Cryptic Extensibility in von Willebrand Factor Revealed by Molecular Nanodissection
Abstract
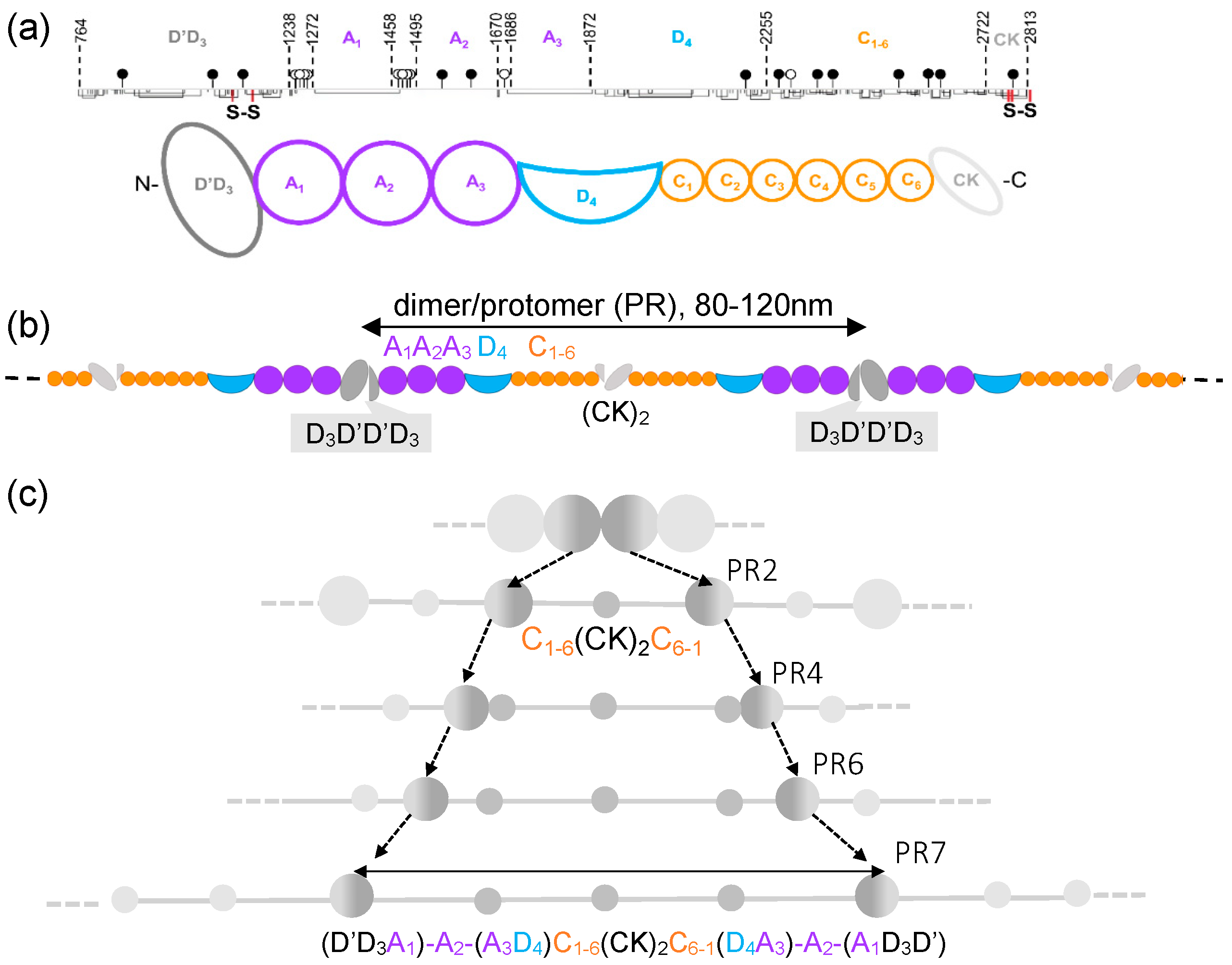
1. Introduction
2. Results and Discussion
2.1. Continuous VWF Loops Reveal Sources of Cryptic Extensibility

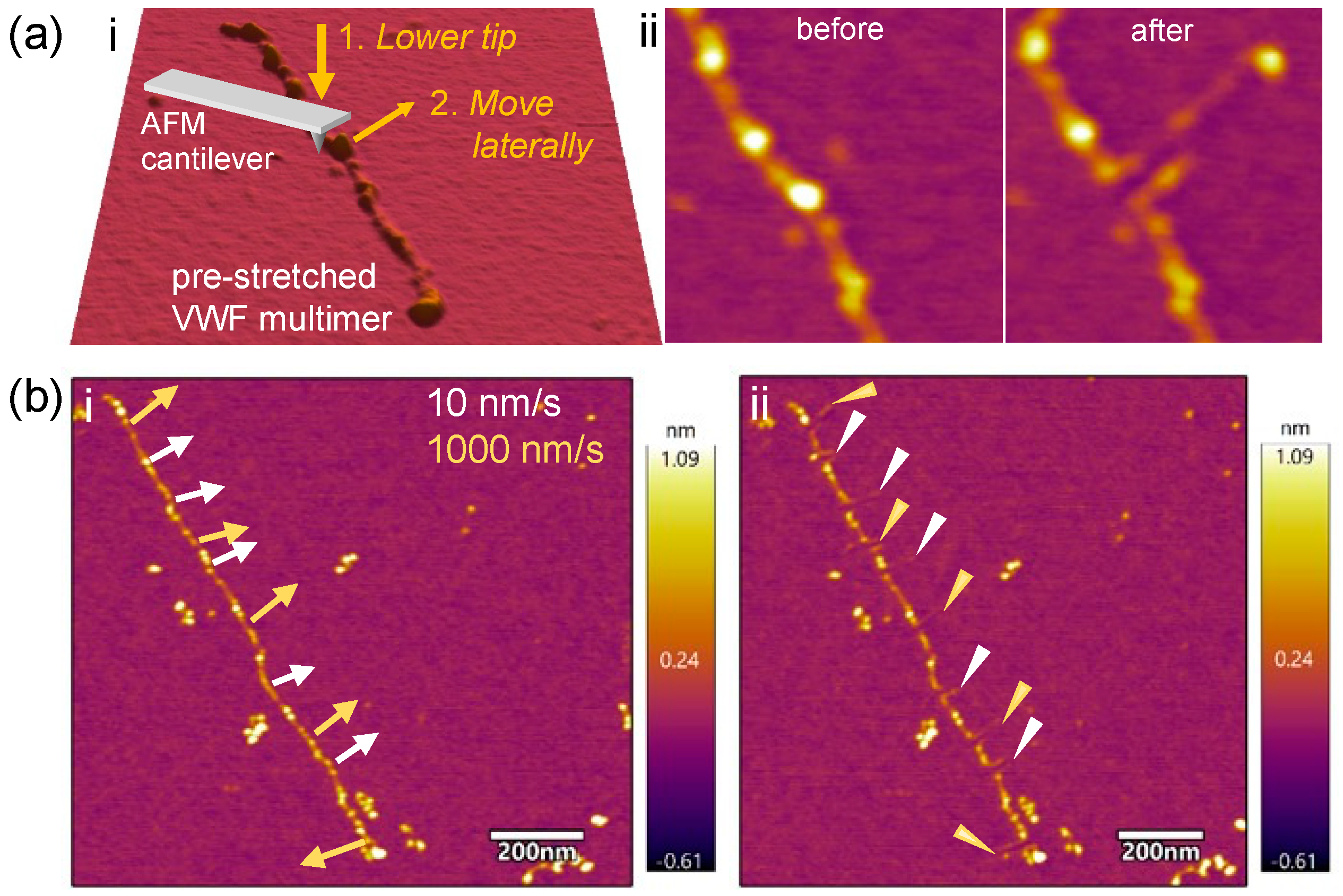
2.2. Ruptured VWF Loops Uncover Mechanically Weak Regions
3. Materials and Methods
3.1. Sample Preparation
3.2. Pre-Stretching VWF Multimers
3.3. AFM Imaging
3.4. VWF Nanodissection
3.5. Image Processing and Data Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bryckaert, M.; Rosa, J.P.; Denis, C.V.; Lenting, P.J. Of von Willebrand factor and platelets. Cell Mol. Life Sci. 2015, 72, 307–326. [Google Scholar] [CrossRef] [PubMed]
- Pham, O.L.; Feher, S.E.; Nguyen, Q.T.; Papavassiliou, D.V. Distribution and history of extensional stresses on vWF surrogate molecules in turbulent flow. Sci. Rep. 2022, 12, 171. [Google Scholar] [CrossRef]
- Lancellotti, S.; Sacco, M.; Basso, M.; De Cristofaro, R. Mechanochemistry of von Willebrand factor. Biomol. Concepts 2019, 10, 194–208. [Google Scholar] [CrossRef] [PubMed]
- Springer, T.A. von Willebrand factor, Jedi knight of the bloodstream. Blood 2014, 124, 1412–1425. [Google Scholar] [CrossRef] [PubMed]
- Csanyi, M.C.; Salamon, P.; Feller, T.; Bozo, T.; Harsfalvi, J.; Kellermayer, M.S.Z. Structural hierarchy of mechanical extensibility in human von Willebrand factor multimers. Protein Sci. 2023, 32, e4535. [Google Scholar] [CrossRef]
- Choi, H.; Aboulfatova, K.; Pownall, H.J.; Cook, R.; Dong, J.F. Shear-induced disulfide bond formation regulates adhesion activity of von Willebrand factor. J. Biol. Chem. 2007, 282, 35604–35611. [Google Scholar] [CrossRef]
- Zhou, Y.F.; Eng, E.T.; Zhu, J.; Lu, C.; Walz, T.; Springer, T.A. Sequence and structure relationships within von Willebrand factor. Blood 2012, 120, 449–458. [Google Scholar] [CrossRef]
- Zhou, Y.F.; Springer, T.A. Highly reinforced structure of a C-terminal dimerization domain in von Willebrand factor. Blood 2014, 123, 1785–1793. [Google Scholar] [CrossRef]
- Fowler, W.E.; Fretto, L.J.; Hamilton, K.K.; Erickson, H.P.; McKee, P.A. Substructure of human von Willebrand factor. J. Clin. Investig. 1985, 76, 1491–1500. [Google Scholar] [CrossRef]
