Unlocking Nature’s Rhythms: Insights into Secondary Metabolite Modulation by the Circadian Clock
Abstract
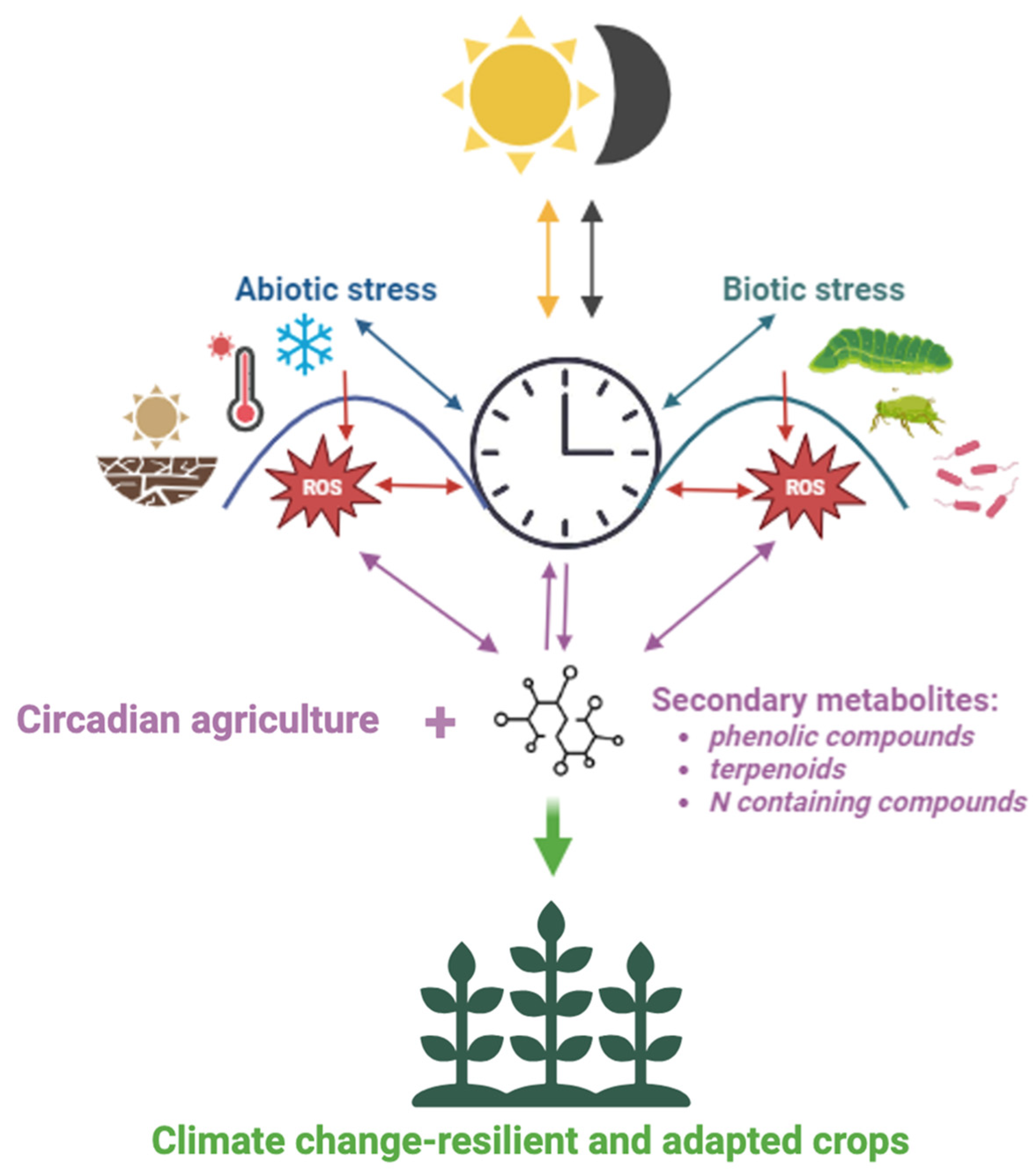
:1. Introduction
2. Regulation of Secondary Metabolites by the Circadian Clock
2.1. Internal Clock and Phenolic Compounds
2.1.1. Phenylpropanoids
2.1.2. Flavonoids
2.2. Internal Clock and Terpenoids
2.2.1. Volatile Terpenes
2.2.2. Sterols
2.2.3. Carotenoids
2.3. Internal Clock and N-Containing Compounds
2.3.1. Non-Protein Amino Acids
2.3.2. Alkaloids
2.3.3. Glucosinolates
2.3.4. Phytoalexins
2.3.5. Cyanogenic Glucosides
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Doherty, C.J.; Kay, S.A. Circadian Control of Global Gene Expression Patterns. Annu. Rev. Genet. 2010, 44, 419–444. [Google Scholar] [CrossRef] [PubMed]
- Gallego, M.; Virshup, D.M. Post-Translational Modifications Regulate the Ticking of the Circadian Clock. Nat. Rev. Mol. Cell Biol. 2007, 8, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Aschoff, J. Exogenous and Endogenous Components in Circadian Rhythms. Cold Spring Harb. Symp. Quant. Biol. 1960, 25, 11–28. [Google Scholar] [CrossRef] [PubMed]
- Lakin-Thomas, P.L. Circadian Rhythms—New Functions for Old Clock Genes. Trends Genet. 2000, 16, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Alabadí, D.; Oyama, T.; Yanovsky, M.J.; Harmon, F.G.; Más, P.; Kay, S.A. Reciprocal Regulation between TOC1 and LHY/CCA1 within the Arabidopsis Circadian Clock. Science 2001, 293, 880–883. [Google Scholar] [CrossRef] [PubMed]
- Dalchau, N.; Hubbard, K.E.; Robertson, F.C.; Hotta, C.T.; Briggs, H.M.; Stan, G.B.; Gonçalves, J.M.; Webb, A.A.R. Correct Biological Timing in Arabidopsis Requires Multiple Light-Signaling Pathways. Proc. Natl. Acad. Sci. USA 2010, 107, 13171–13176. [Google Scholar] [CrossRef] [PubMed]
- Somers, D.E.; Devlin, P.F.; Kay, S.A. Phytochromes and Cryptochromes in the Entrainment of the Arabidopsis Circadian Clock. Science 1998, 282, 1488–1490. [Google Scholar] [CrossRef] [PubMed]
- Devlin, P.F.; Kay, S.A. Cryptochromes Are Required for Phytochrome Signaling to the Circadian Clock but Not for Rhythmicity. Plant Cell 2000, 12, 2499–2509. [Google Scholar] [CrossRef] [PubMed]
- Mas, P.; Devlin, P.F.; Panda, S.; Kay, S.A. Functional Interaction of Phytochrome B and Cryptochrome 2. Nature 2000, 408, 207–211. [Google Scholar] [CrossRef]
- Tóth, R.; Kevei, É.; Hall, A.; Millar, A.J.; Nagy, F.; Kozma-Bognár, L. Circadian Clock-Regulated Expression of Phytochrome and Cryptochrome Genes in Arabidopsis. Plant Physiol. 2001, 127, 1607–1616. [Google Scholar] [CrossRef] [PubMed]
- Staiger, D.; Shin, J.; Johansson, M.; Davis, S.J. The Circadian Clock Goes Genomic. Genome Biol. 2013, 14, 208. [Google Scholar] [CrossRef] [PubMed]
- Harmer, S.L.; Hogenesch, J.B.; Straume, M.; Chang, H.S.; Han, B.; Zhu, T.; Wang, X.; Kreps, J.A.; Kay, S.A. Orchestrated Transcription of Key Pathways in Arabidopsis by the Circadian Clock. Science 2000, 290, 2110–2113. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Tobin, E.M. Constitutive Expression of the CIRCADIAN CLOCK ASSOCIATED 1 (CCA1) Gene Disrupts Circadian Rhythms and Suppresses Its Own Expression. Cell 1998, 93, 1207–1217. [Google Scholar] [CrossRef] [PubMed]
- Gendron, J.M.; Pruneda-Paz, J.L.; Doherty, C.J.; Gross, A.M.; Kang, S.E.; Kay, S.A. Arabidopsis Circadian Clock Protein, TOC1, Is a DNA-Binding Transcription Factor. Proc. Natl. Acad. Sci. USA 2012, 109, 3167–3172. [Google Scholar] [CrossRef] [PubMed]
- Rawat, R.; Takahashi, N.; Hsu, P.Y.; Jones, M.A.; Schwartz, J.; Salemi, M.R.; Phinney, B.S.; Harmer, S.L. REVEILLE8 and PSEUDO-REPONSE REGULATOR5 Form a Negative Feedback Loop within the Arabidopsis Circadian Clock. PLoS Genet. 2011, 7, e1001350. [Google Scholar] [CrossRef] [PubMed]
- Helfer, A.; Nusinow, D.A.; Chow, B.Y.; Gehrke, A.R.; Bulyk, M.L.; Kay, S.A. LUX ARRHYTHMO Encodes a Nighttime Repressor of Circadian Gene Expression in the Arabidopsis Core Clock. Curr. Biol. 2011, 21, 126–133. [Google Scholar] [CrossRef]
- Chow, B.Y.; Helfer, A.; Nusinow, D.A.; Kay, S.A. ELF3 Recruitment to the PRR9 Promoter Requires Other Evening Complex Members in the Arabidopsis Circadian Clock. Plant Signal Behav. 2012, 7, 170–173. [Google Scholar] [CrossRef]
- Huang, W.; Pérez-García, P.; Pokhilko, A.; Millar, A.J.; Antoshechkin, I.; Riechmann, J.L.; Mas, P. Mapping the Core of the Arabidopsis Circadian Clock Defines the Network Structure of the Oscillator. Science 2012, 336, 75–79. [Google Scholar] [CrossRef]
- Pruneda-Paz, J.L.; Breton, G.; Para, A.; Kay, S.A. A Functional Genomics Approach Reveals CHE as a Novel Component of the Arabidopsis Circadian Clock. Science 2009, 323, 1481. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.Y.; Kim, J.; Kim, T.S.; Zeng, Q.; Wang, L.; Lee, S.Y.; Kim, W.Y.; Somers, D.E. GIGANTEA Is a Co-Chaperone Which Facilitates Maturation of ZEITLUPE in the Arabidopsis Circadian Clock. Nat. Commun. 2017, 8, 3. [Google Scholar] [CrossRef]
- Más, P.; Kim, W.Y.; Somers, D.E.; Kay, S.A. Targeted Degradation of TOC1 by ZTL Modulates Circadian Function in Arabidopsis thaliana. Nature 2003, 426, 567–570. [Google Scholar] [CrossRef]
