Quantity and Distribution of Muscle Spindles in Animal and Human Muscles
Abstract
:1. Introduction
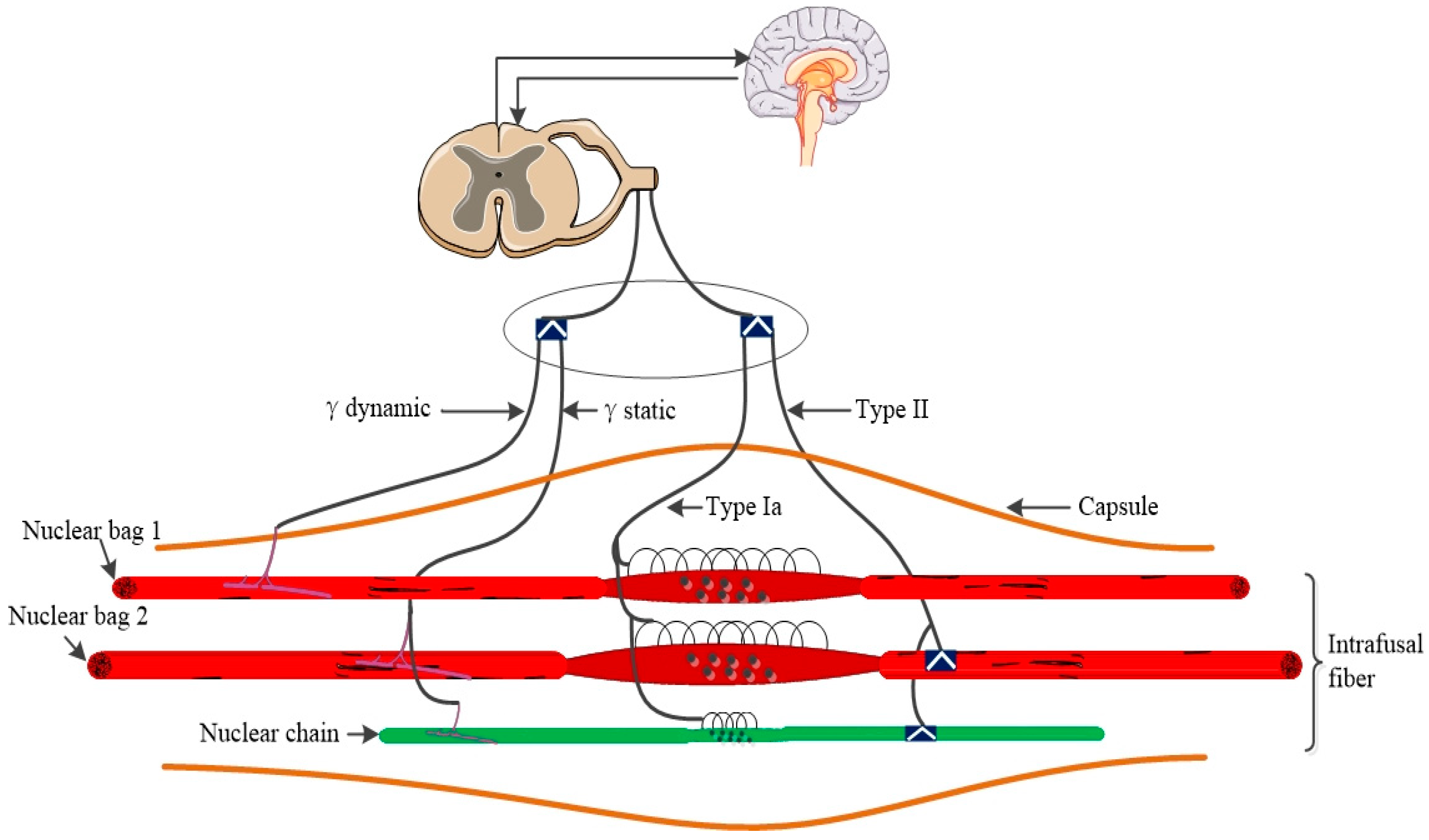
Muscle Spindle Neurophysiology
2. Methods
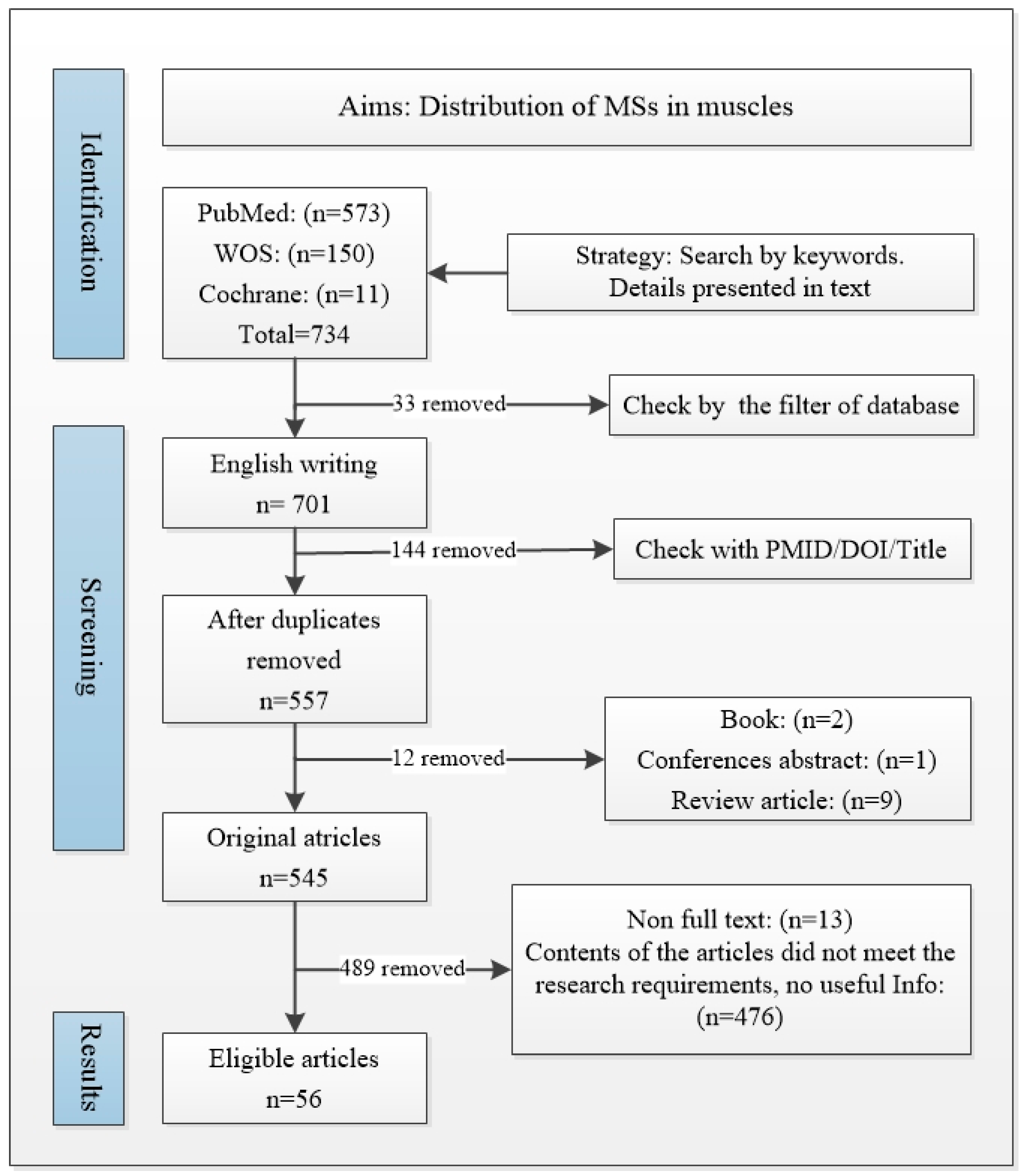
2.1. Searching Strategy for MS Distributions
2.1.1. Databases and Terms
2.1.2. Inclusion and Exclusion Criteria
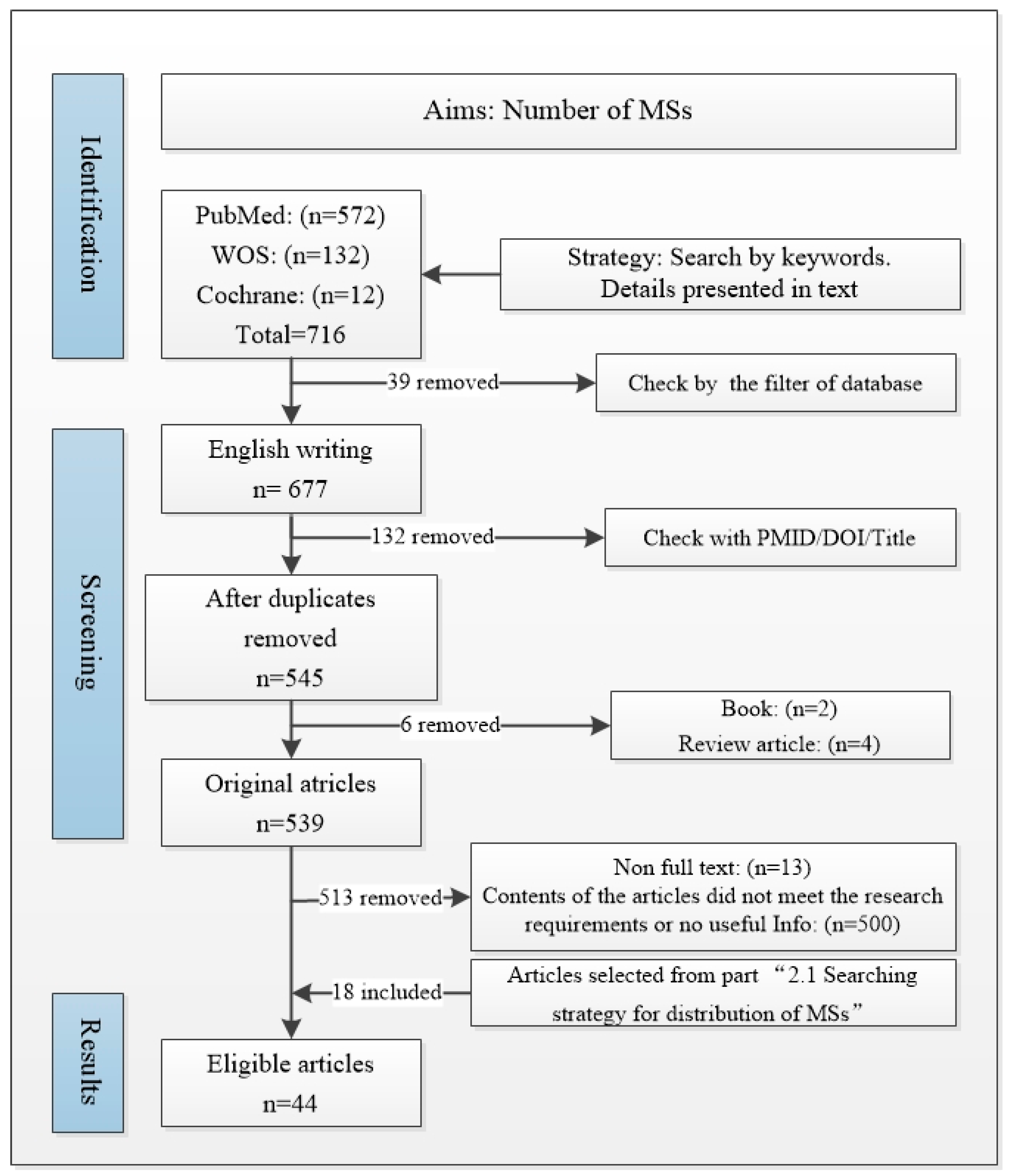
2.2. MS Quantity Search Strategy
2.2.1. Databases and Terms
2.2.2. Inclusion and Exclusion Criteria
3. Results
3.1. Studies in Animals and Humans
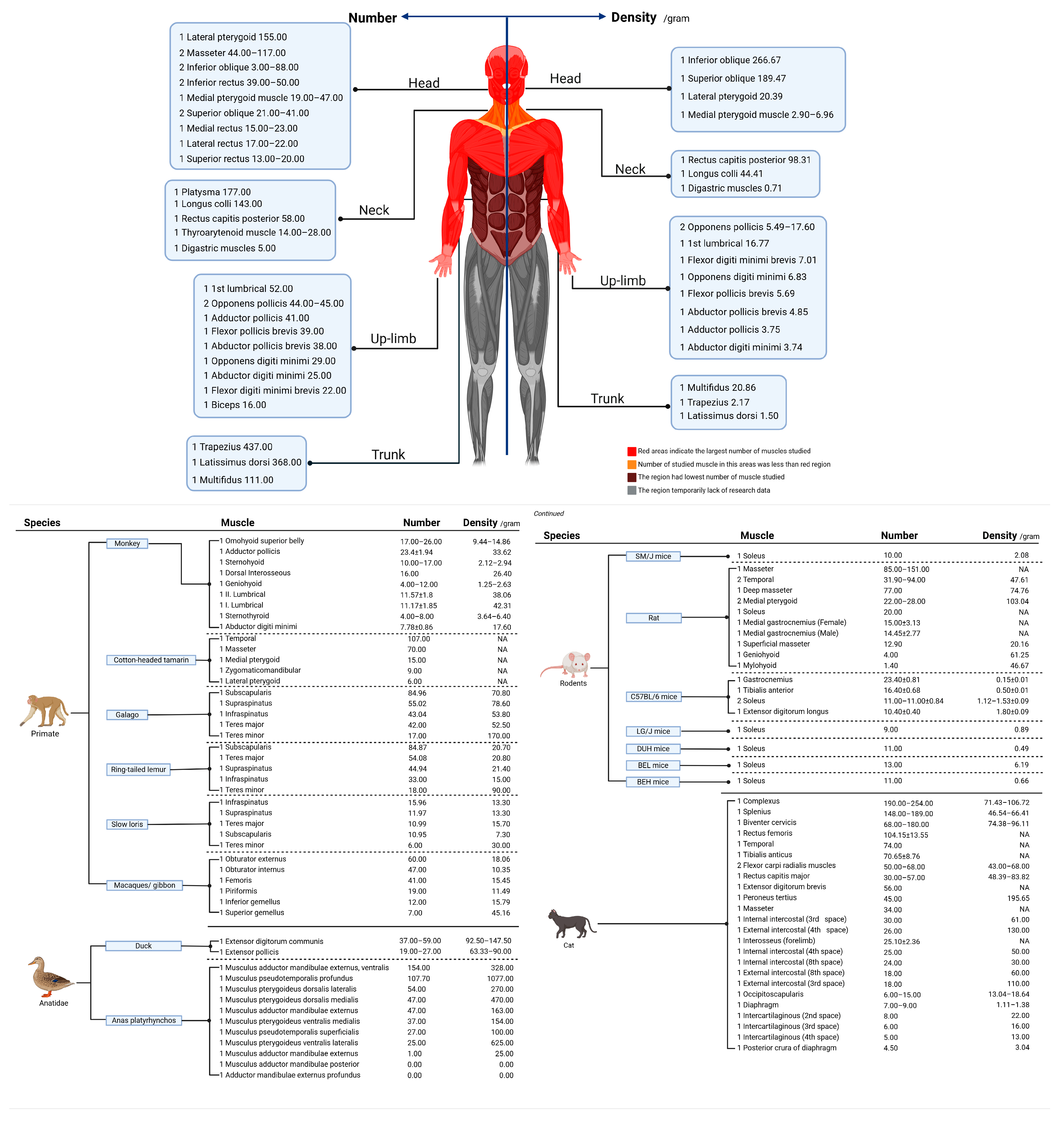
3.2. Muscle Types Studied
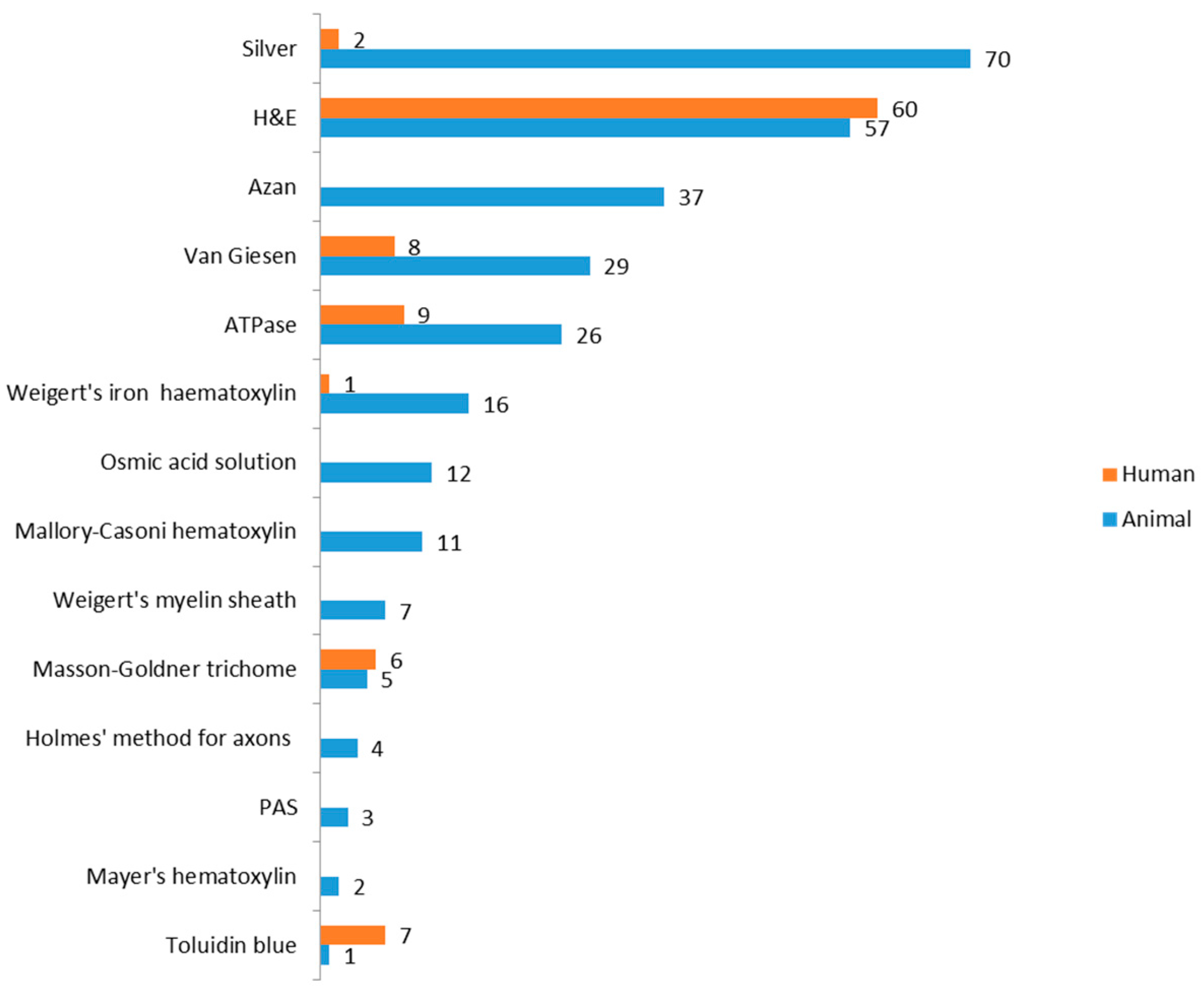
3.3. Analysis of Staining Methods
3.4. Density and Quantity in Different Muscles
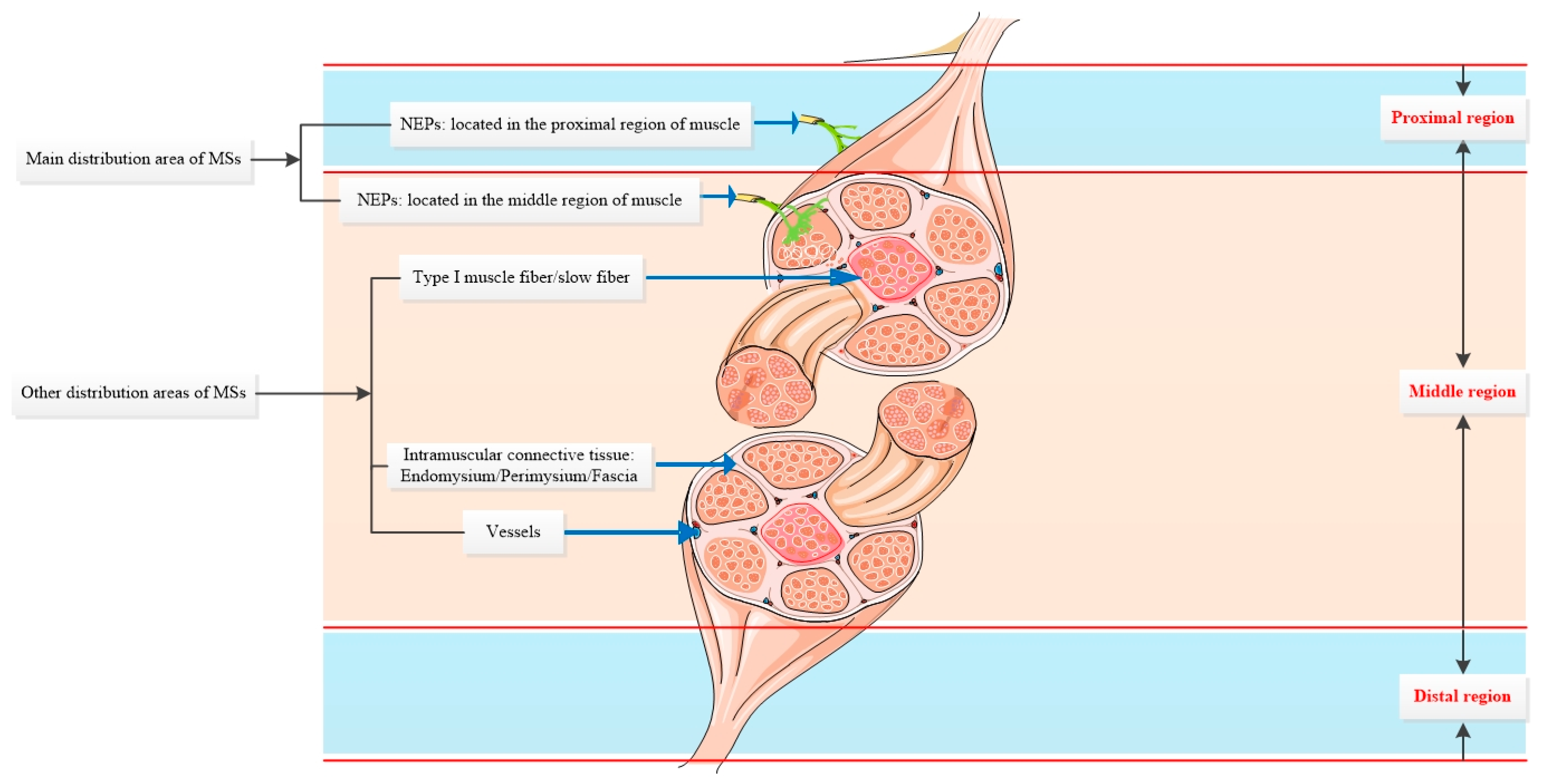
3.5. Distribution and Location Related to the Muscle Fiber, Vessel, NEPs (Nerve Entry Points), Fascia, and Other Anatomical Structures
3.6. MSs Topographic Distribution in Muscle
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Matthews, P.B. Where Anatomy led, Physiology followed: A survey of our developing understanding of the muscle spindle, what it does and how it works. J. Anat. 2015, 227, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Li, Y.; Yang, L.; Li, Y.; Zhang, S.; Yang, S. The highest region of muscle spindle abundance should be the optimal target of botulinum toxin A injection to block muscle spasms in rats. Front. Neurol. 2023, 14, 1061849. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, J.; Kulshreshtha, D.; Almotiri, M.; Jog, M. Muscle Tone Physiology and Abnormalities. Toxins 2021, 13, 282. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Chen, P.; Jia, F.; Wang, M.; Wu, J.; Yang, S. Division of neuromuscular compartments and localization of the center of the highest region of muscle spindles abundance in deep cervical muscles based on Sihlers staining. Front. Neuroanat. 2024, 18, 1340468. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Jia, F.; Chen, P.; Zhou, G.; Wang, M.; Wu, J.; Yang, S. Localisation of the centre of the highest region of muscle spindle abundance of anterior forearm muscles. J. Anat. 2024, 244, 803–814. [Google Scholar] [CrossRef]
- Stecco, A.; Giordani, F.; Fede, C.; Pirri, C.; De Caro, R.; Stecco, C. From Muscle to the Myofascial Unit: Current Evidence and Future Perspectives. Int. J. Mol. Sci. 2023, 24, 4527. [Google Scholar] [CrossRef]
