Bitter Taste Receptor 46 (hTAS2R46) Protects Monocytes/Macrophages from Oxidative Stress
Abstract
:1. Introduction
2. Results
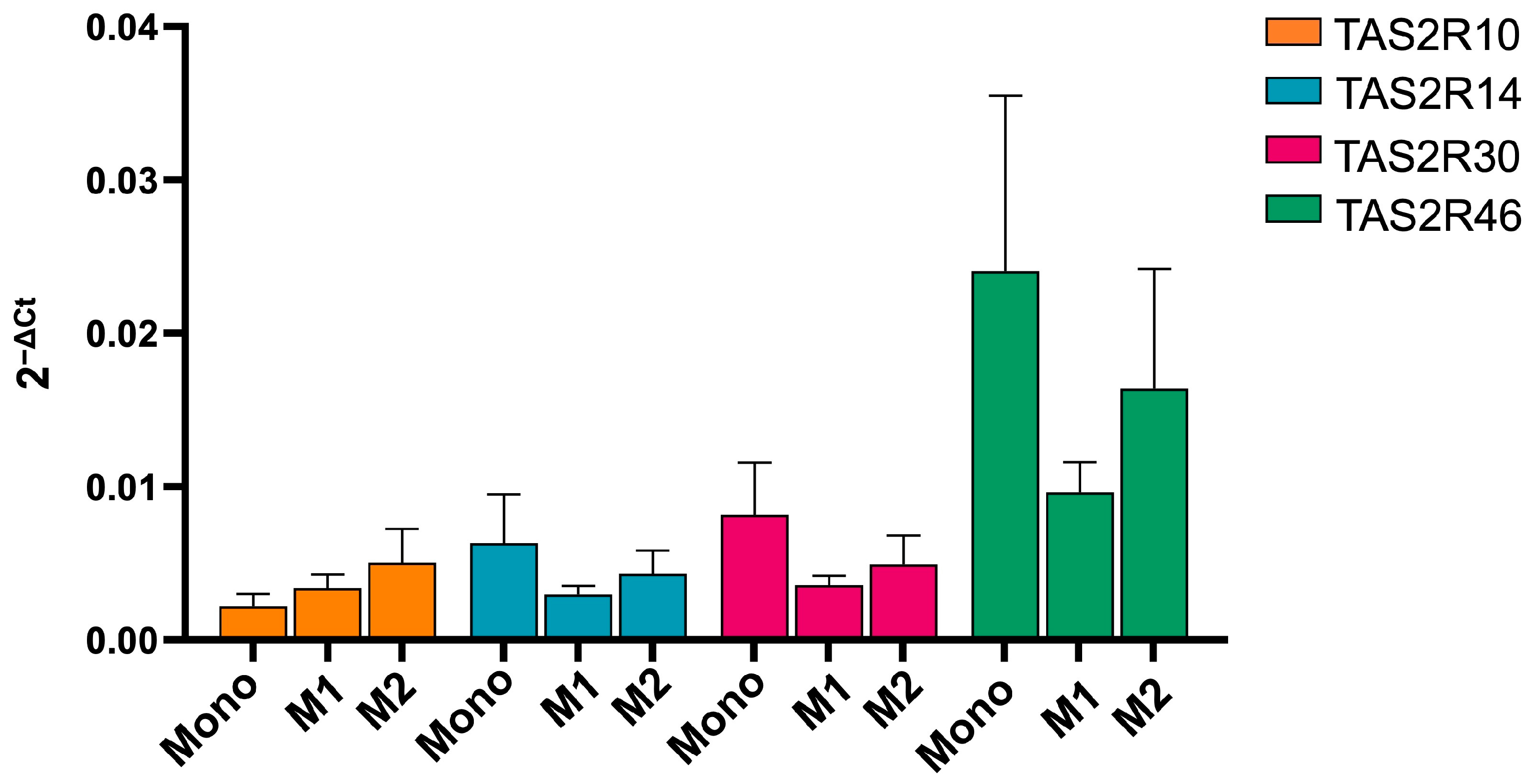
2.1. hTAS2R46 Is Expressed Differently in Monocytes and Macrophages
2.2. Absinthin Modulates the Cell Phenotype
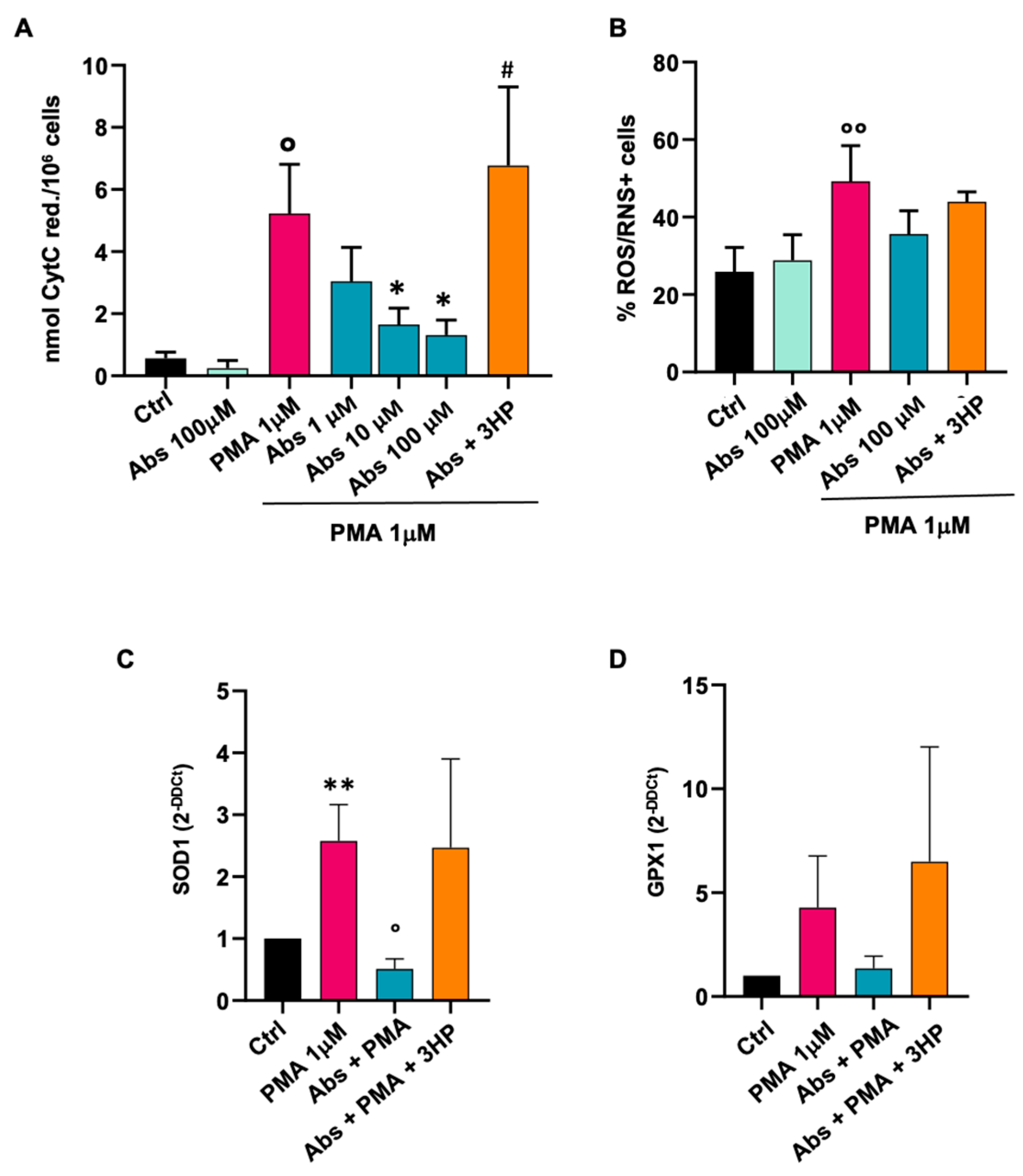
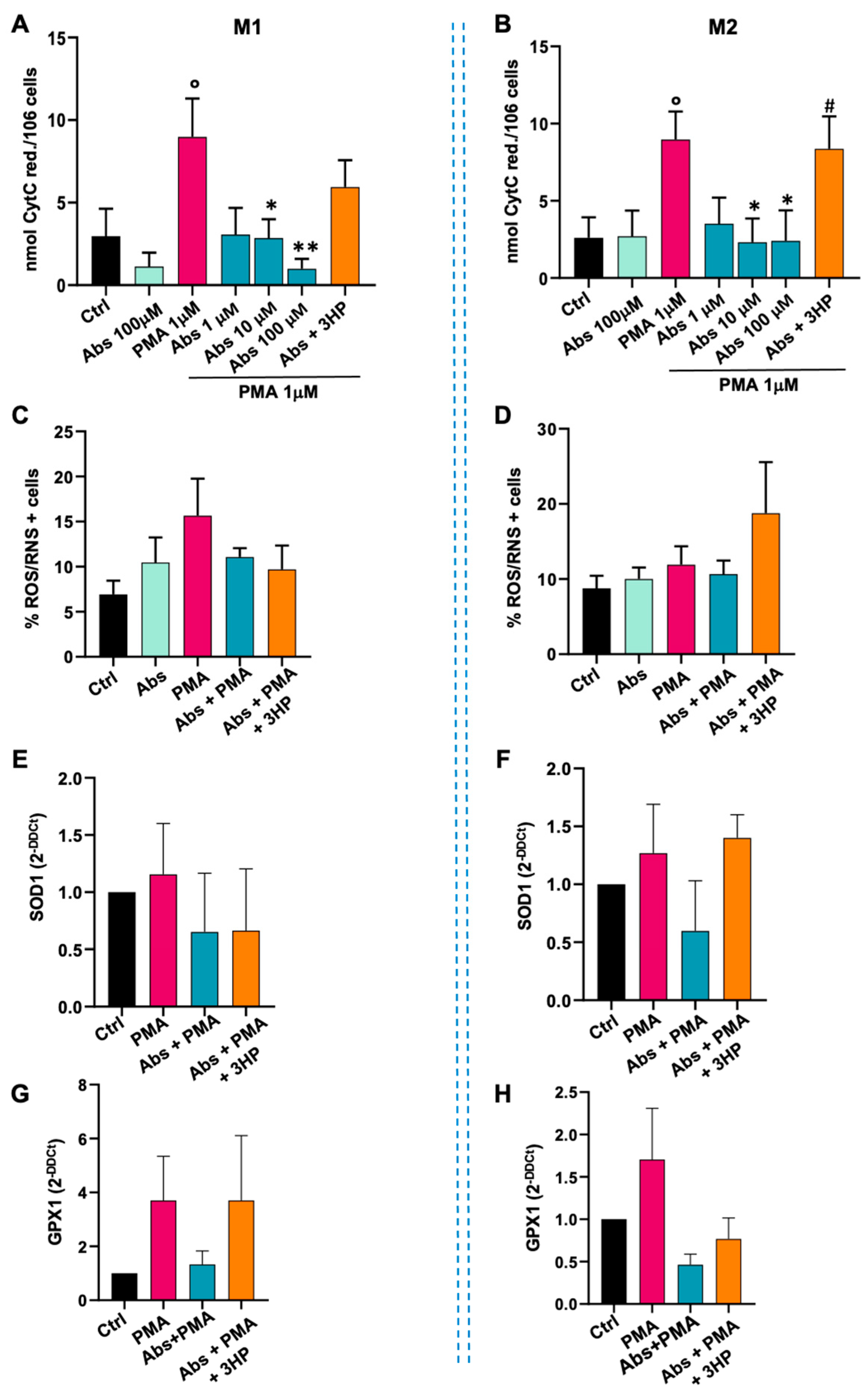
2.3. Absinthin Reduced the PMA-Induced Oxidative Burst in Monocytes and Macrophages
2.3.1. Effect of Absinthin on Monocytes
2.3.2. Effect of Absinthin on M1 and M2 Macrophages
2.3.3. Effect of Absinthin on Monocyte Differentiation
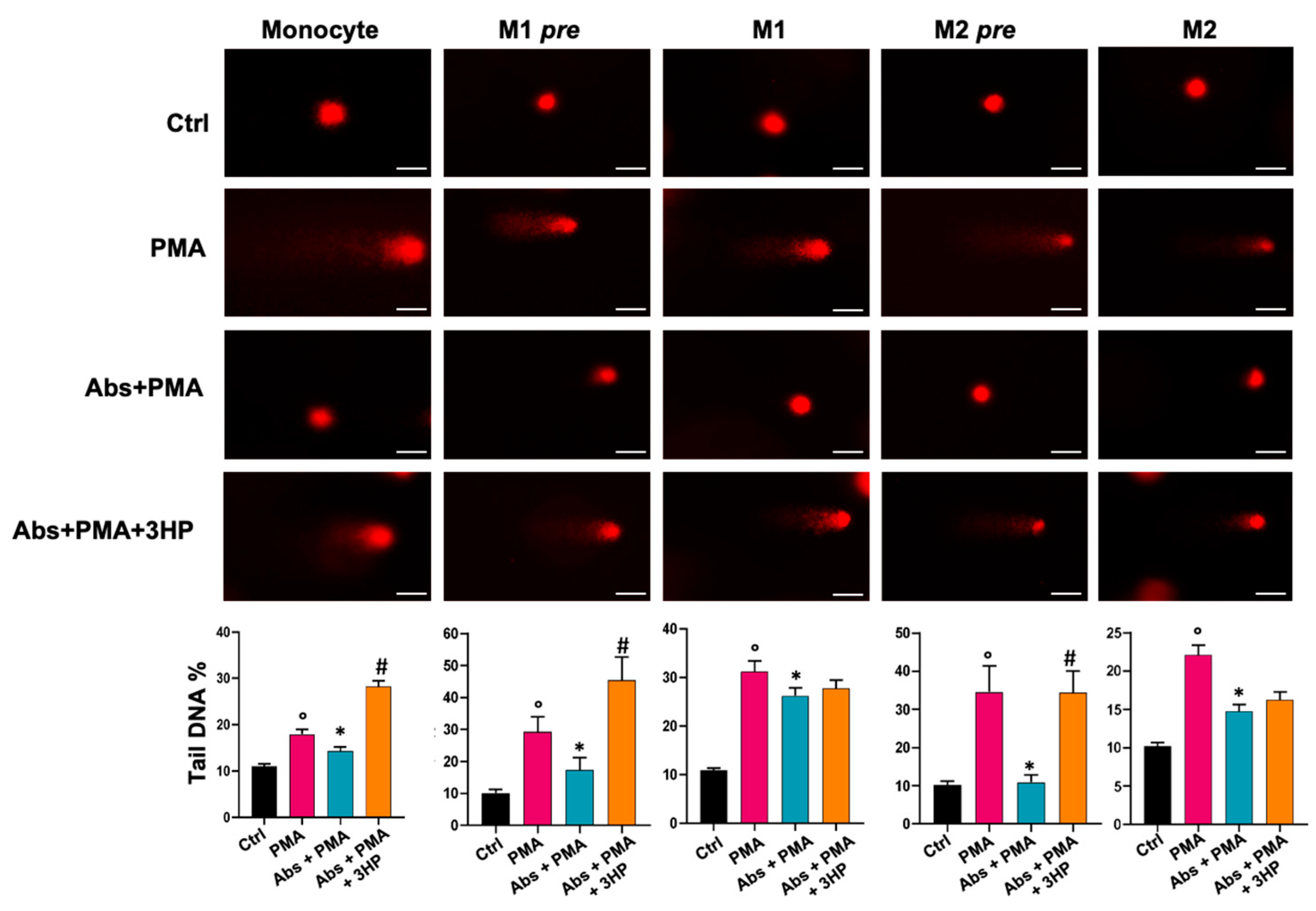
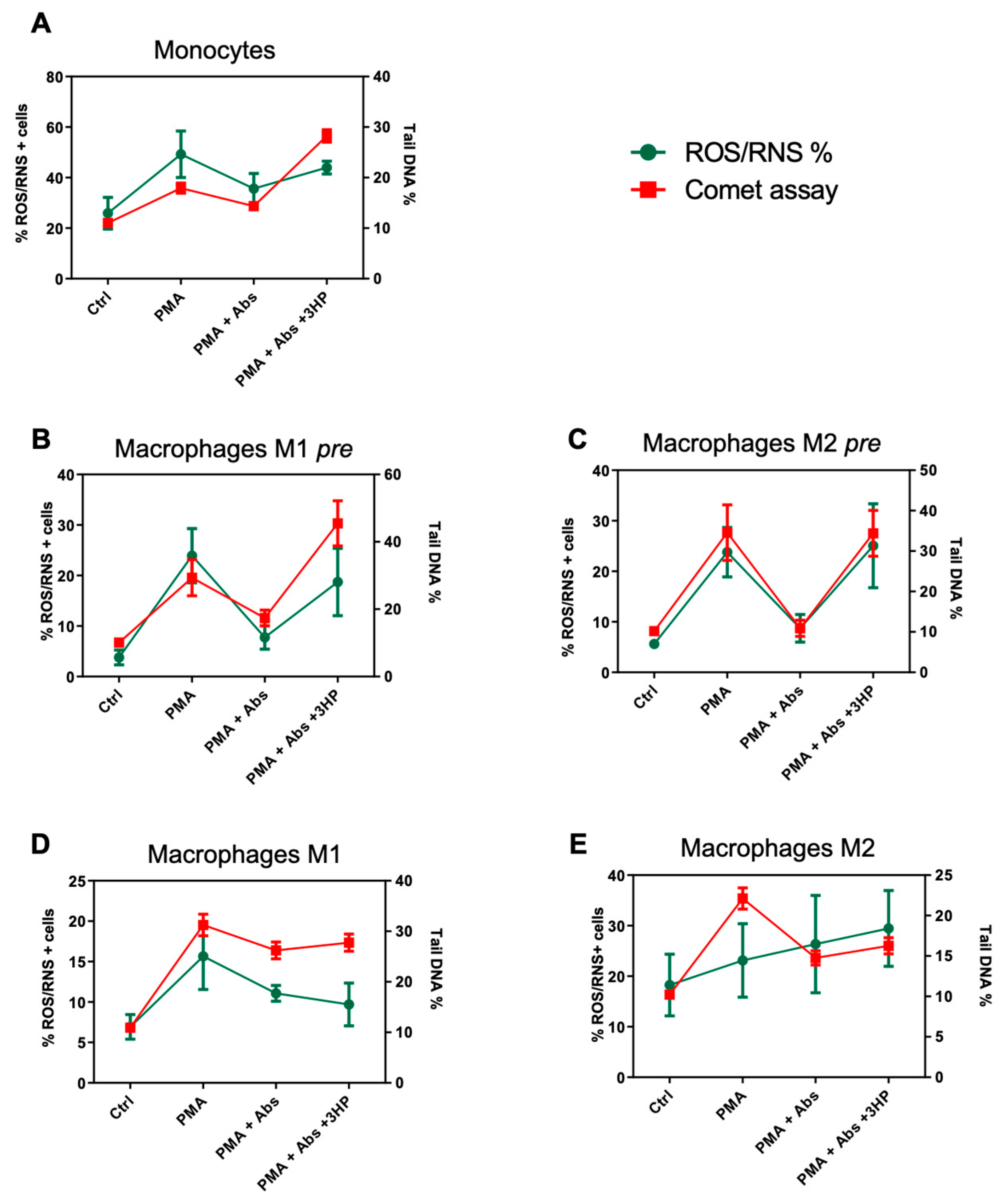
2.4. Absinthin Protects Cells from DNA Damage
2.5. The Effect of Absinthin on ROS/RNS Correlates with DNA Repair
3. Discussion
4. Materials and Methods
4.1. Monocytes’ Isolation and Differentiation
4.2. Flow Cytometry Analysis
4.3. Analysis of Reactive Oxygen and Nitrogen Species (ROS/RNS) Production
4.4. Quantitative Real Time-PCR
4.5. Immunofluorescence Assay
4.6. Single-Cell Gel Electrophoresis
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Behrens, M.; Lang, T. Extra-Oral Taste Receptors—Function, Disease, and Perspectives. Front. Nutr. 2022, 9, 881177. [Google Scholar] [CrossRef] [PubMed]
- Talmon, M.; Pollastro, F.; Fresu, L.G. The Complex Journey of the Calcium Regulation Downstream of TAS2R Activation. Cells 2022, 11, 3638. [Google Scholar] [CrossRef] [PubMed]
