Sex- and Gender-Related Differences in Obesity: From Pathophysiological Mechanisms to Clinical Implications
Abstract
1. Introduction
2. Sex and Gender-Specific Differences Underlying Obesity Pathology
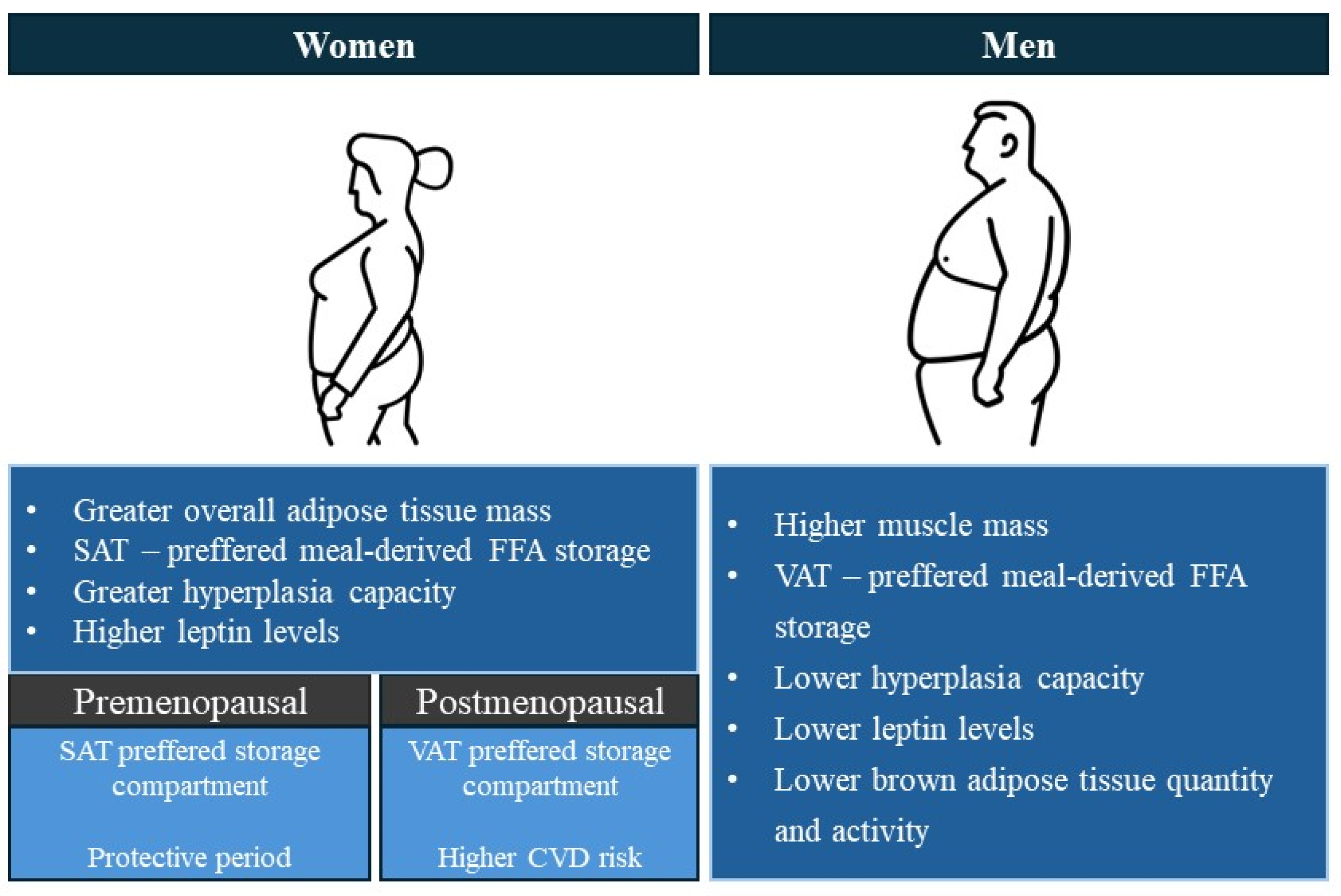
2.1. Differential Body Composition and Fat Distribution in Men and Women
2.2. Differential Energy Expenditure in Men and Women
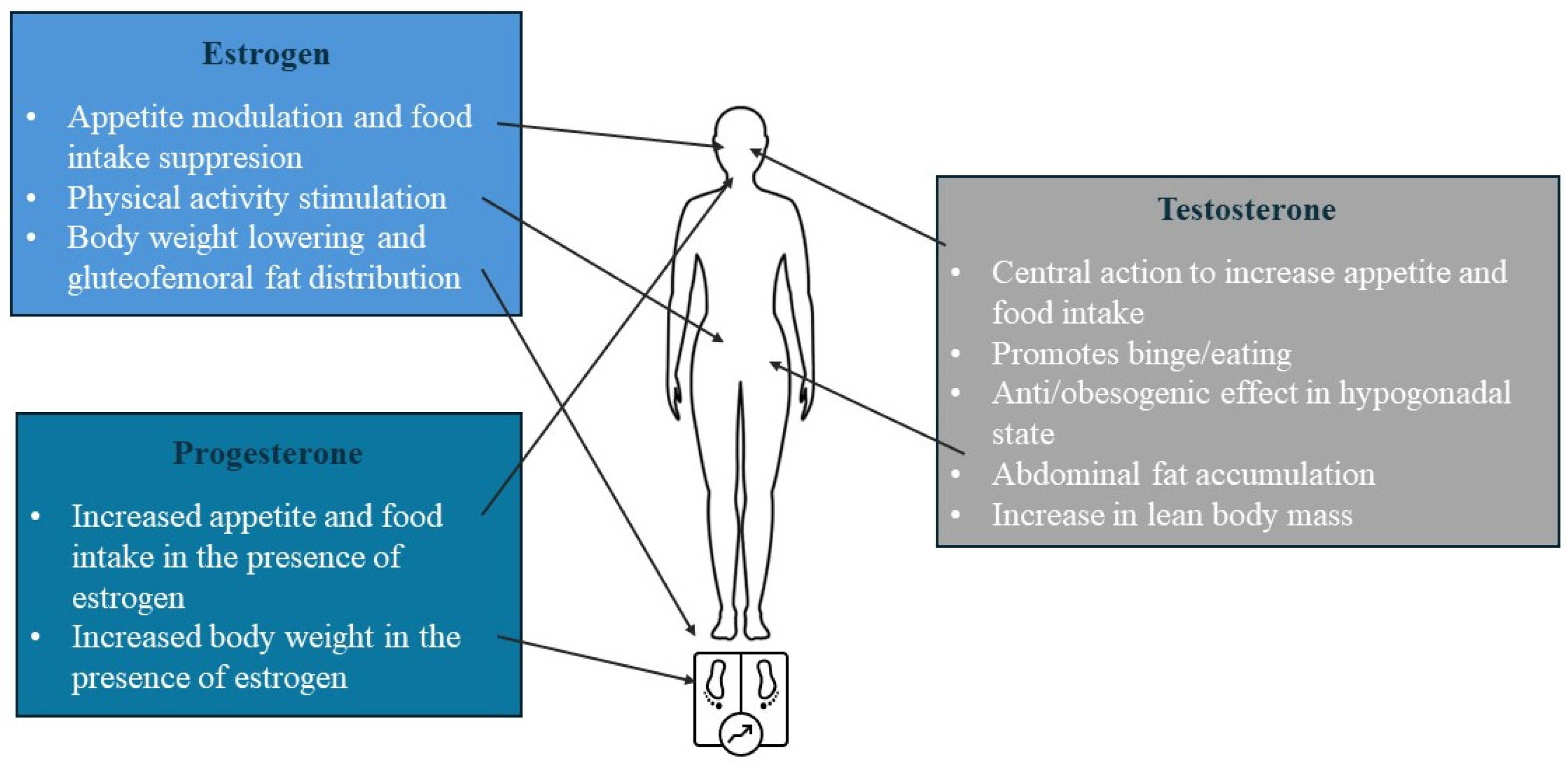
2.3. Sex-Specific Roles of Sex Steroid Hormones
2.3.1. Lessons from Animal Models
2.3.2. Insights from Human Physiology
2.4. Sex Difference in Obesity-Related Genetic Susceptibility
2.5. Sex-Related Differences in Gut Microbiota
3. Sex and Gender—Specific Risk Factors Influencing Overweight/Obesity Pathology
3.1. Behavioural Neuroadaptive Food Intake Preferences
3.2. Sociocultural Role Modelling and Gender-Different Psychological Influence during Development
4. Clinical Implications of Sex-Related Differences in Obesity
4.1. Differential Sex and Gender Risks of Obesity-Related Comorbidities
4.2. Gender-Differential Effectiveness of Weight Loss Interventions
4.2.1. Non-Pharmacological Interventions
4.2.2. Pharmacological Interventions
4.2.3. Bariatric Surgery
5. Challenges and Future Directions
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organisation. Global Health Observatory (GHO) Data: Overweight and Obesity. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 25 May 2024).
- Lostein, T.; Jackson-Leach, R.; Powis, J.; Brinsden, H.; Gray, M. World Obesity Federation, World Obesity Atlas 2023. Available online: https://www.worldobesity.org/resources/resource-library/world-obesity-atlas-2023 (accessed on 25 May 2024).
- Phelps, N.H.; Singleton, R.K.; Zhou, B.; Heap, R.A.; Mishra, A.; Bennett, J.E.; Paciorek, C.J.; Lhoste, V.P.; Carrillo-Larco, R.M.; Stevens, G.A.; et al. Worldwide trends in underweight and obesity from 1990 to 2022: A pooled analysis of 3663 population-representative studies with 222 million children, adolescents, and adults. Lancet 2024, 403, 1027–1050. [Google Scholar] [CrossRef] [PubMed]
- Swinburn, B.A.; Sacks, G.; Hall, K.D.; McPherson, K.; Finegood, D.T.; Moodie, M.L.; Gortmaker, S.L. The global obesity pandemic: Shaped by global drivers and local environments. Lancet 2011, 378, 804–814. [Google Scholar] [CrossRef] [PubMed]
- Hales, C.M.; Carroll, M.D.; Fryar, C.D.; Ogden, C.L. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017–2018. NCHS Data Brief, No. 360. Available online: https://www.cdc.gov/nchs/data/databriefs/db360-h.pdf (accessed on 25 May 2024).
- Lin, X.; Li, H. Obesity: Epidemiology, Pathophysiology, and Therapeutics. Front. Endocrinol. 2021, 12, 706978. [Google Scholar] [CrossRef] [PubMed]
- Allegra, S.; Chiara, F.; Di Grazia, D.; Gaspari, M.; De Francia, S. Evaluation of Sex Differences in Preclinical Pharmacology Research: How Far Is Left to Go? Pharmaceuticals 2023, 16, 786. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Sifuentes, Y.; Maney, D.L. Reporting and misreporting of sex differences in the biological sciences. eLife 2021, 10, e70817. [Google Scholar] [CrossRef]
- Brettle, H.; Tran, V.; Drummond, G.R.; Franks, A.E.; Petrovski, S.; Vinh, A.; Jelinic, M. Sex hormones, intestinal inflammation, and the gut microbiome: Major influencers of the sexual dimorphisms in obesity. Front. Immunol. 2022, 13, 971048. [Google Scholar] [CrossRef] [PubMed]
- Gavin, K.M.; Bessesen, D.H. Sex Differences in Adipose Tissue Function. Endocrinol. Metab. Clin. N. Am. 2020, 49, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Karastergiou, K.; Smith, S.R.; Greenberg, A.S.; Fried, S.K. Sex differences in human adipose tissues—The biology of pear shape. Biol. Sex Differ. 2012, 3, 13. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.; Stanforth, P.; Gagnon, J.; Rankinen, T.; Leon, A.; Rao, D.; Skinner, J.; Bouchard, C.; Wilmore, J. The effect of sex, age and race on estimating percentage body fat from body mass index: The Heritage Family Study. Int. J. Obes. 2002, 26, 789–796. [Google Scholar] [CrossRef]
- Christen, T.; Trompet, S.; Noordam, R.; Van Klinken, J.B.; Van Dijk, K.W.; Lamb, H.J.; Cobbaert, C.M.; Den Heijer, M.; Jazet, I.M.; Jukema, J.W.; et al. Sex differences in body fat distribution are related to sex differences in serum leptin and adiponectin. Peptides 2018, 107, 25–31. [Google Scholar] [CrossRef]
- Arner, P.; Lithell, H.; Wahrenberg, H.; Brönnegard, M. Expression of lipoprotein lipase in different human subcutaneous adipose tissue regions. J. Lipid Res. 1991, 32, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Mauvais-Jarvis, F. Sex differences in energy metabolism: Natural selection, mechanisms and consequences. Nat. Rev. Nephrol. 2024, 20, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Palmer, B.F.; Clegg, D.J. The sexual dimorphism of obesity. Mol. Cell. Endocrinol. 2015, 402, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Manolopoulos, K.N.; Karpe, F.; Frayn, K.N. Gluteofemoral body fat as a determinant of metabolic health. Int. J. Obes. 2010, 34, 949–959. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Z.; Song, Y.; Xie, H.; Dong, M. An update on brown adipose tissue and obesity intervention: Function, regulation and therapeutic implications. Front. Endocrinol. 2023, 13, 1065263. [Google Scholar] [CrossRef] [PubMed]
