1995, 270, 23520–23532. [Google Scholar] [CrossRef]
- Metlitskaya, A.; Kazakov, T.; Kommer, A.; Pavlova, O.; Praetorius-Ibba, M.; Ibba, M.; Krasheninnikov, I.; Kolb, V.; Khmel, I.; Severinov, K. Aspartyl-tRNA synthetase is the target of peptide nucleotide antibiotic Microcin, C. J. Biol. Chem. 2006, 281, 18033–18042. [Google Scholar] [CrossRef] [PubMed]
- Cotter, P.D.; Hill, C.; Ross, R.P. Bacteriocins: Developing innate immunity for food. Nat. Rev. Microbiol. 2005, 3, 777–788. [Google Scholar] [CrossRef] [PubMed]
- Rebuffat, S. Microcins in action: Amazing defence strategies of enterobacteria. Biochem. Soc. Trans. 2012, 40, 1456–1462. [Google Scholar] [CrossRef] [PubMed]
- Nes, I.F. Prokaryotic Antimicrobial Peptides; Springer: New York, NY, USA, 2011; pp. 3–12. [Google Scholar]
- Jack, R.W.; Tagg, J.R.; Ray, B. Bacteriocins of gram-positive bacteria. Microbiol. Rev. 1995, 59, 171–200. [Google Scholar] [CrossRef] [PubMed]
- Riley, M.A.; Wertz, J.E. Bacteriocins: Evolution, ecology, and application. Annu. Rev. Microbiol. 2002, 56, 117–137. [Google Scholar] [CrossRef] [PubMed]
- Drider, D.; Fimland, G.; Héchard, Y.; McMullen, L.M.; Prévost, H. The continuing story of class IIa bacteriocins. Microbiol. Mol. Biol. Rev. 2006, 70, 564–582. [Google Scholar] [CrossRef] [PubMed]
- Piper, C.; Cotter, P.D.; Ross, R.P.; Hill, C. Discovery of medically significant lantibiotics. Curr. Drug Discov. Technol. 2009, 6, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Klaenhammer, T.R. Genetics of bacteriocins produced by lactic acid bacteria. FEMS Microbiol. Rev. 1993, 12, 39–85. [Google Scholar] [CrossRef]
- Meindl, K.; Schmiederer, T.; Schneider, K.; Reicke, A.; Butz, D.; Keller, S.; Gühring, H.; Vértesy, L.; Wink, J.; Hoffmann, H.; et al. Labyrinthopeptins: A new class of carbacyclic lantibiotics. Angew. Chem. Int. Ed. Engl. 2010, 49, 1151–1154. [Google Scholar] [CrossRef]
- Kawulka, K.; Sprules, T.; McKay, R.T.; Mercier, P.; Diaper, C.M.; Zuber, P.; Vederas, J.C. Structure of subtilosin A, an antimicrobial peptide from Bacillus subtilis with unusual posttranslational modifications linking cysteine sulfurs to alpha-carbons of phenylalanine and threonine. J. Am. Chem. Soc. 2003, 125, 4726–4727. [Google Scholar] [CrossRef]
- Bierbaum, G.; Sahl, H.G. Lantibiotics: Mode of action, biosynthesis and bioengineering. Curr. Pharm. Biotechnol. 2009, 10, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Severina, E.; Severin, A.; Tomasz, A. Antibacterial efficacy of Nisin against multidrug-resistant gram-positive pathogens. J. Antimicrob. Chemother. 1998, 41, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.T.; Wu, J.Q.; Xie, F.; Hu, S.H.; Mo, Y. Efficacy of Nisin in treatment of clinical mastitis inlactating dairy cows. J. Dairy Sci. 2007, 90, 3980–3985. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Sieiro, P.; Montalbán-López, M.; Mu, D.; Kuipers, O.P. Bacteriocins of lactic acid bacteria: Extending the family. Appl. Microbiol. Biotechnol. 2016, 100, 2939–2951. [Google Scholar] [CrossRef] [PubMed]
