Self-Assembling Nanoparticle Hemagglutinin Influenza Vaccines Induce High Antibody Response
Abstract
:1. Introduction
2. Results
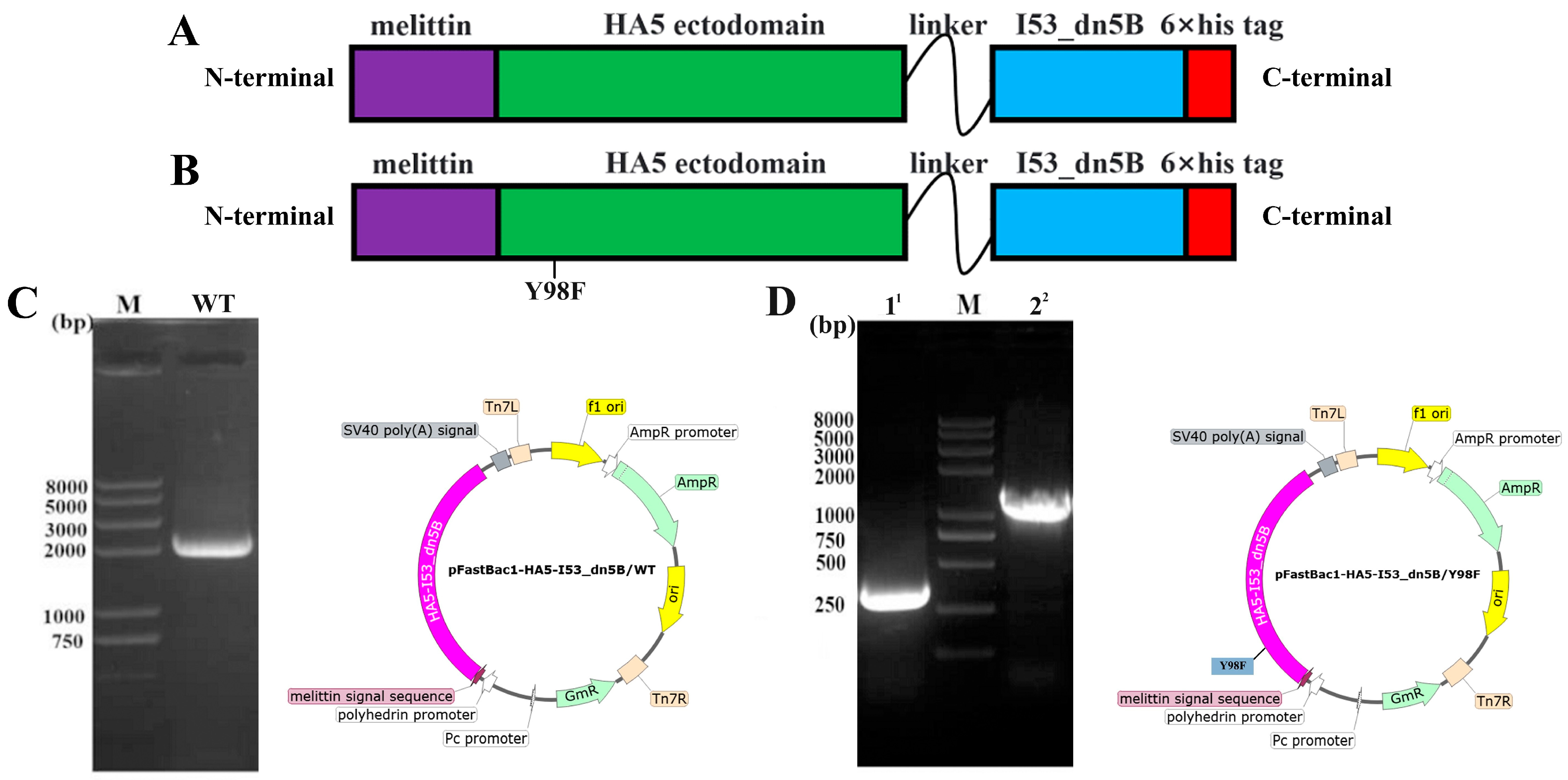
2.1. Construction of Vectors and Expression of HA5-I53_dn5B/WT and HA5-I53_dn5B/Y98F
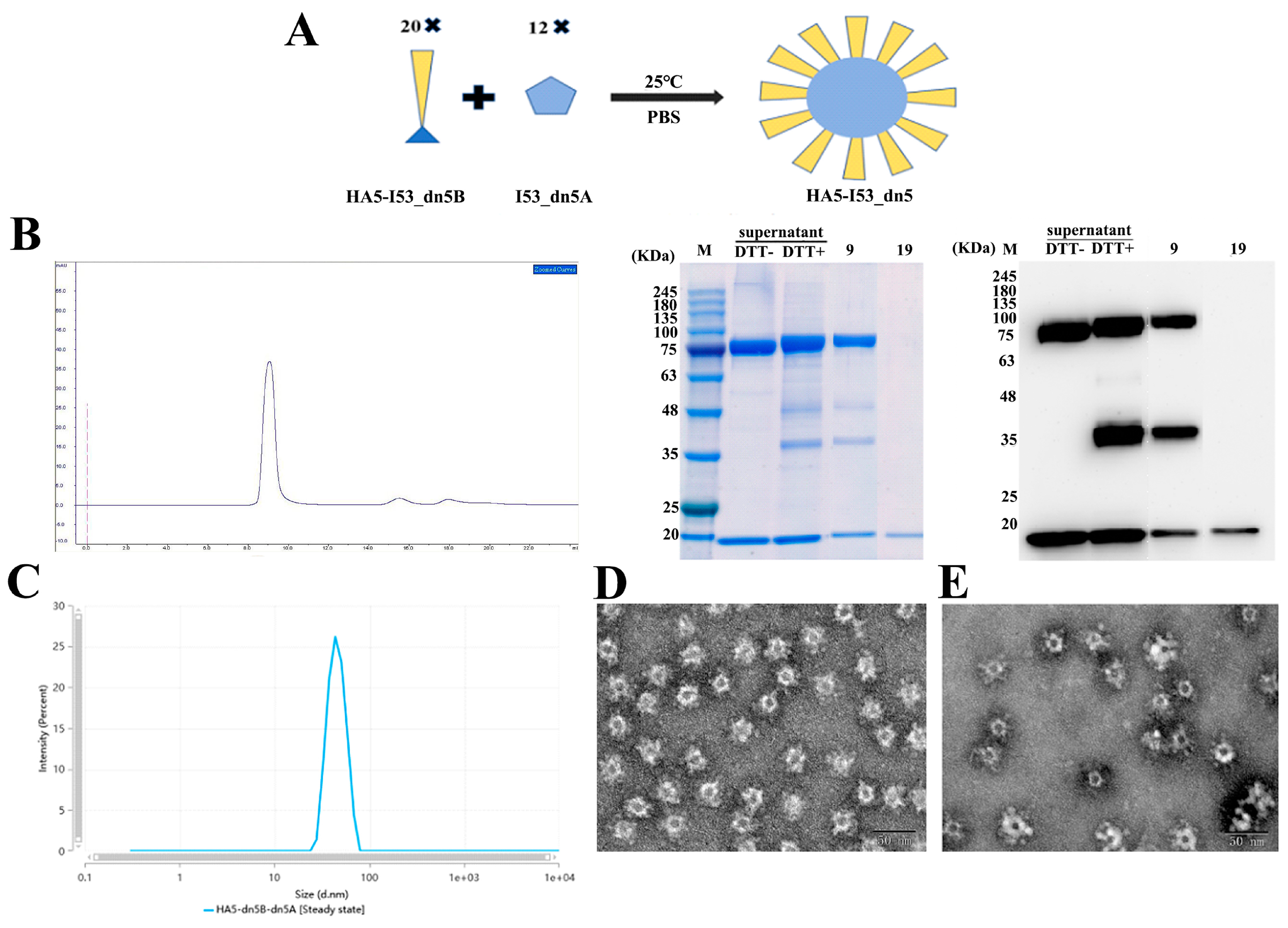
2.2. Expression, Purification, and Verification of HA5-I53_dn5B
2.3. Expression, Purification, and Verification of I53_dn5A
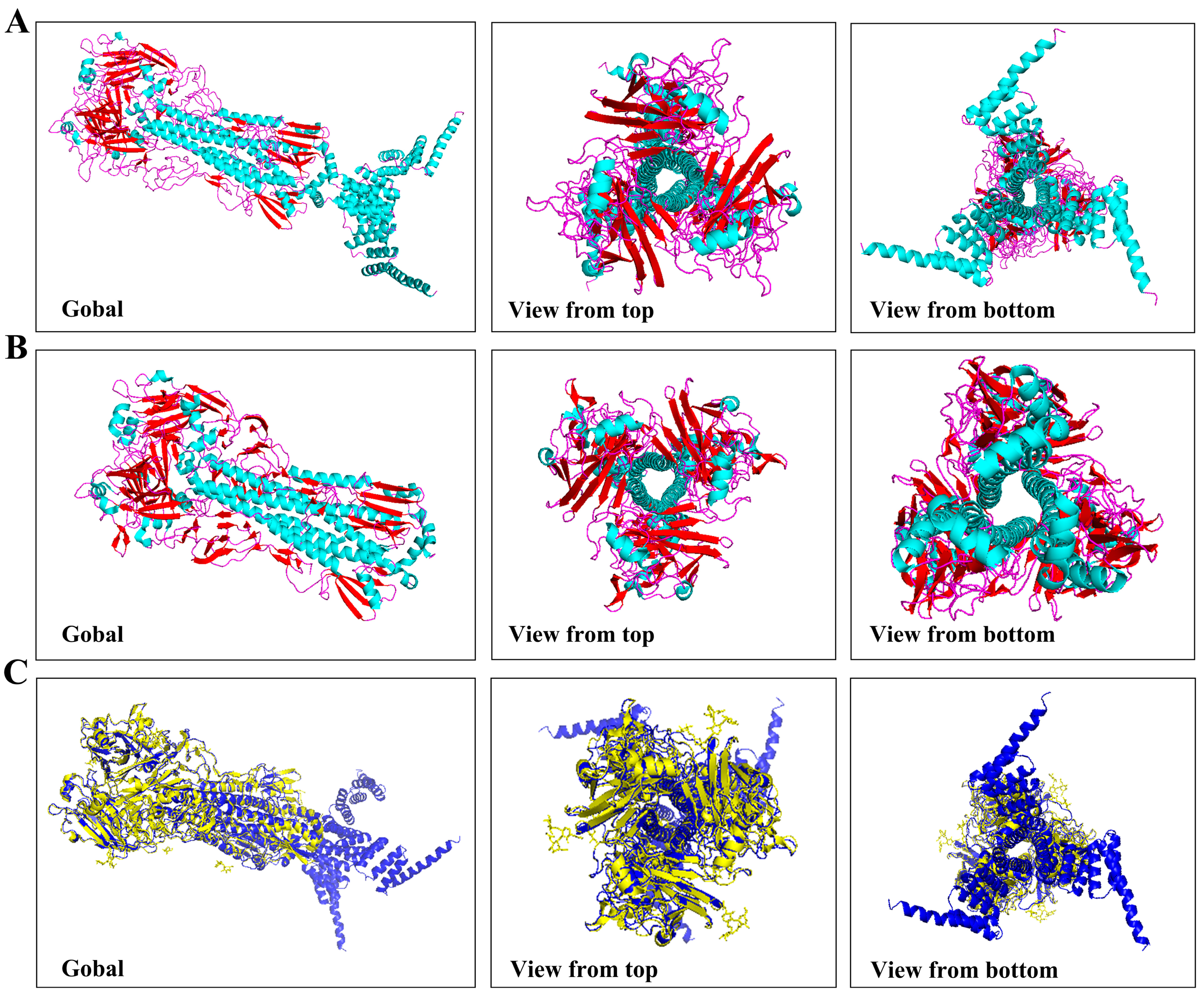
2.4. Assembly of HA5-I53_dn5 Nanoparticles In Vitro
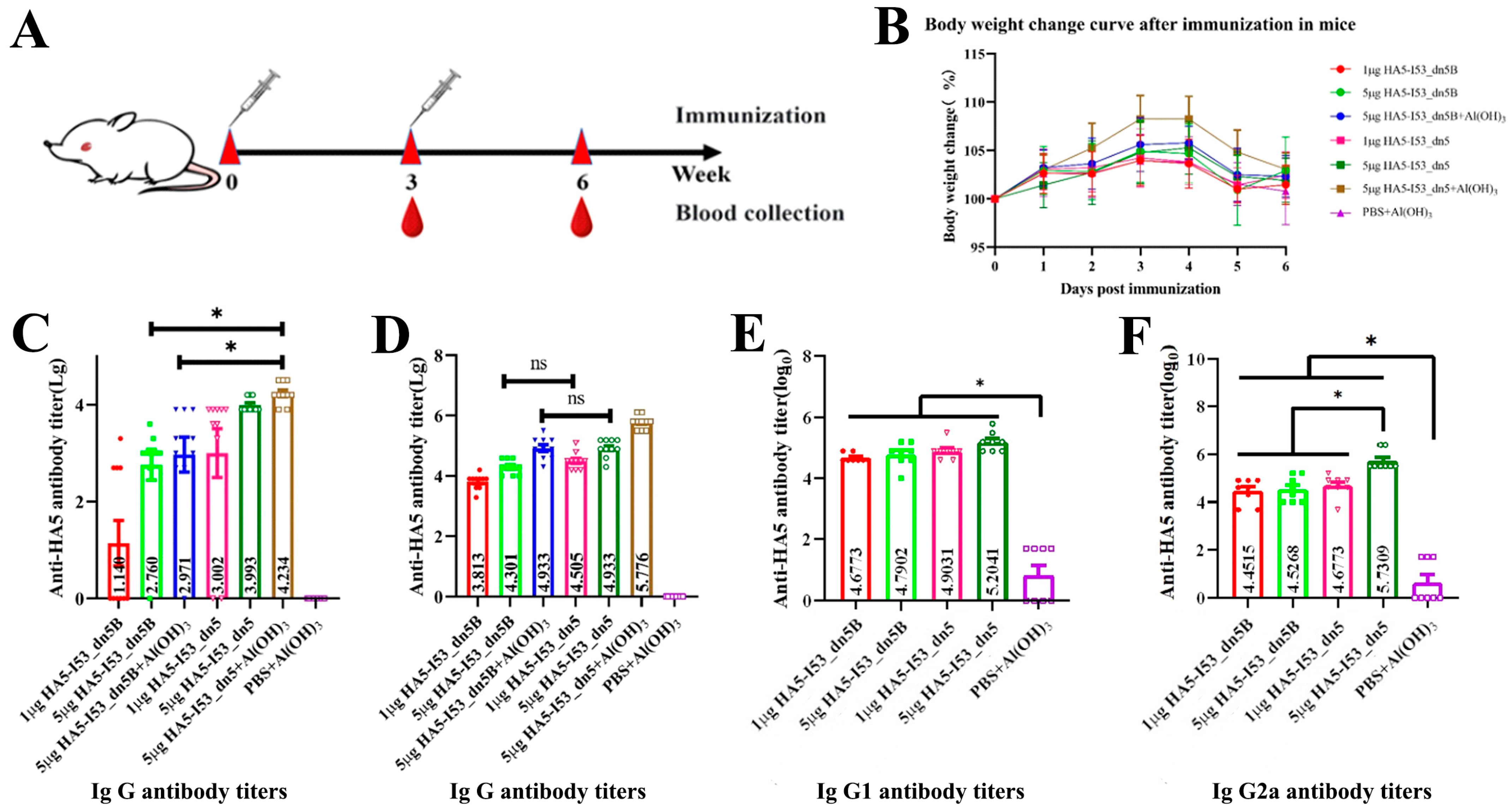
2.5. Evaluation of the Immunogenicity in the Mouse Model
3. Discussion
4. Materials and Methods
4.1. Cell Cultures
4.2. Construction of Recombinant Shuttle Plasmid Bacmid-HA5-I53_dn5B
4.3. Culture and Purification of Recombinant Baculovirus
4.4. Expression and Purification of HA5-I53_dn5B
4.5. Production of I53_dn5A Antigens
4.6. Assembly of Nanoparticles In Vitro
4.7. Experiments with Mice
4.8. Detection of HA5-Specific Total IgG, IgG1, and IgG2a Antibody Titers
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Spackman, E. A Brief Introduction to Avian Influenza Virus. Methods Mol. Biol. 2020, 2123, 83–92. [Google Scholar] [PubMed]
- Krammer, F.; Smith, G.J.D.; Fouchier, R.A.M.; Peiris, M.; Kedzierska, K.; Doherty, P.C.; Palese, P.; Shaw, M.L.; Treanor, J.; Webster, R.G.; et al. Influenza. Nat. Rev. Dis. Primers 2018, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Subbarao, K.; Chen, H.; Swayne, D.; Mingay, L.; Fodor, E.; Brownlee, G.; Xu, X.; Lu, X.; Katz, J.; Cox, N.; et al. Evaluation of a genetically modified reassortant H5N1 influenza A virus vaccine candidate generated by plasmid-based reverse genetics. Virology 2003, 305, 192–200. [Google Scholar] [CrossRef]
