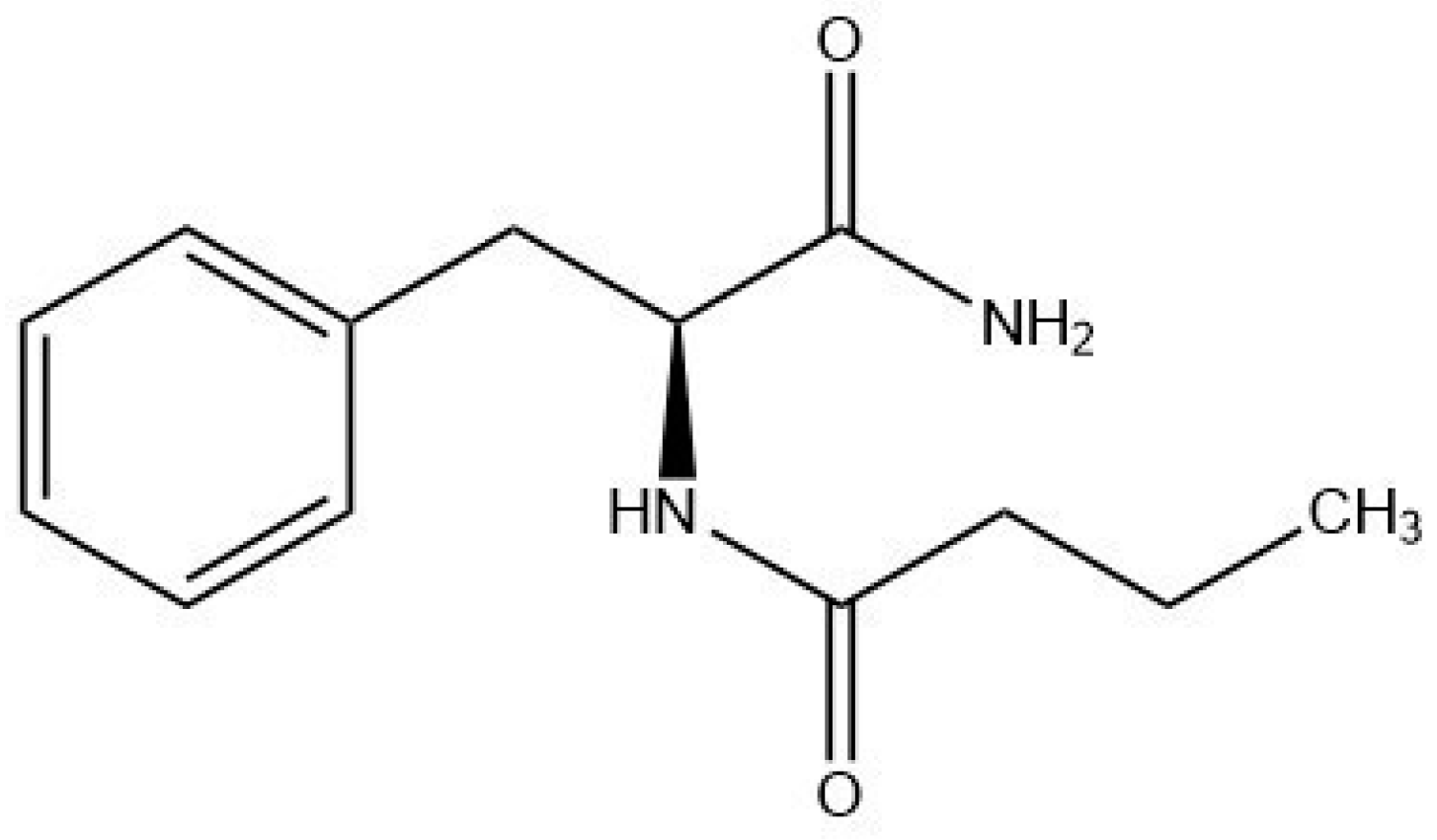
Phenylalanine Butyramide: A Butyrate Derivative as a Novel Inhibitor of Tyrosinase
Abstract
1. Introduction
2. Results
2.1. Tyrosinase Inhibition
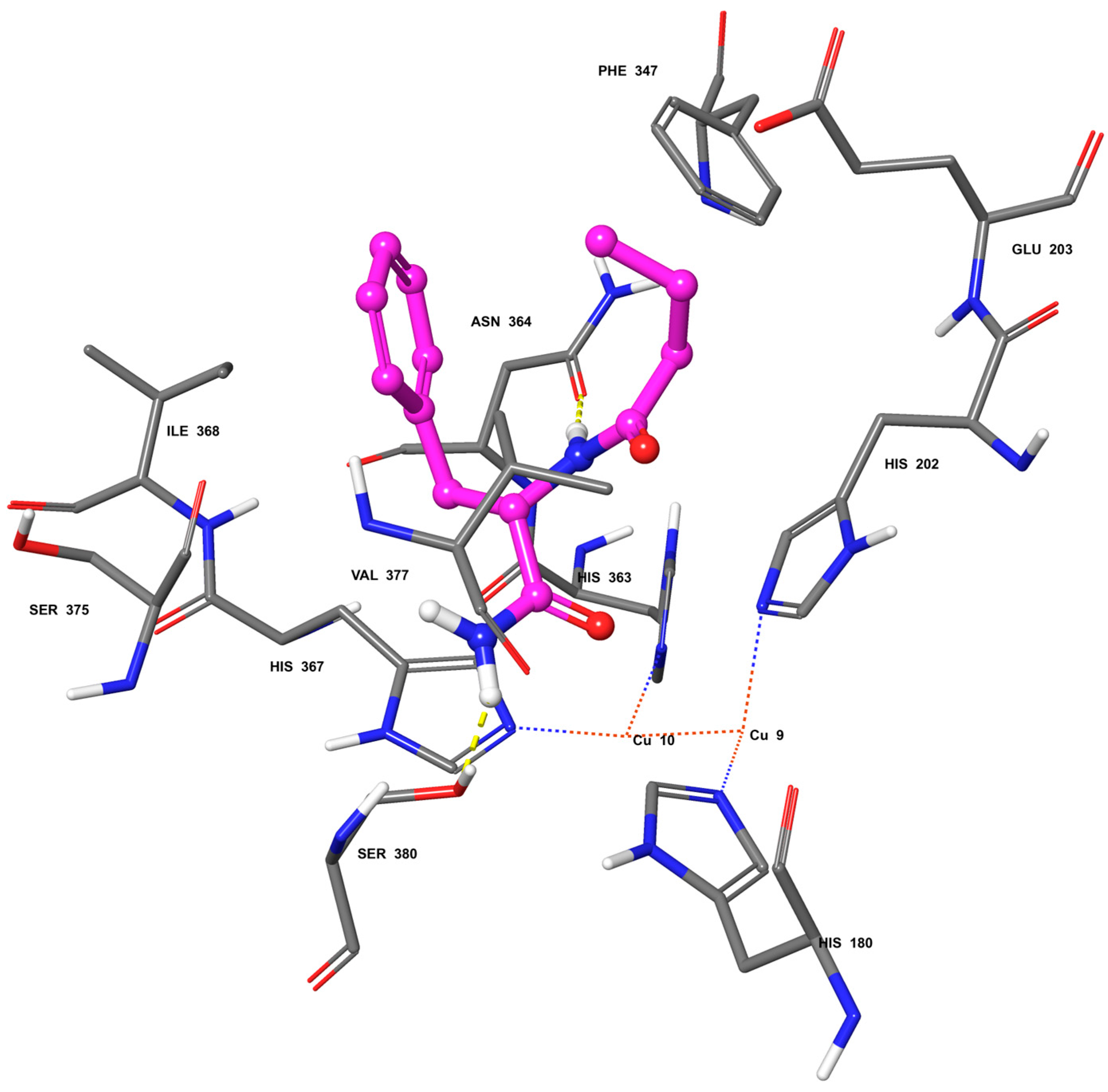
2.2. Molecular Docking
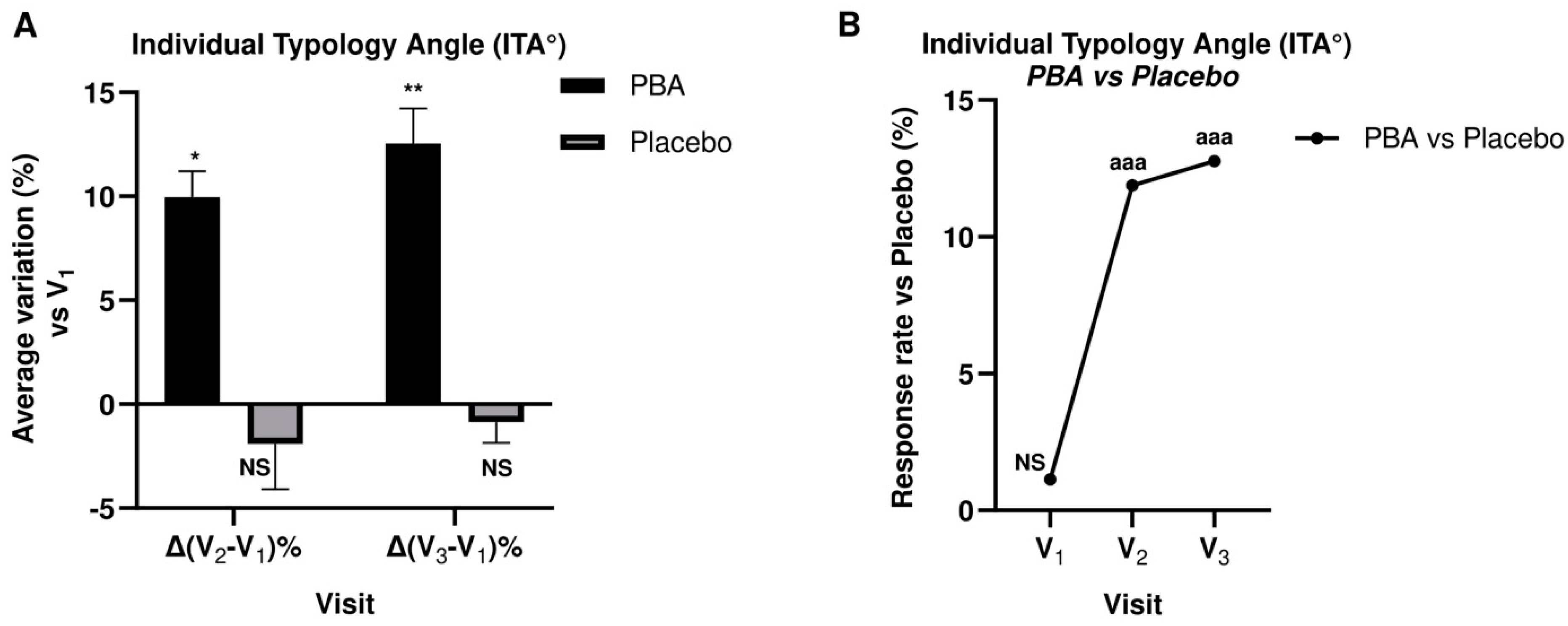
