Alpha 1,3 N-Acetylgalactosaminyl Transferase (GTA) Impairs Invasion Potential of Trophoblast Cells in Preeclampsia
Abstract
1. Introduction
2. Results
2.1. Increased Terminal GalNAc α1,3 Gal in Preeclamptic Placental Tissues
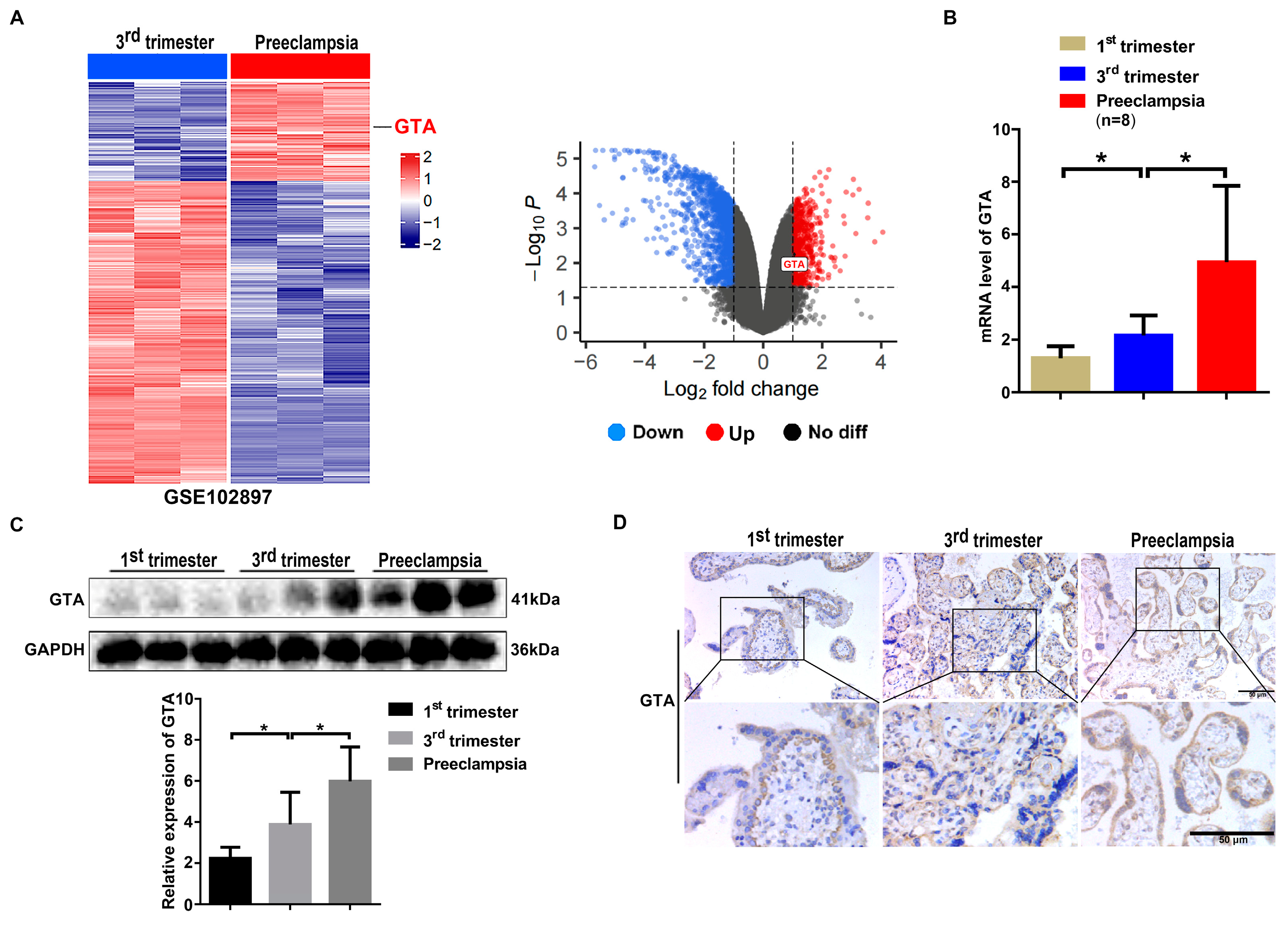
2.2. Expression of GTA Is Upregulated in Preeclamptic Placental Tissues
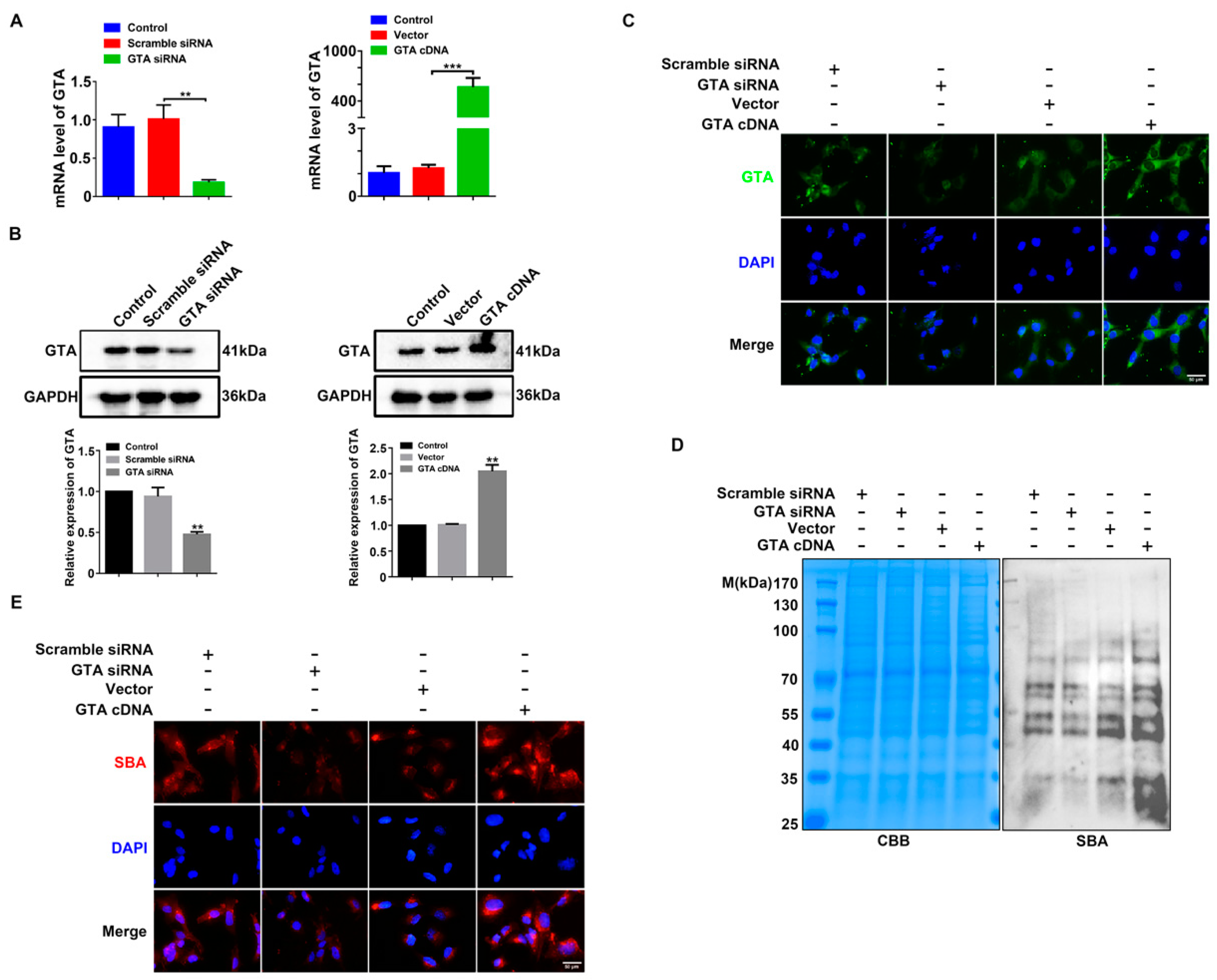
2.3. GTA Promotes the Biosynthesis of Terminal GalNAc α1,3 Gal in Trophoblast Cells
2.4. GTA Affects EMT, Proliferation, and Vascular Remodeling of Trophoblast Cells
2.5. DNA Methylation Status of GTA Promoter in Third Trimester and Preeclamptic Placental Tissues
2.6. Upregulated GTA Expression by Hypomethylation Inhibits Migration/Invasion and Vascularization of Trophoblast Cells
3. Discussion
4. Materials and Methods
4.1. Tissue Collection
4.2. Experimental Reagents and Antibodies
4.3. Cell Culture and Transfection
4.4. Real-Time Quantitative PCR
4.5. Western Blot Assay
4.6. Immunofluorescence
4.7. Transwell Assay
4.8. 5-Ethynyl-20-Deoxyuridine (EdU) Incorporation Assay
4.9. Tube Formation Assay
4.10. Methylation-Specific PCR and Bisulfite Sequencing PCR
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Chaiworapongsa, T.; Chaemsaithong, P.; Yeo, L.; Romero, R. Pre-eclampsia part 1: Current understanding of its pathophysiology. Nat. Rev. Nephrol. 2014, 10, 466–480. [Google Scholar] [CrossRef]
- Phipps, E.A.; Thadhani, R.; Benzing, T.; Karumanchi, S.A. Pre-eclampsia: Pathogenesis, novel diagnostics and therapies. Nat. Rev. Nephrol. 2019, 15, 275–289. [Google Scholar] [CrossRef] [PubMed]
- Armaly, Z.; Jadaon, J.E.; Jabbour, A.; Abassi, Z.A. Preeclampsia: Novel Mechanisms and Potential Therapeutic Approaches. Front. Physiol. 2018, 9, 973. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.M.; Gammill, H.S. Preeclampsia: Recent insights. Hypertension 2005, 46, 1243–1249. [Google Scholar] [CrossRef]
- Pollheimer, J.; Vondra, S.; Baltayeva, J.; Beristain, A.G.; Knöfler, M. Regulation of Placental Extravillous Trophoblasts by the Maternal Uterine Environment. Front. Immunol. 2018, 9, 2597. [Google Scholar] [CrossRef]