- Kim, W.Y.; Fujiwara, S.; Suh, S.S.; Kim, J.; Kim, Y.; Han, L.; David, K.; Putterill, J.; Nam, H.G.; Somers, D.E. ZEITLUPE Is a Circadian Photoreceptor Stabilized by GIGANTEA in Blue Light. Nature 2007, 449, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.X.; Webb, C.J.; Knowles, S.M.; Kim, S.H.J.; Wang, Z.; Tobin, E.M. CCA1 and ELF3 Interact in the Control of Hypocotyl Length and Flowering Time in Arabidopsis. Plant Physiol. 2012, 158, 1079. [Google Scholar] [CrossRef]
- Para, A.; Farré, E.M.; Imaizumi, T.; Pruneda-Paz, J.L.; Harmon, F.G.; Kay, S.A. PRR3 Is a Vascular Regulator of TOC1 Stability in the Arabidopsis Circadian Clock. Plant Cell 2007, 19, 3462–3473. [Google Scholar] [CrossRef] [PubMed]
- Matsushika, A.; Makino, S.; Kojima, M.; Mizuno, T. Circadian Waves of Expression of the APRR1/TOC1 Family of Pseudo-Response Regulators in Arabidopsis thaliana: Insight into the Plant Circadian Clock. Plant Cell Physiol. 2000, 41, 1002–1012. [Google Scholar] [CrossRef]
- Wu, J.F.; Wang, Y.; Wu, S.H. Two New Clock Proteins, LWD1 and LWD2, Regulate Arabidopsis Photoperiodic Flowering. Plant Physiol. 2008, 148, 948–959. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, J.F.; Nakamichi, N.; Sakakibara, H.; Nam, H.G.; Wu, S.H. LIGHT-REGULATED WD1 and PSEUDO-RESPONSE REGULATOR9 Form a Positive Feedback Regulatory Loop in the Arabidopsis Circadian Clock. Plant Cell 2011, 23, 486–498. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Wang, P.; Liu, X.; Yuan, L.; Wang, L.; Zhang, C.; Li, Y.; Xing, H.; Zhi, L.; Yue, Z.; et al. LNK1 and LNK2 Are Transcriptional Coactivators in the Arabidopsis Circadian Oscillator. Plant Cell 2014, 26, 2843. [Google Scholar] [CrossRef]
- Dai, S.; Wei, X.; Pei, L.; Thompson, R.L.; Liu, Y.; Heard, J.E.; Ruff, T.G.; Beachy, R.N. BROTHER OF LUX ARRHYTHMO Is a Component of the Arabidopsis Circadian Clock. Plant Cell 2011, 23, 961–972. [Google Scholar] [CrossRef]
- Michael, T.P.; Mockler, T.C.; Breton, G.; McEntee, C.; Byer, A.; Trout, J.D.; Hazen, S.P.; Shen, R.; Priest, H.D.; Sullivan, C.M.; et al. Network Discovery Pipeline Elucidates Conserved Time-of-Day–Specific Cis-Regulatory Modules. PLoS Genet. 2008, 4, e14. [Google Scholar] [CrossRef]
- Covington, M.F.; Maloof, J.N.; Straume, M.; Kay, S.A.; Harmer, S.L. Global Transcriptome Analysis Reveals Circadian Regulation of Key Pathways in Plant Growth and Development. Genome Biol. 2008, 9, R130. [Google Scholar] [CrossRef] [PubMed]
- Webb, A.A.R.; Seki, M.; Satake, A.; Caldana, C. Continuous Dynamic Adjustment of the Plant Circadian Oscillator. Nat. Commun. 2019, 10, 550. [Google Scholar] [CrossRef] [PubMed]
- Joanito, I.; Chu, J.W.; Wu, S.H.; Hsu, C.P. An Incoherent Feed-Forward Loop Switches the Arabidopsis Clock Rapidly between Two Hysteretic States. Sci. Rep. 2018, 8, 13944. [Google Scholar] [CrossRef] [PubMed]
- Salomé, P.A.; McClung, C.R. PSEUDO-RESPONSE REGULATOR 7 and 9 Are Partially Redundant Genes Essential for the Temperature Responsiveness of the Arabidopsis Circadian Clock. Plant Cell 2005, 17, 791. [Google Scholar] [CrossRef] [PubMed]
- Nakamichi, N.; Kiba, T.; Kamioka, M.; Suzukie, T.; Yamashino, T.; Higashiyama, T.; Sakakibara, H.; Mizuno, T. Transcriptional Repressor PRR5 Directly Regulates Clock-Output Pathways. Proc. Natl. Acad. Sci. USA 2012, 109, 17123–17128. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Han, X.; Yang, J.; Jiang, Y.; Hu, Y. The Arabidopsis Circadian Clock Protein PRR5 Interacts with and Stimulates ABI5 to Modulate Abscisic Acid Signaling during Seed Germination. Plant Cell 2021, 33, 3022. [Google Scholar] [CrossRef] [PubMed]
- Nagel, D.H.; Doherty, C.J.; Pruneda-Paz, J.L.; Schmitz, R.J.; Ecker, J.R.; Kay, S.A. Genome-Wide Identification of CCA1 Targets Uncovers an Expanded Clock Network in Arabidopsis. Proc. Natl. Acad. Sci. USA 2015, 112, E4802–E4810. [Google Scholar] [CrossRef]
- Dong, M.A.; Farré, E.M.; Thomashow, M.F. Circadian Clock-Associated 1 and Late Elongated Hypocotyl Regulate Expression of the C-Repeat Binding Factor (CBF) Pathway in Arabidopsis. Proc. Natl. Acad. Sci. USA 2011, 108, 7241–7246. [Google Scholar] [CrossRef]
- Nusinow, D.A.; Helfer, A.; Hamilton, E.E.; King, J.J.; Imaizumi, T.; Schultz, T.F.; Farré, E.M.; Kay, S.A. The ELF4–ELF3–LUX Complex Links the Circadian Clock to Diurnal Control of Hypocotyl Growth. Nature 2011, 475, 398–402. [Google Scholar] [CrossRef]
- Raschke, A.; Ibañez, C.; Ullrich, K.K.; Anwer, M.U.; Becker, S.; Glöckner, A.; Trenner, J.; Denk, K.; Saal, B.; Sun, X.; et al. Natural Variants of ELF3 Affect Thermomorphogenesis by Transcriptionally Modulating PIF4-Dependent Auxin Response Genes. BMC Plant Biol. 2015, 15, 197. [Google Scholar] [CrossRef]
- Lai, A.G.; Doherty, C.J.; Mueller-Roeber, B.; Kay, S.A.; Schippers, J.H.M.; Dijkwel, P.P. Circadian Clock-Associated 1 Regulates ROS Homeostasis and Oxidative Stress Responses. Proc. Natl. Acad. Sci. USA 2012, 109, 17129–17134. [Google Scholar] [CrossRef] [PubMed]
- Greenham, K.; McClung, C.R. Integrating Circadian Dynamics with Physiological Processes in Plants. Nat. Rev. Genet. 2015, 16, 598–610. [Google Scholar] [CrossRef] [PubMed]