2.3. Skin Complexion and Luminosity
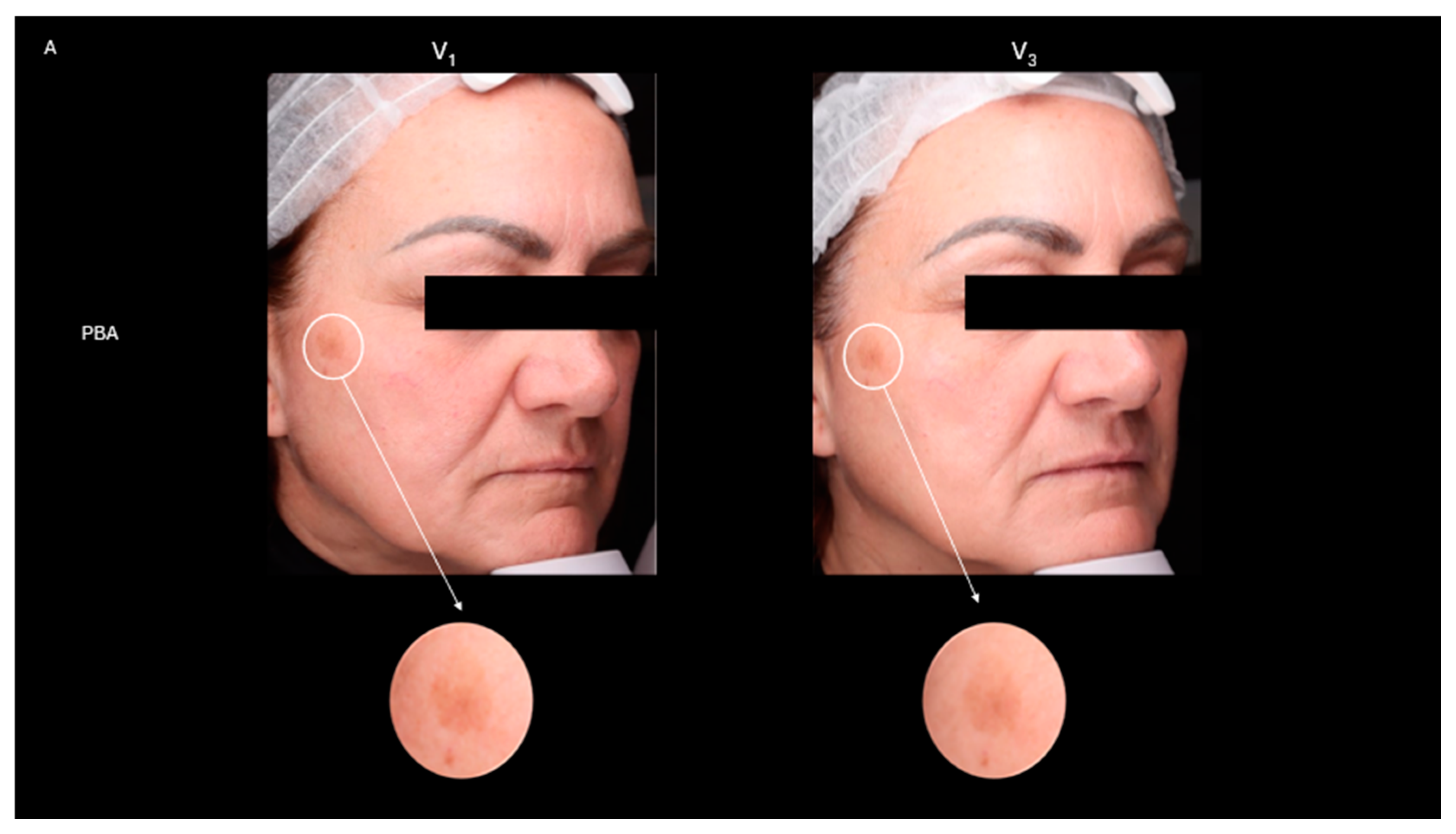
2.4. Skin Depigmentation Activity
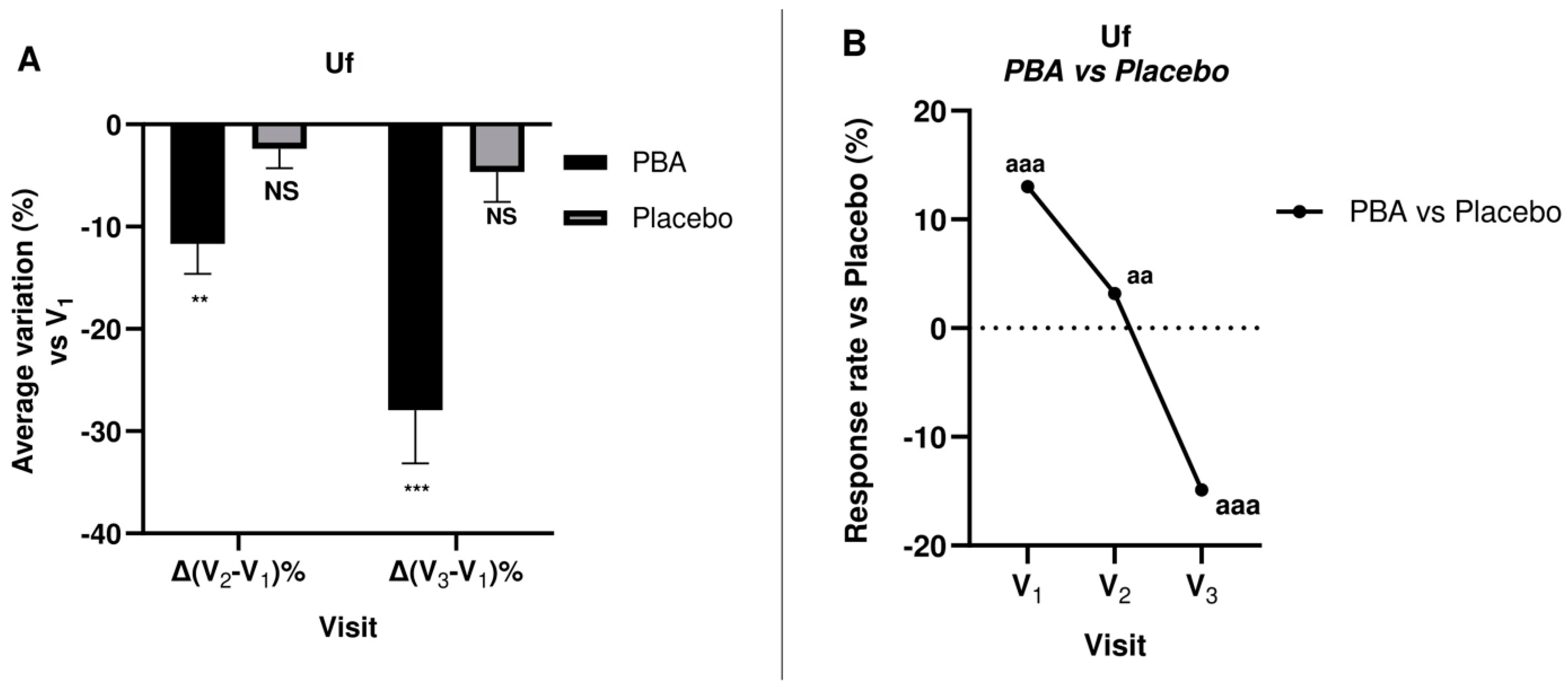
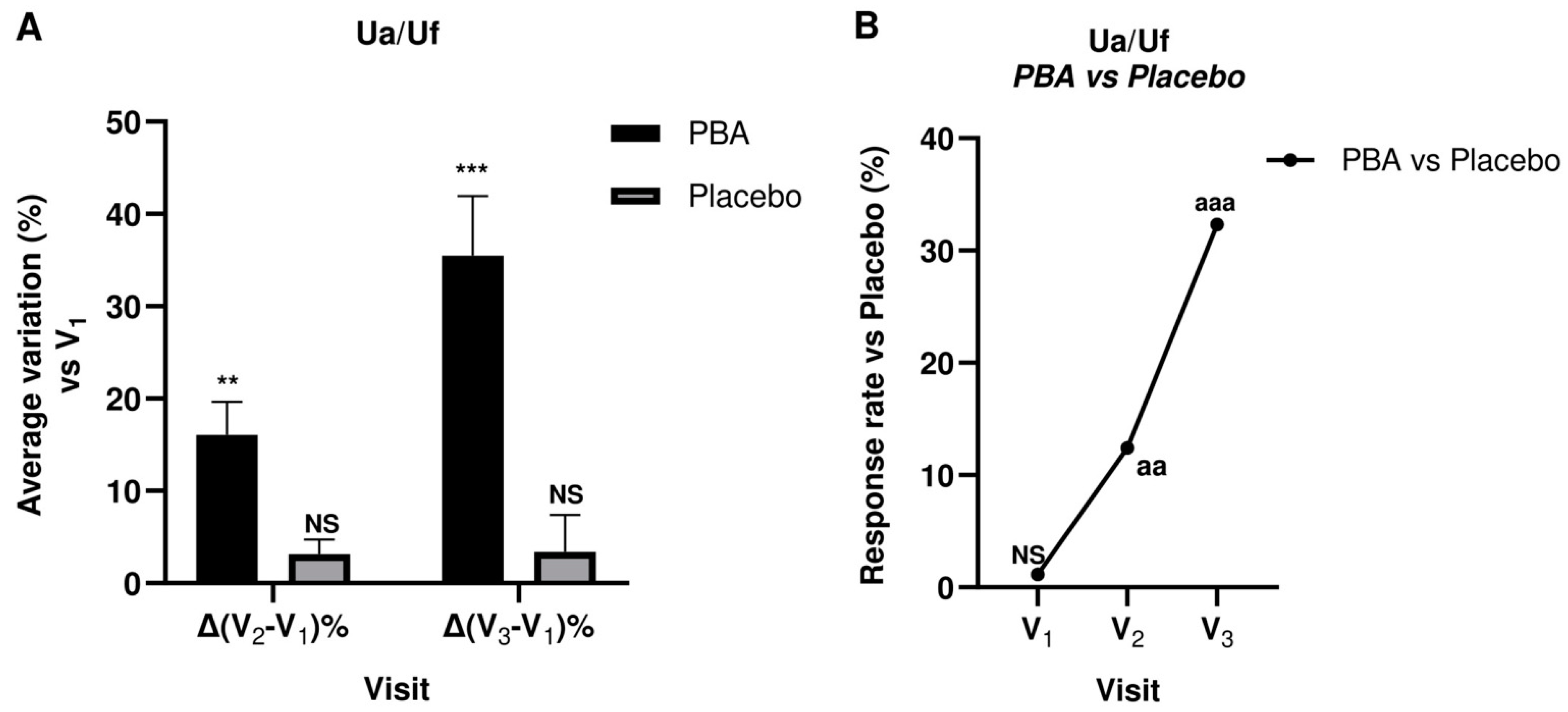
2.5. Skin Firmness and Elasticity (Suction Parameters Uf and Ua/Uf)
3. Discussion
4. Materials and Methods
4.1. Tyrosinase Inhibition Assay
4.2. Molecular Docking Simulation
4.3. Clinical Study
4.4. PBA and Excipients
4.5. PBA-Based Topical Preparation
4.6. Skin Parameters
- Skin complexion color was measured as Individual Typology Angle (ITA°) values with the Skin-colorimeter CL 400 (Courage + Khazaka Electronic GmbH, Köln, Germany).
