Functional Study of the Role of the Methyl Farnesoate Epoxidase Gene in the Ovarian Development of Macrobrachium nipponense
Abstract
:1. Introduction
2. Results
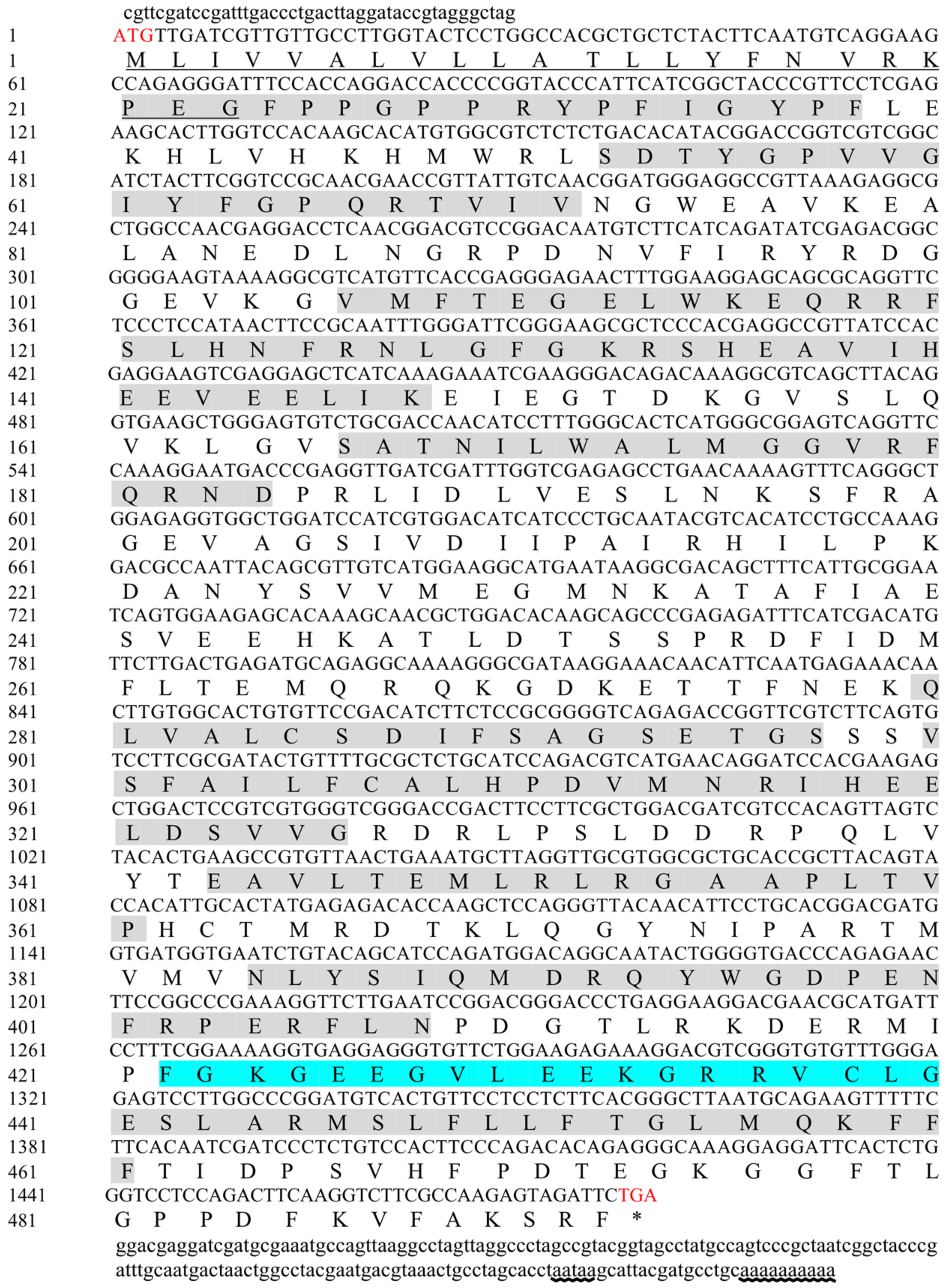
2.1. Sequence Analysis
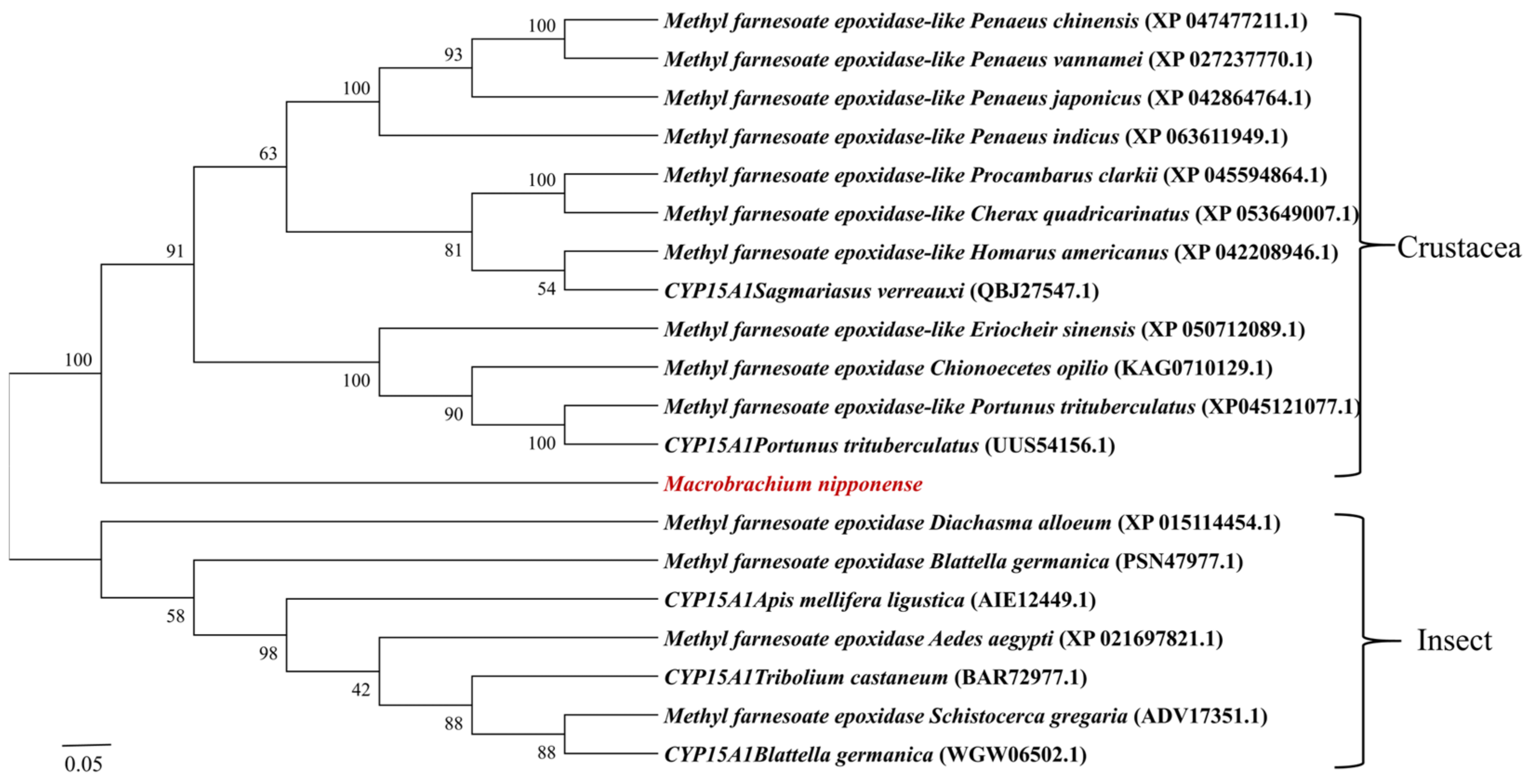
2.2. Sequence Comparison and Phylogenetic Analysis
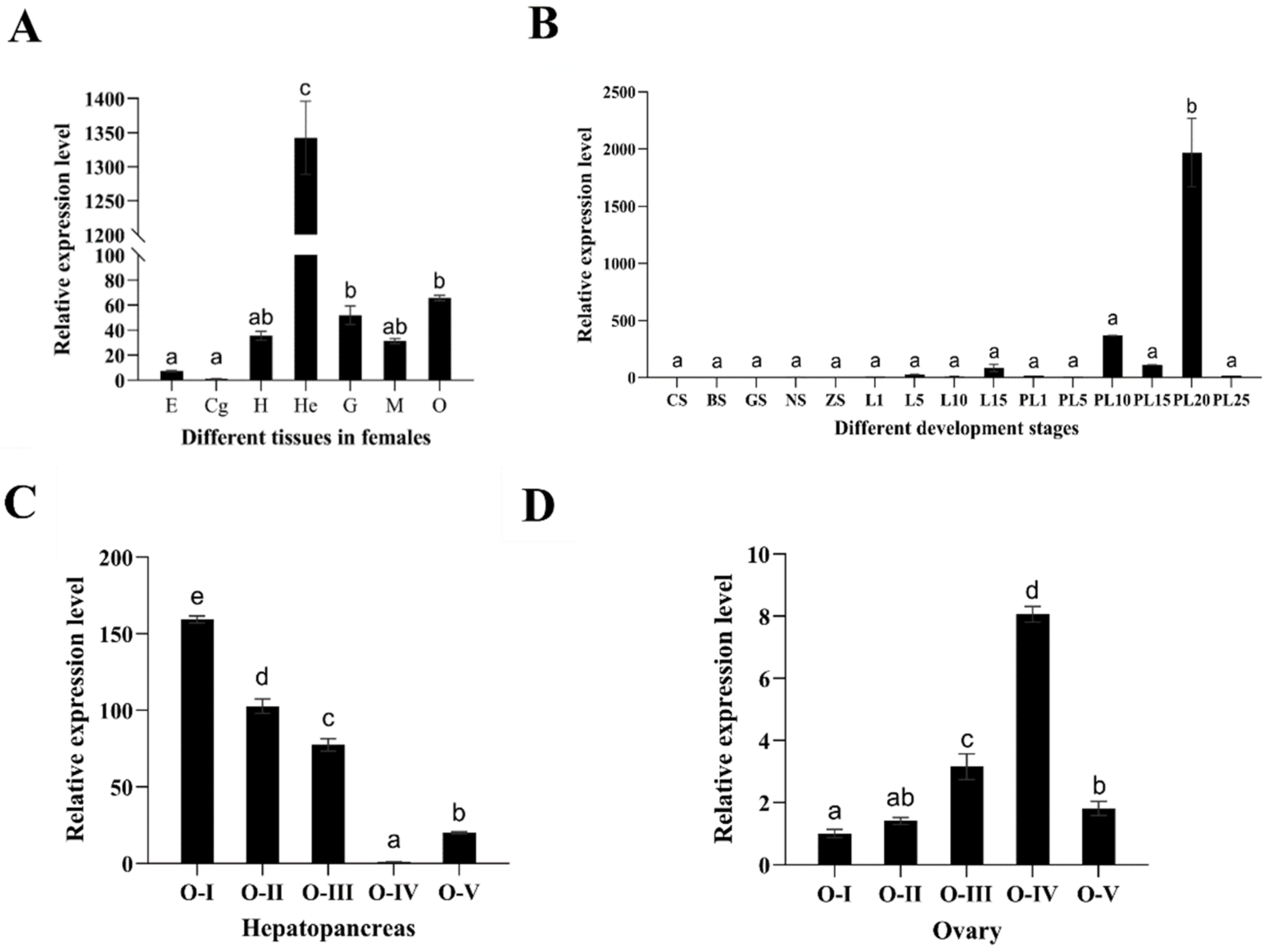
2.3. Expression Analysis of Mn-MFE
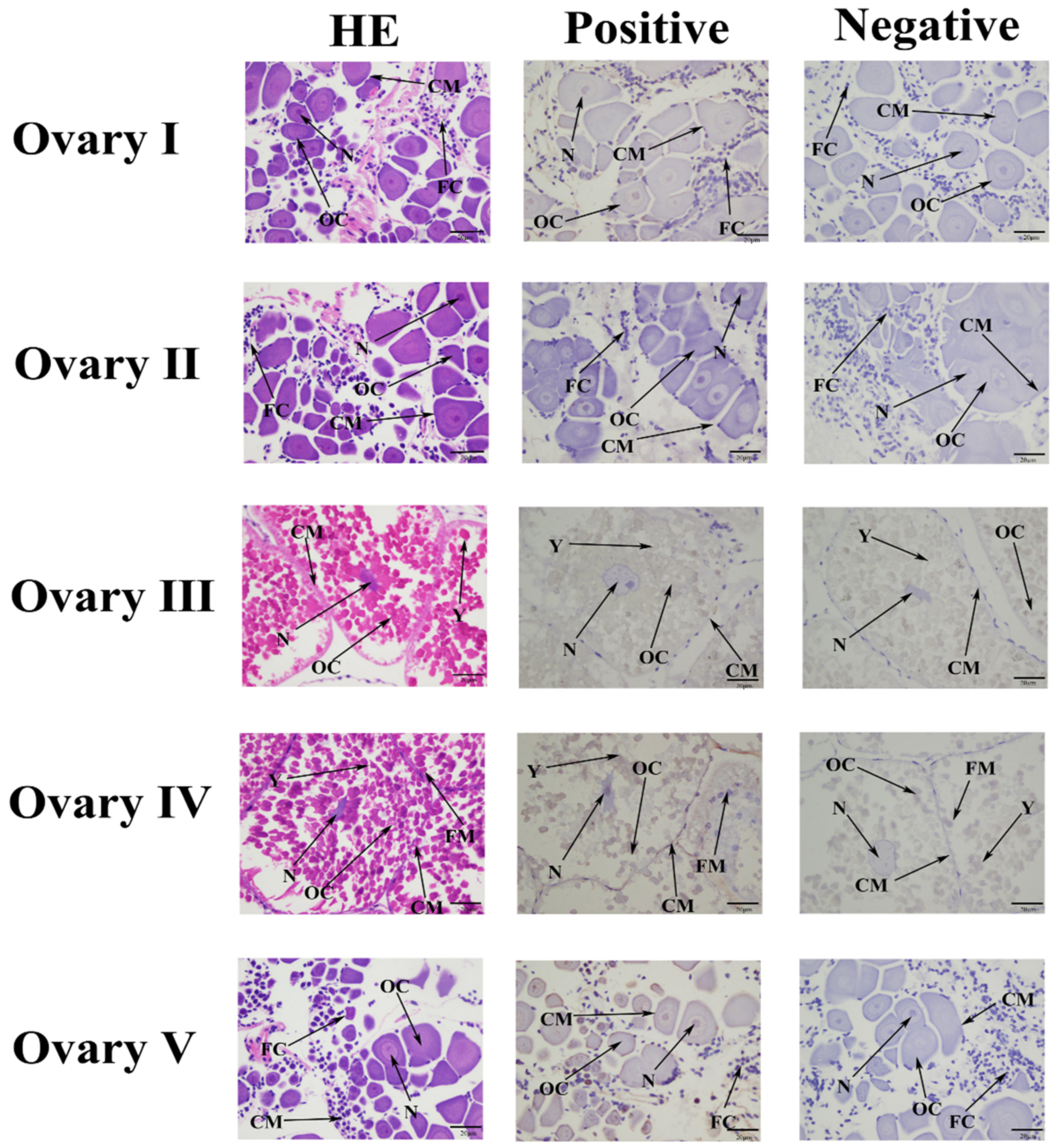
2.4. Localization of Mn-MFE in Different Stages of Ovarian Development
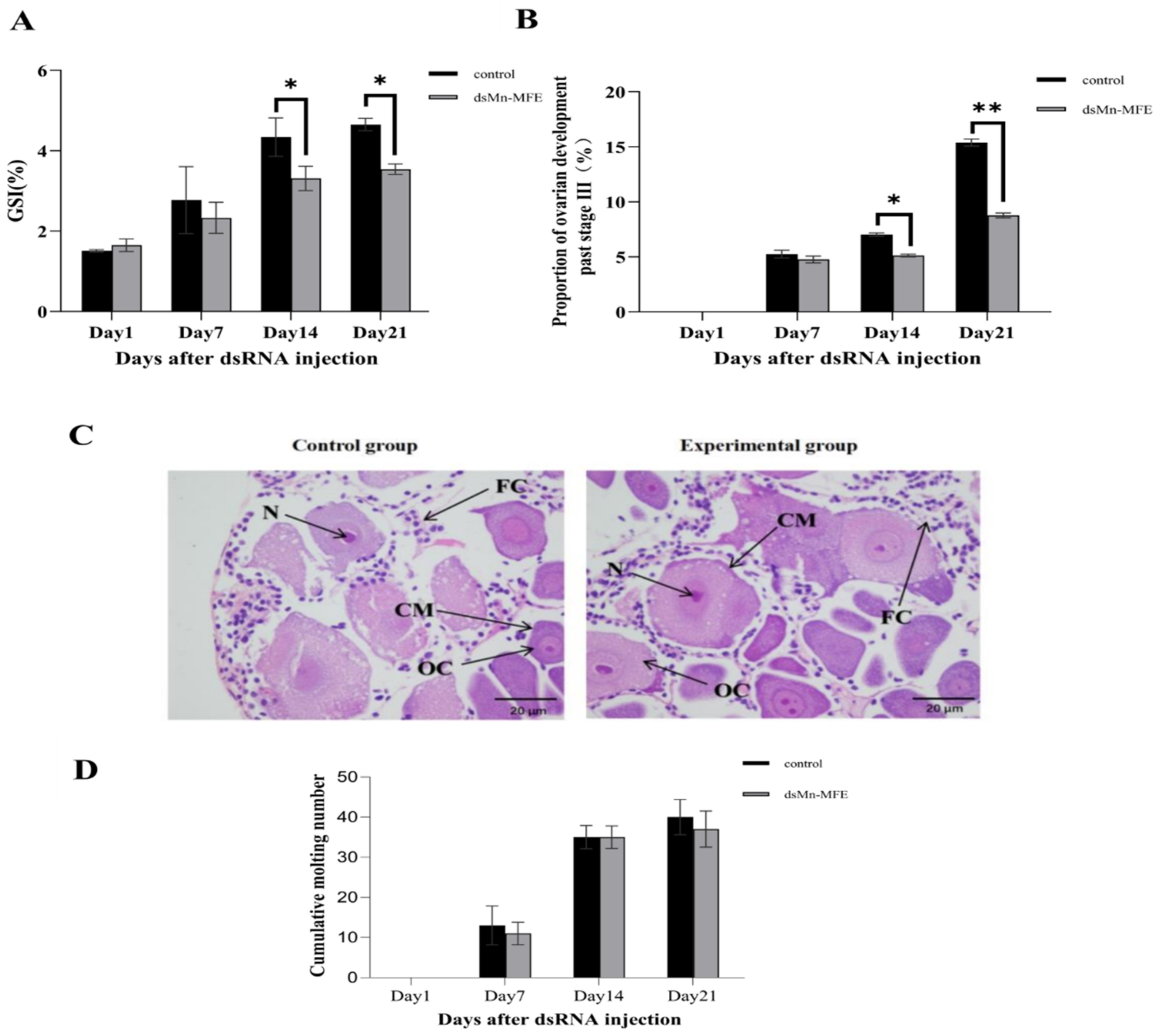
2.5. Functions of Mn-MFE in Regulating Ovarian Development
3. Discussion
4. Materials and Methods
4.1. Experimental Animal
4.2. Sample Collection
4.3. RNA Extraction, cDNA Synthesis, and Sequence Verification
4.4. Sequence Analysis
4.5. In Situ Hybridization (ISH)
4.6. RNA Interference (RNAi)
4.7. Quantitative PCR (qPCR)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, F.; Zhang, S.; Fu, C.; Li, T.; Cui, X. Molecular and functional analysis of the insulin-like peptides gene in the oriental river prawn Macrobrachium nipponense. Gen. Comp. Endocrinol. 2019, 280, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Song, M.S.; Pan, L.Q.; Zhang, M.Y.; Huang, F.; Gao, S.; Tian, C.C. Changes of water, sediment, and intestinal bacterial communities in Penaeus japonicus cultivation and their impacts on shrimp physiological health. Aquac. Int. 2020, 28, 1847–1865. [Google Scholar] [CrossRef]
- Kulesh, V.F.; Alekhnovych, A.V. Forming of size structure and density of the oriental river shrimp Macrobrachium nipponense in the warm-water polyculture of the fishery ponds. Hydrobiol. J. 2018, 54, 46–59. [Google Scholar] [CrossRef]
- Fu, C.P.; Li, F.J.; Wang, L.F.; Li, T.T. Molecular insights into ovary degeneration induced by environmental factors in female oriental river prawns Macrobrachium nipponense. Environ. Pollut. 2019, 253, 882–888. [Google Scholar] [CrossRef]