- Schirinzi, V.; Poli, C.; Berteotti, C.; Leone, A. Browning of Adipocytes: A Potential Therapeutic Approach to Obesity. Nutrients 2023, 15, 2229. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Barrios, A.; Dirakvand, G.; Pervin, S. Human Brown Adipose Tissue and Metabolic Health: Potential for Therapeutic Avenues. Cells 2021, 10, 3030. [Google Scholar] [CrossRef]
- Kaikaew, K.; Grefhorst, A.; Visser, J.A. Sex Differences in Brown Adipose Tissue Function: Sex Hormones, Glucocorticoids, and Their Crosstalk. Front. Endocrinol. 2021, 12, 652444. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.M.; Valencak, T.G. Sex differences and aging: Is there a role of brown adipose tissue? Mol. Cell. Endocrinol. 2021, 531, 111310. [Google Scholar] [CrossRef]
- Keuper, M.; Jastroch, M. The good and the BAT of metabolic sex differences in thermogenic human adipose tissue. Mol. Cell. Endocrinol. 2021, 533, 111337. [Google Scholar] [CrossRef]
- Lee, M.-J.; Fried, S.K. Sex-dependent Depot Differences in Adipose Tissue Development and Function; Role of Sex Steroids. J. Obes. Metab. Syndr. 2017, 26, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Martínez de Morentin, P.B.; González-García, I.; Martins, L.; Lage, R.; Fernández-Mallo, D.; Martínez-Sánchez, N.; Ruíz-Pino, F.; Liu, J.; Morgan, D.A.; Pinilla, L.; et al. Estradiol Regulates Brown Adipose Tissue Thermogenesis via Hypothalamic AMPK. Cell Metab. 2014, 20, 41–53. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Dos Santos, A.R.; de Oliveira Zanuso, B.; Miola, V.F.B.; Barbalho, S.M.; Santos Bueno, P.C.; Flato, U.A.P.; Detregiachi, C.R.P.; Buchaim, D.V.; Buchaim, R.L.; Tofano, R.J.; et al. Adipokines, Myokines, and Hepatokines: Crosstalk and Metabolic Repercussions. Int. J. Mol. Sci. 2021, 22, 2639. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Clegg, D.J. Sex differences in the regulation of body weight. Physiol. Behav. 2009, 97, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Frithioff-Bøjsøe, C.; Lund, M.A.V.; Lausten-Thomsen, U.; Hedley, P.L.; Pedersen, O.; Christiansen, M.; Baker, J.L.; Hansen, T.; Holm, J.-C. Leptin, adiponectin, and their ratio as markers of insulin resistance and cardiometabolic risk in childhood obesity. Pediatr. Diabetes 2020, 21, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Rebordelo, E.; Cunarro, J.; Perez-Sieira, S.; Seoane, L.; Diéguez, C.; Nogueiras, R.; Tovar, S. Regulation of Chemerin and CMKLR1 Expression by Nutritional Status, Postnatal Development, and Gender. Int. J. Mol. Sci. 2018, 19, 2905. [Google Scholar] [CrossRef] [PubMed]
- Lőrincz, H.; Csiha, S.; Ratku, B.; Somodi, S.; Sztanek, F.; Seres, I.; Paragh, G.; Harangi, M. Gender-Dependent Associations between Serum Betatrophin Levels and Lipoprotein Subfractions in Diabetic and Nondiabetic Obese Patients. Int. J. Mol. Sci. 2023, 24, 16504. [Google Scholar] [CrossRef] [PubMed]
- Westerterp, K.R. Changes in physical activity over the lifespan: Impact on body composition and sarcopenic obesity. Obes. Rev. 2018, 19, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Westerterp, K.R. Diet induced thermogenesis. Nutr. Metab. 2004, 1, 5. [Google Scholar] [CrossRef]
- Westerterp, K.R. Physical activity and physical activity induced energy expenditure in humans: Measurement, determinants, and effects. Front. Physiol. 2013, 4, 90. [Google Scholar] [CrossRef]
- Pietiläinen, K.H.; Kaprio, J.; Borg, P.; Plasqui, G.; Yki-Järvinen, H.; Kujala, U.M.; Rose, R.J.; Westerterp, K.R.; Rissanen, A. Physical inactivity and obesity: A vicious circle. Obes. Silver Spring Md 2008, 16, 409–414. [Google Scholar] [CrossRef]
- Sanal, E.; Ardic, F.; Kirac, S. Effects of aerobic or combined aerobic resistance exercise on body composition in overweight and obese adults: Gender differences. A randomized intervention study. Eur. J. Phys. Rehabil. Med. 2013, 49, 1–11. [Google Scholar]
- Aadland, E.; Jepsen, R.; Andersen, J.R.; Anderssen, S.A. Differences in fat loss in response to physical activity among severely obese men and women. J. Rehabil. Med. 2014, 46, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Power, M.L.; Schulkin, J. Sex differences in fat storage, fat metabolism, and the health risks from obesity: Possible evolutionary origins. Br. J. Nutr. 2008, 99, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Mauvais-Jarvis, F.; Clegg, D.J.; Hevener, A.L. The Role of Estrogens in Control of Energy Balance and Glucose Homeostasis. Endocr. Rev. 2013, 34, 309–338. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, M.; Weihua, Z.; Andersson, N.; Moverare, S.; Gao, H.; Vidal, O.; Erlandsson, M.; Windahl, S.; Andersson, G.; Lubahn, D.; et al. Estrogen receptor specificity for the effects of estrogen in ovariectomized mice. J. Endocrinol. 2002, 174, 167–178. [Google Scholar] [CrossRef]