- Before and after photos were also collected to compare results.
- Skin firmness and elasticity were evaluated by the Cutometer® DUAL MPA 580 (Courage + Khazaka electronic GmbH, Köln, Germany) with a standard probe (Ø mm2). The surface of the skin is sucked into the probe opening and a constant 450 mbar vacuum is applied for a preset time of 5 seconds, after which the skin is released following 5 seconds of air depression break (relaxation time). Thus, the skin can return to its original position. Five suction cycles (1 cycle: suction/release) are performed on the same point. The optical detection system inside the probe measured several skin features, such as the penetration depth, the resistance to the negative pressure, which is related to the skin’s firmness, and the ability of the skin to return to its original state (skin elasticity). The optical detection system includes a light source and a light receptor, along with two prisms facing each other, which project the light from transmitter to receptor. During real-time skin measurements, the light intensity changes depending on the skin penetration depth and relaxation time. These data are displayed as curves, which report the height (in mm) reached by the skin during the suction phase and the different levels of “skin return” during the relaxation time in real-time measurement procedures, allowing the calculation of the skin’s visco-elastic properties and skin aging. As described by Dobrev et al. [54], the following regions can be seen in a typical skin deformation curve: Ue, immediate distension; Uv, delayed distension; Uf, final distension; Ur, immediate retraction; and Ua, final retraction. The skin mechanical parameters considered in this study were skin firmness (Uf) and skin elasticity (Ua/Uf).
4.7. Data Analysis and Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gallo, R.L. Human Skin Is the Largest Epithelial Surface for Interaction with Microbes. J. Investig. Dermatol. 2017, 137, 1213–1214. [Google Scholar] [CrossRef]
- Holick, M.F. Skin as the Site of Vitamin D Synthesis and Target Tissue for 1,25-Dihydroxyvitamin D3. Arch. Dermatol. 1987, 123, 1677. [Google Scholar] [CrossRef]
- Grice, E.A.; Segre, J.A. The Skin Microbiome. Nat. Rev. Microbiol. 2011, 9, 244–253. [Google Scholar] [CrossRef]
- Park, Y.J.; Lee, H.K. The Role of Skin and Orogenital Microbiota in Protective Immunity and Chronic Immune-Mediated Inflammatory Disease. Front. Immunol. 2018, 8, 1955. [Google Scholar] [CrossRef]
- Luna, P.C. Skin Microbiome as Years Go By. Am. J. Clin. Dermatol. 2020, 21, 12–17. [Google Scholar] [CrossRef]
- Fredricks, D.N. Microbial Ecology of Human Skin in Health and Disease. J. Investig. Dermatol. Symp. Proc. 2001, 6, 167–169. [Google Scholar] [CrossRef]
- Ogunrinola, G.A.; Oyewale, J.O.; Oshamika, O.O.; Olasehinde, G.I. The Human Microbiome and Its Impacts on Health. Int. J. Microbiol. 2020, 2020, 8045646. [Google Scholar] [CrossRef]
- Prescott, S.L.; Larcombe, D.L.; Logan, A.C.; West, C.; Burks, W.; Caraballo, L.; Levin, M.; Van Etten, E.; Horwitz, P.; Kozyrskyj, A.; et al. The Skin Microbiome: Impact of Modern Environments on Skin Ecology, Barrier Integrity, and Systemic Immune Programming. World Allergy Organ. J. 2017, 10, 29. [Google Scholar] [CrossRef]
- Rea, K.; Dinan, T.G.; Cryan, J.F. The Microbiome: A Key Regulator of Stress and Neuroinflammation. Neurobiol. Stress 2016, 4, 23–33. [Google Scholar] [CrossRef]
- Kapoor, B.; Gulati, M.; Rani, P.; Gupta, R. Psoriasis: Interplay between Dysbiosis and Host Immune System. Autoimmun. Rev. 2022, 21, 103169. [Google Scholar] [CrossRef]
- Stec, A.; Sikora, M.; Maciejewska, M.; Paralusz-Stec, K.; Michalska, M.; Sikorska, E.; Rudnicka, L. Bacterial Metabolites: A Link between Gut Microbiota and Dermatological Diseases. Int. J. Mol. Sci. 2023, 24, 3494. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between Microbiota and Immunity in Health and Disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Neish, A.S. Mucosal Immunity and the Microbiome. Ann. Am. Thorac. Soc. 2014, 11, S28–S32. [Google Scholar] [CrossRef]
- Krejner, A.; Bruhs, A.; Mrowietz, U.; Wehkamp, U.; Schwarz, T.; Schwarz, A. Decreased Expression of G-Protein-Coupled Receptors GPR43 and GPR109a in Psoriatic Skin Can Be Restored by Topical Application of Sodium Butyrate. Arch. Dermatol. Res. 2018, 310, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Kimura, I.; Ichimura, A.; Ohue-Kitano, R.; Igarashi, M. Free Fatty Acid Receptors in Health and Disease. Physiol. Rev. 2020, 100, 171–210. [Google Scholar] [CrossRef]
- De Pessemier, B.; Grine, L.; Debaere, M.; Maes, A.; Paetzold, B.; Callewaert, C. Gut–Skin Axis: Current Knowledge of the Interrelationship between Microbial Dysbiosis and Skin Conditions. Microorganisms 2021, 9, 353. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.N.; Sinha, P.K. Effect of sodium butyrate on mammalian cells in culture: A review. In Vitro 1976, 12, 125–132. [Google Scholar] [CrossRef]
- Karna, E.; Miltyk, W.; Pałka, J.A. Butyrate-Induced Collagen Biosynthesis in Cultured Fibroblasts Is Independent on A2β1 Integrin Signalling and Undergoes through IGF-I Receptor Cascade. Mol. Cell Biochem. 2006, 286, 147–152. [Google Scholar] [CrossRef]
- Karna, E.; Trojan, S.; Pałka, J.A. The Mechanism of Butyrate-Induced Collagen Biosynthesis in Cultured Fibroblasts. Acta Pol. Pharm. 2009, 66, 129–134. [Google Scholar]
- Qi, C.; Zhu, Y.; Reddy, J.K. Peroxisome Proliferator-Activated Receptors, Coactivators, and Downstream Targets. Cell Biochem. Biophys. 2000, 32, 187–204. [Google Scholar] [CrossRef]
- Alex, S.; Lange, K.; Amolo, T.; Grinstead, J.S.; Haakonsson, A.K.; Szalowska, E.; Koppen, A.; Mudde, K.; Haenen, D.; Al-Lahham, S.; et al. Short-Chain Fatty Acids Stimulate Angiopoietin-Like 4 Synthesis in Human Colon Adenocarcinoma Cells by Activating Peroxisome Proliferator-Activated Receptor γ. Mol. Cell Biol. 2013, 33, 1303–1316. [Google Scholar] [CrossRef]