- Zhang, W.Y.; Xiong, Y.W.; Wang, P.C.; Chen, T.Y.; Jiang, S.F.; Qiao, H.; Gong, Y.S.; Wu, Y.; Jin, S.B.; Fu, H.T. RNA interference analysis of potential functions of cyclin A in the reproductive development of male oriental river prawns (Macrobrachium nipponense). Front. Genet. 2022, 13, 1053826. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.P.; Fu, H.T.; Qiao, H.; Jin, S.B.; Zhang, W.Y.; Jiang, S.F.; Gong, Y.S.; Xiong, Y.W. Expression and functional analysis of cathepsin L1 in ovarian development of the oriental river prawn, Macrobrachium nipponense. Aquac. Rep. 2021, 20, 100724. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, M.Q.; Liu, P.; Li, J.T.; Gao, B.Q.; Meng, X.L. The miRNAs let-7b and miR-141 coordinately regulate vitellogenesis by modulating methyl farnesoate degradation in the swimming crab Portunus trituberculatus. Int. J. Mol. Sci. 2023, 25, 279. [Google Scholar] [CrossRef]
- Nagaraju, G.P.C. Reproductive regulators in decapod crustaceans: An overview. J. Exp. Biol. 2011, 214, 3–16. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, F.Y.; Wang, W.; Liu, Z.Q.; Ma, C.Y.; Fu, Y.; Chen, W.; Ma, L.B. Identification and evolution analysis of the complete methyl farnesoate biosynthesis and related pathway genes in the mud crab, Scylla paramamosain. Int. J. Mol. Sci. 2022, 23, 9451. [Google Scholar] [CrossRef]
- Rotllant, G.; Pascual, N.; Sarda, F.; Takac, P.; Laufer, H. Identification of methyl farnesoate in the hemolymph of the Mediterranean deep-sea species Norway lobster, Nephrops norvegicus. J. Crustac. Biol. 2001, 21, 328–333. [Google Scholar] [CrossRef]
- Reddy, P.R.; Nagaraju, G.P.C.; Reddy, P.S. Involvement of methyl farnesoate in the regulation of molting and reproduction in the freshwater crab Oziotelphusa senex. J. Crustac. Biol. 2004, 24, 511–515. [Google Scholar] [CrossRef]
- Roe, R.M.; Donahue, K.V.; Khalil, S.M.; Bissinger, B.W.; Zhu, J.; Sonenshine, D.E. Hormonal regulation of metamorphosis and reproduction in ticks. Infect. Dis. Clin. N. Am. 2013, 1, 416. [Google Scholar] [CrossRef] [PubMed]
