Direct Current Electrical Stimulation Shifts THP-1-Derived Macrophage Polarization towards Pro-Regenerative M2 Phenotype
Abstract
:1. Introduction
2. Results
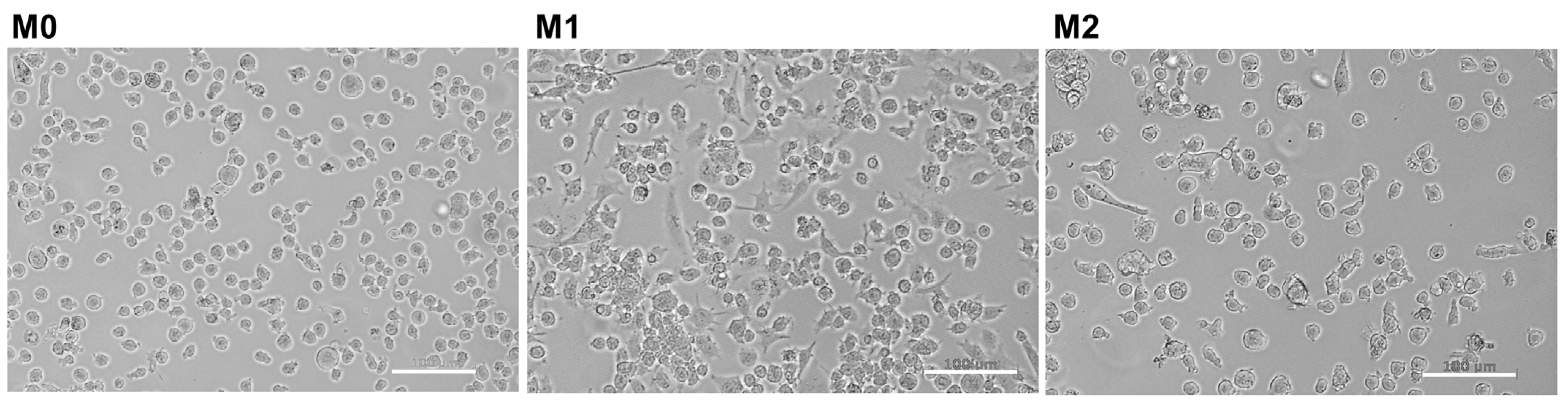
2.1. Induction of THP-1 Differentiation and Polarization
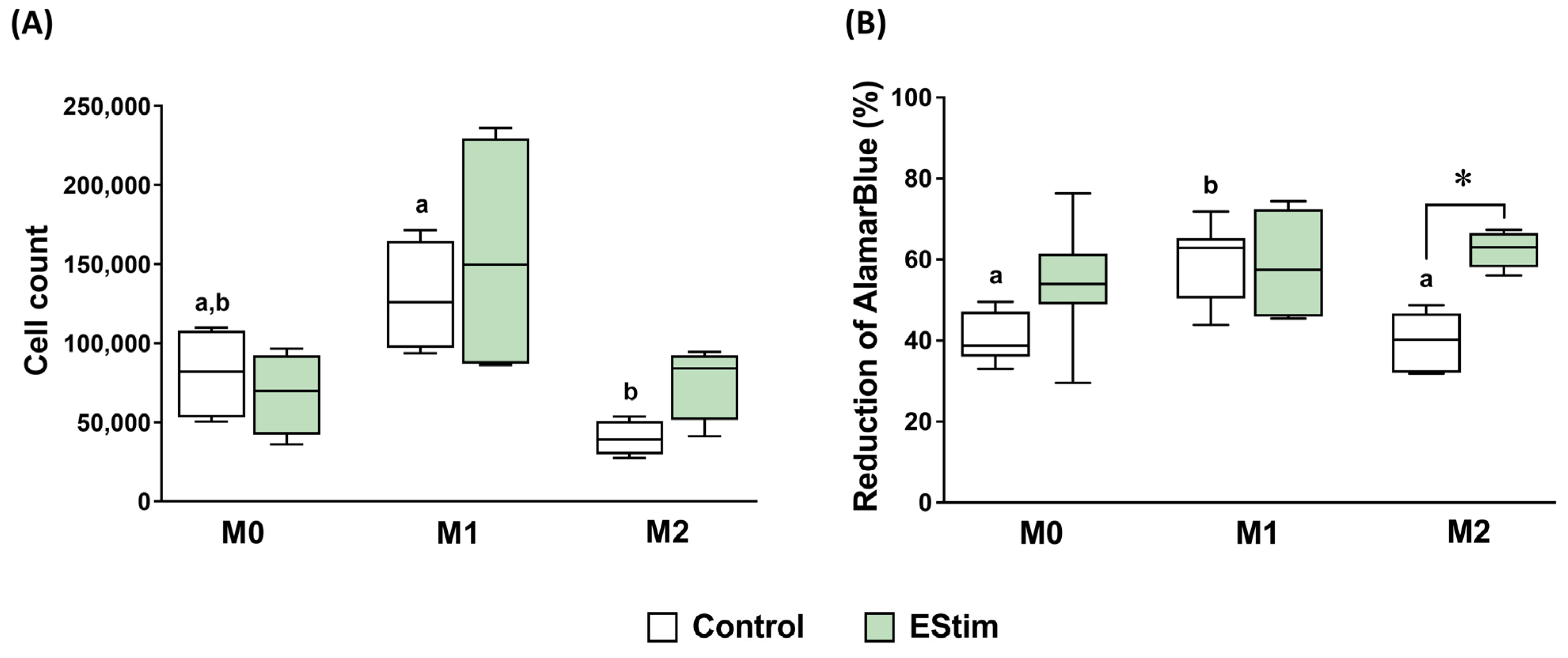
2.2. DC EStim Does Not Affect Cell Count and Viability of M0, M1, and M2 Macrophages
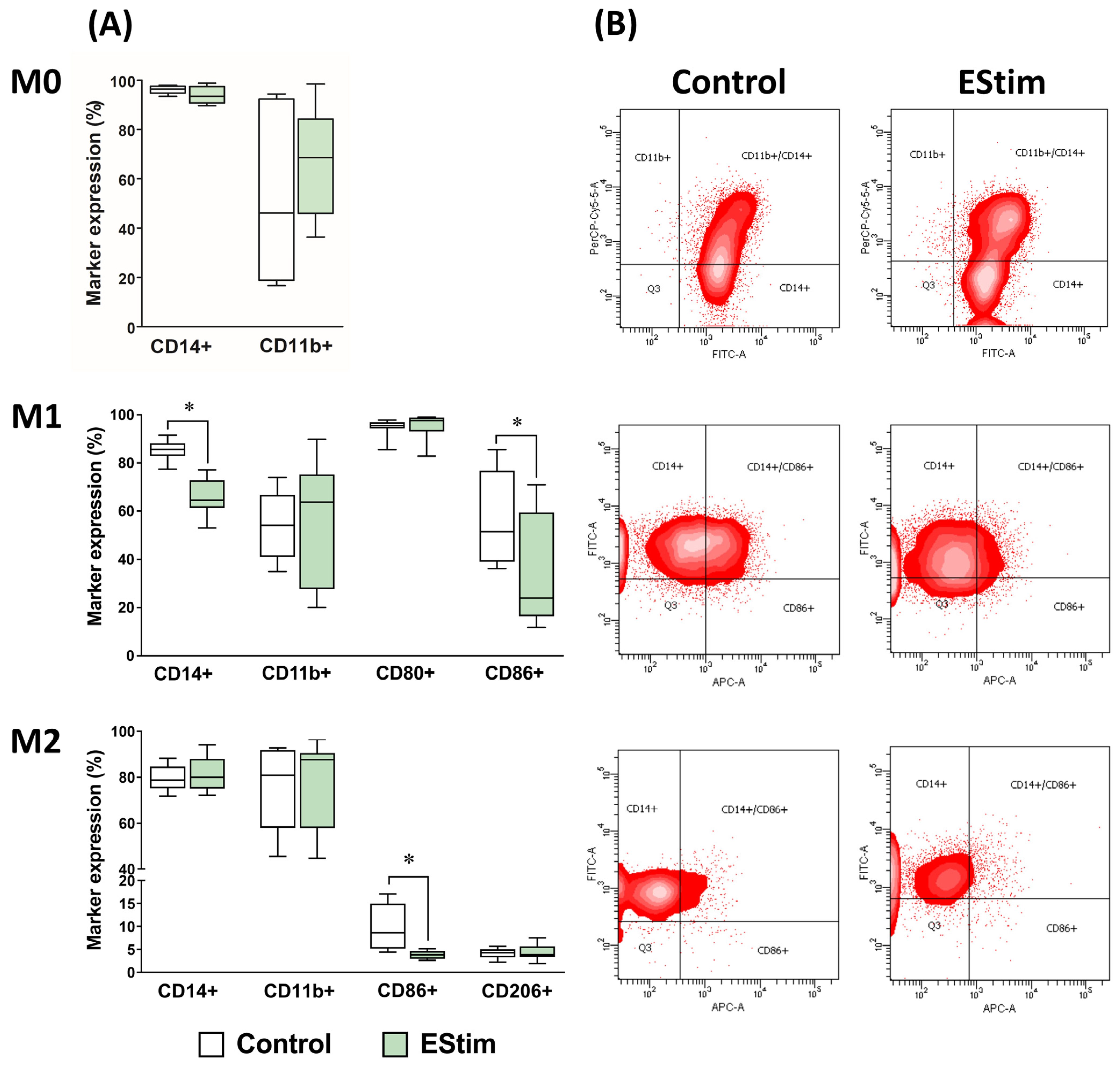
2.3. DC EStim Reduces Expressions of Cell Surface Markers CD14 in M1 and CD86 in M1 and M2 Macrophages
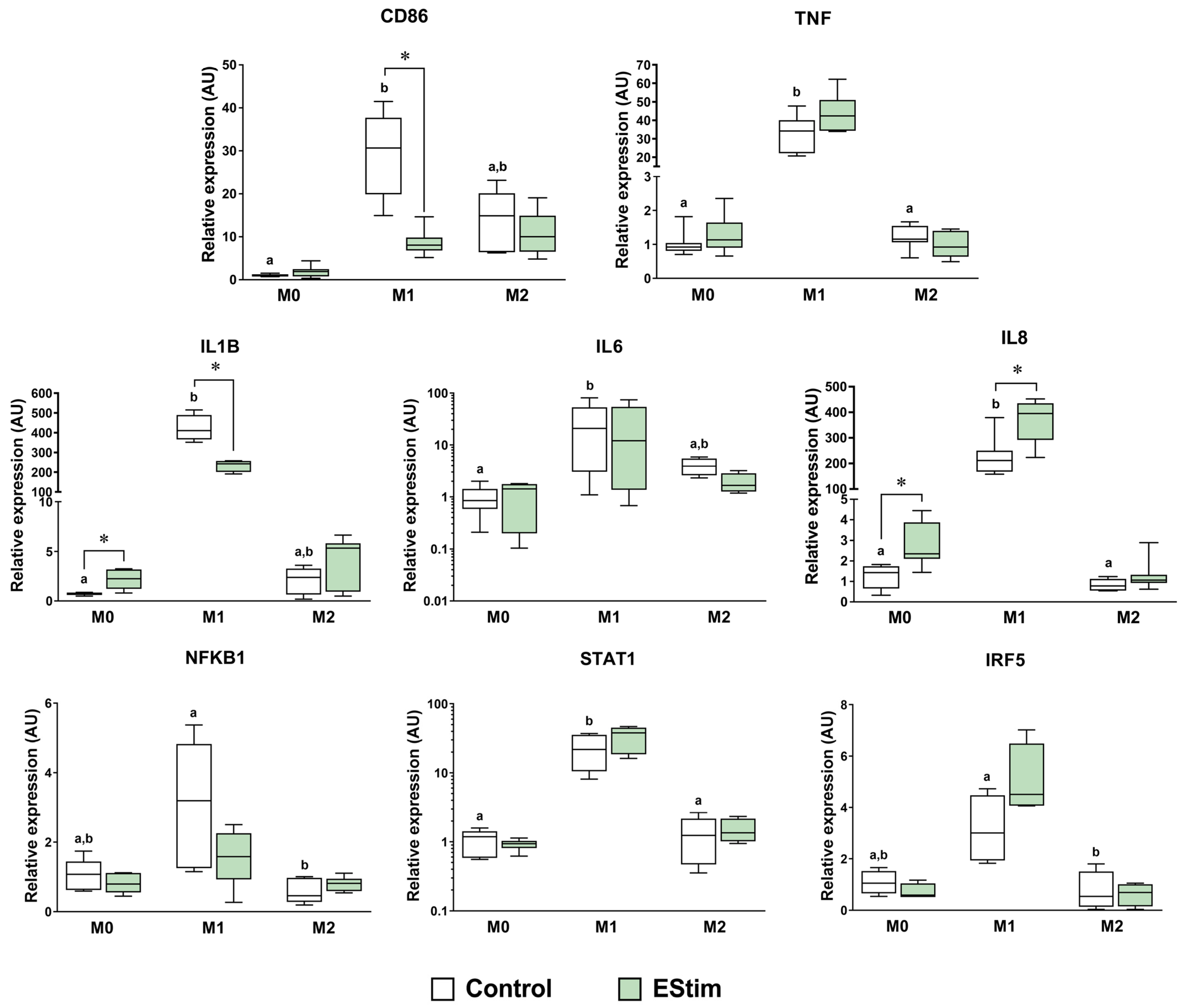
2.4. DC EStim Downregulates CD86 and IL1B Gene Expressions in M1 Cells While Upregulating Expressions of M2 Phenotype Markers in M0 and M1 Macrophages
2.5. DC EStim Reduces Secretion of IL-1β and IL-6 in M1 Cells While Increasing Secretion of IL-8 in M0, M1, and M2 Cells
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Cell Priming and Polarization
4.3. Direct Current Electrical Stimulation
4.4. Cell Viability
- O1 = E of oxidized alamarBlue at 570 nm (value: 80,586);
- O2 = E of oxidized alamarBlue at 600 nm (value: 117,216);
- R1 = E of reduced alamarBlue at 570 nm (value: 155,677);
- R2 = E of reduced alamarBlue at 600 nm (value: 14,652);
- A1 = absorbance of test wells at 570 nm;
- A2 = absorbance of test wells at 600 nm;
- N1 = absorbance of negative control well (medium plus alamarBlue but no cells) at 570 nm;
- N2 = absorbance of negative control well (medium plus alamarBlue but no cells) at 600 nm.
4.5. Recovery of Adherent Cells
4.6. Cell Surface Marker Analysis Using Flow Cytometry
4.7. Reverse Transcription Quantitative Polymerase Chain Reaction (RT-qPCR)
4.8. Cytokines in Cell-Conditioned Medium
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schlundt, C.; Fischer, H.; Bucher, C.H.; Rendenbach, C.; Duda, G.N.; Schmidt-Bleek, K. The multifaceted roles of macrophages in bone regeneration: A story of polarization, activation and time. Acta Biomater. 2021, 133, 46–57. [Google Scholar] [CrossRef]
- Hoare, J.I.; Rajnicek, A.M.; McCaig, C.D.; Barker, R.N.; Wilson, H.M. Electric fields are novel determinants of human macrophage functions. J. Leukoc. Biol. 2016, 99, 1141–1151. [Google Scholar] [CrossRef]
- Rőszer, T. Understanding the mysterious M2 macrophage through activation markers and effector mechanisms. Mediat. Inflamm. 2015, 2015, 816460. [Google Scholar] [CrossRef]