- Kawada, T.; Goto, T.; Takahashi, N.; Hirai, S. Various Terpenoids Derived from Herbal and Dietary Plants Function as PPAR Modulators and Regulate Carbohydrate and Lipid Metabolism. PPAR Res. 2010, 2010, 483958. [Google Scholar] [CrossRef]
- Peters, J.M.; Shah, Y.M.; Gonzalez, F.J. The Role of Peroxisome Proliferator-Activated Receptors in Carcinogenesis and Chemoprevention. Nat. Rev. Cancer 2012, 12, 181–195. [Google Scholar] [CrossRef]
- Schmuth, M.; Jiang, Y.J.; Dubrac, S.; Elias, P.M.; Feingold, K.R. Thematic Review Series: Skin Lipids. Peroxisome Proliferator-Activated Receptors and Liver X Receptors in Epidermal Biology. J. Lipid Res. 2008, 49, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Mahajan, V.K.; Mehta, K.S.; Chauhan, P.S.; Rawat, R. Peroxisome Proliferator-Activated Receptors (PPARs) and PPAR Agonists: The ‘Future’ in Dermatology Therapeutics? Arch. Dermatol. Res. 2015, 307, 767–780. [Google Scholar] [CrossRef]
- Grabacka, M.; Wieczorek, J.; Michalczyk-Wetula, D.; Malinowski, M.; Wolan, N.; Wojcik, K.; Plonka, P.M. Peroxisome Proliferator-Activated Receptor α (PPARα) Contributes to Control of Melanogenesis in B16 F10 Melanoma Cells. Arch. Dermatol. Res. 2017, 309, 141–157. [Google Scholar] [CrossRef]
- Darvin, M.E.; Salazar, A.; Schleusener, J.; Lademann, J.; von Hagen, J. Topical Peroxisome Proliferator-Activated Receptor Agonist Induces Molecular Alterations Enhancing Barrier Function and Water-Holding Capacity of the Human Stratum Corneum In Vivo. Cosmetics 2024, 11, 44. [Google Scholar] [CrossRef]
- Wiechers, J.W.; Rawlings, A.V.; Garcia, C.; Chesné, C.; Balaguer, P.; Nicolas, J.C.; Corre, S.; Galibert, M.D. A New Mechanism of Action for Skin Whitening Agents: Binding to the Peroxisome Proliferator-Activated Receptor. Int. J. Cosmet. Sci. 2005, 27, 123–132. [Google Scholar] [CrossRef]
- Di Lorenzo, R.; Bernardi, A.; Grumetto, L.; Sacchi, A.; Avagliano, C.; Coppola, S.; de Giovanni di Santa Severina, A.F.; Bruno, C.; Paparo, L.; Laneri, S.; et al. Phenylalanine Butyramide Is a New Cosmetic Ingredient with Soothing and Anti-Reddening Potential. Molecules 2021, 26, 6611. [Google Scholar] [CrossRef]
- Russo, R.; Santarcangelo, C.; Badolati, N.; Sommella, E.; De Filippis, A.; Dacrema, M.; Campiglia, P.; Stornaiuolo, M.; Daglia, M. In Vivo Bioavailability and in Vitro Toxicological Evaluation of the New Butyric Acid Releaser N-(1-Carbamoyl-2-Phenyl-Ethyl) Butyramide. Biomed. Pharmacother. 2021, 137, 111385. [Google Scholar] [CrossRef]
- Coppola, S.; Avagliano, C.; Sacchi, A.; Laneri, S.; Calignano, A.; Voto, L.; Luzzetti, A.; Canani, R.B. Potential Clinical Applications of the Postbiotic Butyrate in Human Skin Diseases. Molecules 2022, 27, 1849. [Google Scholar] [CrossRef] [PubMed]
- Oyama, T.; Yoshimori, A.; Ogawa, H.; Shirai, Y.; Abe, H.; Kamiya, T.; Tanuma, S. ichi The Structural Differences between Mushroom and Human Tyrosinase Cleared by Investigating the Inhibitory Activities of Stilbenes. J. Mol. Struct. 2023, 1272, 134180. [Google Scholar] [CrossRef]
- Neagu, N.; Conforti, C.; Agozzino, M.; Marangi, G.F.; Morariu, S.H.; Pellacani, G.; Persichetti, P.; Piccolo, D.; Segreto, F.; Zalaudek, I.; et al. Melasma Treatment: A Systematic Review. J. Dermatol. Treat. 2022, 33, 1816–1837. [Google Scholar] [CrossRef] [PubMed]
- Boo, Y.C. Metabolic Basis and Clinical Evidence for Skin Lightening Effects of Thiol Compounds. Antioxidants 2022, 11, 503. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Liu, W.; Zhu, D.; Cao, Y.; Tang, A.; Gong, G.; Su, H. Natural Skin-whitening Compounds for the Treatment of Melanogenesis (Review). Exp. Ther. Med. 2020, 20, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Tenore, G.C.; Carotenuto, A.; Caruso, D.; Buonomo, G.; D’Avino, M.; Brancaccio, D.; Ciampaglia, R.; Maisto, M.; Schisano, C.; Novellino, E. A Nutraceutical Formulation Based on Annurca Apple Polyphenolic Extract Is Effective on Intestinal Cholesterol Absorption: A Randomised, Placebo-Controlled, Crossover Study. PharmaNutrition 2018, 6, 85–94. [Google Scholar] [CrossRef]
- Annunziata, G.; Ciampaglia, R.; Maisto, M.; D’avino, M.; Caruso, D.; Tenore, G.C.; Novellino, E. Taurisolo®, a Grape Pomace Polyphenol Nutraceutical Reducing the Levels of Serum Biomarkers Associated with Atherosclerosis. Front. Cardiovasc. Med. 2021, 8, 697272. [Google Scholar] [CrossRef] [PubMed]
- Schiano, E.; Piccolo, V.; Novellino, E.; Maisto, M.; Iannuzzo, F.; Summa, V.; Tenore, G.C. Thinned Nectarines, an Agro-Food Waste with Antidiabetic Potential: HPLC-HESI-MS/MS Phenolic Characterization and In Vitro Evaluation of Their Beneficial Activities. Foods 2022, 11, 1010. [Google Scholar] [CrossRef]
- Gillbro, J.M.; Olsson, M.J. The Melanogenesis and Mechanisms of Skin-lightening Agents—Existing and New Approaches. Int. J. Cosmet. Sci. 2011, 33, 210–221. [Google Scholar] [CrossRef]
- Muto, J.; Kuroda, K.; Wachi, H.; Hirose, S.; Tajima, S. Accumulation of Elafin in Actinic Elastosis of Sun-Damaged Skin: Elafin Binds to Elastin and Prevents Elastolytic Degradation. J. Investig. Dermatol. 2007, 127, 1358–1366. [Google Scholar] [CrossRef]
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Characteristics of the Aging Skin. Adv. Wound Care 2013, 2, 5–10. [Google Scholar] [CrossRef]
- Knott, A.; Reuschlein, K.; Lucius, R.; Stäb, F.; Wenck, H.; Gallinat, S. Deregulation of Versican and Elastin Binding Protein in Solar Elastosis. Biogerontology 2009, 10, 181–190. [Google Scholar] [CrossRef]
- Kolakul, P.; Sripanidkulchai, B. Phytochemicals and Anti-Aging Potentials of the Extracts from Lagerstroemia Speciosa and Lagerstroemia Floribunda. Ind. Crops Prod. 2017, 109, 707–716. [Google Scholar] [CrossRef]
- Faure, C.; Ng, Y.M.; Belle, C.; Soler-Lopez, M.; Khettabi, L.; Saidi, M.; Berthet, N.; Maresca, M.; Philouze, C.; Rachidi, W.; et al. Interactions of Phenylalanine Derivatives with Human Tyrosinase: Lessons from Experimental and Theoretical Studies. ChemBioChem 2024, 25, e202400235. [Google Scholar] [CrossRef]
- UNI EN ISO 9001:2015; Quality Management Systems—Requirements. ISO: Geneva, Switzerland, 2015.
