Dissimilar Changes in Serum Cortisol after Epileptic and Psychogenic Non-Epileptic Seizures: A Promising Biomarker in the Differential Diagnosis of Paroxysmal Events?
Abstract
1. Introduction
2. Results
2.1. Characteristics of Patients at Admission
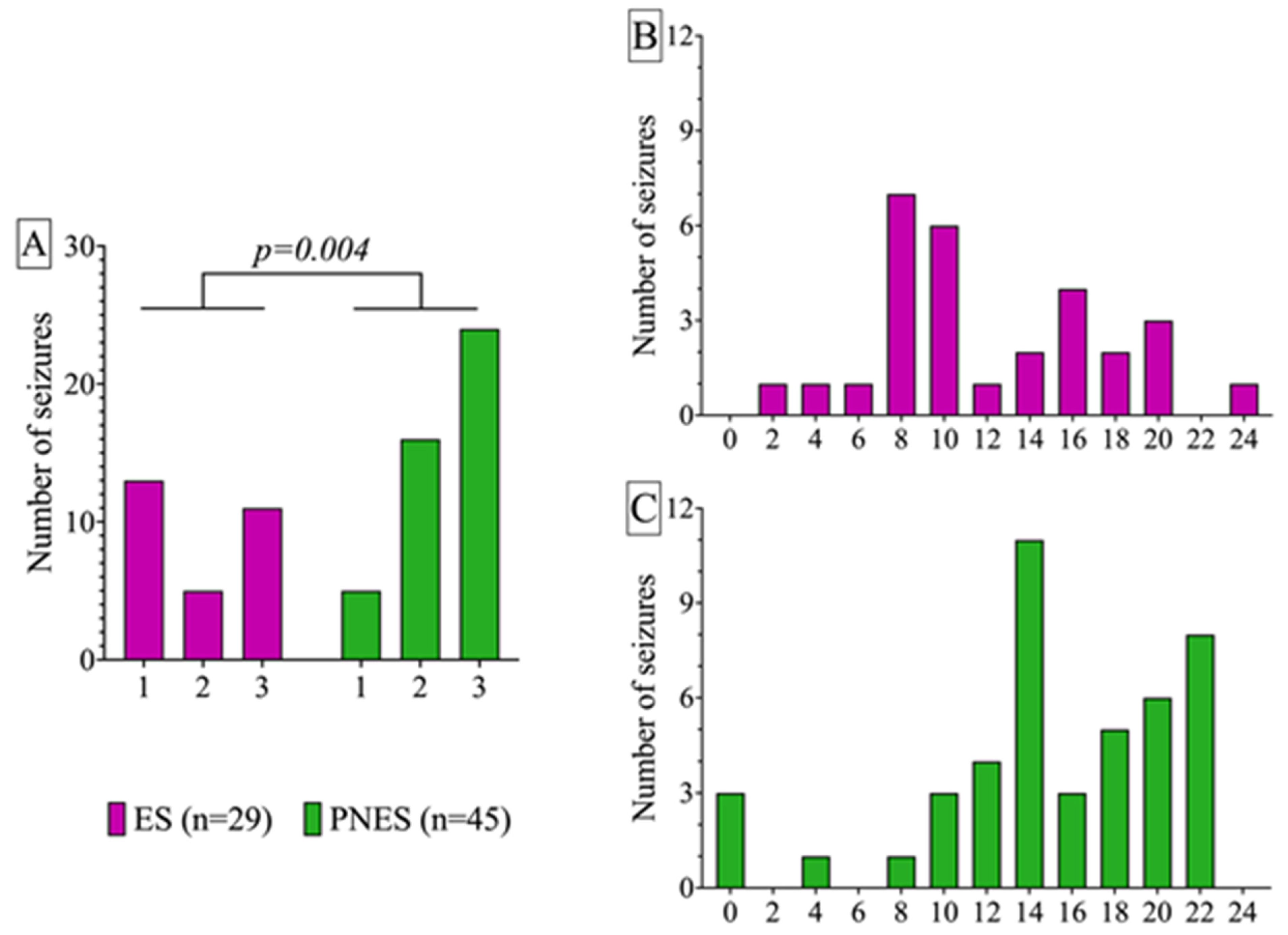
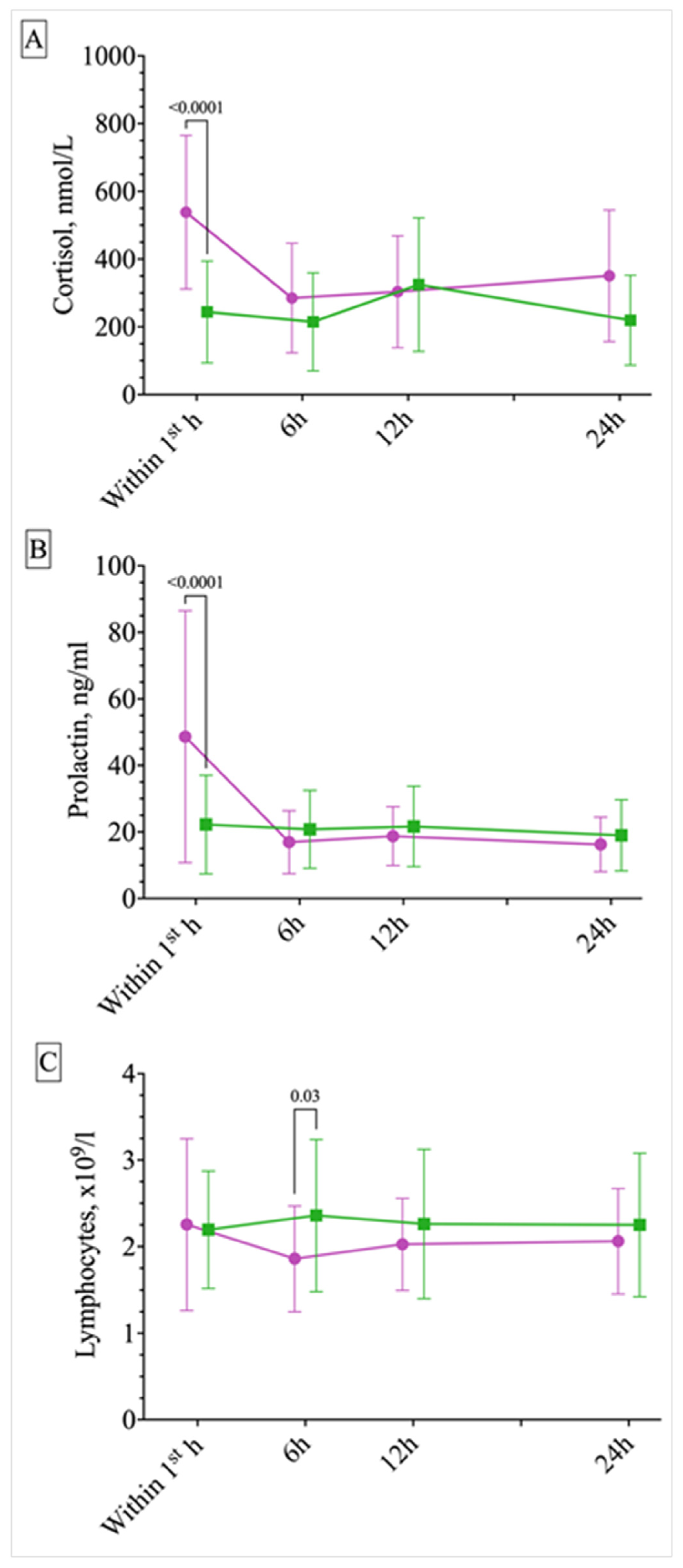
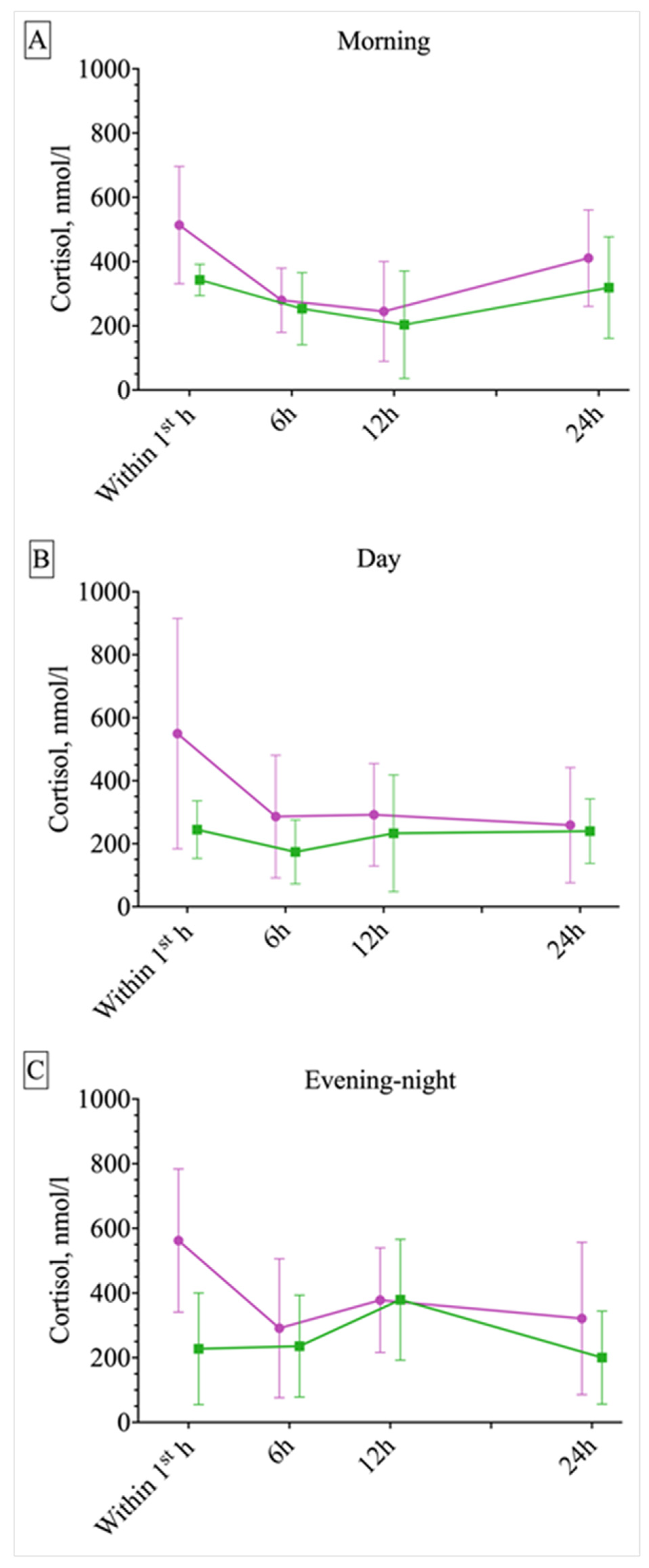
2.2. Circadian Differences in ES and PNES Occurrence and Response of Serum Cortisol, Prolactin, and Lymphocytes to Different Paroxysmal Events
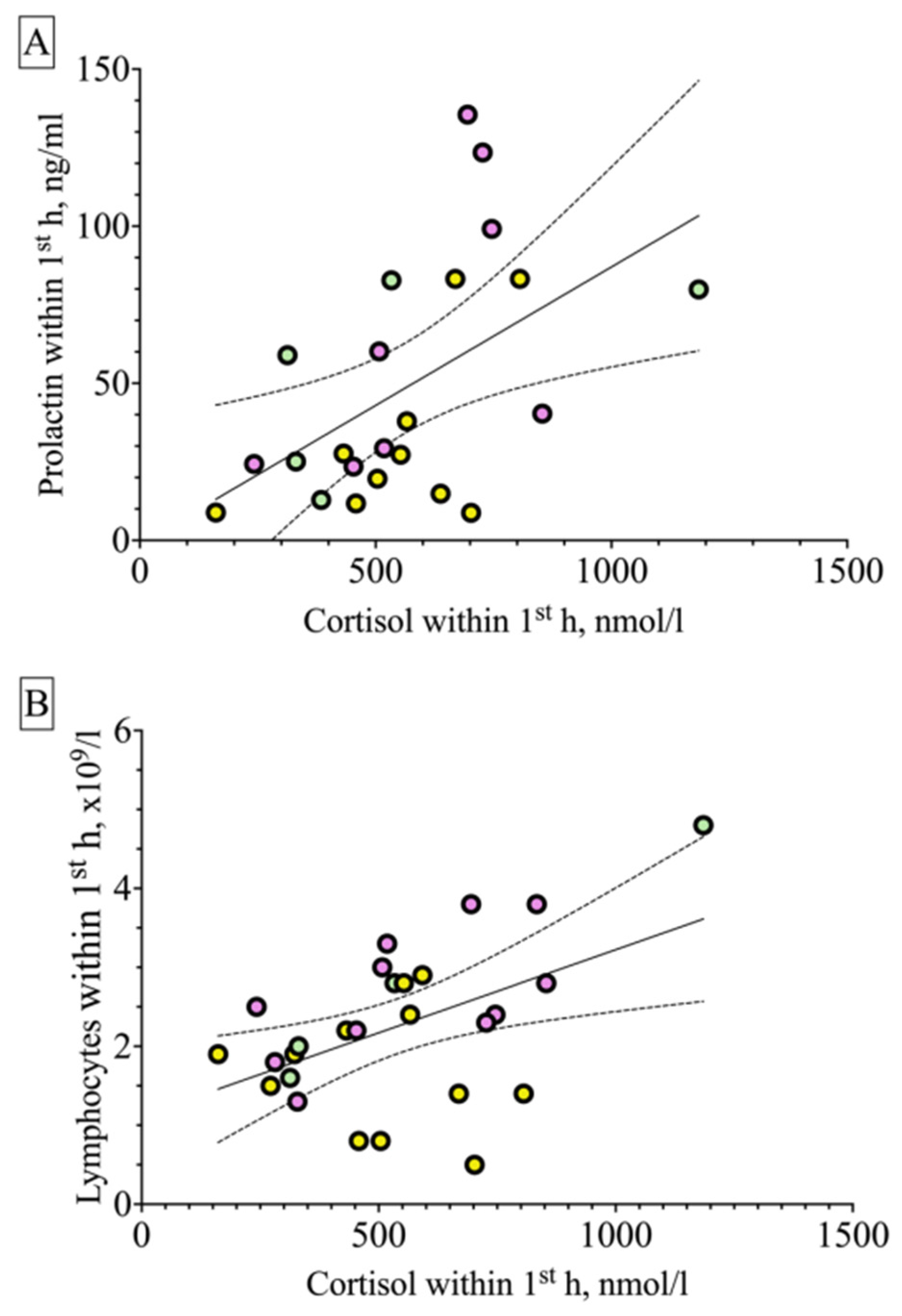
2.3. Correlations
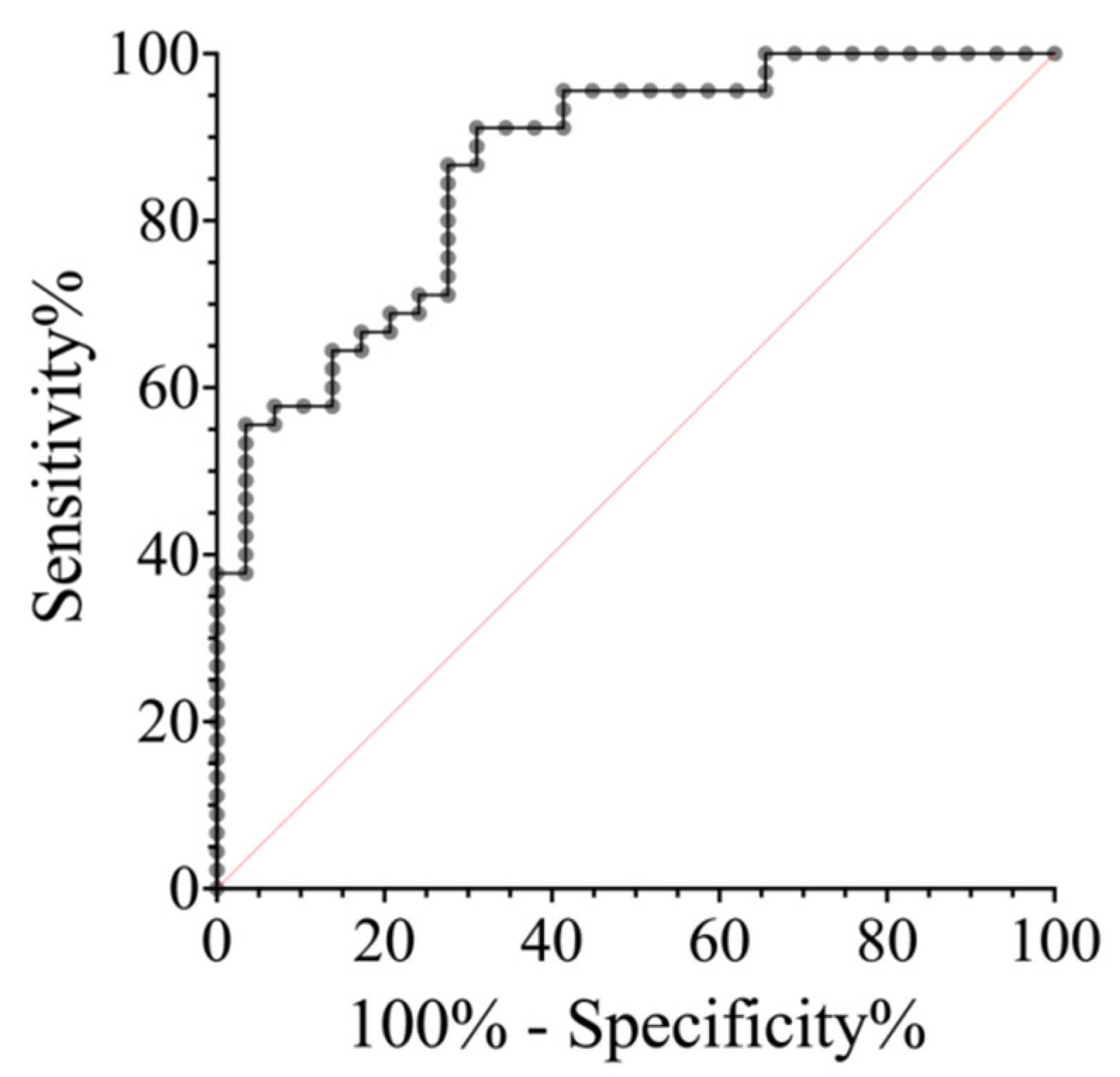
2.4. ROC Analysis
3. Discussion
4. Materials and Methods
4.1. Subjects, Procedure, and Instruments
4.2. Blood Sampling, Assessment of Hormones, and Hemogram
