Emerging Synthetic Bioluminescent Reactions for Non-Invasive Imaging of Freely Moving Animals
Abstract
:1. Introduction
2. Bioluminescence Imaging of Freely Moving Animals
3. Near-Infrared Firefly Bioluminescent Reactions
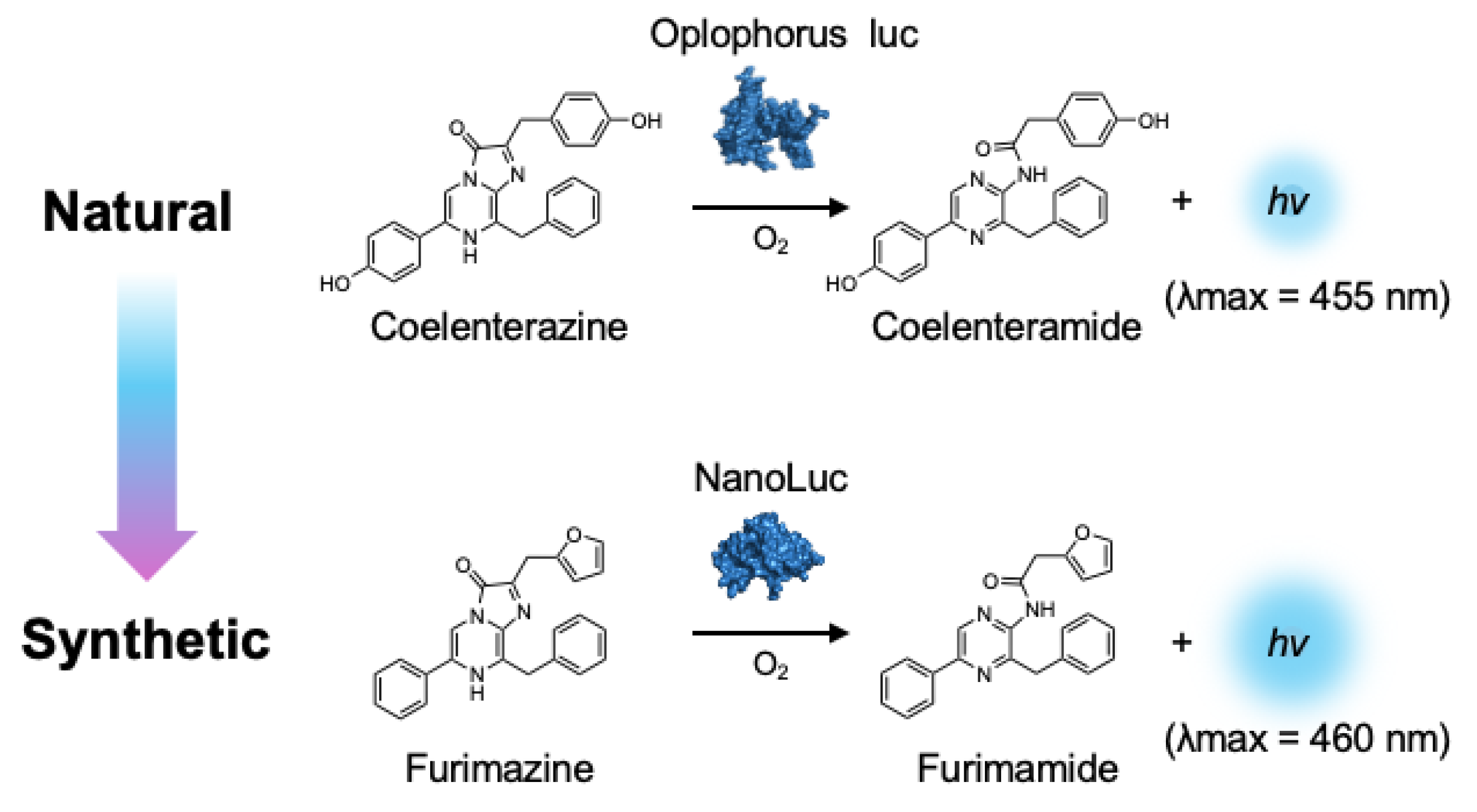
4. Bright Marine Bioluminescent Reactions
5. Bioluminescence Indicators
6. Outlook and Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Miyawaki, A. Fluorescence imaging in the last two decades. Microscopy 2013, 62, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Mezzanotte, L.; Root, M.V.; Karatas, H.; Goun, E.A.; Lowik, C.W.G.M. In Vivo Molecular Bioluminescence Imaging: New Tools and Applications. Trends Biotechnol. 2017, 35, 640–652. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.C.; Legant, W.R.; Wang, K.; Shao, L.; Kikie, D.; Davidson, M.W.; Janetopoulos, C.; Wu, X.S.; Hammer, J.A., III; Liu, Z.; et al. Lattice light-sheet microscopy: Imaging molecules to embryos at high spatiotemporal resolution. Science 2014, 346, 1257998. [Google Scholar] [CrossRef] [PubMed]
- Avci, P.; Karimi, M.; Sadasivam, M.; Antunes-Melo, W.C.; Carrasco, E.; Hamblin, M.R. In-vivo monitoring of infectious diseases in living animals using bioluminescence imaging. Virulence 2018, 9, 28–63. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Kowada, T.; Kikuta, J.; Furuya, M.; Shirazaki, M.; Mizukami, S.; Ishii, M.; Kikuchi, K. Real-time intravital imaging of pH variation associated with osteoclast activity. Nat. Chem. Biol. 2016, 12, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Takai, A.; Nakano, M.; Saito, K.; Haruno, R.; Watanabe, T.M.; Ohyanagi, T.; Jin, T.; Okada, Y.; Nagai, T. Expanded palette of Nano-lanterns for real-time multicolor luminescence imaging. Proc. Natl. Acad. Sci. USA 2015, 112, 4352–4356. [Google Scholar] [CrossRef]
- Tsien, R.Y. The geen fluorescent protein. Annu. Rev. Biochem. 1998, 67, 509–544. [Google Scholar] [CrossRef] [PubMed]
- Wet, J.R.; Wood, K.V.; Deluca, M.; Helinski, D.R.; Subramani, S. Firefly luciferase gene: Structure and expression in mammalian cells. Mol. Cell Biol. 1987, 7, 725–737. [Google Scholar]
- Kricka, L.J. Clinical and biochemical applications of luciferases and luciferins. Anal. Biochem. 1988, 175, 14–21. [Google Scholar] [CrossRef]
- Marques, S.M.; Esteves da Silva, J.C.G. Firefly bioluminescence: A mechanistic approach of luciferase catalyzed reactions. IUBMB Life 2009, 61, 6–17. [Google Scholar] [CrossRef]
- Cheong, W.F.; Prahl, S.A.; Welch, A.J. A review of the optical properties of biological tissues. IEEE J. Quant. Electron. 1990, 26, 2166–2185. [Google Scholar] [CrossRef]