- Welcome, M.O.; Dogo, D.; Nikos, E. Mastorakis Cellular Mechanisms and Molecular Pathways Linking Bitter Taste Receptor Signalling to Cardiac Inflammation, Oxidative Stress, Arrhythmia and Contractile Dysfunction in Heart Diseases. Inflammopharmacology 2023, 31, 89–117. [Google Scholar] [CrossRef] [PubMed]
- Foster, S.R.; Blank, K.; Hoe, L.E.S.; Behrens, M.; Meyerhof, W.; Peart, J.N.; Thomas, W.G. Bitter Taste Receptor Agonists Elicit G-protein-dependent Negative Inotropy in the Murine Heart. FASEB J. 2014, 28, 4497–4508. [Google Scholar] [CrossRef]
- Kim, K.-S.; Egan, J.M.; Jang, H.-J. Denatonium Induces Secretion of Glucagon-like Peptide-1 through Activation of Bitter Taste Receptor Pathways. Diabetologia 2014, 57, 2117–2125. [Google Scholar] [CrossRef] [PubMed]
- Iven, J.; Biesiekierski, J.R.; Zhao, D.; Deloose, E.; O’Daly, O.G.; Depoortere, I.; Tack, J.; Van Oudenhove, L. Intragastric Quinine Administration Decreases Hedonic Eating in Healthy Women through Peptide-Mediated Gut-Brain Signaling Mechanisms. Nutr. Neurosci. 2019, 22, 850–862. [Google Scholar] [CrossRef] [PubMed]
- Feng, P.; Chai, J.; Yi, H.; Redding, K.; Margolskee, R.F.; Huang, L.; Wang, H. Aggravated Gut Inflammation in Mice Lacking the Taste Signaling Protein α-Gustducin. Brain. Behav. Immun. 2018, 71, 23–27. [Google Scholar] [CrossRef] [PubMed]
- An, S.S.; Liggett, S.B. Taste and Smell GPCRs in the Lung: Evidence for a Previously Unrecognized Widespread Chemosensory System. Cell. Signal. 2018, 41, 82–88. [Google Scholar] [CrossRef]