- Brandl, A.; Egner, C.; Reer, R.; Schmidt, T.; Schleip, R. Associations between Deformation of the Thoracolumbar Fascia and Activation of the Erector Spinae and Multifidus Muscle in Patients with Acute Low Back Pain and Healthy Controls: A Matched Pair Case-Control Study. Life 2022, 12, 1735. [Google Scholar] [CrossRef]
- Fan, C.; Pirri, C.; Fede, C.; Guidolin, D.; Biz, C.; Petrelli, L.; Porzionato, A.; Macchi, V.; De Caro, R.; Stecco, C. Age-Related Alterations of Hyaluronan and Collagen in Extracellular Matrix of the Muscle Spindles. J. Clin. Med. 2021, 11, 86. [Google Scholar] [CrossRef]
- Schiller, B.; Colgan, W., 3rd; Calderon, B.; Johnson, B.R. Muscle Spindles and Our Sense of Physical Self: Kinesthetic Illusions of Limb Position and Posture. J. Undergrad. Neurosci. Educ. 2018, 16, A282–A288. [Google Scholar]
- Papaioannou, S.; Dimitriou, M. Goal-dependent tuning of muscle spindle receptors during movement preparation. Sci. Adv. 2021, 7, eabe0410. [Google Scholar] [CrossRef]
- Banks, R.W.; Barker, D. The muscle spindle. In Myology, 3rd ed.; Engel, A.G., Franzini-Armstrong, C., Eds.; McGraw-Hill: New York, NY, USA, 2004; pp. 489–509. [Google Scholar]
- Osterlund, C.; Liu, J.X.; Thornell, L.E.; Eriksson, P.O. Muscle spindle composition and distribution in human young masseter and biceps brachii muscles reveal early growth and maturation. Anat. Rec. 2011, 294, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Lian, W.X.; Rao, J.S.; Hao, L.F.; Wang, Z.J.; Duan, H.M.; Yang, Z.Y.; Li, X.G. Research progress on muscle spindle morphology. Sheng Li Xue Bao 2022, 74, 1039–1047. (In Chinese) [Google Scholar]
- Radovanovic, D.; Peikert, K.; Lindstrom, M.; Domellof, F.P. Sympathetic innervation of human muscle spindles. J. Anat. 2015, 226, 542–548. [Google Scholar] [CrossRef]
- Macefield, V.G.; Knellwolf, T.P. Functional properties of human muscle spindles. J. Neurophysiol. 2018, 120, 452–467. [Google Scholar] [CrossRef] [PubMed]
- Kröger, S.; Watkins, B. Muscle spindle function in healthy and diseased muscle. Skelet. Muscle 2021, 11, 3. [Google Scholar] [CrossRef]
- Thornell, L.E.; Carlsson, L.; Eriksson, P.O.; Liu, J.X.; Osterlund, C.; Stal, P.; Pedrosa-Domellof, F. Fibre typing of intrafusal fibres. J. Anat. 2015, 227, 136–156. [Google Scholar] [CrossRef]
- Proske, U.; Gandevia, S.C. The proprioceptive senses: Their roles in signaling body shape, body position and movement, and muscle force. Physiol. Rev. 2012, 92, 1651–1697. [Google Scholar] [CrossRef]
- Banks, R.W. The innervation of the muscle spindle: A personal history. J. Anat. 2015, 227, 115–135. [Google Scholar] [CrossRef] [PubMed]