- Franca, M.S.; Brown, J.D. Influenza pathobiology and pathogenesis in avian species. Curr. Top. Microbiol. Immunol. 2014, 385, 221–242. [Google Scholar] [PubMed]
- Pereira, H.G.; Tumova, B.; Law, V.G. Avian influenza A viruses. Bull. World Health Organ. 1965, 32, 855–860. [Google Scholar] [PubMed]
- Lang, G.; Narayan, O.; Rouse, B.T.; Ferguson, A.E.; Connell, M.C. A new influenza A virus infection in turkeys II. A highly pathogenic variant, a/turkey/ontario 772/66. Can. Vet. J. 1968, 9, 151–160. [Google Scholar] [PubMed]
- Harfoot, R.; Webby, R.J. H5 influenza, a global update. J. Microbiol. 2017, 55, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Poehling, K.A.; Caspard, H.; Peters, T.R.; Belongia, E.A.; Congeni, B.; Gaglani, M.; Griffin, M.R.; Irving, S.A.; Kavathekar, P.K.; McLean, H.Q.; et al. 2015–2016 Vaccine Effectiveness of Live Attenuated and Inactivated Influenza Vaccines in Children in the United States. Clin. Infect. Dis. 2018, 66, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, J.; Zhang, J.; Ly, H. Advances in Development and Application of Influenza Vaccines. Front. Immunol. 2021, 12, 711997. [Google Scholar] [CrossRef]
- Sabbaghi, A.; Miri, S.M.; Keshavarz, M.; Zargar, M.; Ghaemi, A. Inactivation methods for whole influenza vaccine production. Rev. Med. Virol. 2019, 29, e2074. [Google Scholar] [CrossRef]