- Tu, S.S.; Tuo, P.; Xu, D.J.; Wang, Z.Y.; Wang, M.G.; Xie, X.; Zhu, D.F. Molecular characterization of the cytochrome P450 epoxidase (CYP15) in the swimming crab Portunus trituberculatus and its putative roles in methyl farnesoate metabolism. Biol. Bull. 2022, 242, 75–86. [Google Scholar] [CrossRef]
- Swetha, C.H.; Girish, B.P.; Hemalatha, M.; Reddy, P.S. Induction of vitellogenesis, methyl farnesoate synthesis and ecdysteroidogenesis in two edible crabs by arachidonic acid and prostaglandins. J. Exp. Biol. 2020, 223, jeb212381. [Google Scholar]
- Borst, D.W.; Laufer, H.; Landau, M.; Chang, E.S.; Hertz, W.A.; Baker, F.C.; Schooley, D.A. Methyl farnesoate and its role in crustacean reproduction and development. Insect Biochem. 1987, 17, 1123–1127. [Google Scholar] [CrossRef]
- Nagaraju, G.P.C. Is methyl farnesoate a crustacean hormone? Aquaculture 2007, 272, 39–54. [Google Scholar] [CrossRef]
- Olmstead, A.W.; Leblanc, G.A. Juvenoid hormone methyl farnesoate is a sex determinant in the crustacean Daphnia magna. J. Exp. Zool. 2002, 293, 736–739. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, F.Y.; Ma, C.Y.; Wang, W.; Liu, Z.Q.; Chen, W.; Zhao, M.; Ma, L.B. Comparative metabolomics and lipidomics of four juvenoids application to Scylla paramamosain hepatopancreas: Implications of lipid metabolism during ovarian maturation. Front. Endocrinol. 2022, 13, 886351. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, S.D.A.; Ayanath, A. Effect of 20-OH ecdysone and methyl farnesoate on moulting in the freshwater crab Travancoriana schirnerae. Invertebr. Reprod. Dev. 2019, 63, 309–318. [Google Scholar] [CrossRef]
- Muhd-Farouk, H.; Nurul, H.A.; Abol-Munafi, A.B.; Mardhiyyah, M.P.; Hasyima-lsmail, N.; Manan, H.; Fatihah, S.N.; Amin-Safwan, A.; Lkhwanuddin, M. Development of ovarian maturations in orange mud crab, Scylla olivacea (Herbst, 1796) through induction of eyestalk ablation and methyl farnesoate. Arab J. Basic App. Sci. 2019, 26, 171–181. [Google Scholar] [CrossRef]
- Brito, D.V.; Silva, C.G.N.; Rêgo, L.C.N.; Carvalho-Zilse, G.A. Expression of methyl farnesoate epoxidase (mfe) and juvenile hormone esterase (jhe) genes and their relation to social organization in the stingless bee Melipona interrupta (Hymenoptera: Apidae). Genet. Mol. Biol. 2021, 44, e20200367. [Google Scholar] [CrossRef]