- Shadrach, J.L.; Gomez-Frittelli, J.; Kaltschmidt, J.A. Proprioception revisited: Where do we stand? Curr. Opin. Physiol. 2021, 21, 23–28. [Google Scholar] [CrossRef]
- Proske, U. The mammalian muscle spindle. News Physiol. Sci. 1997, 12, 37–42. [Google Scholar] [CrossRef]
- Côté, M.-P.; Murray, L.M.; Knikou, M. Spinal control of locomotion: Individual neurons, their circuits and functions. Front. Physiol. 2018, 9, 784. [Google Scholar] [CrossRef] [PubMed]
- De-Doncker, L.; Picquet, F.; Petit, J.; Falempin, M. Characterization of spindle afferents in rat soleus muscle using ramp-and-hold and sinusoidal stretches. J. Neurophysiol. 2003, 89, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, M. Human muscle spindle sensitivity reflects the balance of activity between antagonistic muscles. J. Neurosci. 2014, 34, 13644–13655. [Google Scholar] [CrossRef]
- Baethge, C.; Goldbeck-Wood, S.; Mertens, S. SANRA-a scale for the quality assessment of narrative review articles. Res. Integr. Peer Rev. 2019, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Kokkorogiannis, T. Somatic and intramuscular distribution of muscle spindles and their relation to muscular angiotypes. J. Theor. Biol. 2004, 229, 263–280. [Google Scholar] [CrossRef] [PubMed]
- Kissane, R.W.P.; Charles, J.P.; Banks, R.W.; Bates, K.T. The association between muscle architecture and muscle spindle abundance. Sci. Rep. 2023, 13, 2830. [Google Scholar] [CrossRef] [PubMed]
- Papaioannou, S.; Dimitriou, M. Muscle spindle function in muscular dystrophy: A potential target for therapeutic intervention. J. Physiol. 2020, 598, 1433–1434. [Google Scholar] [CrossRef]
- Wilkinson, D.J.; Piasecki, M.; Atherton, P.J. The age-related loss of skeletal muscle mass and function: Measurement and physiology of muscle fibre atrophy and muscle fibre loss in humans. Ageing Res. Rev. 2018, 47, 123–132. [Google Scholar] [CrossRef]
- Abuel-Atta, A.A.; DeSantis, M.; Wong, A. Encapsulated Sensory Receptors within Intraorbital Skeletal Muscles of a Camel. Anat. Rec. 1997, 247, 189–198. [Google Scholar] [CrossRef]
- Lian, W.; Hao, F.; Hao, P.; Zhao, W.; Gao, Y.; Rao, J.-S.; Duan, H.; Yang, Z.; Li, X. Distribution Heterogeneity of Muscle Spindles Across Skeletal Muscles of Lower Extremities in C57BL/6 Mice. Front. Neuroanat. 2022, 16, 838951. [Google Scholar] [CrossRef]