- Monici, M. Cell and tissue autofluorescence research and diagnostic applications. Biotechol. Annu. Rev. 2005, 11, 227–256. [Google Scholar]
- Croce, A.C.; Bottiroli, G. Autofluorescence Spectroscopy and Imaging: A Tool for Biomedical Research and Diagnosis. Eur. J. Histochem. 2014, 58, 2461. [Google Scholar] [CrossRef] [PubMed]
- Troy, T.; Jekic-McMullen, D.; Sambucetti, L.; Rice, B. Quantitative comparison of the sensitivity of detection of fluorescent and bioluminescent reporters in animal models. Mol. Imaging 2004, 3, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Contag, P.R.; Olomu, I.N.; Stevenson, D.K.; Contag, C.H. Bioluminescent indicators in living mammals. Nat. Med. 1998, 4, 245–247. [Google Scholar] [CrossRef] [PubMed]
- Bhumik, S.; Gambhir, S.S. Optical imaging of Renilla luciferase reporter gene expression in living mice. Proc. Natl. Acad. Sci. USA 2002, 99, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.; Hingtgen, S.; Kasmieh, R.; Figueiredo, J.L.; Garcia-Garcia, E.; Martinez-Serrano, A.; Breakefield, X.; Weissleder, R. Bimodal viral vectors and in vivo imaging reveal the fate of human neural stem cells in experimental glioma model. J. Neuro Sci. 2008, 28, 4406–4413. [Google Scholar]
- De Almeida, P.E.; Van Rappard, J.R.M.; Wu, J.C. In vivo bioluminescence for tracking cell fate and function. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H663–H671. [Google Scholar] [CrossRef] [PubMed]
- Coleman, S.M.; McGregor, A. A bright future for bioluminescent imaging in viral research. Future Virol. 2015, 10, 169–183. [Google Scholar] [CrossRef]
- Shimomura, O.; Johnson, F.H.; Saiga, Y. Extraction, purification and properties of aequorin, a bioluminescent protein from the luminous hydromedusan, Aequorea. J. Cell Comp. Physiol. 1962, 59, 223–239. [Google Scholar] [CrossRef]
- Shimomura, O.; Johnson, F.H. Properties of the bioluminescent protein aequorin. Biochemistry 1969, 8, 3991–3997. [Google Scholar] [CrossRef] [PubMed]
- Tricoire, L.; Tsuzuki, K.; Courjean, O.; Gibelin, N.; Bourout, G.; Rossier, J.; Lambolez, B. Calcium dependence of aequorin bioluminescence dissected by random mutagenesis. Proc. Natl. Acad. Sci. USA 2006, 103, 9500–9505. [Google Scholar] [CrossRef] [PubMed]
- Baubet, V.; Mouellic, H.L.; Campbell, A.K.; Lucas-Meunier, E.; Fossier, P.; Brúlet, P. Chimeric green fluorescent protein–aequorin as bioluminescent Ca2+ reporters at the single-cell level. Proc. Natl. Acad. Sci. USA 2000, 97, 7260–7265. [Google Scholar] [CrossRef] [PubMed]
- Naumann, E.A.; Kampff, A.R.; Prober, D.A.; Schier, A.F.; Engert, F. Monitoring neural activity with bioluminescence during natural behavior. Nat. Neurosci. 2010, 13, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Mercier, D.; Tsuchimoto, Y.; Ohta, K.; Kazama, H. Oflactory Landmark-Based Communication in Interacting Drosophila. Curr. Biol. 2018, 28, 2624–2631. [Google Scholar] [CrossRef] [PubMed]