- Li, S.; Li, L.; Ai, X.; Yang, L.; Bai, Y.; Wang, Z.; Han, H.; Lu, Q.; Luo, F.; Zhang, Z.; et al. A randomized, controlled, blinded study of the safety, immunogenicity and batch consistency of Aleph inactivated split influenza vaccine made in China in Chinese people. Hum. Vaccin. Immunother. 2014, 10, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Shao, M.; Hu, Y.; Liang, Q.; Jia, N.; Chu, K.; Xu, L.; Li, J.; Li, C.; Zhu, F. Immunogenicity and safety of an inactivated quadrivalent influenza vaccine: A randomized, double-blind, controlled phase III clinical trial in children aged 6–35 months in China. Hum. Vaccin. Immunother. 2020, 16, 1691–1698. [Google Scholar] [CrossRef] [PubMed]
- Cox, M.M.; Izikson, R.; Post, P.; Dunkle, L. Safety, efficacy, and immunogenicity of Flublok in the prevention of seasonal influenza in adults. Ther. Adv. Vaccines 2015, 3, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Dunkle, L.M.; Izikson, R.; Patriarca, P.; Goldenthal, K.L.; Muse, D.; Callahan, J.; Cox, M.M.J.; Team, P.S.C.S. Efficacy of Recombinant Influenza Vaccine in Adults 50 Years of Age or Older. N. Engl. J. Med. 2017, 376, 2427–2436. [Google Scholar] [CrossRef] [PubMed]
- Khalaj-Hedayati, A.; Chua, C.L.L.; Smooker, P.; Lee, K.W. Nanoparticles in influenza subunit vaccine development: Immunogenicity enhancement. Influenza Other Respir. Viruses 2020, 14, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Heng, W.T.; Yew, J.S.; Poh, C.L. Nanovaccines against Viral Infectious Diseases. Pharmaceutics 2022, 14, 2554. [Google Scholar] [CrossRef] [PubMed]