- Valdez, G. Effects of disease-afflicted and aging neurons on the musculoskeletal system. Bone 2019, 122, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Slater, C.R. The Structure of Human Neuromuscular Junctions: Some Unanswered Molecular Questions. Int. J. Mol. Sci. 2017, 18, 2183. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.I.; Ovalle, W.K. A Comparative Study of Muscle Spindles in Slow and Fast Neonatal Muscles of Normal and Dystrophic Mice. Am. J. Anat. 1986, 175, 413–427. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, V.; Chandy, M.J.; Babu, K.S. Quantitative Study of Muscle Spindles in Suboccipital Muscles of Human Foetuses. Neurol. India 2001, 49, 355–359. [Google Scholar] [PubMed]
- Patten, R.M.; Ovalle, W.K. Morphometry and Histoenzymology of the Hamster Tenuissimus and Its Muscle Spindles. Anat. Rec. 1992, 232, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Watkins, B.; Schultheiß, J.; Rafuna, A.; Hintze, S.; Meinke, P.; Schoser, B.; Kröger, S. Degeneration of muscle spindles in a murine model of Pompe disease. Sci. Rep. 2023, 13, 6555. [Google Scholar] [CrossRef] [PubMed]
- Pavan, P.; Monti, E.; Bondí, M.; Fan, C.; Stecco, C.; Narici, M.; Reggiani, C.; Marcucci, L. Alterations of Extracellular Matrix Mechanical Properties Contribute to Age-Related Functional Impairment of Human Skeletal Muscles. Int. J. Mol. Sci. 2020, 21, 3992. [Google Scholar] [CrossRef] [PubMed]
- Giuriati, W.; Ravara, B.; Porzionato, A.; Albertin, G.; Stecco, C.; Macchi, V.; De Caro, R.; Martinello, T.; Gomiero, C.; Patruno, M.; et al. Muscle spindles of the rat sternomastoid muscle. Eur. J. Transl. Myol. 2018, 28, 7904. [Google Scholar] [CrossRef]
- James, G.; Stecco, C.; Blomster, L.; Hall, L.; Schmid, A.B.; Shu, C.C.; Little, C.B.; Melrose, J.; Hodges, P.W. Muscle spindles of the multifidus muscle undergo structural change after intervertebral disc degeneration. Eur. Spine J. 2022, 31, 1879–1888. [Google Scholar] [CrossRef]
- Kubota, K.; Masegi, T. Muscle Spindle Distribution in the Masticatory Muscle of the Japanese Shrew-Mole. J. Dent. Res. 1972, 51, 1080–1091. [Google Scholar] [CrossRef]
- Lund, J.; Richmond, F.J.; Touloumis, C.; Patry, Y.; Lamarre, Y. The Distribution of Golgi Tendon Organs and Muscle Spindles in Masseter and Temporalis Muscles of the Cat. Neuroscience 1978, 3, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Sciote, J.J. Fibre Type Distribution in the Muscle Spindles of Cat Jaw-Elevator Muscles. Arch. Oral Biol. 1993, 38, 685–688. [Google Scholar] [CrossRef] [PubMed]
- May, A.; Bramke, S.; Funk, R.H.W.; May, C.A. The Human Platysma Contains Numerous Muscle Spindles. J. Anat. 2018, 232, 146–151. [Google Scholar] [CrossRef]