- Buckley, C.R.; Li, X.; Martí, M.C.; Haydon, M.J. A Bittersweet Symphony: Metabolic Signals in the Circadian System. Curr. Opin. Plant Biol. 2023, 73, 102333. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.A.; Kim, H.S.; Choi, S.H.; Jang, J.Y.; Jeong, M.J.; Lee, S.I. The Importance of the Circadian Clock in Regulating Plant Metabolism. Int. J. Mol. Sci. 2017, 18, 2680. [Google Scholar] [CrossRef]
- Lu, H.; McClung, C.R.; Zhang, C. Tick Tock: Circadian Regulation of Plant Innate Immunity. Annu. Rev. Phytopathol. 2017, 55, 287–311. [Google Scholar] [CrossRef] [PubMed]
- Quint, M.; Delker, C.; Franklin, K.A.; Wigge, P.A.; Halliday, K.J.; Van Zanten, M. Molecular and Genetic Control of Plant Thermomorphogenesis. Nature Plants 2016, 2, 15190. [Google Scholar] [CrossRef]
- Czemmel, S.; Heppel, S.C.; Bogs, J. R2R3 MYB Transcription Factors: Key Regulators of the Flavonoid Biosynthetic Pathway in Grapevine. Protoplasma 2012, 249, 109–118. [Google Scholar] [CrossRef]
- Liebelt, D.J.; Jordan, J.T.; Doherty, C.J. Only a Matter of Time: The Impact of Daily and Seasonal Rhythms on Phytochemicals. Phytochem. Rev. 2019, 18, 1409–1433. [Google Scholar] [CrossRef]
- Pérez-Llorca, M.; Pollmann, S.; Müller, M. Ethylene and Jasmonates Signaling Network Mediating Secondary Metabolites under Abiotic Stress. Int. J. Mol. Sci. 2023, 24, 5990. [Google Scholar] [CrossRef]
- Erb, M.; Kliebenstein, D.J. Plant Secondary Metabolites as Defenses, Regulators, and Primary Metabolites: The Blurred Functional Trichotomy. Plant Physiol. 2020, 184, 39–52. [Google Scholar] [CrossRef]
- Jeyasri, R.; Muthuramalingam, P.; Karthick, K.; Shin, H.; Choi, S.H.; Ramesh, M. Methyl Jasmonate and Salicylic Acid as Powerful Elicitors for Enhancing the Production of Secondary Metabolites in Medicinal Plants: An Updated Review. Plant Cell Tissue Organ Cult. 2023, 153, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Reshi, Z.A.; Ahmad, W.; Lukatkin, A.S.; Javed, S. Bin from Nature to Lab: A Review of Secondary Metabolite Biosynthetic Pathways, Environmental Influences, and In Vitro Approaches. Metabolites 2023, 13, 895. [Google Scholar] [CrossRef] [PubMed]
- Meena, K.K.; Sorty, A.M.; Bitla, U.M.; Choudhary, K.; Gupta, P.; Pareek, A.; Singh, D.P.; Prabha, R.; Sahu, P.K.; Gupta, V.K.; et al. Abiotic Stress Responses and Microbe-Mediated Mitigation in Plants: The Omics Strategies. Front. Plant Sci. 2017, 8, 172. [Google Scholar] [CrossRef] [PubMed]
- Zagoskina, N.V.; Zubova, M.Y.; Nechaeva, T.L.; Kazantseva, V.V.; Goncharuk, E.A.; Katanskaya, V.M.; Baranova, E.N.; Aksenova, M.A. Polyphenols in Plants: Structure, Biosynthesis, Abiotic Stress Regulation, and Practical Applications (Review). Int. J. Mol. Sci. 2023, 24, 13874. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Fan, R.; Guo, S.; Wang, P.; Zhu, X.; Fan, Y.; Chen, Y.; He, K.; Kumar, A.; Shi, J.; et al. The Arabidopsis MYB Transcription Factor, MYB111 Modulates Salt Responses by Regulating Flavonoid Biosynthesis. Environ. Exp. Bot. 2019, 166, 103807. [Google Scholar] [CrossRef]
- Boncan, D.A.T.; Tsang, S.S.K.; Li, C.; Lee, I.H.T.; Lam, H.-M.; Chan, T.-F.; Hui, J.H.L. Terpenes and Terpenoids in Plants: Interactions with Environment and Insects. Int. J. Mol. Sci. 2020, 21, 7382. [Google Scholar] [CrossRef] [PubMed]
- Ninkuu, V.; Zhang, L.; Yan, J.; Fu, Z.; Yang, T.; Zeng, H. Biochemistry of Terpenes and Recent Advances in Plant Protection. Int. J. Mol. Sci. 2021, 22, 5710. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Xu, Q.-Y.; Zhu, Z.-P.; Liu, P.-Z.; Yu, J.-X.; Guo, Y.-X.; Tang, S.; Yu, Z.-F.; Xiong, A.-S. AgMYB5, an MYB Transcription Factor from Celery, Enhanced β-Carotene Synthesis and Promoted Drought Tolerance in Transgenic Arabidopsis. BMC Plant Biol. 2023, 23, 151. [Google Scholar] [CrossRef]
- Jan, R.; Asaf, S.; Numan, M.; Lubna; Kim, K.-M. Plant Secondary Metabolite Biosynthesis and Transcriptional Regulation in Response to Biotic and Abiotic Stress Conditions. Agronomy 2021, 11, 968. [Google Scholar] [CrossRef]
- Khare, S.; Singh, N.B.; Singh, A.; Hussain, I.; Niharika, K.; Yadav, V.; Bano, C.; Yadav, R.K.; Amist, N. Plant Secondary Metabolites Synthesis and Their Regulations under Biotic and Abiotic Constraints. J. Plant Biol. 2020, 63, 203–216. [Google Scholar] [CrossRef]
- Schluttenhofer, C.; Pattanaik, S.; Patra, B.; Yuan, L. Analyses of Catharanthus Roseus and Arabidopsis Thaliana WRKY Transcription Factors Reveal Involvement in Jasmonate Signaling. BMC Genom. 2014, 15, 502. [Google Scholar] [CrossRef]
- Schluttenhofer, C.; Yuan, L. Regulation of Specialized Metabolism by WRKY Transcription Factors. Plant Physiol. 2015, 167, 295–306. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative Stress, Antioxidants and Stress Tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Reactive Oxygen Species and Antioxidant Machinery in Abiotic Stress Tolerance in Crop Plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Mittler, R. ROS Are Good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef]