- Zhang, Y.; Choksi, S.; Chen, K.; Pobezinskaya, Y.; Linnoila, I.; Liu, Z.G. ROS play a critical role in the differentiation of alternatively activated macrophages and the occurrence of tumor-associated macrophages. Cell Res. 2013, 23, 898–914. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J. Macrophage Polarization. Annu. Rev. Physiol. 2017, 79, 541–566. [Google Scholar] [CrossRef]
- Wynn, T.A.; Vannella, K.M. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 2016, 44, 450–462. [Google Scholar] [CrossRef]
- Mirza, R.; DiPietro, L.A.; Koh, T.J. Selective and specific macrophage ablation is detrimental to wound healing in mice. Am. J. Pathol. 2009, 175, 2454–2462. [Google Scholar] [CrossRef] [PubMed]
- Godwin, J.W.; Rosenthal, N. Scar-free wound healing and regeneration in amphibians: Immunological influences on regenerative success. Differentiation 2014, 87, 66–75. [Google Scholar] [CrossRef]
- Simkin, J.; Sammarco, M.C.; Marrero, L.; Dawson, L.A.; Yan, M.; Tucker, C.; Cammack, A.; Muneoka, K. Macrophages are required to coordinate mouse digit tip regeneration. Development 2017, 144, 3907–3916. [Google Scholar] [CrossRef]
- Henrich, D.; Seebach, C.; Verboket, R.; Schaible, A.; Marzi, I.; Bonig, H. The osteo-inductive activity of bone-marrow-derived mononuclear cells resides within the CD14+ population and is independent of the CD34+ population. Eur. Cells Mater. 2018, 35, 165–177. [Google Scholar] [CrossRef]
- Gordon, S. Alternative activation of macrophages. Nat. Rev. Immunol. 2003, 3, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Biswas, S.K.; Galdiero, M.R.; Sica, A.; Locati, M. Macrophage plasticity and polarization in tissue repair and remodelling. J. Pathol. 2013, 229, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.N.; Londono, R.; Tottey, S.; Zhang, L.; Kukla, K.A.; Wolf, M.T.; Daly, K.A.; Reing, J.E.; Badylak, S.F. Macrophage phenotype as a predictor of constructive remodeling following the implantation of biologically derived surgical mesh materials. Acta Biomater. 2012, 8, 978–987. [Google Scholar] [CrossRef] [PubMed]
- Mi, B.; Chen, L.; Xiong, Y.; Yang, Y.; Panayi, A.C.; Xue, H.; Hu, Y.; Yan, C.; Hu, L.; Xie, X.; et al. Osteoblast/osteoclast and immune cocktail therapy of an exosome/drug delivery multifunctional hydrogel accelerates fracture repair. ACS Nano 2022, 16, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Zhao, Y.; Zhang, Y.; Ruan, Z. The Macrophage Polarization Regulates MSC Osteoblast Differentiation in vitro. Ann. Clin. Lab. Sci. 2016, 46, 65–71. [Google Scholar] [PubMed]