- Davis, K.E.; Neinast, M.D.; Sun, K.; Skiles, W.M.; Bills, J.D.; Zehr, J.A.; Zeve, D.; Hahner, L.D.; Cox, D.W.; Gent, L.M.; et al. The sexually dimorphic role of adipose and adipocyte estrogen receptors in modulating adipose tissue expansion, inflammation, and fibrosis. Mol. Metab. 2013, 2, 227–242. [Google Scholar] [CrossRef] [PubMed]
- Naaz, A.; Zakroczymski, M.; Heine, P.; Taylor, J.; Saunders, P.; Lubahn, D.; Cooke, P.S. Effect of Ovariectomy on Adipose Tissue of Mice in the Absence of Estrogen Receptor Alpha (ERα): A Potential Role for Estrogen Receptor Beta (ERβ). Horm. Metab. Res. 2002, 34, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Stubbins, R.E.; Smith, R.R.; Harvey, A.E.; Núñez, N.P. Differential susceptibility to obesity between male, female and ovariectomized female mice. Nutr. J. 2009, 8, 11. [Google Scholar] [CrossRef]
- Liu, P.; Ji, Y.; Yuen, T.; Rendina-Ruedy, E.; DeMambro, V.E.; Dhawan, S.; Abu-Amer, W.; Izadmehr, S.; Zhou, B.; Shin, A.C.; et al. Blocking FSH induces thermogenic adipose tissue and reduces body fat. Nature 2017, 546, 107–112. [Google Scholar] [CrossRef]
- O’Reilly, M.W.; House, P.J.; Tomlinson, J.W. Understanding androgen action in adipose tissue. J. Steroid Biochem. Mol. Biol. 2014, 143, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Sebo, Z.L.; Rodeheffer, M.S. Testosterone metabolites differentially regulate obesogenesis and fat distribution. Mol. Metab. 2021, 44, 101141. [Google Scholar] [CrossRef] [PubMed]
- Nohara, K.; Zhang, Y.; Waraich, R.S.; Laque, A.; Tiano, J.P.; Tong, J.; Münzberg, H.; Mauvais-Jarvis, F. Early-Life Exposure to Testosterone Programs the Hypothalamic Melanocortin System. Endocrinology 2011, 152, 1661–1669. [Google Scholar] [CrossRef] [PubMed]
- Nohara, K.; Waraich, R.S.; Liu, S.; Ferron, M.; Waget, A.; Meyers, M.S.; Karsenty, G.; Burcelin, R.; Mauvais-Jarvis, F. Developmental androgen excess programs sympathetic tone and adipose tissue dysfunction and predisposes to a cardiometabolic syndrome in female mice. Am. J. Physiol.-Endocrinol. Metab. 2013, 304, E1321–E1330. [Google Scholar] [CrossRef] [PubMed]
- Mauvais-Jarvis, F. Developmental androgenization programs metabolic dysfunction in adult mice: Clinical implications. Adipocyte 2014, 3, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Gavin, K.M.; Cooper, E.E.; Hickner, R.C. Estrogen receptor protein content is different in abdominal than gluteal subcutaneous adipose tissue of overweight-to-obese premenopausal women. Metabolism. 2013, 62, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, S.B.; Kristensen, K.; Hermann, P.A.; Katzenellenbogen, J.A.; Richelsen, B. Estrogen controls lipolysis by up-regulating alpha2A-adrenergic receptors directly in human adipose tissue through the estrogen receptor alpha. Implications for the female fat distribution. J. Clin. Endocrinol. Metab. 2004, 89, 1869–1878. [Google Scholar] [CrossRef] [PubMed]
- Lovejoy, J.C.; Champagne, C.M.; de Jonge, L.; Xie, H.; Smith, S.R. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int. J. Obes. 2008, 32, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Bernasochi, G.B.; Bell, J.R.; Simpson, E.R.; Delbridge, L.M.D.; Boon, W.C. Impact of estrogens on the regulation of white, beige, and brown adipose tissue depots. In Comprehensive Physiology; Prakash, Y.S., Ed.; Wiley: Hoboken, NJ, USA, 2019; pp. 457–475. ISBN 978-0-470-65071-4. [Google Scholar] [CrossRef]
- Anagnostis, P.; Christou, K.; Artzouchaltzi, A.-M.; Gkekas, N.K.; Kosmidou, N.; Siolos, P.; Paschou, S.A.; Potoupnis, M.; Kenanidis, E.; Tsiridis, E.; et al. Early menopause and premature ovarian insufficiency are associated with increased risk of type 2 diabetes: A systematic review and meta-analysis. Eur. J. Endocrinol. 2019, 180, 41–50. [Google Scholar] [CrossRef]
- Kanaya, A.M.; Herrington, D.; Vittinghoff, E.; Lin, F.; Grady, D.; Bittner, V.; Cauley, J.A.; Barrett-Connor, E. Heart and Estrogen/progestin Replacement Study Glycemic effects of postmenopausal hormone therapy: The Heart and Estrogen/progestin Replacement Study. A randomized, double-blind, placebo-controlled trial. Ann. Intern. Med. 2003, 138, 1–9. [Google Scholar] [CrossRef]
- Margolis, K.L.; Bonds, D.E.; Rodabough, R.J.; Tinker, L.; Phillips, L.S.; Allen, C.; Bassford, T.; Burke, G.; Torrens, J.; Howard, B.V.; et al. Effect of oestrogen plus progestin on the incidence of diabetes in postmenopausal women: Results from the Women’s Health Initiative Hormone Trial. Diabetologia 2004, 47, 1175–1187. [Google Scholar] [CrossRef] [PubMed]