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomed 2016, 11, 673–692. [Google Scholar] [CrossRef] [PubMed]
- Lengyel, M.; Kállai-Szabó, N.; Antal, V.; Laki, A.J.; Antal, I. Microparticles, Microspheres, and Microcapsules for Advanced Drug Delivery. Sci. Pharm. 2019, 87, 20. [Google Scholar] [CrossRef]
- Pan, J.; Cui, Z. Self-Assembled Nanoparticles: Exciting Platforms for Vaccination. Biotechnol. J. 2020, 15, e2000087. [Google Scholar] [CrossRef]
- Perotti, M.; Perez, L. Virus-Like Particles and Nanoparticles for Vaccine Development against HCMV. Viruses 2019, 12, 35. [Google Scholar] [CrossRef]
- Bale, J.B.; Gonen, S.; Liu, Y.; Sheffler, W.; Ellis, D.; Thomas, C.; Cascio, D.; Yeates, T.O.; Gonen, T.; King, N.P.; et al. Accurate design of megadalton-scale two-component icosahedral protein complexes. Science 2016, 353, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Ueda, G.; Antanasijevic, A.; Fallas, J.A.; Sheffler, W.; Copps, J.; Ellis, D.; Hutchinson, G.B.; Moyer, A.; Yasmeen, A.; Tsybovsky, Y.; et al. Tailored design of protein nanoparticle scaffolds for multivalent presentation of viral glycoprotein antigens. Elife 2020, 9, e57659. [Google Scholar] [CrossRef] [PubMed]
- Milder, F.J.; Jongeneelen, M.; Ritschel, T.; Bouchier, P.; Bisschop, I.J.M.; de Man, M.; Veldman, D.; Le, L.; Kaufmann, B.; Bakkers, M.J.G.; et al. Universal stabilization of the influenza hemagglutinin by structure-based redesign of the pH switch regions. Proc. Natl. Acad. Sci. USA 2022, 119, e2115379119. [Google Scholar] [CrossRef] [PubMed]