- Bonazza, K.; Rottensteiner, H.; Schrenk, G.; Frank, J.; Allmaier, G.; Turecek, P.L.; Scheiflinger, F.; Friedbacher, G. Shear-Dependent Interactions of von Willebrand Factor with Factor VIII and Protease ADAMTS 13 Demonstrated at a Single Molecule Level by Atomic Force Microscopy. Anal. Chem. 2015, 87, 10299–10305. [Google Scholar] [CrossRef]
- Löf, A.; Walker, P.U.; Sedlak, S.M.; Gruber, S.; Obser, T.; Brehm, M.A.; Benoit, M.; Lipfert, J. Multiplexed protein force spectroscopy reveals equilibrium protein folding dynamics and the low-force response of von Willebrand factor. Proc. Natl. Acad. Sci. USA 2019, 116, 18798–18807. [Google Scholar] [CrossRef] [PubMed]
- Siedlecki, C.A.; Lestini, B.J.; Kottke-Marchant, K.K.; Eppell, S.J.; Wilson, D.L.; Marchant, R.E. Shear-dependent changes in the three-dimensional structure of human von Willebrand factor. Blood 1996, 88, 2939–2950. [Google Scholar] [CrossRef] [PubMed]
- Huizinga, E.G.; Tsuji, S.; Romijn, R.A.; Schiphorst, M.E.; de Groot, P.G.; Sixma, J.J.; Gros, P. Structures of glycoprotein Ibalpha and its complex with von Willebrand factor A1 domain. Science 2002, 297, 1176–1179. [Google Scholar] [CrossRef] [PubMed]
- Henderson, E. Imaging and nanodissection of individual supercoiled plasmids by atomic force microscopy. Nucleic Acids Res. 1992, 20, 445–447. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Guthold, M.; Liu, W.; Stephens, B.; Lord, S.T.; Hantgan, R.R.; Erie, D.A.; Taylor, R.M., Jr.; Superfine, R. Visualization and mechanical manipulations of individual fibrin fibers suggest that fiber cross section has fractal dimension 1.3. Biophys. J. 2004, 87, 4226–4236. [Google Scholar] [CrossRef] [PubMed]
- Kreplak, L.; Bar, H.; Leterrier, J.F.; Herrmann, H.; Aebi, U. Exploring the mechanical behavior of single intermediate filaments. J. Mol. Biol. 2005, 354, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.K.; Goh, M.C. Fibrous long spacing type collagen fibrils have a hierarchical internal structure. Proteins 2006, 64, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Sziklai, D.; Sallai, J.; Papp, Z.; Kellermayer, D.; Martonfalvi, Z.; Pires, R.H.; Kellermayer, M.S.Z. Nanosurgical Manipulation of Titin and Its M-Complex. Nanomaterials 2022, 12, 178. [Google Scholar] [CrossRef] [PubMed]