- Xiong, Y.; Chen, L.; Yan, C.; Zhou, W.; Yu, T.; Sun, Y.; Cao, F.; Xue, H.; Hu, Y.; Chen, D.; et al. M2 macrophagy-derived exosomal miRNA-5106 induces bone mesenchymal stem cells towards osteoblastic fate by targeting salt-inducible kinase 2 and 3. J. Nanobiotechnol. 2020, 18, 66. [Google Scholar] [CrossRef] [PubMed]
- Kloth, L.C. Electrical stimulation for wound healing: A review of evidence from in vitro studies, animal experiments, and clinical trials. Int. J. Low Extrem. Wounds 2005, 4, 23–44. [Google Scholar] [CrossRef] [PubMed]
- Bhavsar, M.B.; Han, Z.; DeCoster, T.; Leppik, L.; Costa Oliveira, K.M.; Barker, J.H. Electrical stimulation-based bone fracture treatment, if it works so well why do not more surgeons use it? Eur. J. Trauma Emerg. Surg. 2020, 46, 245–264. [Google Scholar] [CrossRef] [PubMed]
- Luo, R.; Dai, J.; Zhang, J.; Li, Z. Accelerated Skin Wound Healing by Electrical Stimulation. Adv. Healthc. Mater. 2021, 10, e2100557. [Google Scholar] [CrossRef]
- Sundelacruz, S.; Levin, M.; Kaplan, D.L. Membrane potential controls adipogenic and osteogenic differentiation of mesenchymal stem cells. PLoS ONE 2008, 3, e3737. [Google Scholar] [CrossRef]
- Sundelacruz, S.; Levin, M.; Kaplan, D.L. Depolarization alters phenotype, maintains plasticity of predifferentiated mesenchymal stem cells. Tissue Eng. Part A 2013, 19, 1889–1908. [Google Scholar] [CrossRef] [PubMed]
- Mobini, S.; Leppik, L.; Thottakkattumana Parameswaran, V.; Barker, J.H. In vitro effect of direct current electrical stimulation on rat mesenchymal stem cells. PeerJ 2017, 5, e2821. [Google Scholar] [CrossRef] [PubMed]
- Eischen-Loges, M.; Oliveira, K.M.C.; Bhavsar, M.B.; Barker, J.H.; Leppik, L. Pretreating mesenchymal stem cells with electrical stimulation causes sustained long-lasting pro-osteogenic effects. PeerJ 2018, 6, e4959. [Google Scholar] [CrossRef] [PubMed]
- Leppik, L.; Oliveira, K.M.C.; Bhavsar, M.B.; Barker, J.H. Electrical stimulation in bone tissue engineering treatments. Eur. J. Trauma Emerg. Surg. 2020, 46, 231–244. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, J.H.J.; Bagne, L.; Meneghetti, D.H.; Dos Santos, G.M.T.; Esquisatto, M.A.M.; de Andrade, T.A.M.; do Amaral, M.E.C.; Felonato, M.; Caetano, G.F.; Santamaria, M.J.; et al. Electrical stimulation: Complementary therapy to improve the performance of grafts in bone defects? J. Biom. Mater. Res. B Appl. Biomater. 2019, 107, 924–932. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.K.; Oh, J.Y.; Jeong, G.J.; Lee, T.J.; Im, G.B.; Lee, J.R.; Yoon, J.K.; Kim, D.I.; Kim, B.S.; Bhang, S.H.; et al. A disposable photovoltaic patch controlling cellular microenvironment for wound healing. Int. J. Mol. Sci. 2018, 19, 3025. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, K.M.C.; Barker, J.H.; Berezikov, E.; Pindur, L.; Kynigopoulos, S.; Eischen-Loges, M.; Han, Z.; Bhavsar, M.B.; Henrich, D.; Leppik, L. Electrical stimulation shifts healing/scarring towards regeneration in a rat limb amputation model. Sci. Rep. 2019, 9, 11433. [Google Scholar] [CrossRef] [PubMed]
- Tedesco, S.; De Majo, F.; Kim, J.; Trenti, A.; Trevisi, L.; Fadini, G.P.; Bolego, C.; Zandstra, P.W.; Cignarella, A.; Vitiello, L. Convenience versus biological significance: Are PMA-differentiated THP-1 cells a reliable substitute for blood-derived macrophages when studying in vitro polarization? Front. Pharmacol. 2018, 9, 71. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Levin, M.; Kaplan, D.L. Bioelectric modulation of macrophage polarization. Sci. Rep. 2016, 6, 21044. [Google Scholar] [CrossRef] [PubMed]
- Shiratori, H.; Feinweber, C.; Luckhardt, S.; Linke, B.; Resch, E.; Geisslinger, G.; Weigert, A.; Parnham, M.J. THP-1 and human peripheral blood mononuclear cell-derived macrophages differ in their capacity to polarize in vitro. Mol. Immunol. 2017, 88, 58–68. [Google Scholar] [CrossRef]
- Arnold, C.E.; Rajnicek, A.M.; Hoare, J.I.; Pokharel, S.M.; McCaig, C.D.; Barker, R.N.; Wilson, H.M. Physiological strength electric fields modulate human T cell activation and polarisation. Sci. Rep. 2019, 9, 17604. [Google Scholar] [CrossRef] [PubMed]
- Abadijoo, H.; Khayamian, M.A.; Faramarzpour, M.; Ghaderinia, M.; Simaee, H.; Shalileh, S.; Yazdanparast, S.M.; Ghabraie, B.; Makarem, J.; Sarrami-Forooshani, R.; et al. Healing Field: Using Alternating Electric Fields to Prevent Cytokine Storm by Suppressing Clonal Expansion of the Activated Lymphocytes in the Blood Sample of the COVID-19 Patients. Front. Bioeng. Biotechnol. 2022, 10, 850571. [Google Scholar] [CrossRef] [PubMed]