- Siche, S.; Brett, K.; Möller, L.; Kordyukova, L.V.; Mintaev, R.R.; Alexeevski, A.V.; Veit, M. Two Cytoplasmic Acylation Sites and an Adjacent Hydrophobic Residue, but No Other Conserved Amino Acids in the Cytoplasmic Tail of HA from Influenza A Virus Are Crucial for Virus Replication. Viruses 2015, 7, 6458–6475. [Google Scholar] [CrossRef] [PubMed]
- Boyoglu-Barnum, S.; Ellis, D.; Gillespie, R.A.; Hutchinson, G.B.; Park, Y.J.; Moin, S.M.; Acton, O.J.; Ravichandran, R.; Murphy, M.; Pettie, D.; et al. Quadrivalent influenza nanoparticle vaccines induce broad protection. Nature 2021, 592, 623–628. [Google Scholar] [CrossRef] [PubMed]
- López-Sagaseta, J.; Malito, E.; Rappuoli, R.; Bottomley, M.J. Self-assembling protein nanoparticles in the design of vaccines. Comput. Struct. Biotechnol. J. 2015, 14, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Keller, S.A.; Bauer, M.; Manolova, V.; Muntwiler, S.; Saudan, P.; Bachmann, M.F. Cutting Edge: Limited Specialization of Dendritic Cell Subsets for MHC Class II-Associated Presentation of Viral Particles. J. Immunol. 2010, 184, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Leen, A.M.; Christin, A.; Khalil, M.; Weiss, H.; Gee, A.P.; Brenner, M.K.; Heslop, H.E.; Rooney, C.M.; Bollard, C.M. Identification of Hexon-Specific CD4 and CD8 T-Cell Epitopes for Vaccine and Immunotherapy. J. Virol. 2008, 82, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Morales-Hernández, S.; Ugidos-Damboriena, N.; López-Sagaseta, J. Self-Assembling Protein Nanoparticles in the Design of Vaccines: 2022 Update. Vaccines 2022, 10, 1447. [Google Scholar] [CrossRef]