- Foyer, C.H.; Neukermans, J.; Queval, G.; Noctor, G.; Harbinson, J. Photosynthetic Control of Electron Transport and the Regulation of Gene Expression. J. Exp. Bot. 2012, 63, 1637–1661. [Google Scholar] [CrossRef]
- Jiménez, A.; Sevilla, F.; Martí, M.C. Reactive Oxygen Species Homeostasis and Circadian Rhythms in Plants. J. Exp. Bot. 2021, 72, 5825–5840. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive Oxygen Species Homeostasis and Signaling during Drought and Salinity Stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef]
- Van Ruyskensvelde, V.; Van Breusegem, F.; Van Der Kelen, K. Post-Transcriptional Regulation of the Oxidative Stress Response in Plants. Free Radic. Biol. Med. 2018, 122, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Liu, H.; Kong, Y.; Wen, L.; Zhao, Y.; Zhou, C.; Han, L. Late Elongated Hypocotyl Positively Regulates Salt Stress Tolerance in Medicago truncatula. Int. J. Mol. Sci. 2023, 24, 9948. [Google Scholar] [CrossRef]
- Tuzet, A.; Rahantaniaina, M.-S.; Noctor, G. Analyzing the Function of Catalase and the Ascorbate–Glutathione Pathway in H2O2 Processing: Insights from an Experimentally Constrained Kinetic Model. Antioxid. Redox Signal 2019, 30, 1238–1268. [Google Scholar] [CrossRef]
- Lázaro, J.J.; Jiménez, A.; Camejo, D.; Iglesias-Baena, I.; del Carmen Martí, M.; Lázaro-Payo, A.; Barranco-Medina, S.; Sevilla, F. Dissecting the Integrative Antioxidant and Redox Systems in Plant Mitochondria: Effect of Stress and S-Nitrosylation. Front. Plant Sci. 2013, 4, 460. [Google Scholar] [CrossRef]
- Qaderi, M.M.; Martel, A.B.; Strugnell, C.A. Environmental Factors Regulate Plant Secondary Metabolites. Plants 2023, 12, 447. [Google Scholar] [CrossRef]
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The Effect of Developmental and Environmental Factors on Secondary Metabolites in Medicinal Plants. Plant Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef]
- Soengas, P.; Cartea, M.E.; Velasco, P.; Francisco, M. Endogenous Circadian Rhythms in Polyphenolic Composition Induce Changes in Antioxidant Properties in Brassica Cultivars. J. Agric. Food Chem. 2018, 66, 5984–5991. [Google Scholar] [CrossRef]
- Li, J.; Ou-Lee, T.M.; Raba, R.; Amundson, R.G.; Last, R.L. Arabidopsis Flavonoid Mutants Are Hypersensitive to UV-B Irradiation. Plant Cell 1993, 5, 171–179. [Google Scholar] [CrossRef]
- Landry, L.G.; Chapple, C.C.S.; Last, R.L. Arabidopsis Mutants Lacking Phenolic Sunscreens Exhibit Enhanced Ultraviolet-B Injury and Oxidative Damage. Plant Physiol. 1995, 109, 1159–1166. [Google Scholar] [CrossRef]
- Muhlemann, J.K.; Klempien, A.; Dudareva, N. Floral Volatiles: From Biosynthesis to Function. Plant Cell Environ. 2014, 37, 1936–1949. [Google Scholar] [CrossRef]
- Kolosova, N.; Gorenstein, N.; Kish, C.M.; Dudareva, N. Regulation of Circadian Methyl Benzoate Emission in Diurnally and Nocturnally Emitting Plants. Plant Cell 2001, 13, 2333–2347. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Fu, X.; Mei, X.; Zhou, Y.; Du, B.; Watanabe, N.; Yang, Z. Regulation of Biosynthesis and Emission of Volatile Phenylpropanoids/Benzenoids in Petunia× Hybrida Flowers by Multi-Factors of Circadian Clock, Light, and Temperature. Plant Physiol. Biochem. 2016, 107, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fenske, M.P.; Hewett Hazelton, K.D.; Hempton, A.K.; Shim, J.S.; Yamamoto, B.M.; Riffell, J.A.; Imaizumi, T. Circadian Clock Gene LATE ELONGATED HYPOCOTYL Directly Regulates the Timing of Floral Scent Emission in Petunia. Proc. Natl. Acad. Sci. USA 2015, 112, 9775–9780. [Google Scholar] [CrossRef]
- Petroni, K.; Tonelli, C. Recent Advances on the Regulation of Anthocyanin Synthesis in Reproductive Organs. Plant Science 2011, 181, 219–229. [Google Scholar] [CrossRef]
- Falcone Ferreyra, M.L.; Rius, S.P.; Casati, P. Flavonoids: Biosynthesis, Biological Functions, and Biotechnological Applications. Front. Plant Sci. 2012, 3, 222. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Jeong, C.Y.; Kang, G.; Yoo, S.; Hong, S.; Lee, H. MYBD Employed by HY 5 Increases Anthocyanin Accumulation via Repression of MYBL 2 in Arabidopsis. Plant J. 2015, 84, 1192–1205. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Lee, H. MYB-Related Transcription Factors Function as Regulators of the Circadian Clock and Anthocyanin Biosynthesis in Arabidopsis. Plant Signal Behav. 2016, 11, e1139278. [Google Scholar] [CrossRef]
- Pan, Y.; Michael, T.P.; Hudson, M.E.; Kay, S.A.; Chory, J.; Schuler, M.A. Cytochrome P450 Monooxygenases as Reporters for Circadian-Regulated Pathways. Plant Physiol. 2009, 150, 858–878. [Google Scholar] [CrossRef]
- Pérez-García, P.; Ma, Y.; Yanovsky, M.J.; Mas, P. Time-Dependent Sequestration of RVE8 by LNK Proteins Shapes the Diurnal Oscillation of Anthocyanin Biosynthesis. Proc. Natl. Acad. Sci. USA 2015, 112, 5249–5253. [Google Scholar] [CrossRef]
- Farinas, B.; Mas, P. Functional Implication of the MYB Transcription Factor RVE8/LCL5 in the Circadian Control of Histone Acetylation. Plant J. 2011, 66, 318–329. [Google Scholar] [CrossRef]
