Exploring Molecular Mechanisms and Biomarkers in COPD: An Overview of Current Advancements and Perspectives
Abstract
1. Introduction
2. Molecular Pathogenesis of COPD
2.1. Inflammation
2.2. Oxidative Stress
2.3. Protease–Antiprotease Imbalance
2.4. Genetic Susceptibility
2.5. Lung Tissue Remodeling
3. Molecular Biomarkers in COPD (Table 1)
3.1. Blood-Based Biomarkers
| Biomarker | Function | Clinical Relevance | References |
|---|---|---|---|
| C-reactive protein | Indicator of systemic inflammation | Linked to disease severity and exacerbation risks | [28] |
| Fibrinogen | Role in coagulation cascade | Associated with cardiovascular comorbidities | [31] |
| White blood cells | Immune response indicator | Reflects inflammation levels and disease progression | [32] |
| Malondialdehyde | Marker of oxidative stress | Reflects systemic redox imbalances in COPD | [33] |
| MMPs | Proteins involved in tissue | Extracellular matrix remodeling and lung function | [30] |
3.2. Sputum-Based Biomarkers
3.3. Imaging Biomarkers
4. Therapeutic Targets and Personalized Medicine in COPD (Table 2)
| Therapeutic Target | Mechanism | Potential Clinical Impact | References |
|---|---|---|---|
| PDE4 inhibitors | Anti-inflammatory agent | Reduced exacerbation rates and improved lung function | [41,42] |
| IL-5 monoclonal antibodies | Targeting eosinophilic inflammation | Reduced exacerbation frequency and enhanced lung function | [43] |
| M3 receptor antagonists | Improved bronchodilation | Potency and safety profiles surpassing traditional therapies | [44,45] |
| Biologic agents targeting IL-17 pathway | Modulating immune responses | Potential for treating neutrophilic inflammation | [46] |
| Beta-2 adrenergic receptor | Improved bronchodilation | Long-established, symptomatic, and effective therapeutic target | [47] |
4.1. Molecularly Targeted Therapies
4.2. Precision Medicine Approaches
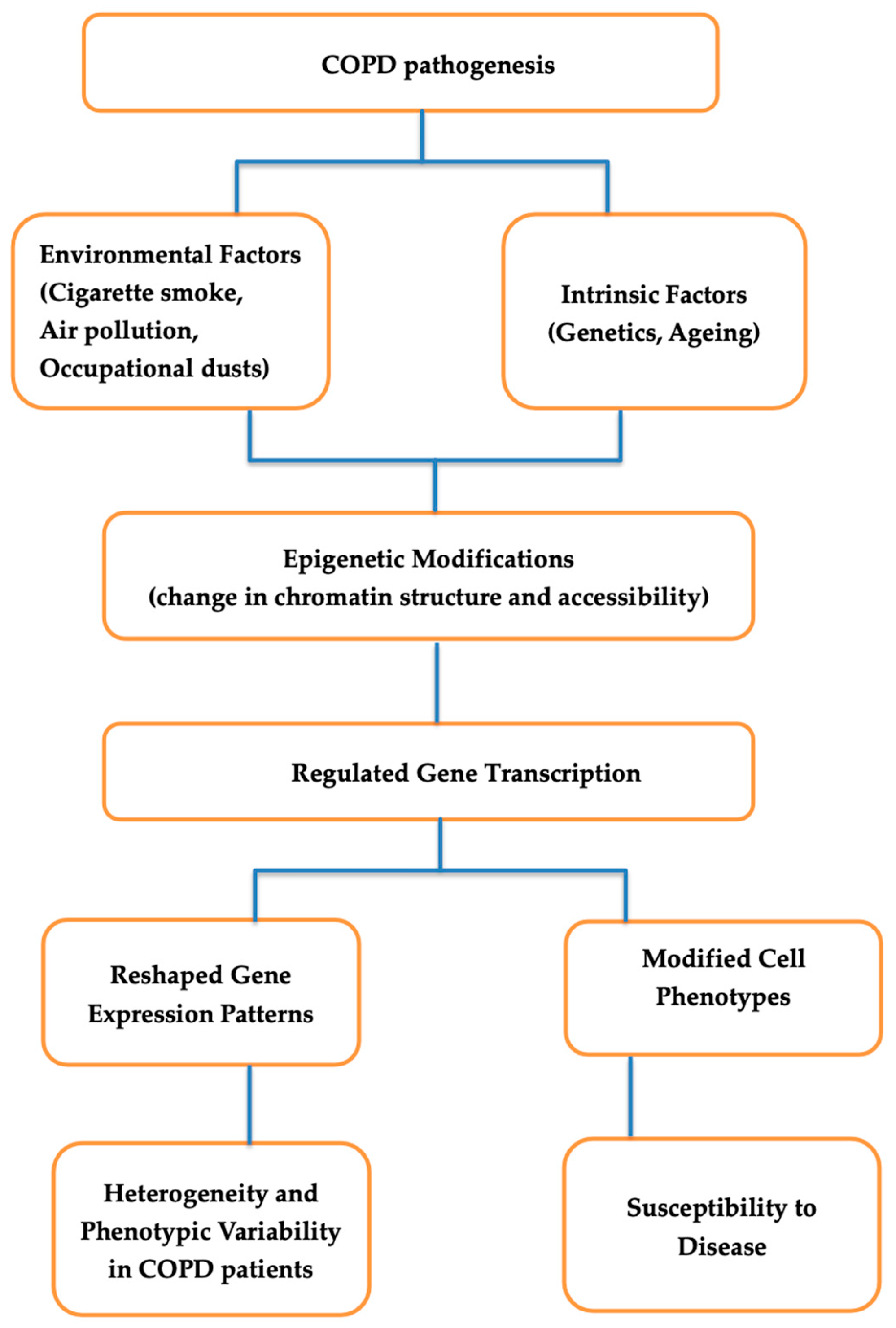
5. Epigenetics and Gene Regulation in COPD
5.1. Epigenetic Modifications
5.2. Gene Regulatory Networks
5.3. Implications for Understanding Disease Heterogeneity and Identifying Therapeutic Targets
6. Emerging Technologies and Omics Approaches in COPD Research
6.1. Genomics
6.2. Transcriptomics
6.3. Proteomics
6.4. Metabolomics
6.5. Integration of Multi-Omics Data
7. Challenges and Future Directions in COPD Research
7.1. Translating Molecular Research into Clinical Applications
7.2. Future Perspectives on Targeted Therapies and Precision Medicine
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, J.; Lee, J.H.; Kim, Y.; Kim, K.; Oh, Y.M.; Yoo, K.H.; Rhee, C.K.; Yoon, H.K.; Kim, Y.S.; Park, Y.B.; et al. Association between chronic obstructive pulmonary disease and gastroesophageal reflux disease: A national cross-sectional cohort study. BMC Pulm. Med. 2013, 13, 51. [Google Scholar] [CrossRef] [PubMed]
- López-Campos, J.L.; Tan, W.; Soriano, J.B. Global burden of COPD. Respirology 2016, 21, 14–23. [Google Scholar] [CrossRef]
- Shaddock, E.; Richards, G. Pharmacological management of chronic obstructive pulmonary disease. S. Afr. Med. J. 2015, 105, 790. [Google Scholar] [CrossRef] [PubMed]