- Jones, C.J.; Aplin, J.D. Glycosylation at the fetomaternal interface: Does the glycocode play a critical role in implantation? Glycoconj. J. 2009, 26, 359–366. [Google Scholar] [CrossRef]
- Keikkala, E.; Vuorela, P.; Laivuori, H.; Romppanen, J.; Heinonen, S.; Stenman, U.H. First trimester hyperglycosylated human chorionic gonadotrophin in serum—A marker of early-onset preeclampsia. Placenta 2013, 34, 1059–1065. [Google Scholar] [CrossRef]
- Moungmaithong, S.; Wang, X.; Lau, C.S.L.; Tse, A.W.T.; Lee, N.M.W.; Leung, H.H.Y.; Poon, L.C.; Sahota, D.S. Glycosylated fibronectin improves first-trimester prediction of pre-eclampsia. Ultrasound Obs. Gynecol. 2023, 62, 512–521. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, S.; Liu, Y.; Wang, Z.; Wang, X.; Yan, Q. Overexpression of fucosyltransferase VII (FUT7) promotes embryo adhesion and implantation. Fertil. Steril. 2009, 91, 908–914. [Google Scholar] [CrossRef]
- Letts, J.A.; Rose, N.L.; Fang, Y.R.; Barry, C.H.; Borisova, S.N.; Seto, N.O.; Palcic, M.M.; Evans, S.V. Differential recognition of the type I and II H antigen acceptors by the human ABO(H) blood group A and B glycosyltransferases. J. Biol. Chem. 2006, 281, 3625–3632. [Google Scholar] [CrossRef]
- Liao, W.-C.; Liu, C.-H.; Chen, C.-H.; Hsu, W.-M.; Liao, Y.-Y.; Chang, H.-M.; Lan, C.-T.; Huang, M.-C.; Shyu, M.-K. β-1,4-Galactosyltransferase III suppresses extravillous trophoblast invasion through modifying β1-integrin glycosylation. Placenta 2015, 36, 357–364. [Google Scholar] [CrossRef]
- Wang, H.; Cui, X.; Wang, L.; Fan, N.; Yu, M.; Qin, H.; Liu, S.; Yan, Q. α1,3-fucosylation of MEST promotes invasion potential of cytotrophoblast cells by activating translation initiation. Cell Death Dis. 2023, 14, 651. [Google Scholar] [CrossRef]
- Zhou, R.; Wang, X.; Liu, H.; Guo, L.; Su, Q.; Wang, H.; Vasiliadis, T.; Ho, W.; Li, J. GalNAc-Specific Soybean Lectin Inhibits HIV Infection of Macrophages through Induction of Antiviral Factors. J. Virol. 2018, 92, e01720-17. [Google Scholar] [CrossRef]