- Bhat, Z.Y.; Mir, J.A.; Yadav, A.K.; Singh, D.; Ashraf, N. CstMYB1R1, a REVEILLE-8-like Transcription Factor, Regulates Diurnal Clock-Specific Anthocyanin Biosynthesis and Response to Abiotic Stress in Crocus sativus L. Plant Cell Rep. 2024, 43, 20. [Google Scholar] [CrossRef]
- Odgerel, K.; Jose, J.; Karsai-Rektenwald, F.; Ficzek, G.; Simon, G.; Végvári, G.; Bánfalvi, Z. Effects of the Repression of GIGANTEA Gene StGI.04 on the Potato Leaf Transcriptome and the Anthocyanin Content of Tuber Skin. BMC Plant Biol. 2022, 22, 249. [Google Scholar] [CrossRef]
- Brandoli, C.; Petri, C.; Egea-Cortines, M.; Weiss, J. Gigantea: Uncovering New Functions in Flower Development. Genes 2020, 11, 1142. [Google Scholar] [CrossRef]
- Thain, S.C.; Murtas, G.; Lynn, J.R.; McGrath, R.B.; Millar, A.J. The Circadian Clock That Controls Gene Expression in Arabidopsis Is Tissue Specific. Plant Physiol. 2002, 130, 102–110. [Google Scholar] [CrossRef]
- Hildreth, S.B.; Littleton, E.S.; Clark, L.C.; Puller, G.C.; Kojima, S.; Winkel, B.S.J. Mutations That Alter Arabidopsis Flavonoid Metabolism Affect the Circadian Clock. Plant J. 2022, 110, 932–945. [Google Scholar] [CrossRef]
- Nakabayashi, R.; Yonekura-Sakakibara, K.; Urano, K.; Suzuki, M.; Yamada, Y.; Nishizawa, T.; Matsuda, F.; Kojima, M.; Sakakibara, H.; Shinozaki, K.; et al. Enhancement of Oxidative and Drought Tolerance in Arabidopsis by Overaccumulation of Antioxidant Flavonoids. Plant J. 2014, 77, 367–379. [Google Scholar] [CrossRef]
- Xie, Y.; Chen, P.; Yan, Y.; Bao, C.; Li, X.; Wang, L.; Shen, X.; Li, H.; Liu, X.; Niu, C.; et al. An Atypical R2R3 MYB Transcription Factor Increases Cold Hardiness by CBF-dependent and CBF-independent Pathways in Apple. New Phytol. 2018, 218, 201–218. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Gao, Z.-Q.; Hou, J.-M.; Tian, S.-K.; Zhang, Z.-X.; Yang, L.; Liu, Y. Identification of Biosynthetic Pathways Involved in Flavonoid Production in Licorice by RNA-Seq Based Transcriptome Analysis. Plant Growth Regul. 2020, 92, 15–28. [Google Scholar] [CrossRef]
- Isah, T. Stress and Defense Responses in Plant Secondary Metabolites Production. Biol. Res. 2019, 52, 39. [Google Scholar] [CrossRef]
- Tholl, D. Biosynthesis and Biological Functions of Terpenoids in Plants. Adv. Biochem. Eng. Biotechnol. 2015, 148, 63–106. [Google Scholar] [CrossRef]
- Pérez-Llorca, M.; Casadesús, A.; Munné-Bosch, S.; Müller, M. Contrasting Patterns of Hormonal and Photoprotective Isoprenoids in Response to Stress in Cistus albidus during a Mediterranean Winter. Planta 2019, 250, 1409–1422. [Google Scholar] [CrossRef]
- Graßmann, J. Terpenoids as Plant Antioxidants. Vitam. Horm. 2005, 72, 505–535. [Google Scholar] [CrossRef]
- Bouwmeester, H.; Schuurink, R.C.; Bleeker, P.M.; Schiestl, F. The Role of Volatiles in Plant Communication. Plant J. 2019, 100, 892. [Google Scholar] [CrossRef]
- Qiao, Z.; Hu, H.; Shi, S.; Yuan, X.; Yan, B.; Chen, L. An Update on the Function, Biosynthesis and Regulation of Floral Volatile Terpenoids. Horticulturae 2021, 7, 451. [Google Scholar] [CrossRef]
- Cordoba, E.; Salmi, M.; León, P. Unravelling the Regulatory Mechanisms That Modulate the MEP Pathway in Higher Plants. J. Exp. Bot. 2009, 60, 2933–2943. [Google Scholar] [CrossRef]
- Vranová, E.; Coman, D.; Gruissem, W. Structure and Dynamics of the Isoprenoid Pathway Network. Mol. Plant 2012, 5, 318–333. [Google Scholar] [CrossRef]
- James, A.B.; Monreal, J.A.; Nimmo, G.A.; Kelly, C.L.; Herzyk, P.; Jenkins, G.I.; Nimmo, H.G. The Circadian Clock in Arabidopsis Roots Is a Simplified Slave Version of the Clock in Shoots. Science 2008, 322, 1832–1835. [Google Scholar] [CrossRef]
- Toledo-Ortiz, G.; Huq, E.; Rodríguez-Concepción, M. Direct Regulation of Phytoene Synthase Gene Expression and Carotenoid Biosynthesis by Phytochrome-Interacting Factors. Proc. Natl. Acad. Sci. USA 2010, 107, 11626–11631. [Google Scholar] [CrossRef]
- Rodríguez-Concepción, M.; Forés, O.; Martínez-García, J.F.; González, V.; Phillips, M.A.; Ferrer, A.; Boronat, A. Distinct Light-Mediated Pathways Regulate the Biosynthesis and Exchange of Isoprenoid Precursors during Arabidopsis Seedling Development. Plant Cell 2004, 16, 144. [Google Scholar] [CrossRef]
- Zeng, L.; Wang, X.; Kang, M.; Dong, F.; Yang, Z. Regulation of the Rhythmic Emission of Plant Volatiles by the Circadian Clock. Int. J. Mol. Sci. 2017, 18, 2408. [Google Scholar] [CrossRef]
- Lu, S.; Xu, R.; Jia, J.W.; Pang, J.; Matsuda, S.P.T.; Chen, X.Y. Cloning and Functional Characterization of a β-Pinene Synthase from Artemisia Annua That Shows a Circadian Pattern of Expression. Plant Physiol. 2002, 130, 477. [Google Scholar] [CrossRef]
- Simkin, A.J.; Underwood, B.A.; Auldridge, M.; Loucas, H.M.; Shibuya, K.; Schmelz, E.; Clark, D.G.; Klee, H.J. Circadian Regulation of the PhCCD1 Carotenoid Cleavage Dioxygenase Controls Emission of Beta-Ionone, a Fragrance Volatile of Petunia Flowers. Plant Physiol. 2004, 136, 3504–3514. [Google Scholar] [CrossRef]
- Yon, F.; Joo, Y.; Cortés Llorca, L.; Rothe, E.; Baldwin, I.T.; Kim, S.G. Silencing Nicotiana Attenuata LHY and ZTL Alters Circadian Rhythms in Flowers. New Phytol. 2016, 209, 1058–1066. [Google Scholar] [CrossRef]