- Marescotti, M.; Lagogiannis, K.; Webb, B.; Davies, R.W.; Armstrong, J.D. Monitoring brain activity and behaviour in freely moving Drosophila larvae using bioluminescence. Sci. Rep. 2018, 8, 9246. [Google Scholar] [CrossRef] [PubMed]
- Curie, T.; Rogers, K.L.; Colasante, C.; Brûlet, P. Red-shifted aequorin-based bioluminescent reporters for in vivo imaging of Ca2+ signaling. Mol. Imaging 2007, 6, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Bakayan, A.; Vaquero, C.F.; Picazo, F.; Llopis, J. Red fluorescent protein–aequorin fusion as improved bioluminescent Ca2+ reporters in single cells and mice. PLoS ONE 2011, 6, e19520. [Google Scholar] [CrossRef] [PubMed]
- Bakayan, A.; Domingo, B.; Miyawaki, A.; Llopis, J. Imaging Ca2+ activity in mammalian cells and zebrafish with a novel red-emitting aequorin variant. Pflugers Arch. 2015, 467, 2031–2042. [Google Scholar] [CrossRef]
- Hamada, T.; Sutherland, K.; Ishikawa, M.; Miyamoto, N.; Honma, S.; Shirato, H.; Honma, K. In vivo imaging of clock gene expression in multiple tissue of freely moving mice. Nat. Commun. 2016, 7, 11705. [Google Scholar] [CrossRef]
- Martin-Burgos, B.; Wang, W.; William, I.; Tir, I.; Mohammad, I.; Javed, R.; Smith, S.; Cui, Y.; Arzavala, J.; Mora, D.; et al. Methods for Detecting PER2: LUCIFERASE Bioluminescence Rhythms in Freely Moving Mice. J. Bio. Rhythms 2022, 37, 78–93. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.; Zavadil, J.A.; Geusz, M. Using bioluminescence to image gene expression and spontaneous behavior in freely moving mice. PLoS ONE 2023, 18, e0279875. [Google Scholar] [CrossRef] [PubMed]
- Prescher, J.A.; Contag, C.H. Guided by the light: Visualizing biomolecular processes in living animals with bioluminescence. Curr. Opin. Chem. Biol. 2010, 14, 80–89. [Google Scholar] [CrossRef]
- Weissleder, R.; Ntziachristos, V. Shedding light onto live molecular targets. Nat. Med. 2003, 9, 123–128. [Google Scholar] [CrossRef]
- Viviani, V.R.; Bechara, E.J.; Ohmiya, Y. Cloning, sequence analysis, and expression of active Phrixothrix railroad-worms luciferases: Relationship between bioluminescence spectra and primary structures. Biochemistry 1999, 38, 8271–8279. [Google Scholar] [CrossRef]
- Ogoh, K.; Akiyoshi, R.; May-Maw-Thet; Sugiyama, T.; Dosaka, S.; Hatta-Ohashi, Y.; Suzuki, H. Bioluminescence microscopy using a short focal-length imaging lens. J. Microsc. 2014, 253, 191–197. [Google Scholar] [CrossRef]