- Loh, N.Y.; Humphreys, E.; Karpe, F.; Tomlinson, J.W.; Noordam, R.; Christodoulides, C. Sex hormones, adiposity, and metabolic traits in men and women: A Mendelian randomisation study. Eur. J. Endocrinol. 2022, 186, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, E.J.; Gianatti, E.; Strauss, B.J.; Wentworth, J.; Lim-Joon, D.; Bolton, D.; Zajac, J.D.; Grossmann, M. Increase in visceral and subcutaneous abdominal fat in men with prostate cancer treated with androgen deprivation therapy. Clin. Endocrinol. 2011, 74, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Stanworth, R.D.; Jones, T.H. Testosterone for the aging male; current evidence and recommended practice. Clin. Interv. Aging 2008, 3, 25–44. [Google Scholar] [PubMed]
- Santosa, S.; Bush, N.C.; Jensen, M.D. Acute Testosterone Deficiency Alters Adipose Tissue Fatty Acid Storage. J. Clin. Endocrinol. Metab. 2017, 102, 3056–3064. [Google Scholar] [CrossRef] [PubMed]
- Eng, P.C.; Phylactou, M.; Qayum, A.; Woods, C.; Lee, H.; Aziz, S.; Moore, B.; Miras, A.D.; Comninos, A.N.; Tan, T.; et al. Obesity-Related Hypogonadism in Women. Endocr. Rev. 2024, 45, 171–189. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.M.; Jones, T.H. Testosterone and obesity. Obes. Rev. 2015, 16, 581–606. [Google Scholar] [CrossRef] [PubMed]
- Tramunt, B.; Smati, S.; Grandgeorge, N.; Lenfant, F.; Arnal, J.-F.; Montagner, A.; Gourdy, P. Sex differences in metabolic regulation and diabetes susceptibility. Diabetologia 2020, 63, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Cherkerzian, S.; Loucks, E.B.; Buka, S.L.; Handa, R.J.; Lasley, B.L.; Bhasin, S.; Goldstein, J.M. Sex Differences in the Prenatal Programming of Adult Metabolic Syndrome by Maternal Androgens. J. Clin. Endocrinol. Metab. 2018, 103, 3945–3953. [Google Scholar] [CrossRef]
- Kyinn, M.; Banks, K.; Leemaqz, S.Y.; Sarkodie, E.; Goldstein, D.; Irwig, M.S. Weight gain and obesity rates in transgender and gender-diverse adults before and during hormone therapy. Int. J. Obes. 2021, 45, 2562–2569. [Google Scholar] [CrossRef]
- Ford, K.; Huggins, E.; Sheean, P. Characterising body composition and bone health in transgender individuals receiving gender-affirming hormone therapy. J. Hum. Nutr. Diet. 2022, 35, 1105–1114. [Google Scholar] [CrossRef]
- Hirschberg, A.L. Sex hormones, appetite and eating behaviour in women. Maturitas 2012, 71, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Leeners, B.; Geary, N.; Tobler, P.N.; Asarian, L. Ovarian hormones and obesity. Hum. Reprod. Update 2017, 23, 300–321. [Google Scholar] [CrossRef] [PubMed]
- Barr, S.I.; Janelle, K.C.; Prior, J.C. Energy intakes are higher during the luteal phase of ovulatory menstrual cycles. Am. J. Clin. Nutr. 1995, 61, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Davidsen, L.; Vistisen, B.; Astrup, A. Impact of the menstrual cycle on determinants of energy balance: A putative role in weight loss attempts. Int. J. Obes. 2007, 31, 1777–1785. [Google Scholar] [CrossRef]
- Kaisinger, L.R.; Kentistou, K.A.; Stankovic, S.; Gardner, E.J.; Day, F.R.; Zhao, Y.; Mörseburg, A.; Carnie, C.J.; Zagnoli-Vieira, G.; Puddu, F.; et al. Large-scale exome sequence analysis identifies sex- and age-specific determinants of obesity. Cell Genom. 2023, 3, 100362. [Google Scholar] [CrossRef]
- Heid, I.M.; Jackson, A.U.; Randall, J.C.; Winkler, T.W.; Qi, L.; Steinthorsdottir, V.; Thorleifsson, G.; Zillikens, M.C.; Speliotes, E.K.; Mägi, R.; et al. Meta-analysis identifies 13 new loci associated with waist-hip ratio and reveals sexual dimorphism in the genetic basis of fat distribution. Nat. Genet. 2010, 42, 949–960. [Google Scholar] [CrossRef]
- Randall, J.C.; Winkler, T.W.; Kutalik, Z.; Berndt, S.I.; Jackson, A.U.; Monda, K.L.; Kilpeläinen, T.O.; Esko, T.; Mägi, R.; Li, S.; et al. Sex-stratified Genome-wide Association Studies Including 270,000 Individuals Show Sexual Dimorphism in Genetic Loci for Anthropometric Traits. PLoS Genet. 2013, 9, e1003500. [Google Scholar] [CrossRef]
- Mauvais-Jarvis, F.; Arnold, A.P.; Reue, K. A Guide for the Design of Pre-clinical Studies on Sex Differences in Metabolism. Cell Metab. 2017, 25, 1216–1230. [Google Scholar] [CrossRef]
- Chen, X.; McClusky, R.; Chen, J.; Beaven, S.W.; Tontonoz, P.; Arnold, A.P.; Reue, K. The Number of X Chromosomes Causes Sex Differences in Adiposity in Mice. PLoS Genet. 2012, 8, e1002709. [Google Scholar] [CrossRef]
- Link, J.C.; Wiese, C.B.; Chen, X.; Avetisyan, R.; Ronquillo, E.; Ma, F.; Guo, X.; Yao, J.; Allison, M.; Chen, Y.-D.I.; et al. X chromosome dosage of histone demethylase KDM5C determines sex differences in adiposity. J. Clin. Investig. 2020, 130, 5688–5702. [Google Scholar] [CrossRef] [PubMed]
- Bernabeu, E.; Canela-Xandri, O.; Rawlik, K.; Talenti, A.; Prendergast, J.; Tenesa, A. Sex differences in genetic architecture in the UK Biobank. Nat. Genet. 2021, 53, 1283–1289. [Google Scholar] [CrossRef] [PubMed]