- Nissen-Meyer, J.; Holo, H.; Håvarstein, L.S.; Sletten, K.; Nes, I.F. A novel lactococcal bacteriocin whose activity depends on the complementary action of two peptides. J. Bacteriol. 1992, 174, 5686–5692. [Google Scholar] [CrossRef] [PubMed]
- Tahara, T.; Oshimura, M.; Umezawa, C.; Kanatani, K. Isolation, partial characterization, and mode of action of acidocin J1132, a two-component bacteriocin produced by Lactobacillus acidophilus JCM 1132. Appl. Environ. Microbiol. 1996, 62, 892–897. [Google Scholar] [CrossRef] [PubMed]
- Simon, L.; Fremaux, C.; Cenatiempo, Y.; Berjeaud, J.M. Sakacin G, a new type of antilisterial bacteriocin. Appl. Environ. Microbiol. 2002, 68, 6416–6420. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Díaz, R.; Ruiz-Barba, J.L.; Cathcart, D.P.; Holo, H.; Nes, I.F.; Sletten, K.H.; Warner, P.J. Purification and partial amino acid sequence of plantaricin S, a bacteriocin produced by Lactobacillus plantarum LPCO10, the activity of which depends on the complementary action of two peptides. Appl. Environ. Microb. 1995, 61, 4459–4463. [Google Scholar] [CrossRef]
- Kawai, Y.; Saitoh, B.; Takahashi, O.; Kitazawa, H.; Saito, T.; Nakajima, H.; Itoh, T. Primary amino acid and DNA sequences of gassericin T, a lactacin F-family bacteriocin produced by Lactobacillus gasseri SBT2055. Biosci. Biotechnol. Biochem. 2000, 64, 2201–2208. [Google Scholar] [CrossRef]
- Vaughan, A.; Eijsink, V.G.; O’Sullivan, T.F.; O’hanlon, K.; van Sinderen, D. An analysis of bacteriocins produced by lactic acid bacteria isolated from malted barley. J. Appl. Microbiol. 2001, 91, 131–138. [Google Scholar] [CrossRef]
- Senes, A.; Engel, D.E.; DeGrado, W.F. Folding of helical membrane proteins: The role of polar, GxxxG-like and proline motifs. Curr. Opin. Struct. Biol. 2004, 14, 465–479. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, M.A.; Barrachi-Saccilotto, A.C.; Valdivia, E.; Maqueda, M.; Rico, M. Design, NMR characterization and activity of a 21-residue peptide fragment of bacteriocin AS-48 containing its putative membrane interacting region. J. Pept. Sci. 2005, 11, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Martin-Visscher, L.A.; Gong, X.D.; Duszyk, M.; Vederas, J.C. The three-dimensional structure of carnocyclin A reveals that many circular bacteriocins share a common structural motif. J. Biol. Chem. 2009, 284, 28674–28681. [Google Scholar] [CrossRef] [PubMed]
- Leer, R.J.; van der Vossen, J.M.; van Giezen, M.; van Noort, J.M.; Pouwels, P.H. Genetic analysis of acidocin B, a novel bacteriocin produced by Lactobacillus acidophilus. Microbiology 1995, 141, 1629–1635. [Google Scholar] [CrossRef] [PubMed]