- Talmon, M.; Bosso, L.; Quaregna, M.; Lopatriello, A.; Rossi, S.; Gavioli, D.; Marotta, P.; Caprioglio, D.; Boldorini, R.; Miggiano, R.; et al. Correction to Anti-Inflammatory Activity of Absinthin and Derivatives in Human Broncho-Epithelial Cells. J. Nat. Prod. 2020, 83, 2815–2816. [Google Scholar] [CrossRef]
- Sharma, P.; Conaway, S.; Deshpande, D. Bitter Taste Receptors in the Airway Cells Functions. In The Pharmacology of Taste; Palmer, R.K., Servant, G., Eds.; Handbook of Experimental Pharmacology; Springer International Publishing: Cham, Switzerland, 2021; Volume 275, pp. 203–227. ISBN 978-3-031-06449-4. [Google Scholar]
- Talmon, M.; Rossi, S.; Lim, D.; Pollastro, F.; Palattella, G.; Ruffinatti, F.A.; Marotta, P.; Boldorini, R.; Genazzani, A.A.; Fresu, L.G. Absinthin, an Agonist of the Bitter Taste Receptor hTAS2R46, Uncovers an ER-to-Mitochondria Ca2+–Shuttling Event. J. Biol. Chem. 2019, 294, 12472–12482. [Google Scholar] [CrossRef]
- Welcome, M.O.; Mastorakis, N.E. The Taste of Neuroinflammation: Molecular Mechanisms Linking Taste Sensing to Neuroinflammatory Responses. Pharmacol. Res. 2021, 167, 105557. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Vrontakis, M.; Parkinson, F.; Chelikani, P. Functional Bitter Taste Receptors Are Expressed in Brain Cells. Biochem. Biophys. Res. Commun. 2011, 406, 146–151. [Google Scholar] [CrossRef]
- Austermann, J.; Roth, J.; Barczyk-Kahlert, K. The Good and the Bad: Monocytes’ and Macrophages’ Diverse Functions in Inflammation. Cells 2022, 11, 1979. [Google Scholar] [CrossRef] [PubMed]