- Rachinsky, A.; Tobe, S.S.; Feldlaufer, F.M. Terminal steps in JH biosynthesis in the honey bee (Apis mellifera L.): Developmental changes in sensitivity to JH precursor and allatotropin. Insect Biochem. Mol. 2000, 30, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Marchal, E.; Zhang, J.; Badisco, L.; Verlinden, H.; Hult, E.F.; Van Wielendaele, P.; Yagi, K.; Tobe, S.S.; Broeck, J.V. Final steps in juvenile hormone biosynthesis in the desert locust, Schistocerca gregaria. Insect Biochem. Mol. 2011, 41, 219–227. [Google Scholar] [CrossRef]
- Maestro, J.L.; Pascual, N.; Treiblmayr, K.; Lozano, J.; Bellés, X. Juvenile hormone and allatostatins in the German cockroach embryo. Insect Biochem. Mol. 2010, 40, 660–665. [Google Scholar] [CrossRef]
- Helvig, C.; Koener, J.F.; Unnithan, G.C.; Feyereisen, R. CYP15A1, the cytochrome P450 that catalyzes epoxidation of methyl farnesoate to juvenile hormone III in cockroach corpora allata. Proc. Natl. Acad. Sci. USA 2004, 101, 4024–4029. [Google Scholar] [CrossRef]
- Minakuchi, C.; Ishii, F.; Washidu, Y.; Ichikawa, A.; Tanaka, T.; Miura, K.; Shinoda, T. Expressional and functional analysis of CYP15A1, a juvenile hormone epoxidase, in the red flour beetle Tribolium castaneum. J. Insect Physiol. 2015, 80, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Lü, S.; Zhang, Y.L. Characterization of juvenile hormone related genes regulating cantharidin biosynthesis in Epicauta chinensis. Sci. Rep. 2017, 7, 2308. [Google Scholar] [CrossRef]
- Hatem, N.E.; Wang, Z.; Nave, K.B.; Koyama, T.; Suzuki, Y. The role of juvenile hormone and insulin/TOR signaling in the growth of Manduca sexta. BMC Biol. 2015, 13, 44. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Wang, W.W.; Dai, Y.X.; Qi, R.N.; Gu, H.Y.; Guo, X.Q.; Liu, X.Y.; Ren, Y.Y.; Li, F.C.; Sun, H.N. JH degradation pathway participates in hormonal regulation of larval development of Bombyx mori following λ-cyhalothrin exposure. Chemosphere 2024, 349, 140871. [Google Scholar] [CrossRef]