- Iheanacho, I.; Zhang, S.; King, D.; Rizzo, M.; Ismaila, A.S. Economic burden of chronic obstructive pulmonary disease (COPD): A systematic literature review. Int. J. Chronic Obstr. Pulm. Dis. 2020, 15, 439–460. [Google Scholar] [CrossRef] [PubMed]
- Akmatov, M.K.; Ermakova, T.; Holstiege, J.; Steffen, A.; von Stillfried, D.; Bätzing, J. Comorbidity profile of patients with concurrent diagnoses of asthma and COPD in Germany. Sci. Rep. 2020, 10, 17945. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Cellular and molecular mechanisms of asthma and COPD. Clin. Sci. 2017, 131, 1541–1558. [Google Scholar] [CrossRef] [PubMed]
- Fischer, B.M.; Pavlisko, E.; Voynow, J.A. Pathogenic triad in COPD: Oxidative stress, protease–antiprotease imbalance, and inflammation. Int. J. Chronic Obstr. Pulm. Dis. 2011, 6, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Hosking, L.; Yeo, A.; Hoffman, J.; Chiano, M.; Fraser, D.; Ghosh, S.; Lipson, D.A.; Martin, N.; Condreay, L.D.; Cox, C. Genetics plays a limited role in predicting chronic obstructive pulmonary disease treatment response and exacerbation. Respir. Med. 2021, 187, 106573. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, I.; Guimarães, M.; Van Zeller, M.; Menezes, F.; Moita, J.; Simão, P. Clinical and molecular markers in COPD. Pulmonology 2018, 24, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Lange, P.; Celli, B.; Agustí, A.; Boje Jensen, G.; Divo, M.; Faner, R.; Guerra, S.; Marott, J.L.; Martinez, F.D.; Martinez-Camblor, P.; et al. Lung-Function Trajectories Leading to Chronic Obstructive Pulmonary Disease. N. Engl. J. Med. 2015, 373, 111–122. [Google Scholar] [PubMed]
- Caro, J.; Marín, L.; Iazbik, M.; Zaldivar-López, S.; Borghese, H.; Couto, C. Markers of iron metabolism in retired racing Greyhounds with and without osteosarcoma. Vet. Clin. Pathol. 2013, 42, 360–363. [Google Scholar] [CrossRef] [PubMed]
- Brandsma, C.A.; Van den Berge, M.; Hackett, T.L.; Brusselle, G.; Timens, W. Recent advances in chronic obstructive pulmonary disease pathogenesis: From disease mechanisms to precision medicine. J. Pathol. 2020, 250, 624–635. [Google Scholar] [CrossRef] [PubMed]
- Molinaro, M.; Ameri, P.; Marone, G.; Petretta, M.; Abete, P.; Di Lisa, F.; De Placido, S.; Bonaduce, D.; Tocchetti, C.G. Recent Advances on Pathophysiology, Diagnostic and Therapeutic Insights in Cardiac Dysfunction Induced by Antineoplastic Drugs. BioMed. Res. Int. 2015, 2015, 138148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Valizadeh, H.; Alipourfard, I.; Bidares, R.; Aghebati-Maleki, L.; Ahmadi, M. Epigenetic modifications and therapy in chronic obstructive pulmonary disease (COPD): An update review. COPD J. Chronic Obstr. Pulm. Dis. 2020, 17, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Darquenne, C. Aerosol deposition in health and disease. J. Aerosol. Med. Pulm. Drug Deliv. 2012, 25, 140–147. [Google Scholar] [CrossRef]
- Barnes, P.J. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2016, 138, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Janciauskiene, S. The beneficial effects of antioxidants in health and diseases. Chronic Obstr. Pulm. Dis. J. COPD Found. 2020, 7, 182. [Google Scholar] [CrossRef]