- Ginhoux, F.; Jung, S. Monocytes and Macrophages: Developmental Pathways and Tissue Homeostasis. Nat. Rev. Immunol. 2014, 14, 392–404. [Google Scholar] [CrossRef] [PubMed]
- Jakubzick, C.V.; Randolph, G.J.; Henson, P.M. Monocyte Differentiation and Antigen-Presenting Functions. Nat. Rev. Immunol. 2017, 17, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Locati, M.; Mantovani, A.; Sica, A. Macrophage Activation and Polarization as an Adaptive Component of Innate Immunity. In Advances in Immunology; Elsevier: Amsterdam, The Netherlands, 2013; Volume 120, pp. 163–184. ISBN 978-0-12-417028-5. [Google Scholar]
- Yang, J.; Zhang, L.; Yu, C.; Yang, X.-F.; Wang, H. Monocyte and Macrophage Differentiation: Circulation Inflammatory Monocyte as Biomarker for Inflammatory Diseases. Biomark. Res. 2014, 2, 1. [Google Scholar] [CrossRef] [PubMed]
- Maurer, S.; Wabnitz, G.H.; Kahle, N.A.; Stegmaier, S.; Prior, B.; Giese, T.; Gaida, M.M.; Samstag, Y.; Hänsch, G.M. Tasting Pseudomonas Aeruginosa Biofilms: Human Neutrophils Express the Bitter Receptor T2R38 as Sensor for the Quorum Sensing Molecule N-(3-Oxododecanoyl)-l-Homoserine Lactone. Front. Immunol. 2015, 6, 369. [Google Scholar] [CrossRef] [PubMed]
- Gopallawa, I.; Freund, J.R.; Lee, R.J. Bitter Taste Receptors Stimulate Phagocytosis in Human Macrophages through Calcium, Nitric Oxide, and Cyclic-GMP Signaling. Cell. Mol. Life Sci. 2021, 78, 271–286. [Google Scholar] [CrossRef]