- Miller, D.J.; Henning, T.; Grünbein, W. Phase Inversion of W/O Emulsions by Adding Hydrophilic Surfactant—A Technique for Making Cosmetics Products. Colloids Surf. A Physicochem. Eng. Asp. 2001, 183–185, 681–688. [Google Scholar] [CrossRef]
- Zawodny, P.; Stój, E.; Kulig, P.; Skonieczna-żydecka, K.; Sieńko, J. VISIA Skin Analysis System as a Tool to Evaluate the Reduction of Pigmented Skin and Vascular Lesions Using the 532 Nm Laser. Clin. Cosmet. Investig. Dermatol. 2022, 15, 2187–2195. [Google Scholar] [CrossRef]
- Henseler, H. Validation of the Visia® Camera System for Skin Analysis through Assessment of the Correlations among the Three Offered Measurements—The Percentile, Feature Count and Absolute Score—As Well as the Three Capture Perspectives, from the Left, Front and Right. GMS Interdiscip. Plast. Reconstr. Surg. DGPW 2022, 11, Doc04. [Google Scholar] [CrossRef]
- Goldsberry, A.; Hanke, C.W.; Hanke, K.E. VISIA System: A Possible Tool in the Cosmetic Practice. J. Drugs Dermatol. 2014, 13, 1312–1314. [Google Scholar]
- Piccolo, M.; Ferraro, M.G.; Maione, F.; Maisto, M.; Stornaiuolo, M.; Tenore, G.C.; Santamaria, R.; Irace, C.; Novellino, E. Induction of Hair Keratins Expression by an Annurca Apple-Based Nutraceutical Formulation in Human Follicular Cells. Nutrients 2019, 11, 3041. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, R.; Maisto, M.; Ricci, L.; Piccolo, V.; Marzocchi, A.; Greco, G.; Tenore, G.C.; Laneri, S. Annurca Apple Oleolite as Functional Ingredient for the Formulation of Cosmetics with Skin-Antiaging Activity. Int. J. Mol. Sci. 2024, 25, 1677. [Google Scholar] [CrossRef]
- Di Lorenzo, R.; Forgione, F.; Bernardi, A.; Sacchi, A.; Laneri, S.; Greco, G. Clinical Studies on Topical Curcumin. Ski. Pharmacol. Physiol. 2023, 36, 235–248. [Google Scholar] [CrossRef]
- Rittié, L.; Fisher, G.J. Natural and Sun-Induced Aging of Human Skin. Cold Spring Harb. Perspect. Med. 2015, 5, a015370. [Google Scholar] [CrossRef] [PubMed]