- May, C.A.; Mätz-Rensing, K.; Aschoff, D.; Bramke, S. Muscle spindles in the rhesus monkey platysma. J. Anat. 2022, 240, 936–940. [Google Scholar] [CrossRef]
- Gerwin, L.; Rossmanith, S.; Haupt, C.; Schultheiß, J.; Brinkmeier, H.; Bittner, R.E.; Kröger, S. Impaired muscle spindle function in murine models of muscular dystrophy. J. Physiol. 2020, 598, 1591–1609. [Google Scholar] [CrossRef] [PubMed]
- Oliver, K.M.; Florez-Paz, D.M.; Badea, T.C.; Mentis, G.Z.; Menon, V.; de Nooij, J.C. Molecular correlates of muscle spindle and Golgi tendon organ afferents. Nat. Commun. 2021, 12, 1451. [Google Scholar] [CrossRef] [PubMed]
- Iheanacho, F.; Vellipuram, A.R. Physiology, Mechanoreceptors. In Treasure Island; StatPearls Publishing: St. Petersburg, FL, USA, 2024. [Google Scholar]
- Bout, R.G.; Dubbeldam, J.L. Functional Morphological Interpretation of the Distribution of Muscle Spindles in the Jaw Muscles of the Mallard (Anas Platyrhynchos). J. Morphol. 1991, 210, 215–226. [Google Scholar] [CrossRef]
- Sato, I.; Imura, K.; Miwa, Y.; Ide, Y.; Murata, M.; Sunohara, M. Distribution of Slow Muscle Fiber of Muscle Spindle in Postnatal Rat Masseter Muscle. Okajimas Folia Anat. Jpn. 2007, 84, 99–105. [Google Scholar] [CrossRef]
- Adal, M.N.; Cheng, S.B.C. The Number, Distribution and Density of Muscle Spindles in Two Wing Muscles of the Domestic Duck. J. Anat. 1980, 131 Pt 3, 541–548. [Google Scholar]
- Amonoo-Kuofi, H.S. The Number and Distribution of Muscle Spindles in Human Intrinsic Postvertebral Muscles. J. Anat. 1982, 135 Pt 3, 585–599. [Google Scholar]
- Backenköhler, U.; Halata, Z.; Strasmann, T.J. The Sensory Innervation of the Shoulder Joint of the Mouse. Ann. Anat. Anat. Anz. Off. Organ. Anat. Ges. 1996, 178, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Ovalle, W.K.; Dow, P.R.; Nahirney, P.C. Structure, Distribution and Innervation of Muscle Spindles in Avian Fast and Slow Skeletal Muscle. J. Anat. 1999, 194 Pt 3, 381–394. [Google Scholar] [CrossRef]
- Piotr, M.; Skieresz-Szewczyk, K.; Jackowiak, H.; Celichowski, J. Distribution and Length of Muscle Spindles and Their 3D Visualisation in the Medial Gastrocnemius of Male and Female Rats. J. Anat. 2023, 243, 658–663. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Suzuki, A. Distribution, Density, and Structure of Muscle Spindles in the Vastus Intermedius and the Peroneus Longus Muscles of Sheep. Okajimas Folia Anat. Jpn. 1999, 76, 203–219. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.; Chin, N.K. The Number and Distribution of Muscle-Spindles in Certain Muscles of the Cat. J. Anat. 1960, 94 Pt 4, 473–486. [Google Scholar]