- Lee, R.J.; Cohen, N.A. Taste Receptors in Innate Immunity. Cell. Mol. Life Sci. 2015, 72, 217–236. [Google Scholar] [CrossRef]
- Guilliams, M.; Mildner, A.; Yona, S. Developmental and Functional Heterogeneity of Monocytes. Immunity 2018, 49, 595–613. [Google Scholar] [CrossRef]
- Talmon, M.; Percio, M.; Obeng, J.A.; Ruffinatti, F.A.; Sola, D.; Sainaghi, P.P.; Bellis, E.; Cusinato, S.; Ianniello, A.; Fresu, L.G. Transcriptomic Profile Comparison of Monocytes from Rheumatoid Arthritis Patients in Treatment with Methotrexate, Anti-TNFa, Abatacept or Tocilizumab. PLoS ONE 2023, 18, e0282564. [Google Scholar] [CrossRef] [PubMed]
- Trzebanski, S.; Jung, S. Plasticity of Monocyte Development and Monocyte Fates. Immunol. Lett. 2020, 227, 66–78. [Google Scholar] [CrossRef] [PubMed]
- Rigamonti, A.; Villar, J.; Segura, E. Monocyte Differentiation within Tissues: A Renewed Outlook. Trends Immunol. 2023, 44, 999–1013. [Google Scholar] [CrossRef] [PubMed]
- Xi, R.; Zheng, X.; Tizzano, M. Role of Taste Receptors in Innate Immunity and Oral Health. J. Dent. Res. 2022, 101, 759–768. [Google Scholar] [CrossRef]
- Yasutomi, M.; Ohshima, Y.; Omata, N.; Yamada, A.; Iwasaki, H.; Urasaki, Y.; Mayumi, M. Erythromycin Differentially Inhibits Lipopolysaccharide- or Poly(I:C)-Induced but Not Peptidoglycan-Induced Activation of Human Monocyte-Derived Dendritic Cells1. J. Immunol. 2005, 175, 8069–8076. [Google Scholar] [CrossRef]