- Greco, G.; Di Lorenzo, R.; Ricci, L.; Di Serio, T.; Vardaro, E.; Laneri, S. Clinical Studies Using Topical Melatonin. Int. J. Mol. Sci. 2024, 25, 5167. [Google Scholar] [CrossRef] [PubMed]
- Dobrev, H. Novel Ideas: The Increased Skin Viscoelasticity—A Possible New Fifth Sign for the Very Early Diagnosis of Systemic Sclerosis. Curr. Rheumatol. Rev. 2014, 9, 261–267. [Google Scholar] [CrossRef] [PubMed]

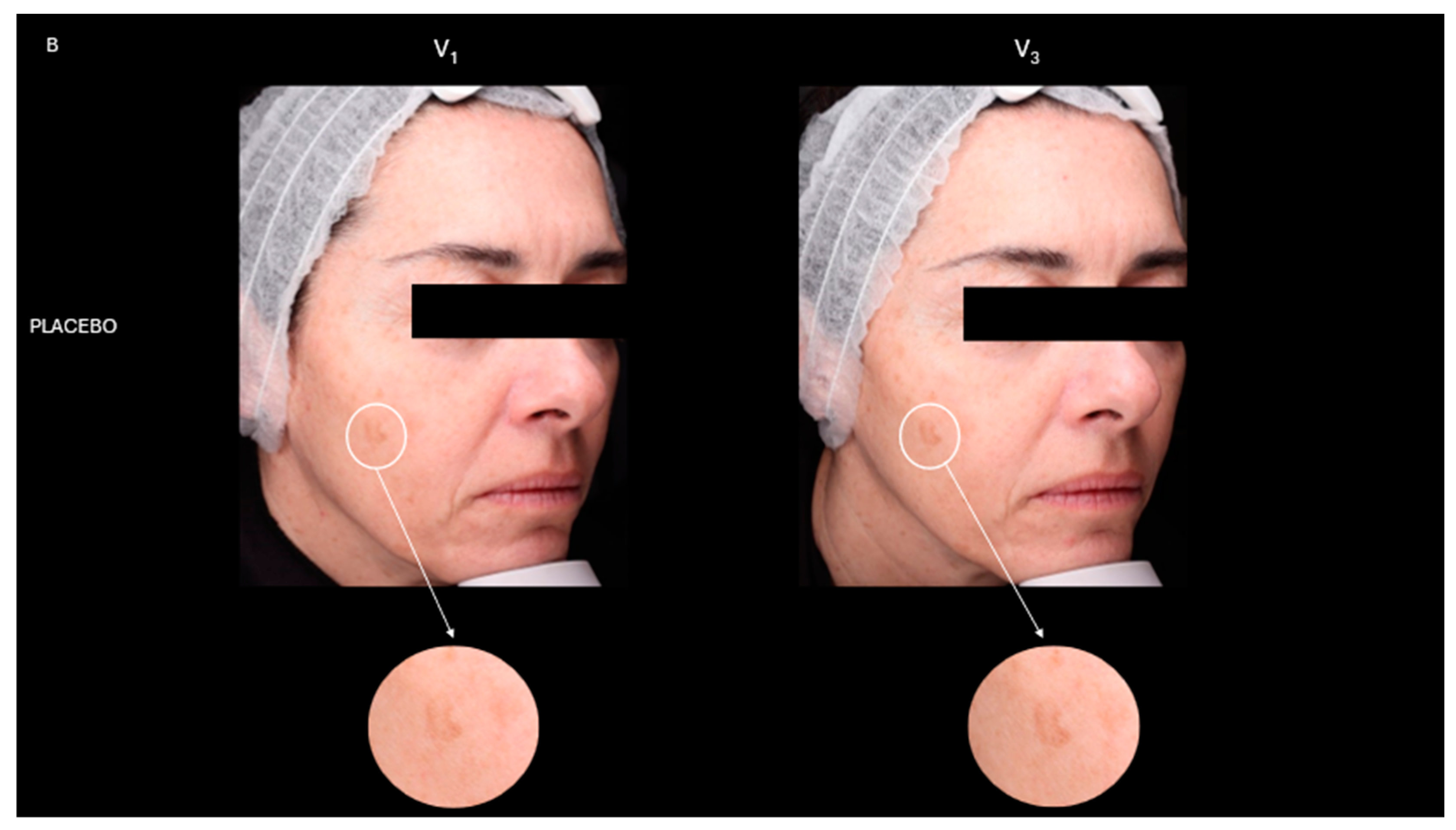
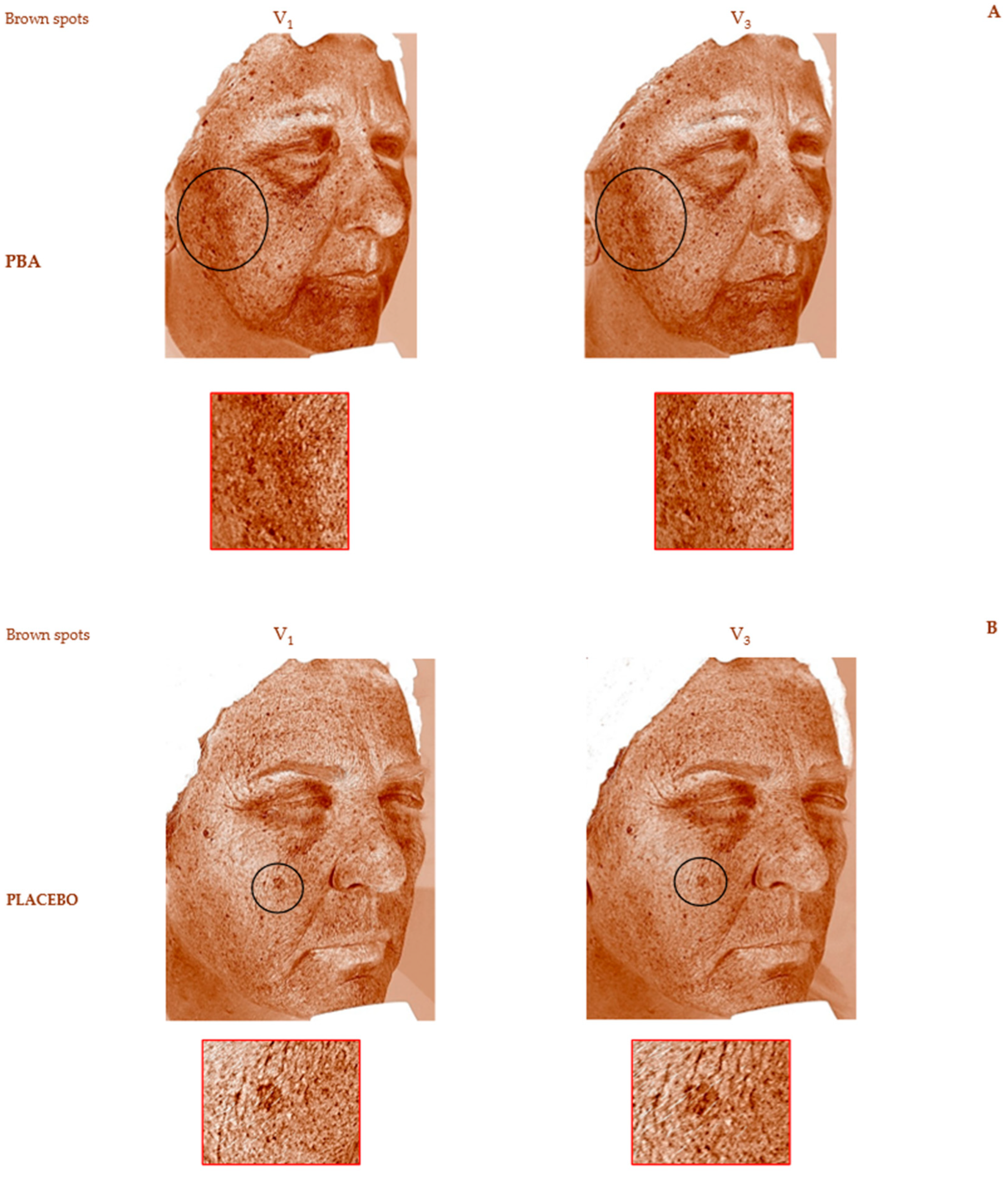
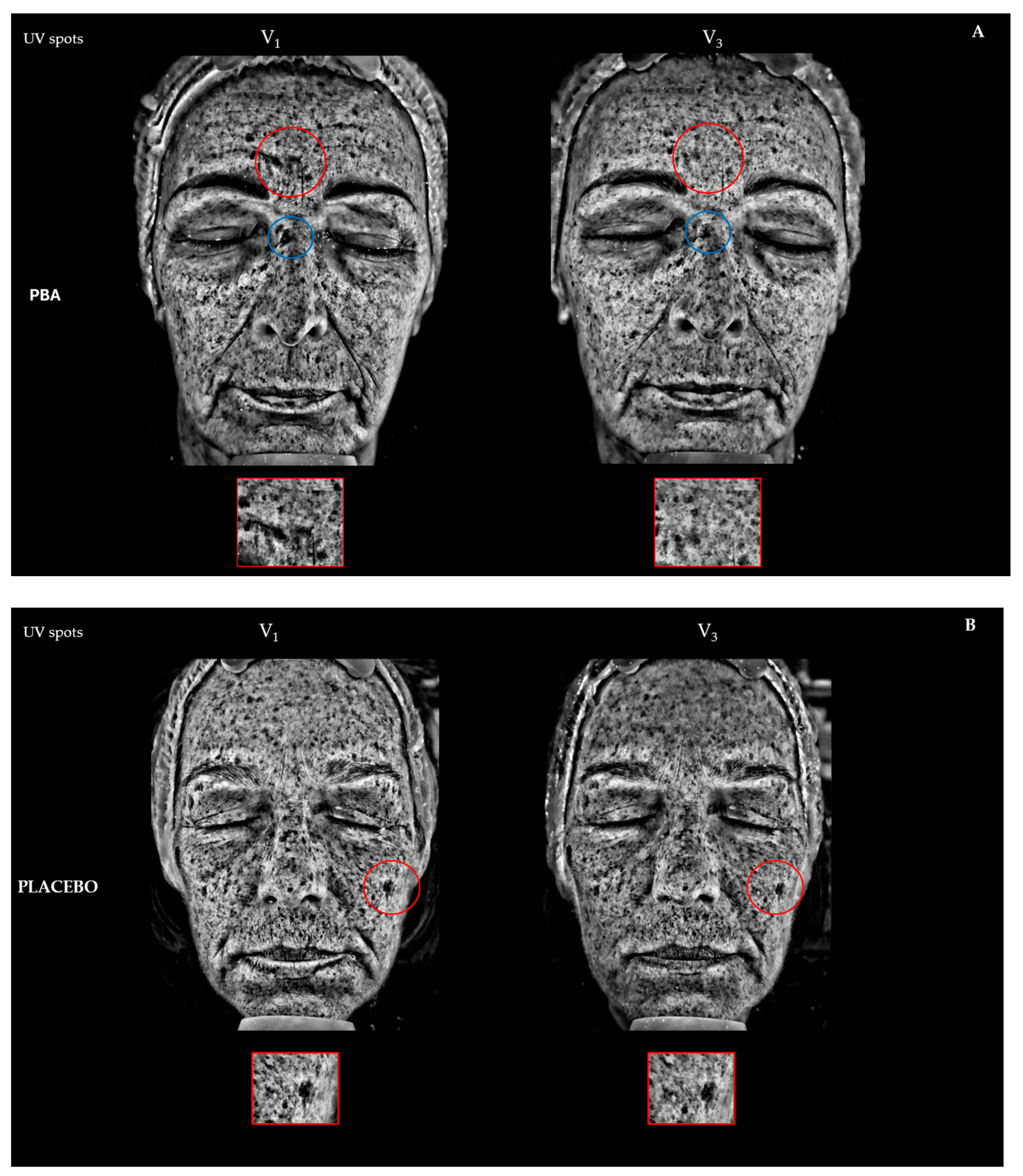

| Placebo | PBA | |||
|---|---|---|---|---|
| Brown spots (Mean ± SD) | Change vs. V1 ± SEM | Change vs. V1 ± SEM | ||
| V1 | 21.5 ± 3.6 | / | 21.5 ± 3.1 | / |
| V2 | 23.5 ± 4.0 | 10.4 ± 0.7 | 19.4 ± 2.5 | −9.2 ± 0.4 * aaa |
| V3 | 22.7 ± 3.6 | 6.4 ± 0.6 | 19.3 ± 3.6 | −10.9 ± 0.4 * aaa |
| UV spots (Mean ± SD) | Change vs. V1 ± SEM | Change vs. V1 ± SEM | ||
| V1 | 24.6 ± 2.9 | / | 24.4 ± 2.1 | / |
| V2 | 25.4 ± 2.5 | 3.6 ± 0.4 | 22.5 ± 1.5 | −7.3 ± 0.3 ** aaa |
| V3 | 22.8 ± 1.6 | 1.7 ± 0.2 | 22.8 ± 1.6 | −6.7 ± 0.2 ** aaa |
| Placebo | PBA | |||
|---|---|---|---|---|
| Uf (Mean ± SD) | Change vs. V1 ± SEM | Change vs. V1 ± SEM | ||
| V1 | 0.270 ± 0.034 | / | 0.310 ± 0.042 | / |
| V2 | 0.264 ± 0.049 | −2.4 ± 0.2 | 0.273 ± 0.052 | −11.7 ± 0.3 * aa |
| V3 | 0.259 ± 0.061 | −4.7 ± 0.4 | 0.226 ± 0.084 | −27.9 ± 0.6 *** aaa |
| Ua/Uf (Mean ± SD) | Change vs. V1 ± SEM | Change vs. V1 ± SEM | ||
| V1 | 0.510 ± 0.055 | / | 0.506 ± 0.056 | / |
| V2 | 0.592 ± 0.104 | 0.012 ± 0.037 | 0.585 ± 0.106 | 16.1 ± 0.4 ** aa |
| V3 | 0.687 ± 0.148 | 0.018 ± 0.094 | 0.678 ± 0.152 | 35.5 ± 0.8 *** aaa |
| Phase | Ingredients RB 1 = 5.00 | Function | % w/w |
|---|---|---|---|
| A | Polyglyceryl-4 Isostearate | Emulsifier | 4.00 |
| A | Cera Alba | Consistency factor | 1.50 |