- Mortaz, E.; Tabarsi, P.; Varahram, M.; Folkerts, G.; Adcock, I.M. The Immune Response and Immunopathology of COVID-19. Front. Immunol. 2020, 11, 2037. [Google Scholar] [CrossRef] [PubMed]
- Uemasu, K.; Tanabe, N.; Tanimura, K.; Hasegawa, K.; Mizutani, T.; Hamakawa, Y.; Sato, S.; Ogawa, E.; Thomas, M.J.; Ikegami, M. Serine protease imbalance in the small airways and development of centrilobular emphysema in chronic obstructive pulmonary disease. Am. J. Respir. Cell. Mol. Biol. 2020, 63, 67–78. [Google Scholar] [CrossRef] [PubMed]
- McKelvey, M.C.; Brown, R.; Ryan, S.; Mall, M.A.; Weldon, S.; Taggart, C.C. Proteases, mucus, and mucosal immunity in chronic lung disease. Int. J. Mol. Sci. 2021, 22, 5018. [Google Scholar] [CrossRef] [PubMed]
- Kelly-Robinson, G.A.; Reihill, J.A.; Lundy, F.T.; McGarvey, L.P.; Lockhart, J.C.; Litherland, G.J.; Thornbury, K.D.; Martin, S.L. The serpin superfamily and their role in the regulation and dysfunction of serine protease activity in COPD and other chronic lung diseases. Int. J. Mol. Sci. 2021, 22, 6351. [Google Scholar] [CrossRef] [PubMed]
- Sze, M.A.; Dimitriu, P.A.; Suzuki, M.; McDonough, J.E.; Campbell, J.D.; Brothers, J.F.; Erb-Downward, J.R.; Huffnagle, G.B.; Hayashi, S.; Elliott, W.M.; et al. Host Response to the Lung Microbiome in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2015, 192, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Kiss, N. Nutrition support and dietary interventions for patients with lung cancer: Current insights. Lung Cancer 2016, 7, 1–9. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, Y.-C.; Tsai, Y.-H.; Wang, C.-C.; Liu, S.-F.; Chen, T.-W.; Fang, W.-F.; Lee, C.-P.; Hsu, P.-Y.; Chao, T.-Y.; Wu, C.-C. Epigenome-wide association study on asthma and chronic obstructive pulmonary disease overlap reveals aberrant DNA methylations related to clinical phenotypes. Sci. Rep. 2021, 11, 5022. [Google Scholar] [CrossRef] [PubMed]
- Demedts, I.K.; Demoor, T.; Bracke, K.R.; Joos, G.F.; Brusselle, G.G. Role of apoptosis in the pathogenesis of COPD and pulmonary emphysema. Respir. Res. 2006, 7, 53. [Google Scholar] [CrossRef] [PubMed]
- May, S.M.; Li, J.T. Burden of chronic obstructive pulmonary disease: Healthcare costs and beyond. Allergy Asthma Proc. 2015, 36, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Rennard, S.I.; Dale, D.C.; Donohue, J.F.; Kanniess, F.; Magnussen, H.; Sutherland, E.R.; Watz, H.; Lu, S.; Stryszak, P.; Rosenberg, E.; et al. CXCR2 Antagonist MK-7123. A Phase 2 Proof-of-Concept Trial for Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2015, 191, 1001–1011. [Google Scholar] [CrossRef] [PubMed]
- Su, B.; Liu, T.; Fan, H.; Chen, F.; Ding, H.; Wu, Z.; Wang, H.; Hou, S. Inflammatory Markers and the Risk of Chronic Obstructive Pulmonary Disease: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0150586. [Google Scholar] [CrossRef] [PubMed]