- Kalmokoff, M.L.; Cyr, T.D.; Hefford, M.A.; Whitford, M.F.; Teather, R.M. Butyrivibriocin AR10, a new cyclic bacteriocin produced by the ruminal anaerobe Butyrivibrio fibrisolvens AR10: Characterization of the gene and peptide. Can. J. Microbiol. 2003, 49, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Kemperman, R.; Kuipers, A.; Karsens, H.; Nauta, A.; Kuipers, O.; Kok, J. Identification and characterization of two novel clostridial bacteriocins, circularin A and closticin 574. Appl. Environ. Microbiol. 2003, 69, 1589–1597. [Google Scholar] [CrossRef] [PubMed]
- Wirawan, R.E.; Swanson, K.M.; Kleffmann, T.; Jack, R.W.; Tagg, J.R. Uberolysin: A novel cyclic bacteriocin produced by Streptococcus uberis. Microbiology 2007, 153, 1619–1630. [Google Scholar] [CrossRef] [PubMed]
- Sawa, N.; Zendo, T.; Kiyofuji, J.; Fujita, K.; Himeno, K.; Nakayama, J.; Sonomoto, K. Identification and characterization of lactocyclicin Q, a novel cyclic bacteriocin produced by Lactococcus sp. strain QU 12. Appl. Environ. Microbiol. 2009, 75, 1552–1558. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.K.; Chittpurna; Ashish; Sharma, V.; Patil, P.B.; Korpole, S. Identification, purification and characterization of laterosporulin, a novel bacteriocin produced by Brevibacillus sp. strain GI-9. PLoS ONE 2012, 7, e31498. [Google Scholar] [CrossRef]
- Singh, P.K.; Solanki, V.; Sharma, S.; Thakur, K.G.; Krishnan, B.; Korpole, S. The intramolecular disulfide-stapled structure of laterosporulin, a class II d bacteriocin, conceals a human defensin-like structural module. FEBS J. 2015, 282, 203–214. [Google Scholar] [CrossRef]
- Joerger, M.C.; Klaenhammer, T.R. Characterization and purification of helveticin J and evidence for a chromosomally determined bacteriocin produced by Lactobacillus helveticus 481. J. Bacteriol. 1986, 167, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Cascales, E.; Buchanan, S.K.; Duché, D.; Kleanthous, C.; Lloubes, R.; Postle, K.; Riley, M.; Slatin, S.; Cavard, D. Colicin biology. Microbiol. Mol. Biol. Rev. 2007, 71, 158–229. [Google Scholar] [CrossRef] [PubMed]
- Moreno, F.; Gónzalez-Pastor, J.E.; Baquero, M.R.; Bravo, D. The regulation of microcin B, C and J operons. Biochimie 2002, 84, 521–529. [Google Scholar] [CrossRef]
- Sharma, S.; Waterfield, N.; Bowen, D.; Rocheleau, T.; Holland, L.; James, R.; Ffrench-Constant, R. The lumicins: Novel bacteriocins from Photorhabdus luminescens with similarity to the uropathogenicspecific protein (USP) from uropathogenic Escherichia coli. FEMS Microbiol. Lett. 2002, 214, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Braun, V.; Patzer, S.I.; Hantke, K. Ton-dependent colicins and microcins: Modular design and evolution. Biochimie 2002, 84, 365–380. [Google Scholar] [CrossRef]
- Drider, D.; Rebuffat, S. Prokaryotic Antimicrobial Peptides: From Genes to Applications; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Arnold, T.; Zeth, K.; Linke, D. Structure and function of colicin S4, a colicin with a duplicated receptor-binding domain. J. Biol. Chem. 2009, 284, 6403–6413. [Google Scholar] [CrossRef]