- Moarefian, M.; Davalos, R.V.; Burton, M.D.; Jones, C.N. Electrotaxis-on-Chip to Quantify Neutrophil Migration Towards Electrochemical Gradients. Front. Immunol. 2021, 12, 674727. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Baldessari, F.; Gyenge, C.C.; Sato, T.; Chambers, R.D.; Santiago, J.G.; Butcher, E.C. Lymphocyte electrotaxis in vitro and in vivo. J. Immunol. 2008, 181, 2465–2471. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Nandagopal, S.; Wu, D.; Romanuik, S.F.; Paul, K.; Thomson, D.J.; Lin, F. Activated T lymphocytes migrate toward the cathode of DC electric fields in microfluidic devices. Lab Chip 2011, 11, 1298–1304. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.R.; Thatte, H.S.; Lee, R.C.; Golan, D.E. Integrin-dependent human macrophage migration induced by oscillatory electrical stimulation. Ann. Biomed. Eng. 2000, 28, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Borovikova, L.V.; Ivanova, S.; Zhang, M.; Yang, H.; Botchkina, G.I.; Watkins, L.R.; Wang, H.; Abumrad, N.; Eaton, J.W.; Tracey, K.J. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 2000, 405, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Roy Barman, S.; Jhunjhunwala, S. Electrical Stimulation for Immunomodulation. ACS Omega 2024, 9, 52–66. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; He, X.; Chen, X.; Dong, L.; Weng, W.; Cheng, K. Effects of electrical stimulation on cytokine-induced macrophage polarization. J. Tissue Eng. Regen. Med. 2022, 16, 448–459. [Google Scholar] [CrossRef]
- Srirussamee, K.; Mobini, S.; Cassidy, N.J.; Cartmell, S.H. Direct electrical stimulation enhances osteogenesis by inducing Bmp2 and Spp1 expressions from macrophages and preosteoblasts. Biotechnol. Bioeng. 2019, 116, 3421–3432. [Google Scholar] [CrossRef]
- Staehlke, S.; Bielfeldt, M.; Zimmermann, J.; Gruening, M.; Barke, I.; Freitag, T.; Speller, S.; Van Rienen, U.; Nebe, B. Pulsed electrical stimulation affects osteoblast adhesion and calcium ion signaling. Cells 2022, 11, 2650. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Titushkin, I.; Cho, M. Regulation of mesenchymal stem cell adhesion and orientation in 3D collagen scaffold by electrical stimulus. Bioelectrochemistry 2006, 69, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K. Effects of electrical stimulation of the cell: Wound healing, cell proliferation, apoptosis, and signal transduction. Med. Sci. 2023, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Viola, A.; Munari, F.; Sánchez-Rodríguez, R.; Scolaro, T.; Castegna, A. The metabolic signature of macrophage responses. Front. Immunol. 2019, 10, 1462. [Google Scholar] [CrossRef] [PubMed]
- Rampersad, S.N. Multiple applications of Alamar Blue as an indicator of metabolic function and cellular health in cell viability bioassays. Sensors 2012, 12, 12347–12360. [Google Scholar] [CrossRef] [PubMed]