- Bartel, S.; Schulz, N.; Alessandrini, F.; Schamberger, A.C.; Pagel, P.; Theis, F.J.; Milger, K.; Noessner, E.; Stick, S.M.; Kicic, A.; et al. Pulmonary microRNA profiles identify involvement of Creb1 and Sec14l3 in bronchial epithelial changes in allergic asthma. Sci. Rep. 2017, 7, 46026. [Google Scholar] [CrossRef] [PubMed]
- Almuntashiri, S.; Alhumaid, A.; Zhu, Y.; Han, Y.; Dutta, S.; Khilji, O.; Zhang, D.; Wang, X. TIMP-1 and its potential diagnostic and prognostic value in pulmonary diseases. Chin. Med. J. Pulm. Crit. Care Med. 2023, 1, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Luyendyk, J.P.; Schoenecker, J.G.; Flick, M.J. The multifaceted role of fibrinogen in tissue injury and inflammation. Blood J. Am. Soc. Hematol. 2019, 133, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Yuan, L.; Wu, K.; Shen, B.; Zhu, C. Association between systemic immune-inflammation index and chronic obstructive pulmonary disease: A population-based study. BMC Pulm. Med. 2023, 23, 295. [Google Scholar] [CrossRef] [PubMed]
- Zinellu, E.; Zinellu, A.; Fois, A.G.; Pau, M.C.; Scano, V.; Piras, B.; Carru, C.; Pirina, P. Oxidative Stress Biomarkers in Chronic Obstructive Pulmonary Disease Exacerbations: A Systematic Review. Antioxidants 2021, 10, 710. [Google Scholar] [CrossRef] [PubMed]
- Goswami, M.T.; Chen, G.; Chakravarthi, B.V.; Pathi, S.S.; Anand, S.K.; Carskadon, S.L.; Giordano, T.J.; Chinnaiyan, A.M.; Thomas, D.G.; Palanisamy, N.; et al. Role and regulation of coordinately expressed de novo purine biosynthetic enzymes PPAT and PAICS in lung cancer. Oncotarget 2015, 6, 23445–23461. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, F.; Trinkmann, F.; Abdo, M.; Kirsten, A.-M.; Rabe, K.F.; Watz, H.; Baraldo, S.; Saetta, M.; Hohlfeld, J.M.; Holz, O. Influence of cell quality on inflammatory biomarkers in COPD sputum supernatant. Int. J. Chronic Obstr. Pulm. Dis. 2021, 16, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, N.; Yi, X.; Wang, Z. The translational potential of the lung microbiome as a biomarker and a therapeutic target for chronic obstructive pulmonary disease. Interdiscip. Med. 2023, 1, e20230023. [Google Scholar] [CrossRef]
- Vasilescu, D.M.; Martinez, F.J.; Marchetti, N.; Galbán, C.J.; Hatt, C.; Meldrum, C.A.; Dass, C.; Tanabe, N.; Reddy, R.M.; Lagstein, A. Noninvasive imaging biomarker identifies small airway damage in severe chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2019, 200, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, S. Quantitative Imaging Biomarkers in Precision Medicine. In Advances in Imaging: Step towards Precision Medicine; Springer: Berlin/Heidelberg, Germany, 2022; pp. 317–326. [Google Scholar]
- Raoof, S.; Shah, M.; Braman, S.; Agrawal, A.; Allaqaband, H.; Bowler, R.; Castaldi, P.; DeMeo, D.; Fernando, S.; Hall, C.S. Lung imaging in COPD Part 2: Emerging concepts. Chest 2023, 164, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Benincasa, G.; DeMeo, D.L.; Glass, K.; Silverman, E.K.; Napoli, C. Epigenetics and pulmonary diseases in the horizon of precision medicine: A review. Eur. Respir. J. 2021, 57, 2003406. [Google Scholar] [CrossRef]