- Severinov, K.; Semenova, E.; Kazakov, A.; Kazakov, T.; Gelfand, M.S. Low-molecular-weight post-translationally modified microcins. Future Microbiol. 2007, 65, 1380–1394. [Google Scholar]
- Vassiliadis, G.; Destoumieux-Garzón, D.; Lombard, C.; Rebuffat, S.; Peduzzi, J. Siderophore microcins form the first family of structure-related antimicrobial peptides from Enterobacteriaceae: Isolation and characterization of microcins M and H47. Antimicrob. Agents Chemother. 2010, 54, 288–297. [Google Scholar] [CrossRef]
- Adelman, K.; Yuzenkova, J.; La Porta, A.; Zenkin, N.; Lee, J.; Lis, J.T.; Borukhov, S.; Wang, M.D.; Severinov, K. Molecular mechanism of transcription inhibition by peptide antibiotic Microcin J25. Mol. Cell 2004, 14, 753–762. [Google Scholar] [CrossRef] [PubMed]
- García-Bustos, J.F.; Pezzi, N.; Asensio, C. Microcin 7: Purification and properties. Biochem. Biophys. Res. Commun. 1984, 119, 779–785. [Google Scholar] [CrossRef]
- Novoa, M.A.; Díaz-Guerra, L.; San Millán, J.L.; Moreno, F. Cloning and mapping of the genetic determinants for microcin C7 production and immunity. J. Bacteriol. 1986, 168, 1384–1391. [Google Scholar] [CrossRef] [PubMed]
- Kazakov, T.; Vondenhoff, G.H.; Datsenko, K.A.; Novikova, M.; Metlitskaya, A.; Wanner, B.L.; Severinov, K. Escherichia coli peptidase A, B, or N can process translation inhibitor microcin C. J. Bacteriol. 2008, 190, 2607–2610. [Google Scholar] [CrossRef]
- Vondenhoff, G.H.M.; Blanchaert, B.; Geboers, S.; Kazakov, T.; Datsenko, K.A.; Wanner, B.L.; Rozenski, J.; Severinow, K.; Aerschot, A.V. Characterization of peptide chain length and constituency requirements for YejABEF-mediated uptake of Microcin C analogues. J. Bacteriol. 2011, 193, 3618–3623. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; de la Fuente-Nunez, C.; Ou, R.W.; Torres, M.D.T.; Pande, S.G.; Sinskey, A.J.; Lu, T.K. Yeast-based synthetic biology platform for antimicrobial peptide production. ACS. Synth. Biol. 2018, 7, 896–902. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, F.M.; Cao, Y.H.; Qiao, S.Y. Novel expression vector for secretion of Ceropin AD in Bacillus subtilis with enhanced antimicrobial activity. Antimicrob. Agents Chemother. 2009, 53, 3683–3689. [Google Scholar] [CrossRef] [PubMed]
- Herbel, V.; Schäfer, H.; Wink, M. Recombinant production of Snakin-2 (an antimicrobial peptide from tomato) in E.coli and analysis of its bioactivity. Molecules 2015, 20, 14889–14901. [Google Scholar] [CrossRef]
- Wei, X.B.; Wu, R.J.; Zhang, L.L.; Ahmad, B.; Si, D.Y.; Zhang, R.J. Expression, purification, and characterization of a novel hybrid peptide with potent antibacterial activity. Molecules 2018, 23, 1491. [Google Scholar] [CrossRef]
- González-Pastor, J.E.; San Millán, J.L.; Castilla, M.A.; Moreno, F. Structure and organization of plasmid genes required to produce the translation inhibitor microcin C7. J. Bacteriol. 1995, 177, 7131–7140. [Google Scholar] [CrossRef]