- Bhojwani, V.; Ghabriel, M.N.; Mihailidis, S.; Townsend, G.C. The Human Medial Pterygoid Muscle: Attachments and Distribution of Muscle Spindles. Clin. Anat. 2017, 30, 1064–1071. [Google Scholar] [CrossRef]
- Blumer, R.; Wasicky, R.; Brugger, P.C.; Hoetzenecker, W.; Wicke, W.L.; Lukas, J.R. Number, Distribution, and Morphologic Particularities of Encapsulated Proprioceptors in Pig Extraocular Muscles. Investig. Ophthalmol. Vis. Sci. 2001, 42, 3085–3094. [Google Scholar]
- Boyd-Clark, L.C.; Briggs, C.A.; Galea, M.P. Muscle Spindle Distribution, Morphology, and Density in Longus Colli and Multifidus Muscles of the Cervical Spine. Spine 2002, 27, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Bredman, J.J.; Weijs, W.A.; Brugman, P. Relationships between Spindle Density, Muscle Architecture and Fibre Type Composition in Different Parts of the Rabbit Masseter. Eur. J. Morphol. 1991, 29, 297–307. [Google Scholar]
- Bridgman, C.F.; Eldred, E.; Eldred, B. Distribution and Structure of Muscle Spindles in the Extensor Digitorum Brevis of the Cat. Anat. Rec. 1962, 143, 219–227. [Google Scholar] [CrossRef]
- Burhanudin, R.; McDonald, F.; Rowlerson, A. Muscle Spindles in the Jaw-Closer Muscles of the Domestic Cat. J. Anat. 1996, 188 Pt 2, 299–309. [Google Scholar]
- Eldred, E.; Yung, L.; Roy, R.R. Spindle Representation Relative to Distribution of Muscle Fiber Types in the Cat Capsularis Muscle. Acta Anat. 1997, 159, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, P.O.; Thornell, L.E. Relation to Extrafusal Fibre-Type Composition in Muscle-Spindle Structure and Location in the Human Masseter Muscle. Arch. Oral. Biol. 1987, 32, 483–491. [Google Scholar] [CrossRef]
- Gonyea, W.J.; Ericson, G.C. Morphological and Histochemical Organization of the Flexor Carpi Radialis Muscle in the Cat. Am. J. Anat. 1977, 148, 329–344. [Google Scholar] [CrossRef]
- Hermanson, J.W.; Lennard, P.R.; Takamoto, R.L. Morphology and Histochemistry of the Ambiens Muscle of the Red-Eared Turtle (Pseudemys Scripta). J. Morphol. 1986, 187, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Karlsen, K. The Location of Motor End Plates and the Distribution and Histological Structure of Muscle Spindles in Jaw Muscles of the Rat. Acta Odontol. Scand. 1965, 23, 521–547. [Google Scholar] [CrossRef]