- Octafia, I.; Sari, D.O.; Wulandari, N.; Annisa, S.; Wongkar, L.W.; Bahari, F.K.; Farikhah, F.; Firmansah, M.; Midhiawati, E.; Herawati, F. Roflumilast: A Review of Chronic Obstructive Pulmonary Disease (COPD) Treatment. Qanun Med. 2021, 5, 35–47. [Google Scholar] [CrossRef]
- Phillips, J.E. Inhaled phosphodiesterase 4 (PDE4) inhibitors for inflammatory respiratory diseases. Front. Pharmacol. 2020, 11, 259. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Targeting cytokines to treat asthma and chronic obstructive pulmonary disease. Nat. Rev. Immunol. 2018, 18, 454–466. [Google Scholar] [CrossRef] [PubMed]
- Bonini, M.; Usmani, O.S. Drugs for airway disease. Medicine 2020, 48, 314–322. [Google Scholar] [CrossRef]
- Cazzola, M.; Page, C.; Matera, M.G. Long-acting muscarinic receptor antagonists for the treatment of respiratory disease. Pulm. Pharmacol. Ther. 2013, 26, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Henen, C.; Johnson, E.A.; Wiesel, S. Unleashing the Power of IL-17: A Promising Frontier in Chronic Obstructive Pulmonary Disease (COPD) Treatment. Cureus 2023, 15, e41977. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.; Yu, G.; Wu, Y.; Wang, H. Role of β2-adrenergic receptors in chronic obstructive pulmonary disease. Life Sci. 2021, 265, 118864. [Google Scholar] [CrossRef] [PubMed]
- Miravitlles, M.; Soler-Cataluña, J.J.; Calle, M.; Molina, J.; Almagro, P.; Quintano, J.A.; Trigueros, J.A.; Piñera, P.; Simón, A.; Riesco, J.A.; et al. A new approach to grading and treating COPD based on clinical phenotypes: Summary of the Spanish COPD guidelines (GesEPOC). Prim. Care Respir. J. 2013, 22, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Greulich, T.; Vogelmeier, C.F. Alpha-1-antitrypsin deficiency: Increasing awareness and improving diagnosis. Ther. Adv. Respir. Dis. 2016, 10, 72–84. [Google Scholar] [CrossRef]
- Hu, Y.; Cheng, X.; Qiu, Z.; Chen, X. Identification of metabolism-associated molecular subtypes of chronic obstructive pulmonary disease. Int. J. Chronic Obstr. Pulm. Dis. 2021, 16, 2351–2362. [Google Scholar] [CrossRef] [PubMed]
- Pantazopoulos, I.; Magounaki, K.; Kotsiou, O.; Rouka, E.; Perlikos, F.; Kakavas, S.; Gourgoulianis, K. Incorporating Biomarkers in COPD Management: The Research Keeps Going. J. Pers. Med. 2022, 12, 379. [Google Scholar] [CrossRef]
- Zong, D.D.; Ouyang, R.Y.; Chen, P. Epigenetic mechanisms in chronic obstructive pulmonary disease. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 844–856. [Google Scholar] [PubMed]
- Barnes, P.J. Oxidative stress-based therapeutics in COPD. Redox Biol. 2020, 33, 101544. [Google Scholar] [CrossRef] [PubMed]
- Morrow, J.D.; Qiu, W.; Chhabra, D.; Rennard, S.I.; Belloni, P.; Belousov, A.; Pillai, S.G.; Hersh, C.P. Identifying a gene expression signature of frequent COPD exacerbations in peripheral blood using network methods. BMC Med. Genom. 2015, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Nezhad, A.F. The Function of Histone Modifications in Chronic Obstructive Pulmonary Disease. Preprints 2022, 2022080265. [Google Scholar] [CrossRef]