- Baxter, E.W.; Graham, A.E.; Re, N.A.; Carr, I.M.; Robinson, J.I.; Mackie, S.L.; Morgan, A.W. Standardized protocols for differentiation of THP-1 cells to macrophages with distinct M(IFNγ+LPS), M(IL-4) and M(IL-10) phenotypes. J. Immunol. Methods 2020, 478, 112721. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.X.; Zhang, S.X.; Wu, H.J.; Rong, X.L.; Guo, J. M2b macrophage polarization and its roles in diseases. J. Leukoc. Biol. 2019, 106, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.O.; Gordon, S. The M1 and M2 paradigm of macrophage activation: Time for reassessment. F1000Prime Rep. 2014, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef]
- de Groot, A.E.; Pienta, K.J. Epigenetic control of macrophage polarization: Implications for targeting tumor-associated macrophages. Oncotarget 2018, 9, 20908–20927. [Google Scholar] [CrossRef]
- Wang, P.H.; Huang, B.S.; Horng, H.C.; Yeh, C.C.; Chen, Y.J. Wound healing. J. Chin. Med. Assoc. 2018, 81, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Mirza, R.E.; Fang, M.M.; Ennis, W.J.; Koh, T.J. Blocking interleukin-1β induces a healing-associated wound macrophage phenotype and improves healing in type 2 diabetes. Diabetes 2013, 62, 2579–2587. [Google Scholar] [CrossRef] [PubMed]
- Weber, B.; Sturm, R.; Henrich, D.; Lupu, L.; Rottluff, K.; Marzi, I.; Leppik, L. Diagnostic and prognostic potential of exosomal cytokines IL-6 and IL-10 in polytrauma patients. Int. J. Mol. Sci. 2023, 24, 11830. [Google Scholar] [CrossRef] [PubMed]
- Vlahopoulos, S.; Boldogh, I.; Casola, A.; Brasier, A.R. Nuclear factor-kappaB-dependent induction of interleukin-8 gene expression by tumor necrosis factor alpha: Evidence for an antioxidant sensitive activating pathway distinct from nuclear translocation. Blood 1999, 94, 1878–1889. [Google Scholar] [CrossRef] [PubMed]
- Harada, A.; Sekido, N.; Akahoshi, T.; Wada, T.; Mukaida, N.; Matsushima, K. Essential involvement of interleukin-8 (IL-8) in acute inflammation. J. Leukoc. Biol. 1994, 56, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Koch, A.E.; Polverini, P.J.; Kunkel, S.L.; Harlow, L.A.; DiPietro, L.A.; Elner, V.M.; Elner, S.G.; Strieter, R.M. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science 1992, 258, 1798–1801. [Google Scholar] [CrossRef]
- Leppik, L.P.; Froemel, D.; Slavici, A.; Ovadia, Z.N.; Hudak, L.; Henrich, D.; Marzi, I.; Barker, J.H. Effects of electrical stimulation on rat limb regeneration, a new look at an old model. Sci. Rep. 2015, 5, 18353. [Google Scholar] [CrossRef]