- Iwano, S.; Obata, R.; Miura, C.; Kiyama, M.; Hama, K.; Nakamura, M.; Amano, Y.; Kojima, S.; Hirano, T.; Maki, S.; et al. Development of simple firefly luciferin analogs emitting blue, green, red, and near-infrared biological window light. Tetrahedron 2013, 69, 3847–3856. [Google Scholar] [CrossRef]
- Kojima, R.; Takakura, H.; Ozawa, T.; Tada, Y.; Nagano, T.; Urano, Y. Rational design and development of near-infrared-emitting firefly luciferins available in vivo. Angew. Chem. Int. Ed. Engl. 2013, 52, 1175–1179. [Google Scholar] [CrossRef] [PubMed]
- Koide, Y.; Urano, Y.; Hanaoka, K.; Piao, W.; Kusakabe, M.; Saito, N.; Terai, T.; Okabe, T.; Nagano, T. Development of NIR fluorescent dyes based on Si-rhodamine for in vivo imaging. J. Am. Chem. Soc. 2012, 134, 5029–5031. [Google Scholar] [CrossRef]
- Jathoul, A.P.; Grounds, H.; Anderson, J.C.; Pule, M.A. A dual-color far-red to near-infrared firefly luciferin analogue designed for multiparametric bioluminescence imaging. Angew. Chem. Int. Ed. Engl. 2014, 53, 13059–13063. [Google Scholar] [CrossRef]
- Saito, R.; Kuchimaru, T.; Higashi, S.; Lu, S.W.; Kiyama, M.; Iwano, S.; Obata, R.; Hirano, T.; Kizaka-Kondoh, S.; Maki, S.A. Synthesis and Luminescence Properties of Near-Infrared N-Heterocyclic Luciferin Analogues for In Vivo Optical Imaging. Bull. Chem. Soc. Jpn. 2019, 92, 608–618. [Google Scholar] [CrossRef]
- Ikeda, Y.; Nomoto, T.; Hiruta, Y.; Nishiyama, N.; Citterio, D.I. Ring-Fused Firefly Luciferins: Expanded Palette of Near-Infrared Emitting Bioluminescent Substrates. Anal. Chem. 2020, 92, 4235–4243. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, G.; Kitada, N.; Saito-Moriya, R.; Obata, R.; Iwano, S.; Miyawaki, A.; Hirano, T.; Maki, S.A. Development of Phenyl Oligoene-type Firefly Luciferin Analogues with Extended π-Electronic Conjugation for Near-infrared Bioluminescence. Chem. Lett. 2021, 50, 1523–1525. [Google Scholar] [CrossRef]
- Hall, M.P.; Woodroofe, C.C.; Wood, M.G.; Que, I.; Rood, M.V.; Ridwan, Y.; Shi, C.; Kirkland, T.A.; Encell, L.P.; Wood, K.V.; et al. Click beetle luciferase mutant and near infrared napthyl-luciferins for improved bioluminescence imaging. Nat. Commun. 2018, 9, 132. [Google Scholar] [CrossRef] [PubMed]
- Love, A.C.; Caldwell, D.R.; Kolbaba-Kartchner, B.; Townsend, K.M.; Halbers, L.P.; Yao, Z.; Brennan, C.K.; Ivanic, J.; Hadjian, T.; Mills, J.H.; et al. Red-Shifted Coumarin Luciferins for Improved Bioluminescence Imaging. J. Am. Chem. Soc. 2023, 145, 3335–3345. [Google Scholar] [CrossRef] [PubMed]