- Lewis, C.L.; Fitzgibbon, Q.P.; Smith, G.G.; Elizur, A.; Ventura, T. Ontogeny of the Cytochrome P450 Superfamily in the Ornate Spiny Lobster (Panulirus ornatus). Int. J. Mol. Sci. 2024, 25, 1070. [Google Scholar] [CrossRef]
- Shen, C.; Li, X. Genome-wide analysis of the P450 gene family in tea plant (Camellia sinensis) reveals functional diversity in abiotic stress. BMC Genom. 2023, 24, 535. [Google Scholar] [CrossRef]
- Ranson, H.; Nikou, D.; Hutchinson, M.; Wang, X.; Roth, C.W.; Hemingway, J.; Collins, F.H. Molecular analysis of multiple cytochrome P450 genes from the malaria vector, Anopheles gambiae. Insect Mol. Biol. 2002, 11, 409–418. [Google Scholar] [CrossRef]
- Werck-Reichhart, D.; Feyereisen, R. Cytochromes P450: A success story. Genome Biol. 2000, 1, 1–9. [Google Scholar] [CrossRef]
- Wilczek, G.; Kramarz, P.; Babczyńska, A. Activity of carboxylesterase and glutathione S-transferase in different life-stages of carabid beetle (Poecilus cupreus) exposed to toxic metal concentrations. Comp. Biochem. Phys. C 2003, 134, 501–512. [Google Scholar] [CrossRef]
- Zhu, J.; Khalil, S.M.; Mitchell, R.D.; Bissinger, B.W.; Egekwu, N.; Sonenshine, D.E.; Roe, R.M. Mevalonate-farnesal biosynthesis in ticks: Comparative synganglion transcriptomics and a new perspective. PLoS ONE 2016, 11, e0141084. [Google Scholar] [CrossRef]
- Daimon, T.; Kozaki, T.; Niwa, R.; Kobayashi, I.; Furuta, K.; Namiki, T.; Uchino, K.; Banno, Y.; Katsuma, S.; Shinoda, T. Precocious metamorphosis in the juvenile hormone–deficient mutant of the silkworm, Bombyx mori. PLoS Genet. 2012, 8, e1002486. [Google Scholar] [CrossRef]
- Zheng, Y.L.; Zhang, W.Y.; Xiong, Y.W.; Wang, J.S.; Jin, S.B.; Qiao, H.; Jiang, S.F.; Fu, H.T. Dual roles of CYP302A1 in regulating ovarian maturation and molting in Macrobrachium nipponense. J. Steroid Biochem. 2023, 232, 106336. [Google Scholar] [CrossRef]