- Apicella, C.; Ruano, C.S.M.; Méhats, C.; Miralles, F.; Vaiman, D. The Role of Epigenetics in Placental Development and the Etiology of Preeclampsia. Int. J. Mol. Sci. 2019, 20, 2837. [Google Scholar] [CrossRef]
- Kamrani, A.; Alipourfard, I.; Ahmadi-Khiavi, H.; Yousefi, M.; Rostamzadeh, D.; Izadi, M.; Ahmadi, M. The role of epigenetic changes in preeclampsia. Biofactors 2019, 45, 712–724. [Google Scholar] [CrossRef]
- Ye, W.; Shen, L.; Xiong, Y.; Zhou, Y.; Gu, H.; Yang, Z. Preeclampsia is Associated with Decreased Methylation of the GNA12 Promoter. Ann. Hum. Genet. 2016, 80, 7–10. [Google Scholar]
- Zhou, Y.; Zhang, X.; Liu, Z.; Wang, N.; Zhao, X.; Guo, R. DNMT1 mediates proliferation, migration and invasion of extravillous trophoblasts by regulating the methylation level of APLNR. Placenta 2023, 138, 33–43. [Google Scholar] [CrossRef]
- Ma, M.; Zhou, Q.J.; Xiong, Y.; Li, B.; Li, X.T. Preeclampsia is associated with hypermethylation of IGF-1 promoter mediated by DNMT1. Am. J. Transl. Res. 2018, 10, 16–39. [Google Scholar]
- Yamamoto, E.; Ino, K.; Miyoshi, E.; Inamori, K.I.; Abe, A.; Sumigama, S.; Iwase, A.; Kajiyama, H.; Shibata, K.; Nawa, A.; et al. N-acetylglucosaminyltransferase V regulates extravillous trophoblast invasion through glycosylation of alpha5beta1 integrin. Endocrinology 2009, 150, 990–999. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yu, M.; Cui, X.; Wang, H.; Liu, J.; Qin, H.; Liu, S.; Yan, Q. FUT8 drives the proliferation and invasion of trophoblastic cells via IGF-1/IGF-1R signaling pathway. Placenta 2019, 75, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.-C.; Liu, C.-H.; Chen, C.-W.; Hung, J.-S.; Chou, C.-H.; Che, M.-I.; Chang, H.-M.; Lan, C.-T.; Huang, H.-C.; Tseng, G.-F.; et al. Expression of GALNT2 in human extravillous trophoblasts and its suppressive role in trophoblast invasion. Placenta 2012, 33, 1005–1011. [Google Scholar] [CrossRef]