- Iwano, S.; Sugiyama, M.; Hama, H.; Watakabe, A.; Hasegawa, N.; Kuchimaru, T.; Tanaka, K.Z.; Takahashi, M.; Ishida, Y.; Hata, J.; et al. Single-cell bioluminescence imaging of deep tissue in freely moving animals. Science 2018, 359, 935–939. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Kayamori, K.; Koide, S.; Shinoda, D.; Oshima, M.; Nakajima-Takagi, Y.; Nagai, Y.; Mimura, N.; Sakaida, E.; Yamazaki, S.; et al. Efficacy of the novel tubulin polymerization inhibitor PTC-028 for myelodysplastic syndrome. Cancer Sci. 2020, 111, 4336–4347. [Google Scholar] [CrossRef] [PubMed]
- Bozec, D.; Sattiraju, A.; Bouras, A.; Jesu Raj, J.G.; Rivera, D.; Huang, Y.; Junqueira Alves, C.; Tejero, R.; Tsankova, N.M.; Zou, H.; et al. Akaluc bioluminescence offers superior sensitivity to track in vivo glioma expansion. Neurooncol. Adv. 2020, 10, vdaa134. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, T.; Xu, H.; Iwasaki, M.; Karigane, D.; Saavedra, B.; Takahashi, Y.; Suchy, F.P.; Monobe, S.; Martin, R.M.; Ohtaka, M.; et al. Sufficiency for inducible Caspase-9 safety switch in human pluripotent stem cells and disease cells. Gene Ther. 2019, 27, 525–534. [Google Scholar] [CrossRef]
- Schuijs, M.J.; Png, S.; Richard, A.C.; Tsyben, A.; Hamm, G.; Stockis, J.; Garcia, C.; Pinaud, S.; Nicholls, A.; Ros, X.R.; et al. ILC2-driven innate immune checkpoint mechanism antagonizes NK cell antimetastatic function in the lung. Nat. Immunol. 2020, 21, 998–1009. [Google Scholar] [CrossRef]
- Ichise, H.; Tsukamoto, S.; Hirashima, T.; Konishi, Y.; Oki, C.; Tsukiji, S.; Iwano, S.; Miyawaki, A.; Sumiyama, K.; Terai, K.; et al. Functional visualization of NK cell-medicated killing of metastatic single tumor cells. Elife 2022, 11, e76269. [Google Scholar] [CrossRef] [PubMed]
- Ago, K.; Nagoshi, N.; Imaizumi, K.; Kitagawa, T.; Kawai, M.; Kajikawa, K.; Shibata, R.; Kamata, Y.; Kojima, K.; Shinozaki, M.; et al. A non-invasive system to monitor in vivo neural graft activity after spinal cord injury. Commun. Biol. 2022, 5, 803. [Google Scholar] [CrossRef] [PubMed]
- Nakashiba, T.; Ogoh, K.; Iwano, S.; Sugiyama, T.; Mizuno-Iijima, S.; Nakashima, K.; Mizuno, S.; Sugiyama, F.; Yoshiki, A.; Miyawaki, A.; et al. Development of two mouse strains conditionally expressing bright luciferases with distinct emission spectra as new tools for in vivo imaging. Lab. Anim. 2023, 52, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Matsuda, N.; Ukita, Y.; Okumura, M.; Chihara, T. Akaluc/AkaLumine bioluminescence system enables highly sensitive, non-invasive and temporal monitoring of gene expression in Drosophila. Commun. Biol. 2023, 6, 1270. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Kang, Y.; Luo, X.; Dai, S.; Shi, Y.; Li, Z.; Tang, Z.; Chen, Z.; Zhu, R.; Yang, P.; et al. Long-term in vivo chimeric cells tracking in non-human primate. Protein Cell 2024, 15, 207–222. [Google Scholar] [CrossRef] [PubMed]