- Rewitz, K.F.; O’Connor, M.B.; Gilbert, L.I. Molecular evolution of the insect Halloween family of cytochrome P450s: Phylogeny, gene organization and functional conservation. Insect Biochem. Mol. 2007, 37, 741–753. [Google Scholar] [CrossRef] [PubMed]
- Weirich, G.F.; Culver, M.G. S-Adenosylmethionine: Juvenile hormone acid methyltransferase in male accessory reproductive glands of Hyalophora cecropia (L.). Arch. Biochem. Biophys. 1979, 198, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Borovsky, D.; Carlson, D.A.; Hancock, R.G.; Rembold, H.; Van Handel, E. De novo biosynthesis of juvenile hormone III and I by the accessory glands of the male mosquito. Insect Biochem. Mol. 1995, 8, 967. [Google Scholar] [CrossRef] [PubMed]
- Steinmann, N.; Corona, M.; Neumann, P.; Dainat, B. Overwintering is associated with reduced expression of immune genes and higher susceptibility to virus infection in honey bees. PLoS ONE 2015, 10, e0129956. [Google Scholar] [CrossRef]
- Tian, L.; Ji, B.Z.; Liu, S.W.; He, C.L.; Jin, F.; Gao, J.; Stanley, D.; Li, S. JH biosynthesis by reproductive tissues and corpora allata in adult longhorned beetles, Apriona germari. Arch. Insect Biochem. 2010, 75, 275–286. [Google Scholar] [CrossRef]
- Cardoso-Júnior, C.A.M.; Silva, R.P.; Borges, N.A.; de Carvalho, W.J.; Walter, S.L.; Simões, Z.L.P.; Bitondi, M.M.G.; Vieira, C.U.; Bonetti, A.M.; Hartfelder, K. Methyl farnesoate epoxidase (mfe) gene expression and juvenile hormone titers in the life cycle of a highly eusocial stingless bee, Melipona scutellaris. J. Insect Physiol. 2017, 101, 185–194. [Google Scholar] [CrossRef]
- Wang, J.S.; Jiang, S.F.; Zhang, W.Y.; Xiong, Y.W.; Jin, S.B.; Cheng, D.; Zheng, Y.L.; Qiao, H.; Fu, H.T. Function Analysis of Cholesterol 7-Desaturase in Ovarian Maturation and Molting in Macrobrachium nipponense: Providing Evidence for Reproductive Molting Progress. Int. J. Mol. Sci. 2023, 24, 6940. [Google Scholar] [CrossRef]
- Santos, R.C.; Nogueira, C.S.; Jaconis, M.S.; Davanso, T.M.; Costa, R.C.; Hirose, G.L. New insights into the male morphotypes of the amphidromous shrimp Macrobrachium olfersii (Weigmann, 1836) (Caridea: Palaemonidae) and a discussion on social dominance hierarchies. Zool. Stud. 2022, 61, 83. [Google Scholar]