- Hu, Y.; Blair, J.D.; Yuen, R.K.; Robinson, W.P.; von Dadelszen, P. Genome-wide DNA methylation identifies trophoblast invasion-related genes: Claudin-4 and Fucosyltransferase IV control mobility via altering matrix metalloproteinase activity. Mol. Hum. Reprod. 2015, 21, 452–465. [Google Scholar] [CrossRef]
- Han, W.; Li, W.; Zhang, X.; Du, Z.; Liu, X.; Zhao, X.; Wen, X.; Wang, G.; Hu, J.-F.; Cui, J. Targeted breast cancer therapy by harnessing the inherent blood group antigen immune system. Oncotarget 2017, 8, 15034–15046. [Google Scholar] [CrossRef]
- Sainty, R.; Silver, M.J.; Prentice, A.M.; Monk, D. The influence of early environment and micronutrient availability on developmental epigenetic programming: Lessons from the placenta. Front. Cell Dev. Biol. 2023, 11, 1212199. [Google Scholar] [CrossRef]
- Zheng, X.; Lian, Y.; Zhou, J.; Zhou, Q.; Zhu, Y.; Tang, C.; Zhang, P.; Zhao, X. Placental ischemia disrupts DNA methylation patterns in distal regulatory regions in rats. Life Sci. 2023, 321, 121623. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, H.; Li, H.; Peng, T.; Gu, W.; Li, X. Hypermethylation of the HLA-G promoter is associated with preeclampsia. Mol. Hum. Reprod. 2015, 21, 736–744. [Google Scholar] [CrossRef]
- Zhang, L.; Leng, M.; Li, Y.; Yuan, Y.; Yang, B.; Li, Y.; Yuan, E.; Shi, W.; Yan, S.; Cui, S. Altered DNA methylation and transcription of WNT2 and DKK1 genes in placentas associated with early-onset preeclampsia. Clin. Chim. Acta. 2019, 490, 154–160. [Google Scholar] [CrossRef]
- Cruz, J.O.; Conceição, I.M.C.A.; Sandrim, V.C.; Luizon, M.R. Comprehensive analyses of DNA methylation of the TIMP3 promoter in placentas from early-onset and late-onset preeclampsia. Placenta 2022, 117, 118–121. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, F.; Li, C.; Li, H.; Tang, Q.; Chen, Y.; Ding, Z.; Xu, Y.; Chen, A.; Liu, S. Hypomethylation of DNA promoter upregulates ADAMTS7 and contributes to HTR-8/SVneo and JEG-3 cells abnormalities in pre-eclampsia. Placenta 2020, 93, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Kominato, Y.; Tsuchiya, T.; Hata, N.; Takizawa, H.; Yamamoto, F. Transcription of human ABO histo-blood group genes is dependent upon binding of transcription factor CBF/NF-Y to minisatellite sequence. J. Biol. Chem. 1997, 272, 25890–25898. [Google Scholar] [CrossRef] [PubMed]