- Wilkinson, M.J.; Owen, S.M.; Possell, M.; Hartwell, J.; Gould, P.; Hall, A.; Vickers, C.; Nicholas Hewitt, C. Circadian Control of Isoprene Emissions from Oil Palm (Elaeis guineensis). Plant J. 2006, 47, 960–968. [Google Scholar] [CrossRef]
- Loivamäki, M.; Louis, S.; Cinege, G.; Zimmer, I.; Fischbach, R.J.; Schnitzler, J.P. Circadian Rhythms of Isoprene Biosynthesis in Grey Poplar Leaves. Plant Physiol. 2007, 143, 540–551. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Fu, X.; Chu, Y.; Wu, P.; Liu, Y.; Ma, L.; Tian, H.; Zhu, B. Biosynthesis and the Roles of Plant Sterols in Development and Stress Responses. Int. J. Mol. Sci. 2022, 23, 2332. [Google Scholar] [CrossRef] [PubMed]
- Fantini, E.; Sulli, M.; Zhang, L.; Aprea, G.; Jiménez-Gómez, J.M.; Bendahmane, A.; Perrotta, G.; Giuliano, G.; Facella, P. Pivotal Roles of Cryptochromes 1a and 2 in Tomato Development and Physiology. Plant Physiol. 2019, 179, 732–748. [Google Scholar] [CrossRef]
- Ince, Y.Ç.; Krahmer, J.; Fiorucci, A.S.; Trevisan, M.; Galvão, V.C.; Wigger, L.; Pradervand, S.; Fouillen, L.; Van Delft, P.; Genva, M.; et al. A Combination of Plasma Membrane Sterol Biosynthesis and Autophagy Is Required for Shade-Induced Hypocotyl Elongation. Nat. Commun. 2022, 13, 5659. [Google Scholar] [CrossRef]
- Edge, R.; McGarvey, D.J.; Truscott, T.G. The Carotenoids as Anti-Oxidants—A Review. J. Photochem. Photobiol. B 1997, 41, 189–200. [Google Scholar] [CrossRef]
- Fukushima, A.; Kusano, M.; Nakamichi, N.; Kobayashi, M.; Hayashi, N.; Sakakibara, H.; Mizuno, T.; Saito, K. Impact of Clock-Associated Arabidopsis Pseudoresponse Regulators in Metabolic Coordination. Proc. Natl. Acad. Sci. USA 2009, 106, 7251–7256. [Google Scholar] [CrossRef]
- Lindgren, L.O.; Stålberg, K.G.; Höglund, A.S. Seed-Specific Overexpression of an Endogenous Arabidopsis Phytoene Synthase Gene Results in Delayed Germination and Increased Levels of Carotenoids, Chlorophyll, and Abscisic Acid. Plant Physiol. 2003, 132, 779. [Google Scholar] [CrossRef]
- Toledo-Ortiz, G.; Johansson, H.; Lee, K.P.; Bou-Torrent, J.; Stewart, K.; Steel, G.; Rodríguez-Concepción, M.; Halliday, K.J. The HY5-PIF Regulatory Module Coordinates Light and Temperature Control of Photosynthetic Gene Transcription. PLoS Genet. 2014, 10, e1004416. [Google Scholar] [CrossRef] [PubMed]
- Eskling, M.; Arvidsson, P.-O.; Åkerlund, H.-E. The Xanthophyll Cycle, Its Regulation and Components. Physiol. Plant 1997, 100, 806–816. [Google Scholar] [CrossRef]
- Rawat, R.; Schwartz, J.; Jones, M.A.; Sairanen, I.; Cheng, Y.; Andersson, C.R.; Zhao, Y.; Ljung, K.; Harmer, S.L. REVEILLE1, a Myb-like Transcription Factor, Integrates the Circadian Clock and Auxin Pathways. Proc. Natl. Acad. Sci. USA 2009, 106, 16883–16888. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.S.; Choi, H.; Kim, J.K.; Baek, S.A.; You, M.K.; Lee, D.; Lim, S.H.; Ha, S.H. Overexpression of OsMYBR22/OsRVE1 Transcription Factor Simultaneously Enhances Chloroplast-Dependent Metabolites in Rice Grains. Metab. Eng. 2022, 70, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Averesch, N.J.H.; Krömer, J.O. Metabolic Engineering of the Shikimate Pathway for Production of Aromatics and Derived Compounds-Present and Future Strain Construction Strategies. Front. Bioeng. Biotechnol. 2018, 6, 32. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, R.A.; Stokes, T.L.; Thum, K.; Xu, X.; Obertello, M.; Katari, M.S.; Tanurdzic, M.; Dean, A.; Nero, D.C.; McClung, C.R.; et al. Systems Approach Identifies an Organic Nitrogen-Responsive Gene Network That Is Regulated by the Master Clock Control Gene CCA1. Proc. Natl. Acad. Sci. USA 2008, 105, 4939–4944. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, G.A. Nonprotein Amino Acids as Protective Allelochemicals. In Herbivores: Their Interactions with Secondary Plant Metabolites; Academic Press: Cambridge, MA, USA, 1991; pp. 1–34. [Google Scholar] [CrossRef]
- Bell, E.A. Nonprotein Amino Acids of Plants: Significance in Medicine, Nutrition, and Agriculture. J. Agric. Food Chem. 2003, 51, 2854–2865. [Google Scholar] [CrossRef] [PubMed]
- Ishaaya, I.; Hirashima, A.; Yablonski, S.; Tawata, S.; Eto, M. Mimosine, a Nonprotein Amino Acid, Inhibits Growth and Enzyme Systems in Tribolium Castaneum. Pestic. Biochem. Physiol. 1991, 39, 35–42. [Google Scholar] [CrossRef]
- Rosenthal, G.A. Investigations of Canavanine Biochemistry in the Jack Bean Plant, Canavalia Ensiformis (L.) DC: II. Canavanine Biosynthesis in the Developing Plant 1. Plant Physiol. 1972, 50, 328. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, C.; Degenkolbe, T.; Caldana, C.; Zuther, E.; Leisse, A.; Willmitzer, L.; Hincha, D.K.; Hannah, M.A. Interaction with Diurnal and Circadian Regulation Results in Dynamic Metabolic and Transcriptional Changes during Cold Acclimation in Arabidopsis. PLoS ONE 2010, 5, e14101. [Google Scholar] [CrossRef]
- Xu, B.; Long, Y.; Feng, X.; Zhu, X.; Sai, N.; Chirkova, L.; Betts, A.; Herrmann, J.; Edwards, E.J.; Okamoto, M.; et al. GABA Signalling Modulates Stomatal Opening to Enhance Plant Water Use Efficiency and Drought Resilience. Nat. Commun. 2021, 12, 1952. [Google Scholar] [CrossRef]