- Cheng, D.; Zhang, W.Y.; Jiang, S.F.; Xiong, Y.W.; Jin, S.B.; Pan, F.Y.; Zhu, J.P.; Gong, Y.S.; Qiao, H.; Fu, H.T. Cathepsin D plays a vital role in Macrobrachium nipponense of ovary maturation: Identification, characterization, and function analysis. Genes 2022, 13, 1495. [Google Scholar] [CrossRef]
- Jiang, S.F.; Qiao, H.; Fu, H.T.; Gu, Z.M. Hepatopancreas Proteomic Analysis Reveals Key Proteins and Pathways in Regulatory of Ovary Maturation of Macrobrachium nipponense. Animals 2023, 13, 977. [Google Scholar] [CrossRef]
- Fang, Z.Y.; Pazienza, L.T.; Zhang, J.; Tam, C.P.; Szostak, J.W. Catalytic Metal Ion-Substrate Coordination during Nonenzymatic RNA Primer Extension. J. Am. Chem. Soc. 2024, 146, 10632–10639. [Google Scholar] [CrossRef]
- Ji, L.Y.; Zhou, A.P.; Yu, X.Y.; Dong, Z.H.; Zhao, H.P.; Xue, H.; Wu, W.J. Differential expression analysis of the SRB1 gene in fluconazole-resistant and susceptible strains of Candida albicans. J. Antibiot. 2020, 73, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.N.; Fu, H.T.; Qiao, H.; Sun, S.M.; Zhang, W.Y.; Jin, S.B.; Jiang, S.F.; Gong, Y.S.; Xiong, Y.W.; Wu, Y. Validation and Evaluation of Reference Genes for Quantitative Real-Time PCR in Macrobrachium nipponense. Int. J. Mol. Sci. 2018, 19, 2258. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.B.; Jiang, P.; Wang, Z.Q.; Long, S.R.; Liu, R.D.; Zhang, X.; Yang, W.; Ren, H.J.; Cui, J. DsRNA-mediated silencing of Nudix hydrolase in Trichinella spiralis inhibits the larval invasion and survival in mice. Exp. Parasitol. 2016, 162, 35–42. [Google Scholar] [CrossRef]
- Gava, S.G.; Tavares, N.C.; de Matos Salim, A.C.; Gomes de Araujo, F.M.; Oliveira, G.; Mourao, M.M. Schistosoma mansoni: Off-target analyses using nonspecific double-stranded RNAs as control for RNAi experiments in schistosomula. Exp. Parasitol. 2017, 177, 98–103. [Google Scholar] [CrossRef]