- Kilpeläinen, T.O.; Zillikens, M.C.; Stančákova, A.; Finucane, F.M.; Ried, J.S.; Langenberg, C.; Zhang, W.; Beckmann, J.S.; Luan, J.; Vandenput, L.; et al. Genetic variation near IRS1 associates with reduced adiposity and an impaired metabolic profile. Nat. Genet. 2011, 43, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Bäckhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef] [PubMed]
- Koliada, A.; Syzenko, G.; Moseiko, V.; Budovska, L.; Puchkov, K.; Perederiy, V.; Gavalko, Y.; Dorofeyev, A.; Romanenko, M.; Tkach, S.; et al. Association between body mass index and Firmicutes/Bacteroidetes ratio in an adult Ukrainian population. BMC Microbiol. 2017, 17, 120. [Google Scholar] [CrossRef] [PubMed]
- Palmas, V.; Pisanu, S.; Madau, V.; Casula, E.; Deledda, A.; Cusano, R.; Uva, P.; Vascellari, S.; Loviselli, A.; Manzin, A.; et al. Gut microbiota markers associated with obesity and overweight in Italian adults. Sci. Rep. 2021, 11, 5532. [Google Scholar] [CrossRef]
- Shin, J.-H.; Park, Y.-H.; Sim, M.; Kim, S.-A.; Joung, H.; Shin, D.-M. Serum level of sex steroid hormone is associated with diversity and profiles of human gut microbiome. Res. Microbiol. 2019, 170, 192–201. [Google Scholar] [CrossRef]
- Qin, Y.; Roberts, J.D.; Grimm, S.A.; Lih, F.B.; Deterding, L.J.; Li, R.; Chrysovergis, K.; Wade, P.A. An obesity-associated gut microbiome reprograms the intestinal epigenome and leads to altered colonic gene expression. Genome Biol. 2018, 19, 7. [Google Scholar] [CrossRef]
- He, J.; Wang, W.; Wu, Z.; Pan, D.; Guo, Y.; Cai, Z.; Lian, L. Effect of Lactobacillus reuteri on intestinal microbiota and immune parameters: Involvement of sex differences. J. Funct. Foods 2019, 53, 36–43. [Google Scholar] [CrossRef]
- Hu, S.; Ding, Q.; Zhang, W.; Kang, M.; Ma, J.; Zhao, L. Gut microbial beta-glucuronidase: A vital regulator in female estrogen metabolism. Gut Microbes 2023, 15, 2236749. [Google Scholar] [CrossRef]
- Colldén, H.; Landin, A.; Wallenius, V.; Elebring, E.; Fändriks, L.; Nilsson, M.E.; Ryberg, H.; Poutanen, M.; Sjögren, K.; Vandenput, L.; et al. The gut microbiota is a major regulator of androgen metabolism in intestinal contents. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E1182–E1192. [Google Scholar] [CrossRef]
- Flores, R.; Shi, J.; Fuhrman, B.; Xu, X.; Veenstra, T.D.; Gail, M.H.; Gajer, P.; Ravel, J.; Goedert, J.J. Fecal microbial determinants of fecal and systemic estrogens and estrogen metabolites: A cross-sectional study. J. Transl. Med. 2012, 10, 253. [Google Scholar] [CrossRef]
- Kroll, D.S.; Feldman, D.E.; Biesecker, C.L.; McPherson, K.L.; Manza, P.; Joseph, P.V.; Volkow, N.D.; Wang, G.-J. Neuroimaging of Sex/Gender Differences in Obesity: A Review of Structure, Function, and Neurotransmission. Nutrients 2020, 12, 1942. [Google Scholar] [CrossRef] [PubMed]
- Stice, E.; Spoor, S.; Bohon, C.; Small, D.M. Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science 2008, 322, 449–452. [Google Scholar] [CrossRef]
- Horstmann, A.; Busse, F.P.; Mathar, D.; Müller, K.; Lepsien, J.; Schlögl, H.; Kabisch, S.; Kratzsch, J.; Neumann, J.; Stumvoll, M.; et al. Obesity-Related Differences between Women and Men in Brain Structure and Goal-Directed Behavior. Front. Hum. Neurosci. 2011, 5, 58. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Wang, G.-J.; Telang, F.; Fowler, J.S.; Thanos, P.K.; Logan, J.; Alexoff, D.; Ding, Y.-S.; Wong, C.; Ma, Y.; et al. Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: Possible contributing factors. NeuroImage 2008, 42, 1537–1543. [Google Scholar] [CrossRef]
- Chao, A.M.; Loughead, J.; Bakizada, Z.M.; Hopkins, C.M.; Geliebter, A.; Gur, R.C.; Wadden, T.A. Sex/gender differences in neural correlates of food stimuli: A systematic review of functional neuroimaging studies. Obes. Rev. 2017, 18, 687–699. [Google Scholar] [CrossRef] [PubMed]
- Lafay, L.; Thomas, F.; Mennen, L.; Charles, M.A.; Eschwege, E.; Borys, J.M.; Basdevant, A.; Fleurbaix Laventie Ville Santé Study Group. Gender differences in the relation between food cravings and mood in an adult community: Results from the fleurbaix laventie ville santé study. Int. J. Eat. Disord. 2001, 29, 195–204. [Google Scholar] [CrossRef]
- Cepeda-Benito, A.; Fernandez, M.C.; Moreno, S. Relationship of gender and eating disorder symptoms to reported cravings for food: Construct validation of state and trait craving questionnaires in Spanish. Appetite 2003, 40, 47–54. [Google Scholar] [CrossRef]
- Imperatori, C.; Innamorati, M.; Tamburello, S.; Continisio, M.; Contardi, A.; Tamburello, A.; Fabbricatore, M. Gender differences in food craving among overweight and obese patients attending low energy diet therapy: A matched case-control study. Eat. Weight Disord. EWD 2013, 18, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Hallam, J.; Boswell, R.G.; DeVito, E.E.; Kober, H. Gender-related Differences in Food Craving and Obesity. Yale J. Biol. Med. 2016, 89, 161–173. [Google Scholar] [PubMed]