- González-Pastor, J.E.; San Millán, J.L.; Moreno, F. The smallest known gene. Nature 1994, 369, 281–282. [Google Scholar] [CrossRef]
- Roush, R.F.; Nolan, E.M.; Löhr, F.; Walsh, C.T. Maturation of an Escherichia coli ribosomal peptide antibiotic by ATP-consuming N-P bond formation in microcin C7. J. Am. Chem. Soc. 2008, 130, 3603–3609. [Google Scholar] [CrossRef]
- Severinov, K.; Nair, S.K. Microcin C: Biosynthesis and mechanisms of bacterial resistance. Future Microbiol. 2012, 7, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.X.; Shang, L.J.; Liu, L.; Li, Z.Q.; Zeng, X.F.; Ding, X.L.; Huang, J.X.; Qiao, S.Y.; Yu, H.T. Engineering and Purification of microcin C7 Variants Resistant to Trypsin and Analysis of Their Biological Activity. Antibiotics 2023, 12, 1346. [Google Scholar] [CrossRef] [PubMed]
- Lopez, F.E.; Vincent, P.A.; Zenoff, A.M.; Salomón, R.A.; Farías, R.N. Efficacy of microcin J25 in biomatrices and in a mouse model of Salmonella infection. J. Antimicrob. Chemother. 2007, 59, 676–680. [Google Scholar] [CrossRef] [PubMed]
- Palmer, J.D.; Mortzfeld, B.M.; Piattelli, E.; Silby, M.W.; McCormick, B.A.; Bucci, V. Microcin H47: A Class IIb Microcin with Potent Activity Against Multidrug Resistant Enterobacteriaceae. ACS Infect. Dis. 2020, 6, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Palmer, J.D.; Piattelli, E.; McCormick, B.A.; Silby, M.W.; Brigham, C.J.; Bucci, V. Engineered Probiotic for the Inhibition of Salmonella via Tetrathionate-Induced Production of Microcin H47. ACS Infect. Dis. 2018, 4, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Kuipers, O.P.; Bierbaum, G.; Ottenwälder, B.; Dodd, H.M.; Horn, N.; Metzger, J.; Kupke, T.; Gnau, V.; Bongers, R.; van den Bogaard, P.; et al. Protein engineering of lantibiotics. Antonie Van Leeuwenhoek 1996, 69, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Kuipers, O.P.; Rollema, H.S.; Yap, W.M.; Boot, H.J.; Siezen, R.J.; de Vos, W.M. Engineering dehydrated amino acid residues in the antimicrobial peptide nisin. J. Biol. Chem. 1992, 267, 24340–24346. [Google Scholar] [CrossRef]
- Yuan, J.; Zhang, Z.Z.; Chen, X.Z.; Yang, W.; Huan, L.D. Site-directed mutagenesis of the hingeregion of Nisin Z and properties of Nisin Z mutants. Appl. Microbiol. Biotechnol. 2004, 64, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Rink, R.; Wierenga, J.; Kuipers, A.; Kluskens, L.D.; Driessen, A.J.M.; Kuipers, O.P.; Moll, G.N. Dissection and modulation of the four distinct activities of Nisin by mutagenesis of rings A and B and by C-terminal truncation. Appl. Environ. Microbiol. 2007, 73, 5809–5816. [Google Scholar] [CrossRef]
- Bierbaum, G.; Szekat, C.; Josten, M.; Heidrich, C.; Kempter, C.; Jung, G.; Sahl, H.G. Engineering of a novel thioether bridge and role of modified residues in the lantibiotic Pep5. Appl. Environ. Microb. 1996, 62, 385–392. [Google Scholar] [CrossRef]