- Fotiadis, D.; Scheuring, S.; Muller, S.A.; Engel, A.; Muller, D.J. Imaging and manipulation of biological structures with the AFM. Micron 2002, 33, 385–397. [Google Scholar] [CrossRef]
- Martonfalvi, Z.; Kellermayer, M. Individual globular domains and domain unfolding visualized in overstretched titin molecules with atomic force microscopy. PLoS ONE 2014, 9, e85847. [Google Scholar] [CrossRef]
- Zhou, Y.F.; Eng, E.T.; Nishida, N.; Lu, C.; Walz, T.; Springer, T.A. A pH-regulated dimeric bouquet in the structure of von Willebrand factor. EMBO J. 2011, 30, 4098–4111. [Google Scholar] [CrossRef] [PubMed]
- Jakobi, A.J.; Mashaghi, A.; Tans, S.J.; Huizinga, E.G. Calcium modulates force sensing by the von Willebrand factor A2 domain. Nat. Commun. 2011, 2, 385. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Halvorsen, K.; Zhang, C.Z.; Wong, W.P.; Springer, T.A. Mechanoenzymatic cleavage of the ultralarge vascular protein von Willebrand factor. Science 2009, 324, 1330–1334. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.C.; Kutzki, F.; Mojzisch, A.; Simon, B.; Xu, E.R.; Aponte-Santamaría, C.; Horny, K.; Jeffries, C.; Schneppenheim, R.; Wilmanns, M.; et al. Structure and dynamics of the von Willebrand Factor C6 domain. J. Struct. Biol. 2022, 214, 107923. [Google Scholar] [CrossRef] [PubMed]
- Kutzki, F.; Butera, D.; Lay, A.J.; Maag, D.; Chiu, J.; Woon, H.-G.; Kubař, T.; Elstner, M.; Aponte-Santamaría, C.; Hogg, P.J.; et al. Dynamic Disulfide Bond Topologies in von-Willebrand-Factor’s C4-Domain Undermine Platelet Binding. bioRxiv 2022, 2022.2008.2020.504523. [Google Scholar] [CrossRef]
- Xu, E.R.; von Bülow, S.; Chen, P.C.; Lenting, P.J.; Kolšek, K.; Aponte-Santamaría, C.; Simon, B.; Foot, J.; Obser, T.; Schneppenheim, R.; et al. Structure and dynamics of the platelet integrin-binding C4 domain of von Willebrand factor. Blood 2019, 133, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Emsley, J.; Cruz, M.; Handin, R.; Liddington, R. Crystal structure of the von Willebrand Factor A1 domain and implications for the binding of platelet glycoprotein Ib. J Biol Chem 1998, 273, 10396–10401. [Google Scholar] [CrossRef]
- Buehler, M.J.; Ackbarow, T. Fracture mechanics of protein materials. Mater. Today 2007, 10, 46–58. [Google Scholar] [CrossRef]