- Wang, G.-J.; Volkow, N.D.; Telang, F.; Jayne, M.; Ma, Y.; Pradhan, K.; Zhu, W.; Wong, C.T.; Thanos, P.K.; Geliebter, A.; et al. Evidence of gender differences in the ability to inhibit brain activation elicited by food stimulation. Proc. Natl. Acad. Sci. USA 2009, 106, 1249–1254. [Google Scholar] [CrossRef] [PubMed]
- Stea, T.H.; Nordheim, O.; Bere, E.; Stornes, P.; Eikemo, T.A. Fruit and vegetable consumption in Europe according to gender, educational attainment and regional affiliation—A cross-sectional study in 21 European countries. PLoS ONE 2020, 15, e0232521. [Google Scholar] [CrossRef] [PubMed]
- Emanuel, A.S.; McCully, S.N.; Gallagher, K.M.; Updegraff, J.A. Theory of Planned Behavior explains gender difference in fruit and vegetable consumption. Appetite 2012, 59, 693–697. [Google Scholar] [CrossRef] [PubMed]
- Wansink, B.; Cheney, M.; Chan, N. Exploring comfort food preferences across age and gender1. Physiol. Behav. 2003, 79, 739–747. [Google Scholar] [CrossRef]
- Lombardo, M.; Aulisa, G.; Padua, E.; Annino, G.; Iellamo, F.; Pratesi, A.; Caprio, M.; Bellia, A. Gender differences in taste and foods habits. Nutr. Food Sci. 2019, 50, 229–239. [Google Scholar] [CrossRef]
- Feraco, A.; Armani, A.; Amoah, I.; Guseva, E.; Camajani, E.; Gorini, S.; Strollo, R.; Padua, E.; Caprio, M.; Lombardo, M. Assessing gender differences in food preferences and physical activity: A population-based survey. Front. Nutr. 2024, 11, 1348456. [Google Scholar] [CrossRef]
- Hill, J.P.; Lynch, M.E. The Intensification of Gender-Related Role Expectations during Early Adolescence. In Girls at Puberty; Brooks-Gunn, J., Petersen, A.C., Eds.; Springer: Boston, MA, USA, 1983; pp. 201–228. ISBN 978-1-4899-0356-3. [Google Scholar] [CrossRef]
- Bearman, S.K.; Presnell, K.; Martinez, E.; Stice, E. The Skinny on Body Dissatisfaction: A Longitudinal Study of Adolescent Girls and Boys. J. Youth Adolesc. 2006, 35, 217–229. [Google Scholar] [CrossRef]
- McCuen-Wurst, C.; Ruggieri, M.; Allison, K.C. Disordered eating and obesity: Associations between binge-eating disorder, night-eating syndrome, and weight-related comorbidities. Ann. N. Y. Acad. Sci. 2018, 1411, 96–105. [Google Scholar] [CrossRef]
- Keel, P.K.; Fulkerson, J.A.; Leon, G.R. Disordered Eating Precursors in Pre- and Early Adolescent Girls and Boys. J. Youth Adolesc. 1997, 26, 203–216. [Google Scholar] [CrossRef]
- Ata, R.N.; Ludden, A.B.; Lally, M.M. The Effects of Gender and Family, Friend, and Media Influences on Eating Behaviors and Body Image During Adolescence. J. Youth Adolesc. 2007, 36, 1024–1037. [Google Scholar] [CrossRef]
- Taylor, N.L. “Guys, She’s Humongous!”: Gender and Weight-Based Teasing in Adolescence. J. Adolesc. Res. 2011, 26, 178–199. [Google Scholar] [CrossRef]
- Dougherty, E.N.; Goldschmidt, A.B.; Johnson, N.K.; Badillo, K.; Engel, S.G.; Haedt-Matt, A.A. Gender differences in the relation between interpersonal stress and momentary shape and weight concerns in youth with overweight/obesity. Body Image 2022, 40, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.-T.; Minh Nguyet, N.T.; Nga, V.T.; Thai Lien, N.V.; Vo, D.D.; Lien, N.; Nhu Ngoc, V.T.; Son, L.H.; Le, D.-H.; Nga, V.B.; et al. An update on obesity: Mental consequences and psychological interventions. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Herva, A.; Laitinen, J.; Miettunen, J.; Veijola, J.; Karvonen, J.T.; Läksy, K.; Joukamaa, M. Obesity and depression: Results from the longitudinal Northern Finland 1966 Birth Cohort Study. Int. J. Obes. 2006, 30, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Pratt, L.A.; Brody, D.J. Depression and Obesity in the U.S. Adult Household Population, 2005–2010; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2014. [Google Scholar]
- De França, G.V.A.; Gigante, D.P.; Olinto, M.T.A. Binge eating in adults: Prevalence and association with obesity, poor self-rated health status and body dissatisfaction. Public Health Nutr. 2014, 17, 932–938. [Google Scholar] [CrossRef]
- Yang, Y.; Smith, D.L.; Keating, K.D.; Allison, D.B.; Nagy, T.R. Variations in body weight, food intake and body composition after long-term high-fat diet feeding in C57BL/6J mice: Variations in Diet-Induced Obese C57BL/6J Mice. Obesity 2014, 22, 2147–2155. [Google Scholar] [CrossRef] [PubMed]
- Hwang, L.; Wang, C.; Li, T.; Chang, S.; Lin, L.; Chen, C.; Chen, C.; Liang, K.; Ho, I.; Yang, W.; et al. Sex Differences in High-fat Diet-induced Obesity, Metabolic Alterations and Learning, and Synaptic Plasticity Deficits in Mice. Obesity 2010, 18, 463–469. [Google Scholar] [CrossRef]
- Narayan, K.M.V.; Boyle, J.P.; Thompson, T.J.; Gregg, E.W.; Williamson, D.F. Effect of BMI on Lifetime Risk for Diabetes in the U.S. Diabetes Care 2007, 30, 1562–1566. [Google Scholar] [CrossRef]