- Tamura, T.; Ito, H.; Torii, S.; Wang, L.; Suzuki, R.; Tsujino, S.; Kamiyama, A.; Oda, Y.; Tsuda, M.; Morioka, Y.; et al. Akaluc bioluminescence offers superior sensitivity to track in vivo dynamics of SARS-CoV-2 infection. iScience 2024, 27, 109647. [Google Scholar] [CrossRef]
- Stowe, C.L.; Burle, T.A.; Allan, H.; Vinci, M.; Kramer-Marek, G.; Ciobota, D.M.; Parkinson, G.N.; Southworth, T.L.; Agliardi, G.; Hotblack, A.; et al. Near-infrared dual bioluminescence imaging in mouse models of cancer using infraluciferin. Elife 2019, 8, e45801. [Google Scholar] [CrossRef] [PubMed]
- Fukuchi, M.; Saito, R.; Maki, S.; Hagiwara, N.; Nakajima, Y.; Mitazaki, S.; Izumi, H.; Mori, H. Visualization of activity-regulated BDNF expression in the living mouse brain using non-invasive near-infrared bioluminescence imaging. Mol. Brain 2020, 13, 122. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, J.; Saito, R.; Hayashi, Y.; Kitada, N.; Tamaki, S.; Han, Y.; Semba, K.; Maki, S.A. High Sensitivity In Vivo Imaging of Cancer Metastasis Using a Near-infrared Luciferin Analogue seMpai. Int. J. Mol. Sci. 2020, 21, 7896. [Google Scholar] [CrossRef]
- Kuchimaru, T.; Iwano, S.; Kiyama, M.; Mitsumata, S.; Kadonosono, T.; Niwa, H.; Maki, S.; Kizaka-Kondoh, S. A luciferin analogue generating near-infrared bioluminescence achieves highly sensitive deep-tissue imaging. Nat. Commun. 2016, 7, 11856. [Google Scholar] [CrossRef]
- Hall, M.P.; Unch, J.; Binkowski, B.F.; Valley, M.P.; Butler, B.L.; Wood, M.G.; Otto, P.; Zimmerman, K.; Vidugiris, G.; Machleidt, T.; et al. Engineered luciferase reporter from a deep sea shrimp utilizing a novel imidazopyrazinone substrate. ACS Chem. Biol. 2012, 7, 1848–1857. [Google Scholar] [CrossRef] [PubMed]
- England, C.G.; Ehlerding, E.B.; Cai, W. NanoLuc: A small Luciferase Is Brightening Up the Field of Bioluminescence. Bioconjug Chem. 2016, 27, 1175–1187. [Google Scholar] [CrossRef] [PubMed]
- Inouye, S.; Watanabe, K.; Nakamura, H.; Shimomura, O. Secretional luciferase of the luminous shrimp Oplophorus gracilirostris: cDNA cloning of a novel imidazopyrazinone luciferase(1). FEBS Lett. 2000, 481, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Inouye, S.; Sasaki, S. Overexpression, purification and characterization of the catalytic component of Oplophorus luciferase in the deep-sea shrimp, Oplophorus gracilirostris. Protein Expr. Purif. 2007, 56, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.A.; Lazarev, S.; Gervasi, C.; Cowan, C.; Machleidt, T.; Ohana, R.F. Luciferase Calibrants Enable Absolute Quantitation of Bioluminescence Power. ACS Meas. Sci. Au 2023, 3, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Nemergut, M.; Pluskal, D.; Horackova, J.; Sustrova, T.; Tulis, J.; Barta, T.; Baatallah, R.; Gagnot, G.; Novakova, V.; Majerova, M.; et al. Illuminating the mechanism and allosteric behavior of NanoLuc luciferase. Nat. Commun. 