- Dolan, C.P.; Dawson, L.A.; Muneoka, K. Digit tip regeneration: Merging regeneration biology with regenerative medicine. Stem Cells Transl. Med. 2018, 7, 262–270. [Google Scholar] [CrossRef]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone tissue engineering: Recent advances and challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef]
- Park, E.K.; Jung, H.S.; Yang, H.I.; Yoo, M.C.; Kim, C.; Kim, K.S. Optimized THP-1 differentiation is required for the detection of responses to weak stimuli. Inflamm. Res. 2007, 56, 45–50. [Google Scholar] [CrossRef]
- Bezold, V.; Rosenstock, P.; Scheffler, J.; Geyer, H.; Horstkorte, R.; Bork, K. Glycation of macrophages induces expression of pro-inflammatory cytokines and reduces phagocytic efficiency. Aging 2019, 11, 5258–5275. [Google Scholar] [CrossRef] [PubMed]
- Mobini, S.; Leppik, L.; Barker, J.H. Direct current electrical stimulation chamber for treating cells in vitro. Biotechniques 2016, 60, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Leppik, L.; Bhavsar, M.B.; Oliveira, K.M.C.; Eischen-Loges, M.; Mobini, S.; Barker, J.H. Construction and Use of an Electrical Stimulation Chamber for Enhancing Osteogenic Differentiation in Mesenchymal Stem/Stromal Cells In Vitro. J. Vis. Exp. 2019, 143. [Google Scholar] [CrossRef]
- Bianconi, S.; Oliveira, K.M.C.; Klein, K.-L.; Wolf, J.; Schaible, A.; Schröder, K.; Barker, J.; Marzi, I.; Leppik, L.; Henrich, D. Pretreatment of Mesenchymal Stem Cells with Electrical Stimulation as a Strategy to Improve Bone Tissue Engineering Outcomes. Cells 2023, 12, 2151. [Google Scholar] [CrossRef] [PubMed]
- Leppik, L.; Gempp, A.; Kuçi, Z.; Kuçi, S.; Bader, P.; Bönig, H.; Marzi, I.; Henrich, D. A New Perspective for Bone Tissue Engineering: Human Mesenchymal Stromal Cells Well-Survive Cryopreservation on β-TCP Scaffold and Show Increased Ability for Osteogenic Differentiation. Int. J. Mol. Sci. 2022, 23, 1425. [Google Scholar] [CrossRef] [PubMed]
- Maciorowski, Z.; Chattopadhyay, P.K.; Jain, P. Basic Multicolor Flow Cytometry. Curr. Protoc. Immunol. 2017, 117, 5.4.1–5.4.38. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
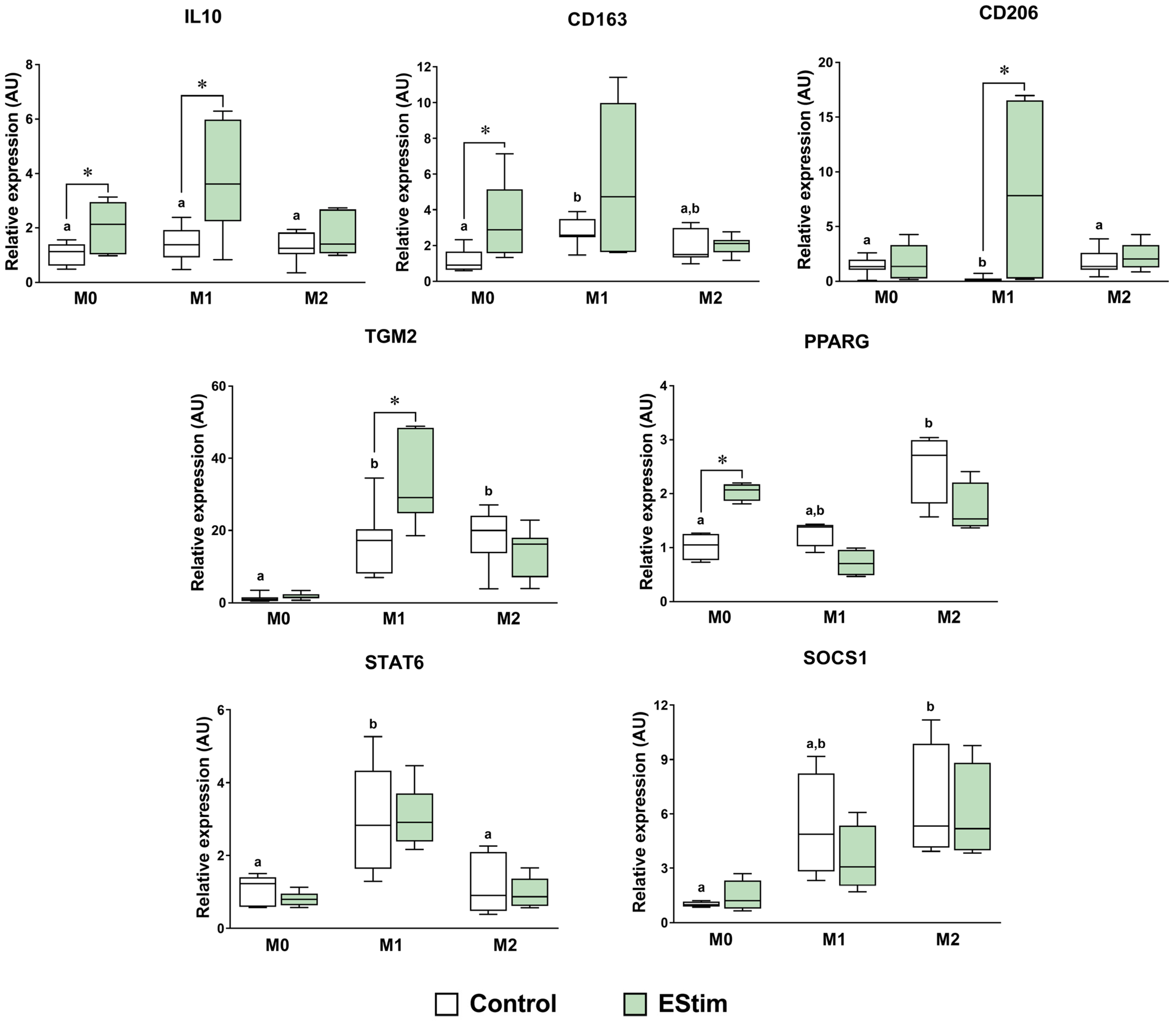

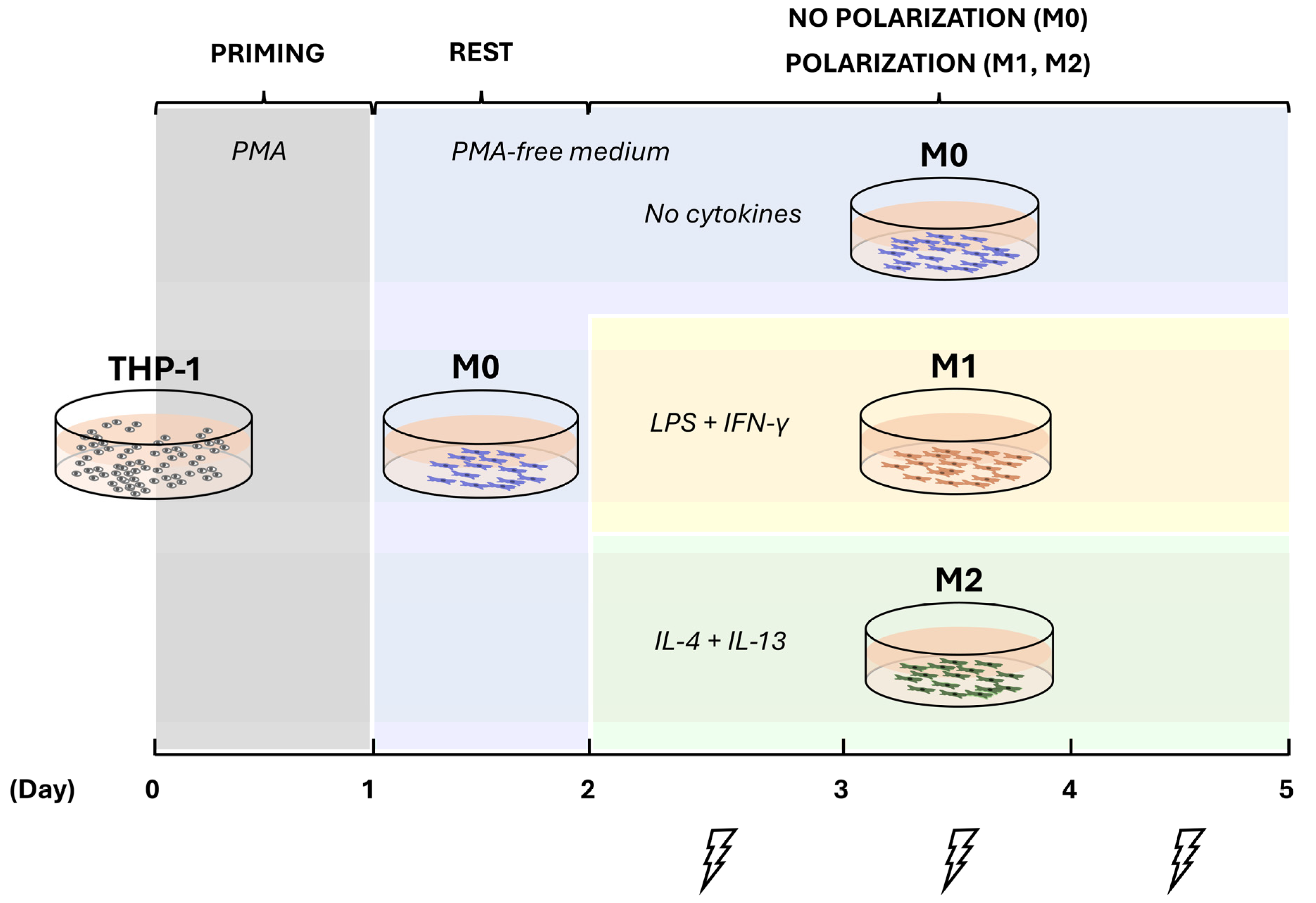
| Group | Priming (24 h) | Cytokines (Days 2–5) | DC EStim (Days 2–4) | Analysis (Day 5) |
|---|---|---|---|---|