- Guh, D.P.; Zhang, W.; Bansback, N.; Amarsi, Z.; Birmingham, C.L.; Anis, A.H. The incidence of co-morbidities related to obesity and overweight: A systematic review and meta-analysis. BMC Public Health 2009, 9, 88. [Google Scholar] [CrossRef] [PubMed]
- Logue, J.; Walker, J.J.; Colhoun, H.M.; Leese, G.P.; Lindsay, R.S.; McKnight, J.A.; Morris, A.D.; Pearson, D.W.; Petrie, J.R.; Philip, S.; et al. Do men develop type 2 diabetes at lower body mass indices than women? Diabetologia 2011, 54, 3003–3006. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Thomas, G.; Majeed, A.; Khunti, K.; Klein, K. Women develop type 2 diabetes at a higher body mass index than men. Diabetologia 2012, 55, 1556–1557. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.K. Women Are Diagnosed with Type 2 Diabetes at Higher Body Mass Indices and Older Ages than Men: Korea National Health and Nutrition Examination Survey 2007–2010. Diabetes Metab. J. 2014, 38, 74. [Google Scholar] [CrossRef] [PubMed]
- Cooper, A.J.; Gupta, S.R.; Moustafa, A.F.; Chao, A.M. Sex/Gender Differences in Obesity Prevalence, Comorbidities, and Treatment. Curr. Obes. Rep. 2021, 10, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Wong, J.; Brooks, V.L. Obesity: Sex and sympathetics. Biol. Sex Differ. 2020, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Cassaglia, P.A.; Pelletier, N.E.; Brooks, V.L. Sex differences in the sympathoexcitatory response to insulin in obese rats: Role of neuropeptide Y. J. Physiol. 2019, 597, 1757–1775. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, G.E.; Ballard, T.P.; Beske, S.D.; Davy, K.P. Subcutaneous obesity is not associated with sympathetic neural activation. Am. J. Physiol.-Heart Circ. Physiol. 2004, 287, H414–H418. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Lin, B.M.; Markt, S.C.; Stampfer, M.J.; Laden, F.; Hu, F.B.; Tworoger, S.S.; Redline, S. Sex differences in the associations of obstructive sleep apnoea with epidemiological factors. Eur. Respir. J. 2018, 51, 1702421. [Google Scholar] [CrossRef]
- Sung, H.; Siegel, R.L.; Torre, L.A.; Pearson-Stuttard, J.; Islami, F.; Fedewa, S.A.; Goding Sauer, A.; Shuval, K.; Gapstur, S.M.; Jacobs, E.J.; et al. Global patterns in excess body weight and the associated cancer burden. CA. Cancer J. Clin. 2019, 69, 88–112. [Google Scholar] [CrossRef]
- Kyrgiou, M.; Kalliala, I.; Markozannes, G.; Gunter, M.J.; Paraskevaidis, E.; Gabra, H.; Martin-Hirsch, P.; Tsilidis, K.K. Adiposity and cancer at major anatomical sites: Umbrella review of the literature. BMJ 2017, 356, j477. [Google Scholar] [CrossRef] [PubMed]
- Argyrakopoulou, G.; Dalamaga, M.; Spyrou, N.; Kokkinos, A. Gender Differences in Obesity-Related Cancers. Curr. Obes. Rep. 2021, 10, 100–115. [Google Scholar] [CrossRef] [PubMed]
- Greaney, M.L.; Cohen, S.A.; Xu, F.; Ward-Ritacco, C.L.; Riebe, D. Healthcare provider counselling for weight management behaviours among adults with overweight or obesity: A cross-sectional analysis of National Health and Nutrition Examination Survey, 2011–2018. BMJ Open 2020, 10, e039295. [Google Scholar] [CrossRef] [PubMed]
- Susanto, A.; Burk, J.; Hocking, S.; Markovic, T.; Gill, T. Differences in weight loss outcomes for males and females on a low-carbohydrate diet: A systematic review. Obes. Res. Clin. Pract. 2022, 16, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.L.; Wood, L.G.; Collins, C.E.; Callister, R. Effectiveness of weight loss interventions—Is there a difference between men and women: A systematic review. Obes. Rev. 2015, 16, 171–186. [Google Scholar] [CrossRef] [PubMed]
- Chakhtoura, M.; Haber, R.; Ghezzawi, M.; Rhayem, C.; Tcheroyan, R.; Mantzoros, C.S. Pharmacotherapy of obesity: An update on the available medications and drugs under investigation. eClinicalMedicine 2023, 58, 101882. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, R.; Tian, Y.; Kong, S.X.; Hersloev, M.; Hobbs, T.; Smolarz, B.G.; Ramasamy, A.; Haase, C.L.; Weng, W. Persistence of newer anti-obesity medications in a real-world setting. Diabetes Res. Clin. Pract. 2018, 143, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Wilding, J.P.H.; Batterham, R.L.; Calanna, S.; Davies, M.; Van Gaal, L.F.; Lingvay, I.; McGowan, B.M.; Rosenstock, J.; Tran, M.T.D.; Wadden, T.A.; et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N. Engl. J. Med. 2021, 384, 989–1002. [Google Scholar] [CrossRef] [PubMed]
- Wadden, T.A.; Bailey, T.S.; Billings, L.K.; Davies, M.; Frias, J.P.; Koroleva, A.; Lingvay, I.; O’Neil, P.M.; Rubino, D.M.; Skovgaard, D.; et al. Effect of Subcutaneous Semaglutide vs Placebo as an Adjunct to Intensive Behavioral Therapy on Body Weight in Adults With Overweight or Obesity: The STEP 3 Randomized Clinical Trial. JAMA 2021, 325, 1403. [Google Scholar] [CrossRef]