2023, 14, 7864. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Kimura, T.; Shinoda, H.; Bai, G.; Daniels, M.J.; Arai, Y.; Nakano, M.; Nagai, T. Five colour variants of bright luminescent protein for real-time multicolour bioimaging. Nat. Commun. 2016, 14, 13718. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.; Oh, Y.; Sens, A.; Ataie, N.; Dana, H.; Macklin, J.J.; Laviv, T.; Welf, E.S.; Dean, K.M.; Zhang, F.; et al. A bright cyan-excitable orange fluorescent protein facilitates dual-emission microscopy and enhances bioluminescence imaging in vivo. Nat. Biotechnol. 2016, 34, 760–767. [Google Scholar] [CrossRef] [PubMed]
- Yeh, H.W.; Xiong, Y.; Wu, T.; Chen, M.; Ji, A.; Li, X.; Ai, H.W. ATP-Independent Bioluminescence Reporter Variants to Improve In Vivo Imaging. ACS Chem. Biol. 2019, 14, 959–965. [Google Scholar] [CrossRef]
- Su, Y.; Walker, J.R.; Park, Y.; Smith, T.P.; Liu, L.X.; Hall, M.P.; Labanieh, L.; Hurst, R.; Wang, D.C.; Encell, L.P.; et al. Novel NanoLuc substrates enable bright two-population bioluminescence imaging in animals. Nat. Methods 2020, 17, 852–860. [Google Scholar] [CrossRef]
- Su, Y.; Walker, J.R.; Hall, M.P.; Klein, M.A.; Wu, X.; Encell, L.P.; Casey, K.M.; Liu, L.X.; Hong, G.; Lin, M.Z.; et al. An optimized bioluminescent substrate for non-invasive imaging in the brain. Nat. Chem. Biol. 2023, 19, 731–739. [Google Scholar] [CrossRef]
- Inagaki, S.; Agetsuma, M.; Ohara, S.; Iijima, T.; Yokota, H.; Wazawa, T.; Arai, Y.; Nagai, T. Imaging local brain activity of multiple freely moving mice sharing the same environment. Sci. Rep. 2019, 9, 7460. [Google Scholar] [CrossRef]
- Wu, Y.; Walker, J.R.; Westberg, M.; Ning, L.; Monjie, M.; Kirkland, T.A.; Lin, M.Z.; Su, Y. Kinase-Modulated Bioluminescent Indicators Enable Noninvasive Imaging of Drug Activity in the Brain. ACS Cent. Sci. 2023, 9, 719–732. [Google Scholar] [CrossRef] [PubMed]
- Shy, Y.J.; Hu, C.D. Fluorescence complementation: An emerging tool for biological research. Trends Biotech. 2008, 26, 622–630. [Google Scholar] [CrossRef]
- Cieri, D.; Vicario, M.; Giacomello, M.; Vallese, F.; Filadi, R.; Wagner, T.; Pozzan, T.; Pizzo, P.; Scorrano, L.; Brini, M.; et al. SPLICS: A split green fluorescent protein-based contact site sensor for narrow and wide heterotypic organelle juxtaposition. Cell Death Differ. 2018, 25, 1131. [Google Scholar] [CrossRef] [PubMed]