- Kominato, Y.; Hata, Y.; Takizawa, H.; Tsuchiya, T.; Tsukada, J.; Yamamoto, F. Expression of human histo-blood group ABO genes is dependent upon DNA methylation of the promoter region. J. Biol. Chem. 1999, 274, 37240–37250. [Google Scholar] [CrossRef]
- Kominato, Y.; Hata, Y.; Takizawa, H.; Matsumoto, K.; Yasui, K.; Tsukada, J.-I.; Yamamoto, F.-I. Alternative promoter identified between a hypermethylated upstream region of repetitive elements and a CpG island in human ABO histo-blood group genes. J. Biol. Chem. 2002, 277, 37936–37948. [Google Scholar] [CrossRef]
- Kominato, Y.; Hata, Y.; Matsui, K.; Takizawa, H.; Tsukada, J.-I.; Nakajima, T.; Kaneko, Y.; Kishi, K. Transcriptional regulation of the human ABO histo-blood group genes is dependent on the N box upstream of the proximal promoter. Transfusion 2004, 44, 1741–1749. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Wu, H.; Pei, X.; Liu, S.; Yan, Q. Alpha 1,3 N-Acetylgalactosaminyl Transferase (GTA) Impairs Invasion Potential of Trophoblast Cells in Preeclampsia. Int. J. Mol. Sci. 2024, 25, 7287. https://doi.org/10.3390/ijms25137287
Li Y, Wu H, Pei X, Liu S, Yan Q. Alpha 1,3 N-Acetylgalactosaminyl Transferase (GTA) Impairs Invasion Potential of Trophoblast Cells in Preeclampsia. International Journal of Molecular Sciences. 2024; 25(13):7287. https://doi.org/10.3390/ijms25137287
Chicago/Turabian StyleLi, Yaqi, Hongpan Wu, Xiaosong Pei, Shuai Liu, and Qiu Yan. 2024. "Alpha 1,3 N-Acetylgalactosaminyl Transferase (GTA) Impairs Invasion Potential of Trophoblast Cells in Preeclampsia" International Journal of Molecular Sciences 25, no. 13: 7287. https://doi.org/10.3390/ijms25137287
APA StyleLi, Y., Wu, H., Pei, X., Liu, S., & Yan, Q. (2024). Alpha 1,3 N-Acetylgalactosaminyl Transferase (GTA) Impairs Invasion Potential of Trophoblast Cells in Preeclampsia. International Journal of Molecular Sciences, 25(13), 7287. https://doi.org/10.3390/ijms25137287