- Zhuang, Y.; Hobbs, B.D.; Hersh, C.P.; Kechris, K. Identifying miRNA-mRNA networks associated with COPD phenotypes. Front. Genet. 2021, 12, 748356. [Google Scholar] [CrossRef] [PubMed]
- Perry, M.M.; Tsitsiou, E.; Austin, P.J.; Lindsay, M.A.; Gibeon, D.S.; Adcock, I.M.; Chung, K.F. Role of non-coding RNAs in maintaining primary airway smooth muscle cells. Respir. Res. 2014, 15, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, T.; Wadhwa, R.; Thapliyal, N.; Sharma, K.; Rani, V.; Maurya, P.K. Oxidative, inflammatory, genetic, and epigenetic biomarkers associated with chronic obstructive pulmonary disorder. J. Cell. Physiol. 2019, 234, 2067–2082. [Google Scholar] [CrossRef] [PubMed]
- Soni, D.K.; Biswas, R. Role of non-coding RNAs in post-transcriptional regulation of lung diseases. Front. Genet. 2021, 12, 767348. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Todd, N.W.; Liu, Z.; Zhan, M.; Fang, H.; Peng, H.; Alattar, M.; Deepak, J.; Stass, S.A.; Jiang, F. Altered miRNA expression in sputum for diagnosis of non-small cell lung cancer. Lung Cancer 2010, 67, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.H.; McDonald, M.L.; Zhou, X.; Mattheisen, M.; Castaldi, P.J.; Hersh, C.P.; Demeo, D.L.; Sylvia, J.S.; Ziniti, J.; Laird, N.M.; et al. Risk loci for chronic obstructive pulmonary disease: A genome-wide association study and meta-analysis. Lancet Respir. Med. 2014, 2, 214–225. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, A.; Ghosh, D.; Bade, G.; Guleria, R.; Sampath, M.; Talwar, A. Array-based Comparative Genomic Hybridization (aCGH) Reveals Chromosomal Aberrations in Chronic Obstructive Pulmonary Disease (COPD): A Preliminary Study. Eur. J. Med. Health Sci. 2021, 3, 127–133. [Google Scholar] [CrossRef]
- Huang, Q.; Wang, Y.; Zhang, L.; Qian, W.; Shen, S.; Wang, J.; Wu, S.; Xu, W.; Chen, B.; Lin, M. Single-cell transcriptomics highlights immunological dysregulations of monocytes in the pathobiology of COPD. Respir. Res. 2022, 23, 367. [Google Scholar] [CrossRef] [PubMed]
- Thul, P.J.; Åkesson, L.; Wiking, M.; Mahdessian, D.; Geladaki, A.; Ait Blal, H.; Alm, T.; Asplund, A.; Björk, L.; Breckels, L.M.; et al. A subcellular map of the human proteome. Science 2017, 356, eaal3321. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-H.; Hoopmann, M.R.; Castaldi, P.J.; Simonsen, K.A.; Midha, M.K.; Cho, M.H.; Criner, G.J.; Bueno, R.; Liu, J.; Moritz, R.L. Lung proteomic biomarkers associated with chronic obstructive pulmonary disease. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2021, 321, L1119–L1130. [Google Scholar] [CrossRef] [PubMed]
- Birhanu, A.G. Mass spectrometry-based proteomics as an emerging tool in clinical laboratories. Clin. Proteom. 2023, 20, 32. [Google Scholar] [CrossRef] [PubMed]
- Nambiar, S.; Bong How, S.; Gummer, J.; Trengove, R.; Moodley, Y. Metabolomics in chronic lung diseases. Respirology 2020, 25, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Morandi, A.; Taddei, M.L.; Chiarugi, P.; Giannoni, E. Targeting the Metabolic Reprogramming That Controls Epithelial-to-Mesenchymal Transition in Aggressive Tumors. Front. Oncol. 2017, 7, 40. [Google Scholar] [CrossRef] [PubMed]