- Kazakov, T.; Metlitskaya, A.; Severinov, K. Amino acid residues required for maturation, cell uptake, and processing of translation inhibitor microcin C. J. Bacteriol. 2007, 189, 2114–2118. [Google Scholar] [CrossRef] [PubMed]
- Bantysh, O.; Serebryakova, M.; Zukher, I.; Kulikovsky, A.; Tsibulskaya, D.; Dubiley, S.; Severinov, K. Enzymatic synthesis and functional characterization of bioactive Microcin C-like compounds with altered peptide sequence and length. J. Bacteriol. 2015, 197, 3133–3141. [Google Scholar] [CrossRef] [PubMed]
- Stockner, T.; Mullen, A.; MacMillan, F. Investigating the dynamic nature of the ABC transporters: ABCB1 and MsbA as examples for the potential synergies of MD theory and EPR applications. Biochem. Soc. Trans. 2015, 43, 1023–1032. [Google Scholar] [CrossRef] [PubMed]
- Metlitskaya, A.; Kazakov, T.; Vondenhoff, G.H.; Novikova, M.; Shashkov, A.; Zatsepin, T.; Semenova, E.; Zaitseva, N.; Ramensky, V.; Van Aerschot, A.; et al. Maturation of the translation inhibitor microcin C. J. Bacteriol. 2009, 191, 2380–2387. [Google Scholar] [CrossRef] [PubMed]
- Ran, R.; Zeng, H.; Zhao, D.; Liu, R.Y.; Xu, X. The Novel Property of Heptapeptide of Microcin C7 in Affecting the Cell Growth of Escherichia coli. Molecules 2017, 22, 432. [Google Scholar] [CrossRef] [PubMed]
- Novikova, M.; Metlitskaya, A.; Datsenko, K.; Kazakov, T.; Kazakov, A.; Wanner, B.; Severinov, K. The Escherichia coli Yej transporter is required for the uptake of translation inhibitor microcin C. J. Bacteriol. 2007, 189, 8361–8365. [Google Scholar] [CrossRef] [PubMed]
- Novikova, M.; Kazakov, T.; Vondenhoff, G.H.; Semenova, E.; Rozenski, J.; Metlytskaya, A.; Zukher, I.; Tikhonov, A.; Van Aerschot, A.; Severinov, K. MccE provides resistance to protein synthesis inhibitor microcin C by acetylating the processed form of the antibiotic. J. Biol. Chem. 2010, 285, 12662–12669. [Google Scholar] [CrossRef] [PubMed]
- Kazakov, T.; Kuznedelov, K.; Semenova, E.; Mukhamedyarov, D.; Datsenko, K.A.; Metlitskaya, A.; Vondenhoff, G.H.; Tikhonov, A.; Agarwal, V.; Nair, S.; et al. The RimL transacetylase provides resistance to translation inhibitor microcin C. J. Bacteriol. 2014, 196, 3377–3385. [Google Scholar] [CrossRef]
- Tikhonov, A.; Kazakov, T.; Semenova, E.; Serebryakova, M.; Vondenhoff, G.; Aerschot, A.V.; Reader, J.S.; Govorun, V.M.; Severinow, K. The mechanism of Microcin C resistance provided by the MccF peptidase. J. Biol. Chem. 2010, 285, 37944–37952. [Google Scholar] [CrossRef]
- Agarwal, V.; Tikhonov, A.; Metlitskaya, A.; Severinov, K.; Nair, S.K. Structure and function of a serine carboxypeptidase adapted for degradation of the protein synthesis antibiotic Microcin C7. Proc. Natl. Acad. Sci. USA 2012, 109, 4425–4430. [Google Scholar] [CrossRef]
- de Vijver, P.V.; Vondenhoff, G.H.M.; Kazakov, T.S.; Semenova, E.; Kuznedelov, K.; Metlitskaya, A.; Aerschot, A.V.; Severinow, K. Synthetic Microcin C analogs targeting different aminoacyl-tRNA synthetases. J. Bacteriol. 2009, 191, 6273–6280. [Google Scholar] [CrossRef] [PubMed]