- Kubota, K.; Masegi, T. Muscle Spindle Distribution in Snout Musculature of the Japanese Shrew-Mole. Anat. Rec. 1972, 172, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Kubota, K.; Masegi, T. Proprioceptive Innervation of the Masticatory Muscles in Pinché. J. Dent. Res. 1975, 54, 788–796. [Google Scholar] [CrossRef]
- Kubota, K.; Masegi, T.; Osanai, K. Muscle Spindle Distribution in the Masticatory Muscle of the Squirrel Monkey (Saimiri Sciurea). Bull. Tokyo Med. Dent. Univ. 1973, 20, 275–286. [Google Scholar] [PubMed]
- Kubota, K.; Masegi, T.; Quanbunchan, K. Muscle Spindle Distribution in the Masticatory Muscle of the Tree Shrew. J. Dent. Res. 1974, 53, 538–546. [Google Scholar] [CrossRef]
- Lennartsson, B. Number and Distribution of Muscle Spindles in the Masticatory Muscles of the Rat. J. Anat. 1980, 130 Pt 2, 279–288. [Google Scholar]
- Lukas, J.R.; Aigner, M.; Blumer, R.; Heinzl, H.; Mayr, R. Number and Distribution of Neuromuscular Spindles in Human Extraocular Muscles. Investig. Ophthalmol. Vis. Sci. 1994, 35, 4317–4327. [Google Scholar]
- Maass, S.; Baumann, K.I.; Halata, Z. Topography of Muscle Spindles and Golgi Tendon Organs in Shoulder Muscles of “Monodelphis Domestica”. Ann. Anat. Anat. Anz. Off. Organ. Anat. Ges. 2001, 183, 237–242. [Google Scholar] [CrossRef]
- Maier, A. Occurrence and Distribution of Muscle Spindles in Masticatory and Suprahyoid Muscles of the Rat. Am. J. Anat. 1979, 155, 483–505. [Google Scholar] [CrossRef] [PubMed]
- Maier, A.; Simpson, D.R.; Edgerton, V.R. Histological and Histochemical Comparisons of Muscle Spindles in Three Hind Limb Muscles of the Guinea Pig. J. Morphol. 1976, 148, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, A.; Saito, K. Distribution of Muscle Spindles in the Extensor Digitorum and Hallucis Brevis Muscles of the Macaque as Determined by Plastination. Acta Anat. 1997, 158, 59–67. [Google Scholar] [CrossRef]
- Odagiri, N.; Kubota, K.; Shibanai, S. Density of Muscle Spindles in the Jaw Muscles of the Japanese Flying Squirrel and the Guinea Pig. Ann. Anat. Anat. Anz. Off. Organ. Anat. Ges. 1993, 175, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Zenker, W.; Sandoz, P.A.; Neuhuber, W. The Distribution of Anterogradely Labeled I--IV Primary Afferents in Histochemically Defined Compartments of the Rats Sternomastoid Muscle. Anat. Embryol. 1988, 177, 235–243. [Google Scholar] [CrossRef]
- Takahashi, Y.; Ohmichi, Y.; Lee, P.A.L.M.; Naito, M.; Nakano, T.; Kakizaki, H. Muscle Spindles in the Levator Palpebrae Superioris Muscle of Human Adults. J. Craniofacial Surg. 2021, 32, 1532–1534. [Google Scholar] [CrossRef]