- Sliz, E.; Kettunen, J.; Holmes, M.V.; Williams, C.O.; Boachie, C.; Wang, Q.; Männikkö, M.; Sebert, S.; Walters, R.; Lin, K.; et al. Metabolomic consequences of genetic inhibition of PCSK9 compared with statin treatment. Circulation 2018, 138, 2499–2512. [Google Scholar] [CrossRef] [PubMed]
- Gillenwater, L.A.; Helmi, S.; Stene, E.; Pratte, K.A.; Zhuang, Y.; Schuyler, R.P.; Lange, L.; Castaldi, P.J.; Hersh, C.P.; Banaei-Kashani, F. Multi-omics subtyping pipeline for chronic obstructive pulmonary disease. PLoS ONE 2021, 16, e0255337. [Google Scholar] [CrossRef]
- Cruickshank-Quinn, C.I.; Jacobson, S.; Hughes, G.; Powell, R.L.; Petrache, I.; Kechris, K.; Bowler, R.; Reisdorph, N. Metabolomics and transcriptomics pathway approach reveals outcome-specific perturbations in COPD. Sci. Rep. 2018, 8, 17132. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Endo-phenotyping of COPD patients. Expert Rev. Respir. Med. 2021, 15, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Goh, F.; Shaw, J.G.; Savarimuthu Francis, S.M.; Vaughan, A.; Morrison, L.; Relan, V.; Marshall, H.M.; Dent, A.G.; O’Hare, P.E.; Hsiao, A.; et al. Personalizing and targeting therapy for COPD: The role of molecular and clinical biomarkers. Expert Rev. Respir. Med. 2013, 7, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Tanner, L.; Single, A.B. Animal models reflecting chronic obstructive pulmonary disease and related respiratory disorders: Translating pre-clinical data into clinical relevance. J. Innate Immun. 2020, 12, 203–225. [Google Scholar] [CrossRef] [PubMed]
- Meteran, H.; Sivapalan, P.; Stæhr Jensen, J.-U. Treatment response biomarkers in asthma and COPD. Diagnostics 2021, 11, 1668. [Google Scholar] [CrossRef] [PubMed]
- Regard, L.; Roche, N.; Burgel, P.-R. The Ongoing Quest for Predictive Biomarkers in Chronic Obstructive Pulmonary Disease; American Thoracic Society: New York, NY, USA, 2023; Volume 208, pp. 511–513. [Google Scholar]
- Cazzola, M.; Calzetta, L.; Rogliani, P.; Matera, M.G. The challenges of precision medicine in COPD. Mol. Diagn. Ther. 2017, 21, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Xing, F.; Ghosh, D.; Hobbs, B.D.; Hersh, C.P.; Banaei-Kashani, F.; Bowler, R.P.; Kechris, K. Deep learning on graphs for multi-omics classification of COPD. PLoS ONE 2023, 18, e0284563. [Google Scholar]
- Wang, C.; Zhou, J.; Wang, J.; Li, S.; Fukunaga, A.; Yodoi, J.; Tian, H. Progress in the mechanism and targeted drug therapy for COPD. Signal Transduct. Target. Ther. 2020, 5, 248. [Google Scholar] [CrossRef] [PubMed]
- Dailah, H.G. Therapeutic potential of small molecules targeting oxidative stress in the treatment of chronic obstructive pulmonary disease (COPD): A comprehensive review. Molecules 2022, 27, 5542. [Google Scholar] [CrossRef] [PubMed]
- Van den Berge, M.; Faiz, A. Transcriptome-based Signatures: The Future Biomarkers in Obstructive Pulmonary Diseases Such as Asthma and Chronic Obstructive Pulmonary Disease? Am. J. Respir. Crit. Care Med. 2022, 205, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Coutu, F.-A.; Iorio, O.C.; Ross, B.A. Remote patient monitoring strategies and wearable technology in chronic obstructive pulmonary disease. Front. Med. 2023, 10, 1236598. [Google Scholar] [CrossRef] [PubMed]