- Fairbrother, J.M.; Nadeau, É.; Gyles, C.L. Escherichia coli in postweaning diarrhea in pigs: An update on bacterial types, pathogenesis, and prevention strategies. Anim. Health Res. Rev. 2007, 6, 17–39. [Google Scholar] [CrossRef] [PubMed]
- Kotlo, K.L.; Nataro, J.P.; Blackwelder, W.C.; Nasrin, D.; Farag, T.H.; Panchalingam, S.; Wu, Y.K.; Sow, S.O.; Sur, D.; Breiman, R.F.; et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): A prospective, case-control study. Lancet 2013, 382, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Ginn, A.; Ma, Z.; Kang, M.; Jeong, K.C.; Wright, A.C. Application of chitosan microparticles for mitigation of Salmonella in agricultural water. J. Appl. Microbiol. 2017, 123, 1346–1358. [Google Scholar] [CrossRef] [PubMed]
- Wlodarska, M.; Willing, B.; Keeney, K.M.; Menendez, A.; Bergstrom, K.S.; Gill, N.; Russell, S.L.; Vallance, B.A.; Finlay, B.B. Antibiotic treatment alters the colonic mucus layer and predisposes the host to exacerbated Citrobacter rodentium-induced colitis. Infect. Immun. 2011, 79, 1536–1545. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.; Yamanishi, S.; Cox, L.; Methé, B.A.; Zavadil, J.; Li, K.; Gao, Z.; Mahana, D.; Raju, K.; Teitler, I.; et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature 2012, 488, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Falagas, M.E.; Tansarli, G.S.; Rafailidis, P.I.; Kapaskelis, A.; Vardakas, K.Z. Impact of antibiotic MIC on infection outcome in patients with susceptible gram-negative bacteria: A systematic review and meta-analysis. Antimicrob. Agents Chemother. 2012, 56, 4214–4222. [Google Scholar] [CrossRef] [PubMed]
- Boucher, H.W.; Talbot, G.H.; Bradley, J.S.; Edwards, J.E.; Gilbert, D.; Rice, L.B.; Scheld, M.; Spellberg, B.; Bartlett, J. Bad bugs, no drugs: No ESKAPE! an update from the infectious diseases society of america. Clin. Infect. Dis. 2009, 48, 1–12. [Google Scholar] [CrossRef]
- Dai, Z.Q.; Shang, L.J.; Wang, F.M.; Zeng, X.F.; Yu, H.T.; Liu, L.; Zhou, J.; Qiao, S.Y. Effects of antimicrobial peptide microcin C7 on growth performance, immune and intestinal barrier functions, and cecal microbiota of broilers. Front. Vet. Sci. 2022, 8, 813629. [Google Scholar] [CrossRef]
- Shang, L.J.; Zhou, J.Y.; Tu, J.Y.; Zeng, X.F.; Qiao, S.Y. Evaluation of effectiveness and safety of microcin C7 in weaned piglets. Animals 2022, 12, 3267. [Google Scholar] [CrossRef]
- Wehkamp, J.; Harder, J.; Weichenthal, M.; Mueller, O.; Herrlinger, K.R.; Fellermann, K.; Schroeder, J.M.; Stange, E.F. Inducible and constitutive beta-defensins are differentially expressed in Crohn’s disease and ulcerative colitis. Inflamm. Bowel. Dis. 2003, 9, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef] [PubMed]