- Shen, S.; Mahadevappa, G.; Oh, H.L.; Wee, B.Y.; Choi, Y.W.; Hwang, L.A.; Lim, S.G.; Hong, W.; Lal, S.K.; Tan, Y.J. Comparing the antibody responses against recombinant hemagglutinin proteins of avian influenza A (H5N1) virus expressed in insect cells and bacteria. J. Med. Virol. 2008, 80, 1972–1983. [Google Scholar] [CrossRef]
- Limburg, H.; Harbig, A.; Bestle, D.; Stein, D.A.; Moulton, H.M.; Jaeger, J.; Janga, H.; Hardes, K.; Koepke, J.; Schulte, L.; et al. TMPRSS2 Is the Major Activating Protease of Influenza A Virus in Primary Human Airway Cells and Influenza B Virus in Human Type II Pneumocytes. J. Virol. 2019, 93, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Russell, C.J.; Hu, M.; Okda, F.A. Influenza Hemagglutinin Protein Stability, Activation, and Pandemic Risk. Trends Microbiol. 2018, 26, 841–853. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.; Wharton, S.A.; Lin, Y.P.; Takemoto, D.K.; Skehel, J.J.; Wiley, D.C.; Steinhauer, D.A. Studies of the binding properties of influenza hemagglutinin receptor-site mutants. Virology 1998, 241, 101–111. [Google Scholar] [CrossRef]
- Whittle, J.R.; Wheatley, A.K.; Wu, L.; Lingwood, D.; Kanekiyo, M.; Ma, S.S.; Narpala, S.R.; Yassine, H.M.; Frank, G.M.; Yewdell, J.W.; et al. Flow cytometry reveals that H5N1 vaccination elicits cross-reactive stem-directed antibodies from multiple Ig heavy-cHIn lineages. J. Virol. 2014, 88, 4047–4057. [Google Scholar] [CrossRef] [PubMed]