- Dunn, J.; Coravos, A.; Fanarjian, M.; Ginsburg, G.S.; Steinhubl, S.R. Remote digital health technologies for improving the care of people with respiratory disorders. Lancet Digit. Health 2024, 6, e291–e298. [Google Scholar] [CrossRef] [PubMed]
- Van Dijk, M.; Gan, C.T.; Koster, T.D.; Wijkstra, P.J.; Slebos, D.-J.; Kerstjens, H.A.; van der Vaart, H.; Duiverman, M.L. Treatment of severe stable COPD: The multidimensional approach of treatable traits. ERJ Open Res. 2020, 6, 00322-2019. [Google Scholar] [CrossRef] [PubMed]
- Czarnecka-Chrebelska, K.H.; Mukherjee, D.; Maryanchik, S.V.; Rudzinska-Radecka, M. Biological and genetic mechanisms of COPD, its diagnosis, treatment, and relationship with lung cancer. Biomedicines 2023, 11, 448. [Google Scholar] [CrossRef] [PubMed]
- Pavord, I.D.; Beasley, R.; Agusti, A.; Anderson, G.P.; Bel, E.; Brusselle, G.; Cullinan, P.; Custovic, A.; Ducharme, F.M.; Fahy, J.V.; et al. After asthma: Redefining airways diseases. Lancet 2018, 391, 350–400. [Google Scholar] [CrossRef] [PubMed]
- McGregor, M.C.; Krings, J.G.; Nair, P.; Castro, M. Role of Biologics in Asthma. Am. J. Respir. Crit. Care Med. 2019, 199, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Blutt, S.E.; Coarfa, C.; Neu, J.; Pammi, M. Multiomic investigations into lung health and disease. Microorganisms 2023, 11, 2116. [Google Scholar] [CrossRef] [PubMed]
- Lommatzsch, M.; van Eeden, S.F. Immune modulation in chronic respiratory diseases: The path to precision medicine. Respiration 2020, 99, 548–549. [Google Scholar] [CrossRef] [PubMed]
- Berndt, A.; Leme, A.S.; Shapiro, S.D. Emerging genetics of COPD. EMBO Mol. Med. 2012, 4, 1144–1155. [Google Scholar] [CrossRef] [PubMed]
- Shakeel, I.; Ashraf, A.; Afzal, M.; Sohal, S.S.; Islam, A.; Kazim, S.N.; Hassan, M.I. The Molecular Blueprint for Chronic Obstructive Pulmonary Disease (COPD): A New Paradigm for Diagnosis and Therapeutics. Oxid. Med. Cell. Longev. 2023, 2023, 2297559. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.-L.; Liu, S.-F. Exploring Molecular Mechanisms and Biomarkers in COPD: An Overview of Current Advancements and Perspectives. Int. J. Mol. Sci. 2024, 25, 7347. https://doi.org/10.3390/ijms25137347
Li C-L, Liu S-F. Exploring Molecular Mechanisms and Biomarkers in COPD: An Overview of Current Advancements and Perspectives. International Journal of Molecular Sciences. 2024; 25(13):7347. https://doi.org/10.3390/ijms25137347
Chicago/Turabian StyleLi, Chin-Ling, and Shih-Feng Liu. 2024. "Exploring Molecular Mechanisms and Biomarkers in COPD: An Overview of Current Advancements and Perspectives" International Journal of Molecular Sciences 25, no. 13: 7347. https://doi.org/10.3390/ijms25137347
APA StyleLi, C.-L., & Liu, S.-F. (2024). Exploring Molecular Mechanisms and Biomarkers in COPD: An Overview of Current Advancements and Perspectives. International Journal of Molecular Sciences, 25(13), 7347. https://doi.org/10.3390/ijms25137347