- Kamada, N.; Kim, Y.G.; Sham, H.P.; Vallance, B.A.; Puente, J.L.; Martens, E.C.; Núñez, G. Regulated virulence controls the ability of a pathogen to compete with the gut microbiota. Science 2012, 336, 1325–1329. [Google Scholar] [CrossRef]
- Kinnebrew, M.A.; Pamer, E.G. Innate immune signaling in defense against intestinal microbes. Immunol. Rev. 2012, 245, 113–131. [Google Scholar] [CrossRef] [PubMed]
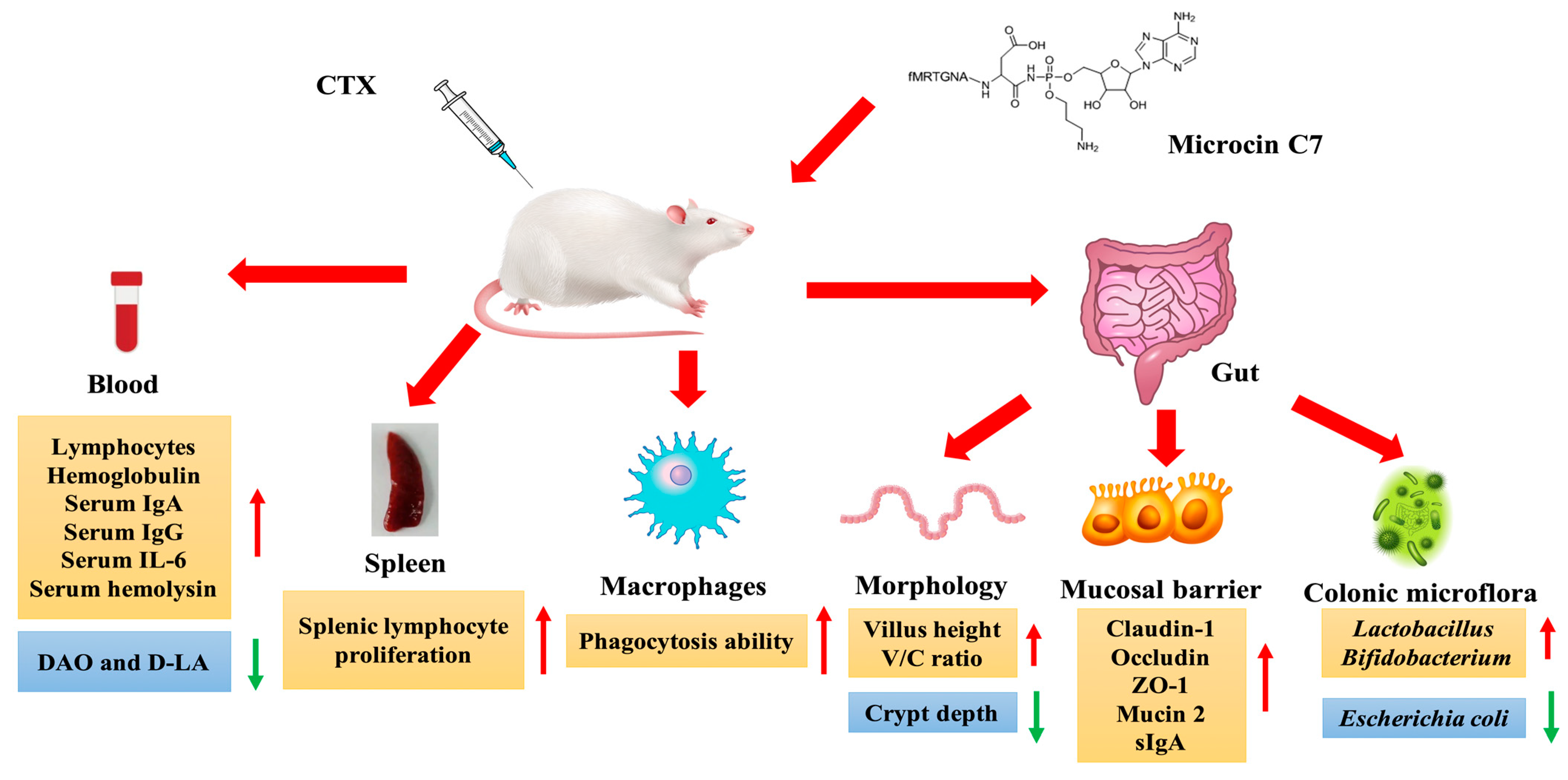
- Dai, Z.Q.; Shang, L.J.; Wei, Y.S.; Li, Z.Q.; Zeng, X.F.; Chen, M.X.; Wang, X.Y.; Li, S.Y.; Qiao, S.Y.; Yu, H.T. Immunomodulatory effects of microcin C7 in cyclophosphamide-induced immunosuppressed mice. J. Agric. Food Chem. 2023, 71, 12700–12714. [Google Scholar] [CrossRef]
- Ma, Z.X.; Kim, D.; Adesogan, A.T.; Ko, S.; Galvao, K.; Jeong, K.C. Chitosan Microparticles Exert Broad-Spectrum Antimicrobial Activity against Antibiotic-Resistant Micro-organisms without Increasing Resistance. ACS Appl. Mater. Interfaces 2016, 8, 10700–10709. [Google Scholar] [CrossRef]

| Microcins | Class | Precursor | Leader Peptide | Mature Microcin | MW, Da | Featured | Bibliography |
|---|---|---|---|---|---|---|---|
| Number of AA | Number of AA | Number of AA | |||||
| MccB17 | I | 69 | 26 | 43 | 3093 | Lasso structure, heterocycle containing or others | [44,77] |
| McC7/51 | I | 7 | 0 | 7 | 1177 | ||
| MccJ25 | I | 58 | 37 | 21 | 2107 | ||
| MccV | IIa | 103 | 15 | 88 | 8733 | Unmodified bacteriocin, linear, non-pediocin-like | |
| MccL | IIa | 105 | 15 | 90 | 8884 | ||
| Mcc24 | IIa | 90 | 17 | 73 | 7475 | ||
| MccE92 | IIb | 103 | 19 | 84 | 8717 | Colicin V-like bacteriocin, and Siderophore-microcin family | |
| MccM | IIb | 92 | 15 | 77 | 7886 | ||
| MccH47 | IIb | 75 | 15 | 60 | 7283 | ||
| MccI47 | IIb | 77 | 15 | 62 | 4865 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, F.; Yang, F.; Huang, J.; Yu, H.; Qiao, S. Microcin C7 as a Potential Antibacterial-Immunomodulatory Agent in the Postantibiotic Era: Overview of Its Bioactivity Aspects and Applications. Int. J. Mol. Sci. 2024, 25, 7213. https://doi.org/10.3390/ijms25137213
Yang F, Yang F, Huang J, Yu H, Qiao S. Microcin C7 as a Potential Antibacterial-Immunomodulatory Agent in the Postantibiotic Era: Overview of Its Bioactivity Aspects and Applications. International Journal of Molecular Sciences. 2024; 25(13):7213. https://doi.org/10.3390/ijms25137213
Chicago/Turabian StyleYang, Fengjuan, Feiyun Yang, Jinxiu Huang, Haitao Yu, and Shiyan Qiao. 2024. "Microcin C7 as a Potential Antibacterial-Immunomodulatory Agent in the Postantibiotic Era: Overview of Its Bioactivity Aspects and Applications" International Journal of Molecular Sciences 25, no. 13: 7213. https://doi.org/10.3390/ijms25137213
APA StyleYang, F., Yang, F., Huang, J., Yu, H., & Qiao, S. (2024). Microcin C7 as a Potential Antibacterial-Immunomodulatory Agent in the Postantibiotic Era: Overview of Its Bioactivity Aspects and Applications. International Journal of Molecular Sciences, 25(13), 7213. https://doi.org/10.3390/ijms25137213