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bodde, N.M.; Brooks, J.L.; Baker, G.A.; Boon, P.A.; Hendriksen, J.G.; Mulder, O.G.; Aldenkamp, A.P. Psychogenic non-epileptic seizures-definition, etiology, treatment and prognostic issues: A critical review. Seizure 2009, 18, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Reuber, M.; Fernández, G.; Helmstaedter, C.; Qurishi, A.; Elger, C.E. Evidence of brain abnormality in patients with psychogenic nonepileptic seizures. Epilepsy Behav. 2002, 3, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli Yaraki, P.; Yu, Y.J.; AlKhateeb, M.; Arevalo Astrada, M.A.; Lapalme-Remis, S.; Mirsattari, S.M. EEG and MRI Abnormalities in Patients with Psychogenic Nonepileptic Seizures. J. Clin. Neurophysiol. 2022, 41, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Kutlubaev, M.A.; Xu, Y.; Hackett, M.L.; Stone, J. Dual diagnosis of epilepsy and psychogenic nonepileptic seizures: Systematic review and meta-analysis of frequency, correlates, and outcomes. Epilepsy Behav. 2018, 89, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Beghi, E.; Tomson, T.; Beghi, M.; Erba, G.; Chang, Z. Mortality in patients with psychogenic non-epileptic seizures a population-based cohort study. J. Neurol. Neurosurg. Psychiatry 2022, 93, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Scevola, L.; Teitelbaum, J.; Oddo, S.; Centurion, E.; Loidl, C.; Kochen, S. Psychiatric disorders in patients with psychogenic nonepileptic seizures and drug-resistant epilepsy: A study of an Argentine population. Epilepsy Behav. 2013, 29, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Kanemoto, K.; LaFrance, W.C.J.; Duncan, R.; Gigineishvili, D.; Park, S.P.; Tadokoro, Y.; Ikeda, H.; Paul, R.; Zhou, D.; Taniguchi, G.; et al. PNES around the world: Where we are