- Minegishi, M.; Kuchimaru, T.; Nishikawa, K.; Isagawa, I.; Iwano, S.; Iida, K.; Hara, H.; Miura, S.; Sato, M.; Watanabe, S.; et al. Secretory GFP reconstitution labeling of neighboring cells interrogates cell–cell interactions in metastatic niches. Nat. Commun. 2023, 14, 8031. [Google Scholar] [CrossRef]
- Ozawa, T.; Kaihara, A.; Sato, M.; Tachihara, K.; Umezawa, Y. Split luciferase as an optical probe for detecting protein–protein interactions in mammalian cells based on protein splicing. Anal. Chem. 2001, 73, 2516–2521. [Google Scholar] [CrossRef]
- Paulmurugan, R.; Gambhir, S.S. Combinatorial library screening for developing an improved split-firefly luciferase fragment-assisted complementation system for studying protein–protein interactions. Anal. Chem. 2007, 79, 2346–2356. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Chuang, Y.; Qin, F.; Zhang, X.E. Near-Infrared Luciferase Complementation Assay with Enhanced Bioluminescence for Studying Protein–Protein Interactions and Drug Evaluation Under Physiological Conditions. Anal. Chem. 2022, 94, 13700–13709. [Google Scholar] [CrossRef]
- Fan, F.; Binkowski, B.F.; Butler, B.L.; Stecha, P.F.; Lewis, M.K.; Wood, K.V. Novel genetically encoded biosensors using firefly luciferase. ACS Chem. Biol. 2008, 3, 346–351. [Google Scholar] [CrossRef]

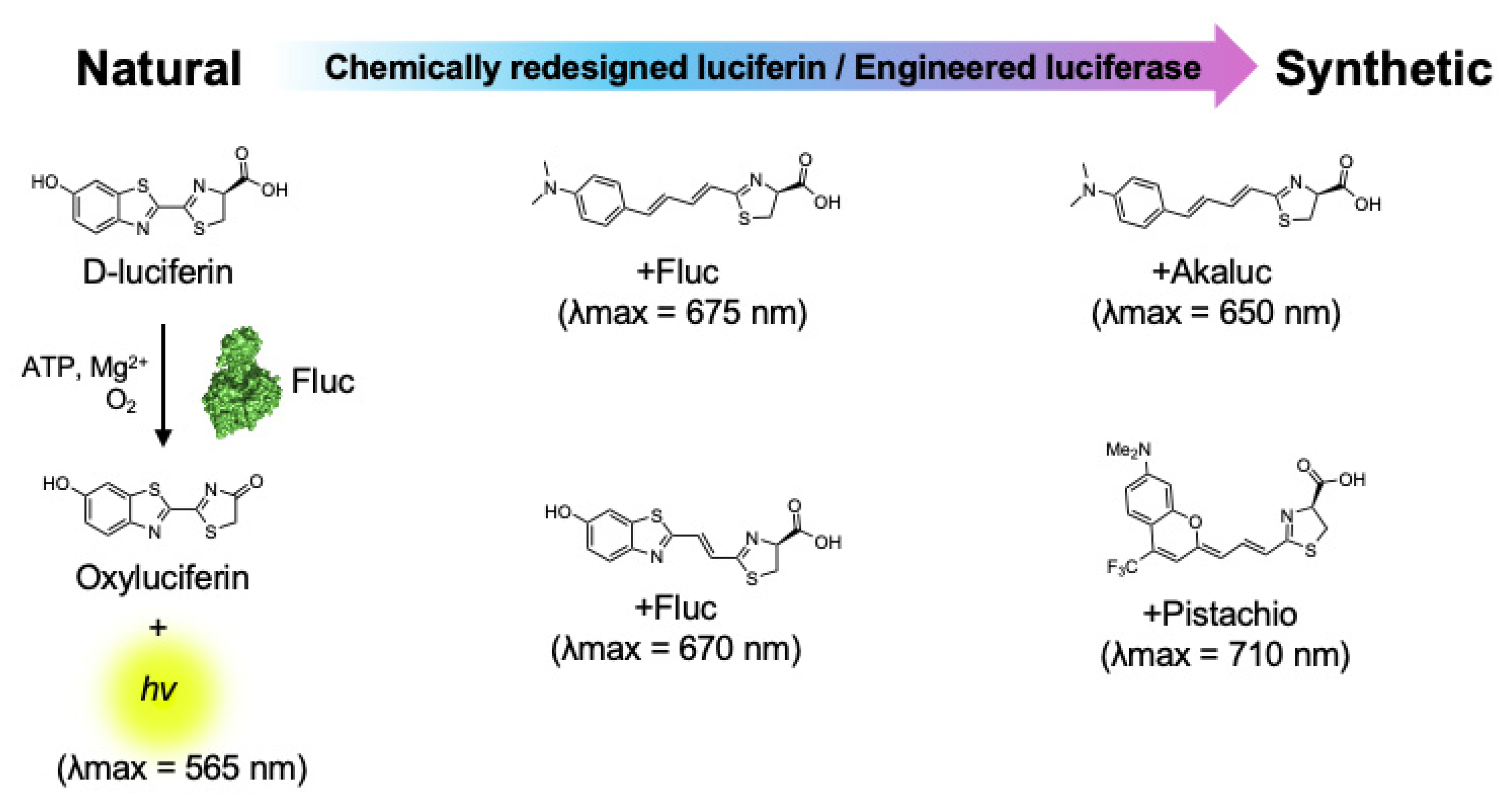
| Luciferin | Luciferase | λmax | Reference |
|---|---|---|---|
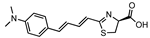 AkaLumine | Fluc Akaluc | 675 650 | [37,46,47,48,49,50,51,52,53,54,55,56] |
 SiR700 X-AL | Fluc | 712 | [38,39] |
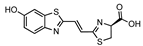 iLH2 | Fluc Fluc S284T | 670 706 | [40,57] |
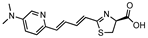 SeMpai | Fluc | 675 | [41,58,59] |
 NIRLuc2 | Fluc | 683 | [42] |
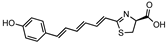 Hexatriene phenol luciferin | Flu | 765 | [43] |
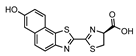 Hydroxy-naphtha[2,1] thiazole luciferin | CBR | 740 | [44] |
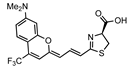 CoLuc-3-NMe2 | Pistachio | 710 | [45] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuchimaru, T. Emerging Synthetic Bioluminescent Reactions for Non-Invasive Imaging of Freely Moving Animals. Int. J. Mol. Sci. 2024, 25, 7338. https://doi.org/10.3390/ijms25137338
Kuchimaru T. Emerging Synthetic Bioluminescent Reactions for Non-Invasive Imaging of Freely Moving Animals. International Journal of Molecular Sciences. 2024; 25(13):7338. https://doi.org/10.3390/ijms25137338
Chicago/Turabian StyleKuchimaru, Takahiro. 2024. "Emerging Synthetic Bioluminescent Reactions for Non-Invasive Imaging of Freely Moving Animals" International Journal of Molecular Sciences 25, no. 13: 7338. https://doi.org/10.3390/ijms25137338