- Pelvan, A.; Bor, M.; Yolcu, S.; Özdemir, F.; Türkan, I. Day and Night Fluctuations in GABA Biosynthesis Contribute to Drought Responses in Nicotiana tabacum L. Plant Signal Behav. 2021, 16, 1899672. [Google Scholar] [CrossRef]
- Huang, T.; Jander, G.; De Vos, M. Non-Protein Amino Acids in Plant Defense against Insect Herbivores: Representative Cases and Opportunities for Further Functional Analysis. Phytochemistry 2011, 72, 1531–1537. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Lu, F.; Li, Y.; Xue, Y.; Kang, Y.; Zhang, S.; Qiu, Q.; Zheng, X.C.; Cui, X.; Zheng, S.; et al. Ubiquitin-Specific Proteases UBP12 and UBP13 Act in Circadian Clock and Photoperiodic Flowering Regulation in Arabidopsis. Plant Physiol. 2013, 162, 897. [Google Scholar] [CrossRef]
- Yan, N.; Doelling, J.H.; Falbel, T.G.; Durski, A.M.; Vierstra, R.D. The Ubiquitin-Specific Protease Family from Arabidopsis.AtUBP1 and 2 Are Required for the Resistance to the Amino Acid Analog Canavanine. Plant Physiol. 2000, 124, 1828–1843. [Google Scholar] [CrossRef]
- Ali, A.H.; Abdelrahman, M.; El-Sayed, M.A. Alkaloid Role in Plant Defense Response to Growth and Stress. In Bioactive Molecules in Plant Defense: Signaling in Growth and Stress; Springer: Berlin/Heidelberg, Germany, 2019; pp. 145–158. [Google Scholar] [CrossRef]
- Bhambhani, S.; Kondhare, K.R.; Giri, A.P. Diversity in Chemical Structures and Biological Properties of Plant Alkaloids. Molecules 2021, 26, 3374. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas, P.D.; Sonawane, P.D.; Heinig, U.; Jozwiak, A.; Panda, S.; Abebie, B.; Kazachkova, Y.; Pliner, M.; Unger, T.; Wolf, D.; et al. Pathways to Defense Metabolites and Evading Fruit Bitterness in Genus Solanum Evolved through 2-Oxoglutarate-Dependent Dioxygenases. Nat. Commun. 2019, 10, 737. [Google Scholar] [CrossRef] [PubMed]
- Carlin, M.G.; Dean, J.R.; Ames, J.M. Opium Alkaloids in Harvested and Thermally Processed Poppy Seeds. Front. Chem. 2020, 8, 539741. [Google Scholar] [CrossRef]
- Bischoff, K.L. Glucosinolates. In Nutraceuticals: Efficacy, Safety and Toxicity; Academic Press: Cambridge, MA, USA, 2016; pp. 551–554. [Google Scholar] [CrossRef]
- Del Carmen Martínez-Ballesta, M.; Moreno, D.A.; Carvajal, M. The Physiological Importance of Glucosinolates on Plant Response to Abiotic Stress in Brassica. Int. J. Mol. Sci. 2013, 14, 11607. [Google Scholar] [CrossRef]
- Baenas, N.; García-Viguera, C.; Moreno, D.A. Biotic Elicitors Effectively Increase the Glucosinolates Content in Brassicaceae Sprouts. J. Agric. Food Chem. 2014, 62, 1881–1889. [Google Scholar] [CrossRef]
- Goodspeed, D.; Liu, J.D.; Chehab, E.W.; Sheng, Z.; Francisco, M.; Kliebenstein, D.J.; Braam, J. Postharvest Circadian Entrainment Enhances Crop Pest Resistance and Phytochemical Cycling. Curr. Biol. 2013, 23, 1235–1241. [Google Scholar] [CrossRef]
- Soengas, P.; Cartea, M.E.; Velasco, P.; Francisco, M. Brassica Glucosinolate Rhythmicity in Response to Light-Dark Entrainment Cycles Is Cultivar-Dependent. Plant Sci. 2018, 275, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Huseby, S.; Koprivova, A.; Lee, B.R.; Saha, S.; Mithen, R.; Wold, A.B.; Bengtsson, G.B.; Kopriva, S. Diurnal and Light Regulation of Sulphur Assimilation and Glucosinolate Biosynthesis in Arabidopsis. J. Exp. Bot. 2013, 64, 1039. [Google Scholar] [CrossRef] [PubMed]
- Ang, L.H.; Chattopadhyay, S.; Wei, N.; Oyama, T.; Okada, K.; Batschauer, A.; Deng, X.W. Molecular Interaction between COP1 and HY5 Defines a Regulatory Switch for Light Control of Arabidopsis Development. Mol. Cell 1998, 1, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.; Kim, S.-H.; Choi, D.-M.; Moon, H.; Kim, J.-I.; Huq, E.; Kim, D.-H. ELONGATED HYPOCOTYL 5 Interacts with HISTONE DEACETYLASE 9 to Suppress Glucosinolate Biosynthesis in Arabidopsis. Plant Physiol. 2024, kiae284. [Google Scholar] [CrossRef]
- Lei, J.; Jayaprakasha, G.K.; Singh, J.; Uckoo, R.; Borrego, E.J.; Finlayson, S.; Kolomiets, M.; Patil, B.S.; Braam, J.; Zhu-Salzman, K. CIRCADIAN CLOCK-ASSOCIATED1 Controls Resistance to Aphids by Altering Indole Glucosinolate Production. Plant Physiol. 2019, 181, 1344. [Google Scholar] [CrossRef]
- Kim, N.S.; Kim, S.J.; Jo, J.S.; Lee, J.G.; Lee, S.I.; Kim, D.H.; Kim, J.A. The Brgi Circadian Clock Gene Is Involved in the Regulation of Glucosinolates in Chinese Cabbage. Genes 2021, 12, 1664. [Google Scholar] [CrossRef]
- Kerwin, R.E.; Jimenez-Gomez, J.M.; Fulop, D.; Harmer, S.L.; Maloof, J.N.; Kliebensteina, D.J. Network Quantitative Trait Loci Mapping of Circadian Clock Outputs Identifies Metabolic Pathway-to-Clock Linkages in Arabidopsis. Plant Cell 2011, 23, 471–485. [Google Scholar] [CrossRef]
- Burow, M.; Halkier, B.A. How Does a Plant Orchestrate Defense in Time and Space? Using Glucosinolates in Arabidopsis as Case Study. Curr. Opin. Plant Biol. 2017, 38, 142–147. [Google Scholar] [CrossRef]
- Klein, A.P.; Sattely, E.S. Biosynthesis of Cabbage Phytoalexins from Indole Glucosinolate. Proc. Natl. Acad. Sci. USA 2017, 114, 1910–1915. [Google Scholar] [CrossRef]