- Kanekiyo, M.; Joyce, M.G.; Gillespie, R.A.; Gallagher, J.R.; Andrews, S.F.; Yassine, H.M.; Wheatley, A.K.; Fisher, B.E.; Ambrozak, D.R.; Creanga, A.; et al. Mosaic nanoparticle display of diverse influenza virus hemagglutinins elicits broad B cell responses. Nat. Immunol. 2019, 20, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Kingsman, S.M.; Kingsman, A.J. Polyvalent recombinant antigens: A new vaccine strategy. Vaccine 1988, 6, 304–306. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Pho, T.; Champion, J.A. Chemical and biological conjugation strategies for the development of multivalent protein vaccine nanoparticles. Biopolymers 2023, 114, e23563. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Qi, Y.; Liu, G.; Song, Y.; Jiang, X.; Du, B. Size-Dependent in vivo Transport of Nanoparticles: Implications for Delivery, Targeting, and Clearance. ACS Nano 2023, 17, 20825–20849. [Google Scholar] [CrossRef]
- Hendin, H.E.; Lavoie, P.O.; Gravett, J.M.; Pillet, S.; Saxena, P.; Landry, N.; D’Aoust, M.A.; Ward, B.J. Elimination of receptor binding by influenza hemagglutinin improves vaccine-induced immunity. NPJ Vaccines 2022, 7, 42. [Google Scholar] [CrossRef]
- Michael, R.G.; Joseph, S. Molecular Cloning: A Laboratory Manual, 4th ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2012; pp. 133–135, 165–166. [Google Scholar]