- Smith, K.K. Histological Demonstration of Muscle Spindles in the Tongue of the Rat. Arch. Oral. Biol. 1989, 34, 529–534. [Google Scholar] [CrossRef]
- Scott, J.J.; Young, H. The Number and Distribution of Muscle Spindles and Tendon Organs in the Peroneal Muscles of the Cat. J. Anat. 1987, 151, 143–155. [Google Scholar] [PubMed]
- Sanders, I.; Han, Y.; Wang, J.; Biller, H. Muscle Spindles Are Concentrated in the Superior Vocalis Subcompartment of the Human Thyroarytenoid Muscle. J. Voice Off. J. Voice Found. 1998, 12, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Saigusa, H.; Yamashita, K.; Tanuma, K.; Saigusa, M.; Niimi, S. Morphological Studies for Retrusive Movement of the Human Adult Tongue. Clin. Anat. 2004, 17, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Sahinen, F.M.; Kennedy, W.R. Distribution of Muscle Spindles in the Human First Dorsal Interosseus. Anat. Rec. 1972, 173, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Richmond, F.J.R.; Stuart, D.G. Distribution of Sensory Receptors in the Flexor Carpi Radialis Muscle of the Cat. J. Morphol. 1985, 183, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Richmond, F.J.R.; Singh, K.; Corneil, B.D. Marked Non-Uniformity of Fiber-Type Composition in the Primate Suboccipital Muscle Obliquus Capitis Inferior. Exp. Brain Res. 1999, 125, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Duron, B.; Jung-Caillol, M.C.; Marlot, D. Myelinated Nerve Fiber Supply and Muscle Spindles in the Respiratory Muscles of Cat: Quantitative Study. Anat. Embryol. 1978, 152, 171–192. [Google Scholar] [CrossRef]
- Rokx, J.T.; van Willigen, J.D.; Jansen, H.W. Muscle Fibre Types and Muscle Spindles in the Jaw Musculature of the Rat. Arch. Oral. Biol. 1984, 29, 25–31. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Fede, C.; Zhao, X.; Del Felice, A.; Pirri, C.; Stecco, C. Quantity and Distribution of Muscle Spindles in Animal and Human Muscles. Int. J. Mol. Sci. 2024, 25, 7320. https://doi.org/10.3390/ijms25137320
Sun Y, Fede C, Zhao X, Del Felice A, Pirri C, Stecco C. Quantity and Distribution of Muscle Spindles in Animal and Human Muscles. International Journal of Molecular Sciences. 2024; 25(13):7320. https://doi.org/10.3390/ijms25137320
Chicago/Turabian StyleSun, Yunfeng, Caterina Fede, Xiaoxiao Zhao, Alessandra Del Felice, Carmelo Pirri, and Carla Stecco. 2024. "Quantity and Distribution of Muscle Spindles in Animal and Human Muscles" International Journal of Molecular Sciences 25, no. 13: 7320. https://doi.org/10.3390/ijms25137320