| A | PEG-40 Hydrogenated Castor Oil | Consistency factor | 1.50 |
| A | Ethylhexyl Palmitate | Emollient | 11.50 |
| A | Caprylic/Capric Triglycerides | Emollient | 11.50 |
| A | PBA | Active ingredient | 1.50 |
| B | Aqua | Solvent | Qs 2 to 100 |
| B | Sodium Gluconate | Chelating agent | 0.20 |
| B | Magnesium Sulfate heptahydrate | Viscosizing agent | 0.50 |
| B | Glycerin | Humectant | 3.00 |
| C | Aqua | Solvent | 3.00 |
| C | Phenoxyethanol (and) Ethylhexylglycerin | Preservative | 0.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Lorenzo, R.; Di Lorenzo, V.; Di Serio, T.; Marzocchi, A.; Ricci, L.; Vardaro, E.; Greco, G.; Maisto, M.; Grumetto, L.; Piccolo, V.; et al. Phenylalanine Butyramide: A Butyrate Derivative as a Novel Inhibitor of Tyrosinase. Int. J. Mol. Sci. 2024, 25, 7310. https://doi.org/10.3390/ijms25137310
Di Lorenzo R, Di Lorenzo V, Di Serio T, Marzocchi A, Ricci L, Vardaro E, Greco G, Maisto M, Grumetto L, Piccolo V, et al. Phenylalanine Butyramide: A Butyrate Derivative as a Novel Inhibitor of Tyrosinase. International Journal of Molecular Sciences. 2024; 25(13):7310. https://doi.org/10.3390/ijms25137310
Chicago/Turabian StyleDi Lorenzo, Ritamaria, Vincenzo Di Lorenzo, Teresa Di Serio, Adua Marzocchi, Lucia Ricci, Eleonora Vardaro, Giovanni Greco, Maria Maisto, Lucia Grumetto, Vincenzo Piccolo, and et al. 2024. "Phenylalanine Butyramide: A Butyrate Derivative as a Novel Inhibitor of Tyrosinase" International Journal of Molecular Sciences 25, no. 13: 7310. https://doi.org/10.3390/ijms25137310
APA StyleDi Lorenzo, R., Di Lorenzo, V., Di Serio, T., Marzocchi, A., Ricci, L., Vardaro, E., Greco, G., Maisto, M., Grumetto, L., Piccolo, V., Morelli, E., & Laneri, S. (2024). Phenylalanine Butyramide: A Butyrate Derivative as a Novel Inhibitor of Tyrosinase. International Journal of Molecular Sciences, 25(13), 7310. https://doi.org/10.3390/ijms25137310