- Chaterjee, O.; Sur, D. Artificially Induced in Situ Macrophage Polarization: An Emerging Cellular Therapy for Immuno-Inflammatory Diseases. Eur. J. Pharmacol. 2023, 957, 176006. [Google Scholar] [CrossRef]
- Ayuso, P.; García-Martín, E.; Agúndez, J.A.G. Variability of the Genes Involved in the Cellular Redox Status and Their Implication in Drug Hypersensitivity Reactions. Antioxidants 2021, 10, 294. [Google Scholar] [CrossRef]
- Pei, J.; Pan, X.; Wei, G.; Hua, Y. Research Progress of Glutathione Peroxidase Family (GPX) in Redoxidation. Front. Pharmacol. 2023, 14, 1147414. [Google Scholar] [CrossRef]
- Brockhoff, A.; Behrens, M.; Roudnitzky, N.; Appendino, G.; Avonto, C.; Meyerhof, W. Receptor Agonism and Antagonism of Dietary Bitter Compounds. J. Neurosci. 2011, 31, 14775–14782. [Google Scholar] [CrossRef] [PubMed]
- Bora, K.S.; Sharma, A. Evaluation of Antioxidant and Free-Radical Scavenging Potential of Artemisia absinthium. Pharm. Biol. 2011, 49, 1216–1223. [Google Scholar] [CrossRef]
- Choodej, S.; Pudhom, K.; Mitsunaga, T. Inhibition of TNF-α-Induced Inflammation by Sesquiterpene Lactones from Saussurea Lappa and Semi-Synthetic Analogues. Planta Med. 2018, 84, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Briegert, M.; Kaina, B. Human Monocytes, but Not Dendritic Cells Derived from Them, Are Defective in Base Excision Repair and Hypersensitive to Methylating Agents. Cancer Res. 2007, 67, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.; Goldstein, M.; Christmann, M.; Becker, H.; Heylmann, D.; Kaina, B. Human