- Arocho, A.; Chen, B.Y.; Ladanyi, M.; Pan, Q.L. Validation of the 2−ΔΔCt Calculation as an Alternate Method of Data Analysis for Quantitative PCR of BCR-ABL P210 Transcripts. Diagn. Mol. Pathol. 2006, 15, 56–61. [Google Scholar] [CrossRef]
- Kang, J.H.; Koo, S.M. Verification of Cervical Cancer Prevention Effect Using SPSS 20.0 WIN Program. Adv. Sci. Lett. 2018, 24, 2166–2170. [Google Scholar] [CrossRef]


| Primer | Primer Sequence (5′-3′) |
|---|---|
| Mn-MFE F (ORF) | CTACTTCAATGTCAGGAAGCCAG |
| Mn-MFE R (ORF) | GACAGAGGGATCGATTGTGAAGA |
| Mn-MFE R (5′) | AACGGGTAGCCGATGAATGGGTA |
| Mn-MFE F (3′) | GCCCGGATGTCACTGTTCCTCCT |
| Mn-MFE F (qPCR) | TGATCGTTGTTGCCTTGGTACTC |
| Mn-MFE R (qPCR) | ACCAAGTGCTTCTCGAGGAACGG |
| EIF-F (qPCR) | CATGGATGTACCTGTGGTGAAAC |
| EIF-R (qPCR) | CTGTCAGCAGAAGGTCCTCATTA |
| Mn-MFE probe | ATGTTGATCGTTGTTGCCTTGGTACTCCTGG |
| Mn-MFE anti-probe | CCAGGAGTACCAAGGCAACAACGATCAACAT |
| Mn-MFE iF (RNAi) | TAATACGACTCACTATAGGGTGATCGTTGTTGCCTTGGTACTC |
| Mn-MFE iR (RNAi) | TAATACGACTCACTATAGGGACCAAGTGCTTCTCGAGGAACGG |
| GFP iF (RNAi) | GATCACTAATACGACTCACTATAGGGTCCTGGTCGAGCTGGACGG |
| GFP iR (RNAi) | GATCACTAATACGACTCACTATAGGGCGCTTCTCGTTGGGGTCTTTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Jiang, S.; Zhang, W.; Xiong, Y.; Jin, S.; Wang, J.; Qiao, H.; Fu, H. Functional Study of the Role of the Methyl Farnesoate Epoxidase Gene in the Ovarian Development of Macrobrachium nipponense. Int. J. Mol. Sci. 2024, 25, 7318. https://doi.org/10.3390/ijms25137318
Zhang M, Jiang S, Zhang W, Xiong Y, Jin S, Wang J, Qiao H, Fu H. Functional Study of the Role of the Methyl Farnesoate Epoxidase Gene in the Ovarian Development of Macrobrachium nipponense. International Journal of Molecular Sciences. 2024; 25(13):7318. https://doi.org/10.3390/ijms25137318
Chicago/Turabian StyleZhang, Mengying, Sufei Jiang, Wenyi Zhang, Yiwei Xiong, Shubo Jin, Jisheng Wang, Hui Qiao, and Hongtuo Fu. 2024. "Functional Study of the Role of the Methyl Farnesoate Epoxidase Gene in the Ovarian Development of Macrobrachium nipponense" International Journal of Molecular Sciences 25, no. 13: 7318. https://doi.org/10.3390/ijms25137318