| M0 control | PMA 5 ng/mL | - | - | Cell count, viability, gene and cell surface marker expressions, and cytokine secretion profiles. |
| M0 EStim | PMA 5 ng/mL | - | 100 mV/mm, 1 h/day | |
| M1 control | PMA 5 ng/mL | IFN-γ 20 ng/mL, LPS 100 ng/mL | - | |
| M1 EStim | PMA 5 ng/mL | IFN-γ 20 ng/mL, LPS 100 ng/mL | 100 mV/mm, 1 h/day | |
| M2 control | PMA 5 ng/mL | IL-4 20 ng/mL, IL-13 20 ng/mL | - | |
| M2 EStim | PMA 5 ng/mL | IL-4 20 ng/mL, IL-13 20 ng/mL | 100 mV/mm, 1 h/day |
| Gene | Forward (5′->3′) | Reverse (5′->3′) | Annealing Temperature (°C) | Amplicon Size |
|---|---|---|---|---|
| CD163 | GCCATTCTGAGCCACACTGA | AGTCCAGGTCTTCATCAAGGT | 61 | 87 |
| CD206 | CGATCCGACCCTTCCTTGAC | TGTCTCCGCTTCATGCCATT | 63 | 120 |
| IL1B | CAGAAGTACCTGAGCTCGCC | AGATTCGTAGCTGGATGCCG | 62 | 153 |
| IL8 | AAGGAACCATCTCACTGTGTGTAAAC | ATCAGGAAGGCTGCCAAGAG | 62 | 70 |
| IRF5 | CCAGCCAGGACGGAGATAAC | AGGTTGGCCTTCCACTTGG | 56 | 106 |
| NFKB1 | GTTTGTCCAGCTTCGGAGGA | CACTACCACCGCCGAAACTA | 59 | 149 |
| SOCS1 | TTTTCGCCCTTAGCGTGAA | CATCCAGGTGAAAGCGGC | 56 | 81 |
| STAT1 | GGAAATCAGACAGTACCTGGCA | ACAGGAGGTCATGAAAACGGA | 56 | 101 |
| STAT6 | AAAGTGCAGCGGCTCTATGT | GGTGCTGGACAGTGTCTGAA | 57 | 151 |
| TNF | CCAGGCAGTCAGATCATCTTCTC | AGCTGGTTATCTCTCAGCTCCAC | 62 | 150 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bianconi, S.; Leppik, L.; Oppermann, E.; Marzi, I.; Henrich, D. Direct Current Electrical Stimulation Shifts THP-1-Derived Macrophage Polarization towards Pro-Regenerative M2 Phenotype. Int. J. Mol. Sci. 2024, 25, 7272. https://doi.org/10.3390/ijms25137272
Bianconi S, Leppik L, Oppermann E, Marzi I, Henrich D. Direct Current Electrical Stimulation Shifts THP-1-Derived Macrophage Polarization towards Pro-Regenerative M2 Phenotype. International Journal of Molecular Sciences. 2024; 25(13):7272. https://doi.org/10.3390/ijms25137272
Chicago/Turabian StyleBianconi, Santiago, Liudmila Leppik, Elsie Oppermann, Ingo Marzi, and Dirk Henrich. 2024. "Direct Current Electrical Stimulation Shifts THP-1-Derived Macrophage Polarization towards Pro-Regenerative M2 Phenotype" International Journal of Molecular Sciences 25, no. 13: 7272. https://doi.org/10.3390/ijms25137272
APA StyleBianconi, S., Leppik, L., Oppermann, E., Marzi, I., & Henrich, D. (2024). Direct Current Electrical Stimulation Shifts THP-1-Derived Macrophage Polarization towards Pro-Regenerative M2 Phenotype. International Journal of Molecular Sciences, 25(13), 7272. https://doi.org/10.3390/ijms25137272