- Rubino, D.; Abrahamsson, N.; Davies, M.; Hesse, D.; Greenway, F.L.; Jensen, C.; Lingvay, I.; Mosenzon, O.; Rosenstock, J.; Rubio, M.A.; et al. Effect of Continued Weekly Subcutaneous Semaglutide vs Placebo on Weight Loss Maintenance in Adults With Overweight or Obesity: The STEP 4 Randomized Clinical Trial. JAMA 2021, 325, 1414. [Google Scholar] [CrossRef]
- Garvey, W.T.; Batterham, R.L.; Bhatta, M.; Buscemi, S.; Christensen, L.N.; Frias, J.P.; Jódar, E.; Kandler, K.; Rigas, G.; Wadden, T.A.; et al. Two-year effects of semaglutide in adults with overweight or obesity: The STEP 5 trial. Nat. Med. 2022, 28, 2083–2091. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.; Færch, L.; Jeppesen, O.K.; Pakseresht, A.; Pedersen, S.D.; Perreault, L.; Rosenstock, J.; Shimomura, I.; Viljoen, A.; Wadden, T.A.; et al. Semaglutide 2·4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): A randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet 2021, 397, 971–984. [Google Scholar] [CrossRef] [PubMed]
- Le Roux, C.W.; Zhang, S.; Aronne, L.J.; Kushner, R.F.; Chao, A.M.; Machineni, S.; Dunn, J.; Chigutsa, F.B.; Ahmad, N.N.; Bunck, M.C. Tirzepatide for the treatment of obesity: Rationale and design of the SURMOUNT clinical development program. Obesity 2023, 31, 96–110. [Google Scholar] [CrossRef] [PubMed]
- Wilding, J.P.H.; Overgaard, R.V.; Jacobsen, L.V.; Jensen, C.B.; le Roux, C.W. Exposure-response analyses of liraglutide 3.0 mg for weight management. Diabetes Obes. Metab. 2016, 18, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Jensterle, M.; Rizzo, M.; Janež, A. Semaglutide in Obesity: Unmet Needs in Men. Diabetes Ther. 2023, 14, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Cataldi, M.; Muscogiuri, G.; Savastano, S.; Barrea, L.; Guida, B.; Taglialatela, M.; Colao, A. Gender-related issues in the pharmacology of new anti-obesity drugs. Obes. Rev. 2019, 20, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Aly, S.; Hachey, K.; Pernar, L.I.M. Gender disparities in weight loss surgery. Mini-Invasive Surg. 2020, 4, 21. [Google Scholar] [CrossRef]
- Libeton, M.; Dixon, J.B.; Laurie, C.; O’Brien, P.E. Patient Motivation for Bariatric Surgery: Characteristics and Impact on Outcomes. Obes. Surg. 2004, 14, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Kochkodan, J.; Telem, D.A.; Ghaferi, A.A. Physiologic and psychological gender differences in bariatric surgery. Surg. Endosc. 2018, 32, 1382–1388. [Google Scholar] [CrossRef]
- Avgerinos, K.I.; Spyrou, N.; Mantzoros, C.S.; Dalamaga, M. Obesity and cancer risk: Emerging biological mechanisms and perspectives. Metabolism 2019, 92, 121–135. [Google Scholar] [CrossRef]
- Zhou, X.; Yu, J.; Li, L.; Gloy, V.L.; Nordmann, A.; Tiboni, M.; Li, Y.; Sun, X. Effects of Bariatric Surgery on Mortality, Cardiovascular Events, and Cancer Outcomes in Obese Patients: Systematic Review and Meta-analysis. Obes. Surg. 2016, 26, 2590–2601. [Google Scholar] [CrossRef] [PubMed]
- Casagrande, D.S.; Rosa, D.D.; Umpierre, D.; Sarmento, R.A.; Rodrigues, C.G.; Schaan, B.D. Incidence of Cancer Following Bariatric Surgery: Systematic Review and Meta-analysis. Obes. Surg. 2014, 24, 1499–1509. [Google Scholar] [CrossRef] [PubMed]
- Sjöström, L.; Gummesson, A.; Sjöström, C.D.; Narbro, K.; Peltonen, M.; Wedel, H.; Bengtsson, C.; Bouchard, C.; Carlsson, B.; Dahlgren, S.; et al. Effects of bariatric surgery on cancer incidence in obese patients in Sweden (Swedish Obese Subjects Study): A prospective, controlled intervention trial. Lancet Oncol. 2009, 10, 653–662. [Google Scholar] [CrossRef] [PubMed]
| Variables Influencing Sex and Gender Dimorphism in Obesity | |
|---|---|
| Sex and Gender—Specific Differences underlying Obesity Pathology |
|
| Sex and Gender—Specific Risk Factors Influencing Obesity Pathology |
|
| Clinical Implications of Sex and Gender—Related Differences in Obesity |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koceva, A.; Herman, R.; Janez, A.; Rakusa, M.; Jensterle, M. Sex- and Gender-Related Differences in Obesity: From Pathophysiological Mechanisms to Clinical Implications. Int. J. Mol. Sci. 2024, 25, 7342. https://doi.org/10.3390/ijms25137342
Koceva A, Herman R, Janez A, Rakusa M, Jensterle M. Sex- and Gender-Related Differences in Obesity: From Pathophysiological Mechanisms to Clinical Implications. International Journal of Molecular Sciences. 2024; 25(13):7342. https://doi.org/10.3390/ijms25137342
Chicago/Turabian StyleKoceva, Andrijana, Rok Herman, Andrej Janez, Matej Rakusa, and Mojca Jensterle. 2024. "Sex- and Gender-Related Differences in Obesity: From Pathophysiological Mechanisms to Clinical Implications" International Journal of Molecular Sciences 25, no. 13: 7342. https://doi.org/10.3390/ijms25137342
APA StyleKoceva, A., Herman, R., Janez, A., Rakusa, M., & Jensterle, M. (2024). Sex- and Gender-Related Differences in Obesity: From Pathophysiological Mechanisms to Clinical Implications. International Journal of Molecular Sciences, 25(13), 7342. https://doi.org/10.3390/ijms25137342