Monocytes Are Severely Impaired in Base and DNA Double-Strand Break Repair That Renders Them Vulnerable to Oxidative Stress. Proc. Natl. Acad. Sci. USA 2011, 108, 21105–21110. [Google Scholar] [CrossRef] [PubMed]
- Ponath, V.; Kaina, B. Death of Monocytes through Oxidative Burst of Macrophages and Neutrophils: Killing in Trans. PLoS ONE 2017, 12, e0170347. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, S.; Wagner, C.; Müller, W.; Brenner-Weiss, G.; Hug, F.; Prior, B.; Obst, U.; Hänsch, G.M. Induction of Neutrophil Chemotaxis by the Quorum-Sensing Molecule N-(3-Oxododecanoyl)-l-Homoserine Lactone. Infect. Immun. 2006, 74, 5687–5692. [Google Scholar] [CrossRef] [PubMed]
- Tateda, K.; Ishii, Y.; Horikawa, M.; Matsumoto, T.; Miyairi, S.; Pechere, J.C.; Standiford, T.J.; Ishiguro, M.; Yamaguchi, K. The Pseudomonas aeruginosa Autoinducer N-3-Oxododecanoyl Homoserine Lactone Accelerates Apoptosis in Macrophages and Neutrophils. Infect. Immun. 2003, 71, 5785–5793. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, A.J.; Jansson, A.; Stallberg, J.; Nilsson, P.; Lysaght, P.; Cooley, M.A. The Pseudomonas aeruginosa Quorum-Sensing Molecule N-3-(Oxododecanoyl)-l-Homoserine Lactone Inhibits T-Cell Differentiation and Cytokine Production by a Mechanism Involving an Early Step in T-Cell Activation. Infect. Immun. 2005, 73, 1648–1655. [Google Scholar] [CrossRef] [PubMed]