| Name | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| HA5-I53_dn5B/Y98F-1 | AAGGCCTACGTCGACGAGCT | AGTCGTTCAGGTTACCAGGGA AGCACAGGTCGTTAGCAGGG |
| HA5-I53_dn5B/Y98F-2 | CCCTGGTAACCTGAACGACT | CTTGGTACCGCATGCCTCGA |
| Serial Number | Grouping | HA (µg) | Al(OH)3 10 mg/mL (µL) | Immune Volume (µL) | Numbers |
|---|---|---|---|---|---|
| 1 | HA5-dn5 Low | 1 | 0 | 100 | 10 |
| 2 | HA5-dn5 High | 5 | 0 | 100 | 10 |
| 3 | HA5-dn5 High + Adjuvant | 5 | 10 | 100 | 10 |
| 4 | HA5-dn5B Low | 1 | 0 | 100 | 10 |
| 5 | HA5-dn5B High | 5 | 0 | 100 | 10 |
| 6 | HA5-dn5B High + Adjuvant | 5 | 10 | 100 | 10 |
| 7 | PBS | 0 | 10 | 100 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, H.; Zhang, B.; Zhang, X.; Wang, T.; Hou, X.; Lan, X.; Pan, C.; Wu, J.; Liu, B. Self-Assembling Nanoparticle Hemagglutinin Influenza Vaccines Induce High Antibody Response. Int. J. Mol. Sci. 2024, 25, 7259. https://doi.org/10.3390/ijms25137259
Ren H, Zhang B, Zhang X, Wang T, Hou X, Lan X, Pan C, Wu J, Liu B. Self-Assembling Nanoparticle Hemagglutinin Influenza Vaccines Induce High Antibody Response. International Journal of Molecular Sciences. 2024; 25(13):7259. https://doi.org/10.3390/ijms25137259
Chicago/Turabian StyleRen, Hongying, Bin Zhang, Xinwei Zhang, Tiantian Wang, Xvchen Hou, Xianyong Lan, Chuanying Pan, Jun Wu, and Bo Liu. 2024. "Self-Assembling Nanoparticle Hemagglutinin Influenza Vaccines Induce High Antibody Response" International Journal of Molecular Sciences 25, no. 13: 7259. https://doi.org/10.3390/ijms25137259