- Ahuja, I.; Kissen, R.; Bones, A.M. Phytoalexins in Defense against Pathogens. Trends Plant Sci. 2012, 17, 73–90. [Google Scholar] [CrossRef]
- Singh, A. GIGANTEA Regulates PAD4 Transcription to Promote Pathogen Defense against Hyaloperonospora Arabidopsidis in Arabidopsis thaliana. Plant Signal Behav. 2022, 17, 2058719. [Google Scholar] [CrossRef] [PubMed]
- Zagrobelny, M.; Bak, S.; Møller, B.L. Cyanogenesis in Plants and Arthropods. Phytochemistry 2008, 69, 1457–1468. [Google Scholar] [CrossRef] [PubMed]
- Zagrobelny, M.; Møller, B.L. Cyanogenic Glucosides in the Biological Warfare between Plants and Insects: The Burnet Moth-Birdsfoot Trefoil Model System. Phytochemistry 2011, 72, 1585–1592. [Google Scholar] [CrossRef] [PubMed]
- Jensen, N.B.; Zagrobelny, M.; Hjernø, K.; Olsen, C.E.; Houghton-Larsen, J.; Borch, J.; Møller, B.L.; Bak, S. Convergent Evolution in Biosynthesis of Cyanogenic Defence Compounds in Plants and Insects. Nat. Commun. 2011, 2, 273. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, F.B.; Cho, S.K.; Olsen, C.E.; Yang, S.W.; Møller, B.L.; Jørgensen, K. Diurnal Regulation of Cyanogenic Glucoside Biosynthesis and Endogenous Turnover in Cassava. Plant Direct 2018, 2, e00038. [Google Scholar] [CrossRef] [PubMed]
- Kongsawadworakul, P.; Viboonjun, U.; Romruensukharom, P.; Chantuma, P.; Ruderman, S.; Chrestin, H. The Leaf, Inner Bark and Latex Cyanide Potential of Hevea Brasiliensis: Evidence for Involvement of Cyanogenic Glucosides in Rubber Yield. Phytochemistry 2009, 70, 730–739. [Google Scholar] [CrossRef]
- Hotta, C.T. From Crops to Shops: How Agriculture Can Use Circadian Clocks. J. Exp. Bot. 2021, 72, 7668–7679. [Google Scholar] [CrossRef]
- Li, C.; Ji, J.; Wang, G.; Li, Z.; Wang, Y.; Fan, Y. Over-Expression of LcPDS, LcZDS, and LcCRTISO, Genes From Wolfberry for Carotenoid Biosynthesis, Enhanced Carotenoid Accumulation, and Salt Tolerance in Tobacco. Front. Plant Sci. 2020, 11, 119. [Google Scholar] [CrossRef]
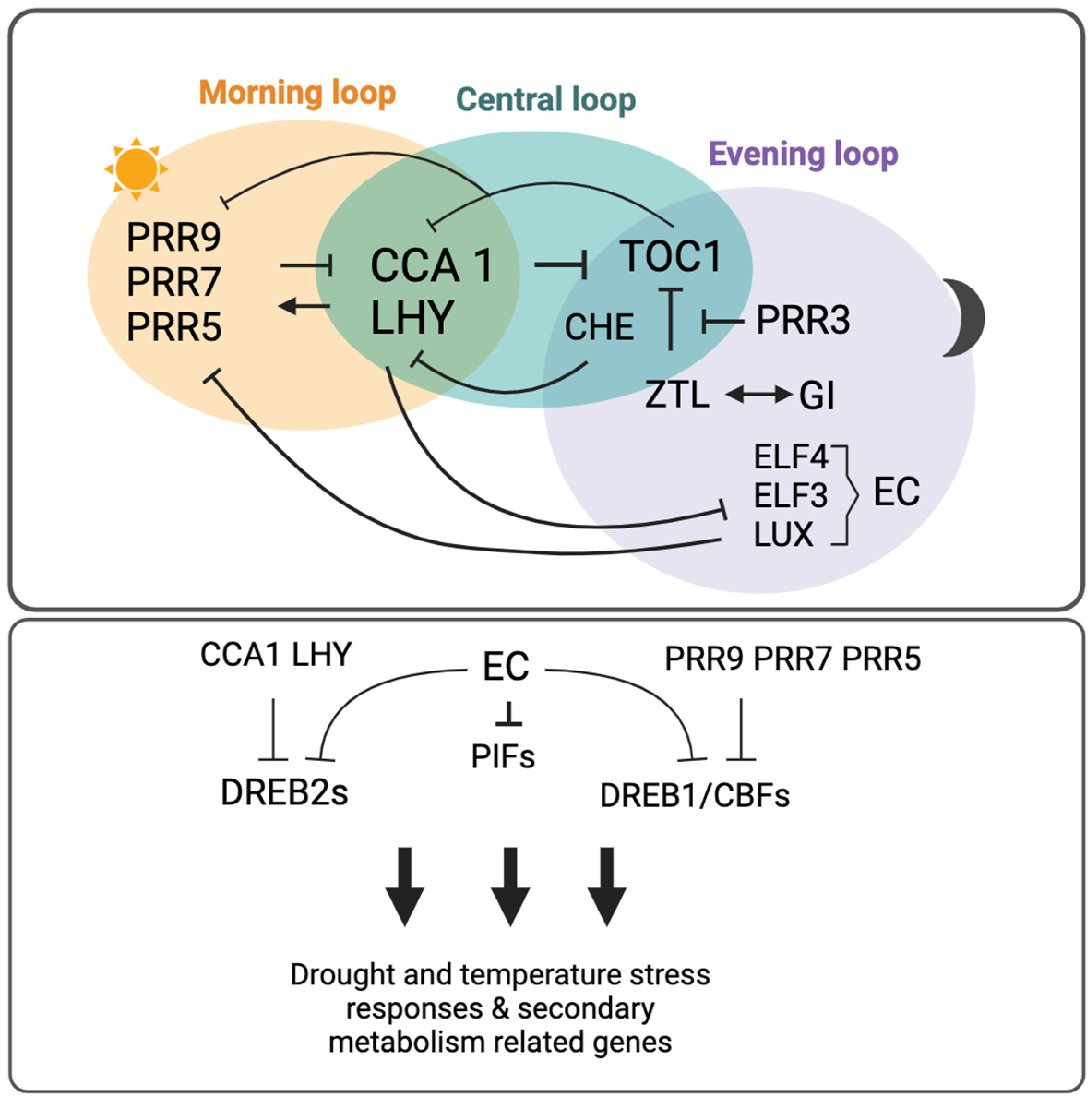
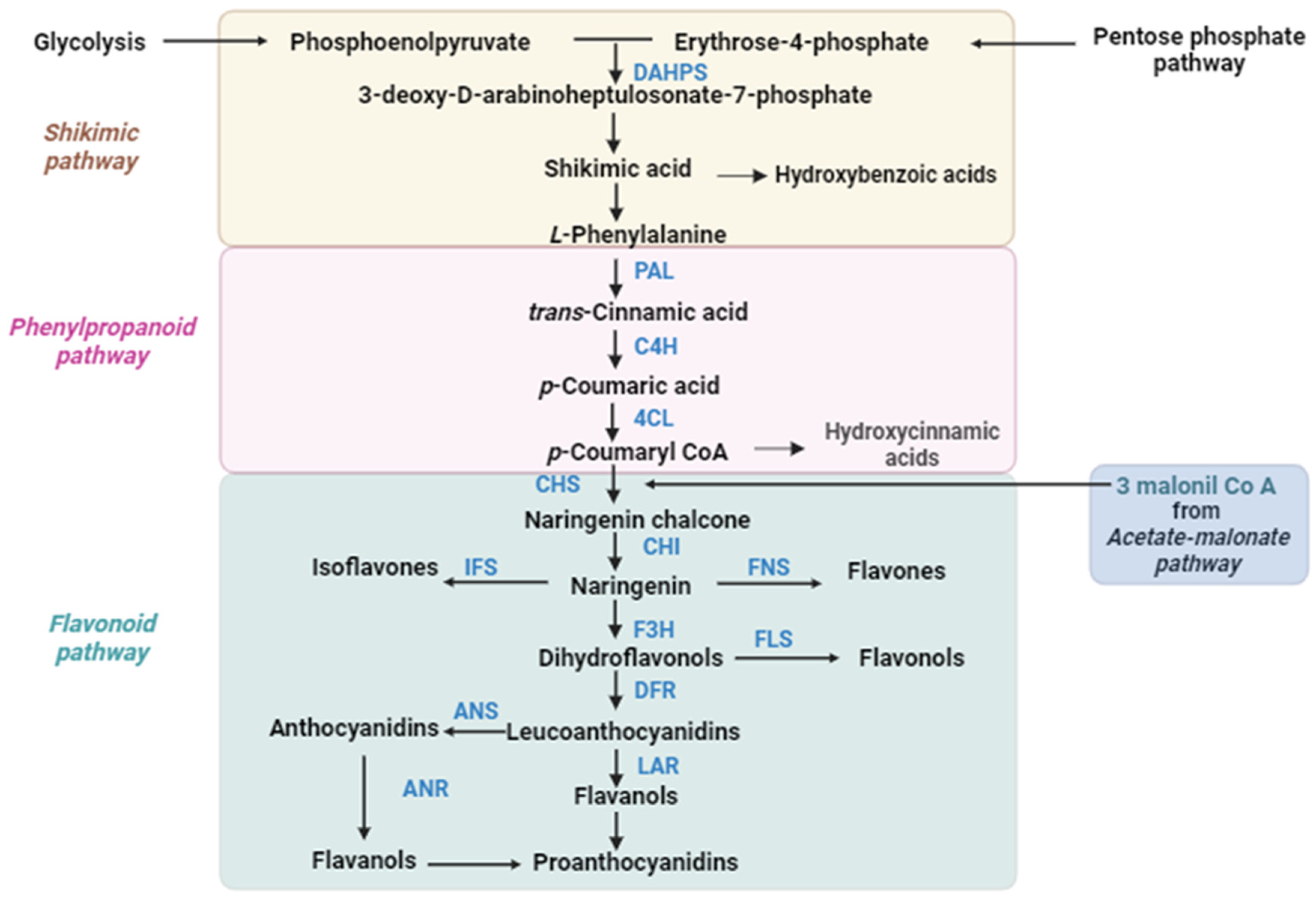
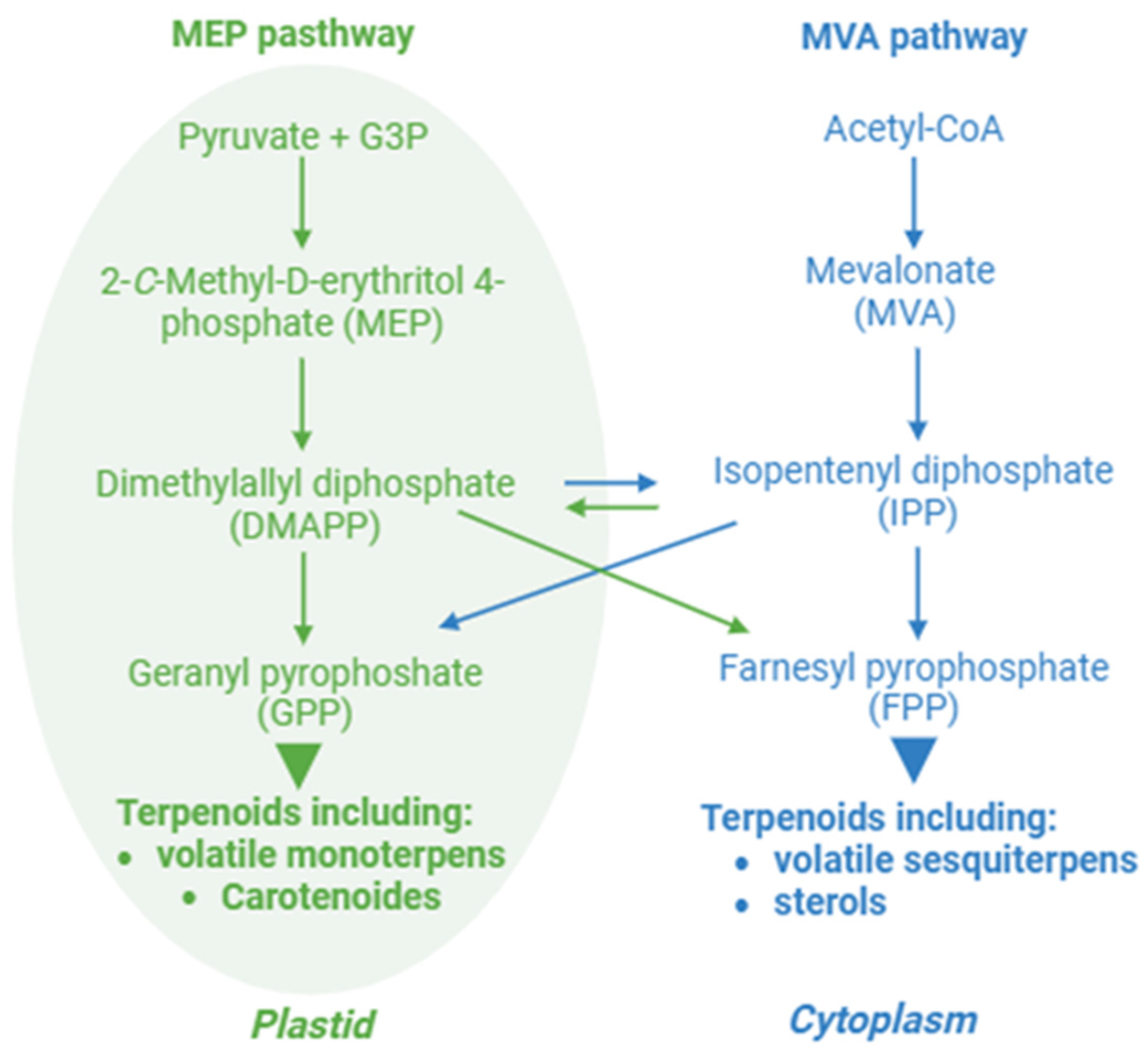
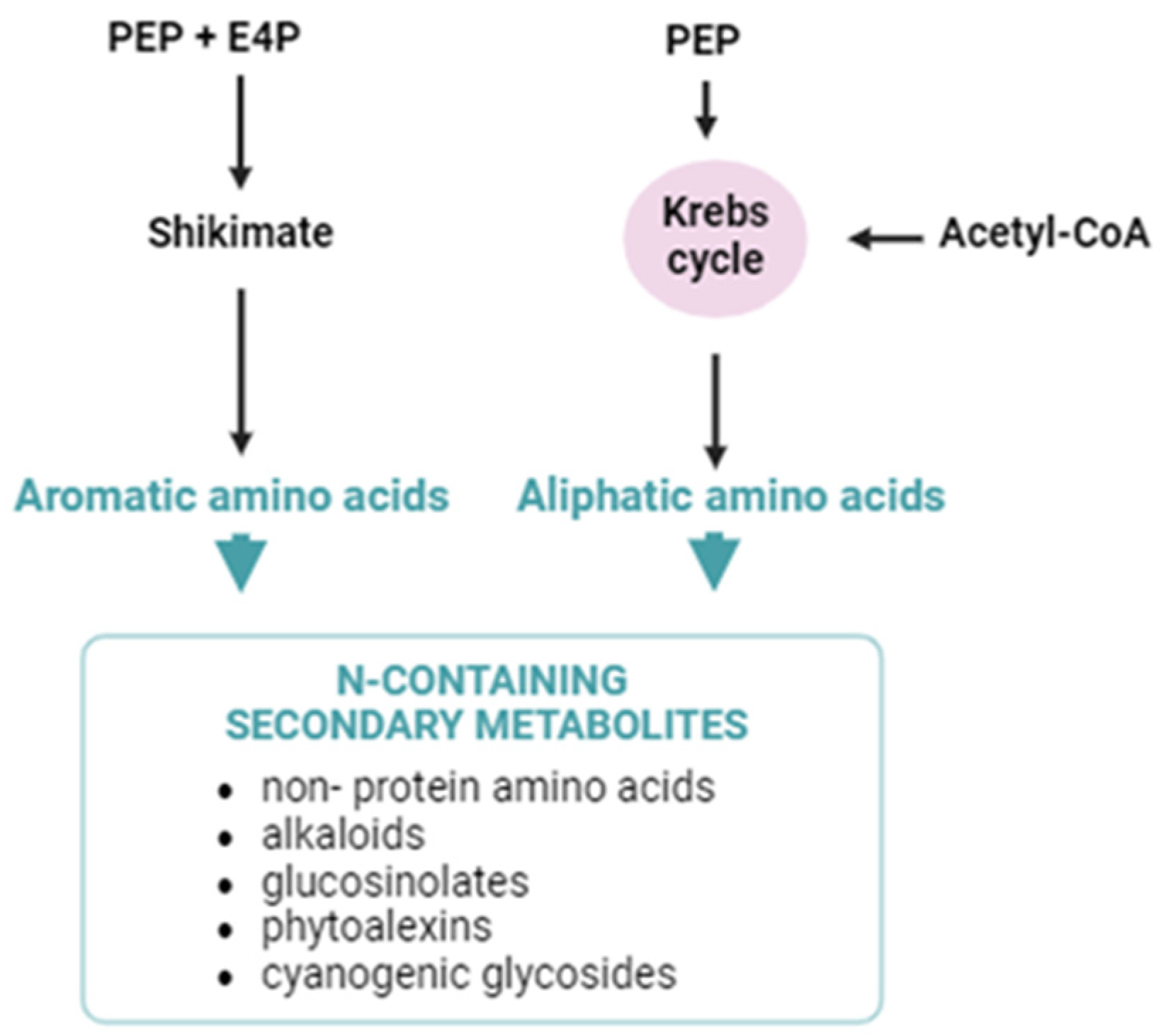
| Plant Species | Clock-Related Genes | Function | Secondary Metabolite | Reference |
|---|---|---|---|---|
| Petunia hybrida | LHY | Regulating timing of floral volatile emission by binding to ODORANT1 | Volatile phenylpropanoid and benzenoid biosynthesis | [81] |
| Arabidopsis | CCA1, TOC1 | Regulating CHS and F3’H activity | Flavonoid biosynthesis | [93] |
| Glycyrrhiza glabra | PRR5, FT, LHY | Enhanced expression observed in flavonoid-hyperaccumulating lines | [96] | |
| Medicago truncatula | LHY | Overexpression of MtLHY resulted in an increased expression of MtFLS, a flavonol synthase gene | [70] | |
| Crocus sativus | CstMYB1R1 | Regulating ANS and LDOX gene expression resulting in enhanced flavonoid and anthocyanin accumulation with peaks at dawn and dusk and minimum contents at night | Flavonoid and anthocyanin biosynthesis | [89] |
| Arabidopsis | MYBD | Thought to act as a regulator of anthocyanin biosynthesis in a circadian-dependent manner | Anthocyanin biosynthesis | [84,85] |
| RVE8/LCL5 | Promoter of anthocyanin-biosynthesis genes | [87] | ||
| LNK | Repressor of anthocyanin-biosynthesis genes | [87] | ||
| Arabidopsis | CCA1, LHY, PRR9 | Co-expression with DXS, HDR from the MEP pathway | MEP pathway | [105] |
| Arabidopsis | TOC1 | Co-expression with AACT2 from the MVA pathway | MVA pathway | [105] |
| Populus x canescens | LHY | Binding to ISPS resulting in peak ISPS expression in the morning | Isoprene | [113] |
| Arabidopsis | PRR 9, PRR7, PRR5 | A triple-knockout mutant showed increased gene expression of carotenoid and ABA biosynthetic pathways | Carotenoid biosynthesis | [118] |
| Oryza sativa | OsRVE1 | Overexpression of OsRVE1 increased carotenoid accumulation | Carotenoids | [123] |
| Arabidopsis | CCA1 | An overexpression line of CCA1 presented enhanced resistance to aphids due to increased levels of indole glucosinolates | Indole glucosinolates | [148] |
| Brassica rapa | BrGI | A GI-knockout mutant showed altered transcripts of glucosinolates as well as reduced accumulation | Aliphatic glucosinolates | [149] |
| Arabidopsis | GI | A GI mutant showed downregulation of PAD4 | Camalexin | [154] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Llorca, M.; Müller, M. Unlocking Nature’s Rhythms: Insights into Secondary Metabolite Modulation by the Circadian Clock. Int. J. Mol. Sci. 2024, 25, 7308. https://doi.org/10.3390/ijms25137308
Pérez-Llorca M, Müller M. Unlocking Nature’s Rhythms: Insights into Secondary Metabolite Modulation by the Circadian Clock. International Journal of Molecular Sciences. 2024; 25(13):7308. https://doi.org/10.3390/ijms25137308
Chicago/Turabian StylePérez-Llorca, Marina, and Maren Müller. 2024. "Unlocking Nature’s Rhythms: Insights into Secondary Metabolite Modulation by the Circadian Clock" International Journal of Molecular Sciences 25, no. 13: 7308. https://doi.org/10.3390/ijms25137308