- Tizzano, M.; Gulbransen, B.D.; Vandenbeuch, A.; Clapp, T.R.; Herman, J.P.; Sibhatu, H.M.; Churchill, M.E.A.; Silver, W.L.; Kinnamon, S.C.; Finger, T.E. Nasal Chemosensory Cells Use Bitter Taste Signaling to Detect Irritants and Bacterial Signals. Proc. Natl. Acad. Sci. USA 2010, 107, 3210–3215. [Google Scholar] [CrossRef]
- Talmon, M.; Rossi, S.; Pastore, A.; Cattaneo, C.I.; Brunelleschi, S.; Fresu, L.G. Vortioxetine Exerts Anti-inflammatory and Immunomodulatory Effects on Human Monocytes/Macrophages. Br. J. Pharmacol. 2018, 175, 113–124. [Google Scholar] [CrossRef]
- Clementi, E.; Garajova, Z.; Markkanen, E. Measuring DNA Damage Using the Alkaline Comet Assay in Cultured Cells. Bio-Protoc. 2021, 11, e4119. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Talmon, M.; Camillo, L.; Vietti, I.; Pollastro, F.; Fresu, L.G. Bitter Taste Receptor 46 (hTAS2R46) Protects Monocytes/Macrophages from Oxidative Stress. Int. J. Mol. Sci. 2024, 25, 7325. https://doi.org/10.3390/ijms25137325
Talmon M, Camillo L, Vietti I, Pollastro F, Fresu LG. Bitter Taste Receptor 46 (hTAS2R46) Protects Monocytes/Macrophages from Oxidative Stress. International Journal of Molecular Sciences. 2024; 25(13):7325. https://doi.org/10.3390/ijms25137325
Chicago/Turabian StyleTalmon, Maria, Lara Camillo, Ilaria Vietti, Federica Pollastro, and Luigia Grazia Fresu. 2024. "Bitter Taste Receptor 46 (hTAS2R46) Protects Monocytes/Macrophages from Oxidative Stress" International Journal of Molecular Sciences 25, no. 13: 7325. https://doi.org/10.3390/ijms25137325