- Beedle, A.E.M.; Mora, M.; Davis, C.T.; Snijders, A.P.; Stirnemann, G.; Garcia-Manyes, S. Forcing the reversibility of a mechanochemical reaction. Nat. Commun. 2018, 9, 3155. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, Y.F.; Zhang, C.Z.; Zhang, X.; Lu, C.; Springer, T.A. Structural specializations of A2, a force-sensing domain in the ultralarge vascular protein von Willebrand factor. Proc. Natl. Acad. Sci. USA 2009, 106, 9226–9231. [Google Scholar] [CrossRef]
- Webster, S.S.; Wong, G.C.L.; O’Toole, G.A. The Power of Touch: Type 4 Pili, the von Willebrand A Domain, and Surface Sensing by Pseudomonas aeruginosa. J. Bacteriol. 2022, 204, e0008422. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xi, Y.; Wang, H.; Sun, A.; Wang, L.; Deng, X.; Chen, Z.; Fan, Y. Development and validation of a mathematical model for evaluating shear-induced damage of von Willebrand factor. Comput. Biol. Med. 2023, 164, 107379. [Google Scholar] [CrossRef] [PubMed]


| Δl [nm] | E | h0 [nm] | Δh [nm] | |
|---|---|---|---|---|
| Continuous VWF loop (n = 29) | 217 * (182–283) | 2.79 * (2.33–3.8) | 0.54 (0.45–0.64) | 0.28 (0.24–0.38) |
| Ruptured VWF loop (n = 11) | 72 * (49–127) | 1.57 * (1.27–1.88) | 0.58 (0.43–0.62) | 0.22 0.20–0.28) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Csányi, M.C.; Sziklai, D.; Feller, T.; Hársfalvi, J.; Kellermayer, M. Cryptic Extensibility in von Willebrand Factor Revealed by Molecular Nanodissection. Int. J. Mol. Sci. 2024, 25, 7296. https://doi.org/10.3390/ijms25137296
Csányi MC, Sziklai D, Feller T, Hársfalvi J, Kellermayer M. Cryptic Extensibility in von Willebrand Factor Revealed by Molecular Nanodissection. International Journal of Molecular Sciences. 2024; 25(13):7296. https://doi.org/10.3390/ijms25137296
Chicago/Turabian StyleCsányi, Mária Csilla, Dominik Sziklai, Tímea Feller, Jolán Hársfalvi, and Miklós Kellermayer. 2024. "Cryptic Extensibility in von Willebrand Factor Revealed by Molecular Nanodissection" International Journal of Molecular Sciences 25, no. 13: 7296. https://doi.org/10.3390/ijms25137296
APA StyleCsányi, M. C., Sziklai, D., Feller, T., Hársfalvi, J., & Kellermayer, M. (2024). Cryptic Extensibility in von Willebrand Factor Revealed by Molecular Nanodissection. International Journal of Molecular Sciences, 25(13), 7296. https://doi.org/10.3390/ijms25137296