now and how we can close the diagnosis and treatment gaps-an ILAE PNES Task Force report. Epilepsia Open 2017, 2, 307–316. [Google Scholar] [CrossRef]
- Brigo, F.; Igwe, S.; Erro, R.; Bongiovanni, L.; Marangi, A.; Nardone, R.; Tinazzi, M.; Trinka, E. Postictal serum creatine kinase for the differential diagnosis of epileptic seizures and psychogenic non-epileptic seizures: A systematic review. J. Neurol. 2014, 262, 251–257. [Google Scholar] [CrossRef]
- Reinsberger, C.; Sarkis, R.; Papadelis, C.; Doshi, C.; Perez, D.; Baslet, G.; Loddenkemper, T.; Dworetzky, B. Autonomic Changes in Psychogenic Nonepileptic Seizures. Clin EEG Neurosci. 2015, 46, 16–25. [Google Scholar] [CrossRef]
- Simani, L.; Elmi, M.; Asadollahi, M. Serum GFAP level: A novel adjunctive diagnostic test in differentiate epileptic seizures from psychogenic attacks. Seizure 2018, 61, 41–44. [Google Scholar] [CrossRef]
- Asadollahi, M.; Simani, L. The diagnostic value of serum UCHL-1 and S100-B levels in differentiate epileptic seizures from psychogenic attacks. Brain Res. 2019, 1704, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Zinchuk, M.; Beghi, M.; Diotti, S.; Pashnin, E.; Kustov, G.; Rider, F.; Urh, L.; Guekht, A.; Cornaggia, C.M. Differential diagnosis between epileptic and psychogenic nonepileptic seizures through conversational analysis: A blinded prospective study in the Russian language. Epilepsy Behav. 2021, 125, 108441. [Google Scholar] [CrossRef] [PubMed]
- Giussani, G.; Erba, G.; Bianchi, E.; Beghi, E. Self-Report questionnaires for the diagnosis of psychogenic non-epileptic seizures in clinical practice. A comprehensive review of the available instruments. Seizure 2020, 79, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Perez, D.L.; LaFrance, W.C., Jr. Nonepileptic seizures: An updated review. CNS Spectr. 2016, 21, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, H.J.; Arima, H.; Mohamed, A. The utility of prolonged outpatient ambulatory EEG. Seizure 2012, 21, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Cragar, D.E.; Berry, D.T.; Fakhoury, T.A.; Cibula, J.E.; Schmitt, F.A. A review of diagnostic techniques in the differential diagnosis of epileptic and nonepileptic seizures. Neuropsychol. Rev. 2002, 12, 31–64. [Google Scholar] [CrossRef] [PubMed]
- Sueri, C.; Gasparini, S.; Balestrini, S.; Labate, A.; Gambardella, A.; Russo, E.; Leo, A.; Casarotto, S.; Pittau, F.; Trimboli, M.; et al. Diagnostic Biomarkers of Epilepsy. Curr. Pharm. Biotechnol. 2018, 19, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.S.; Acevedo, C.; Arzimanoglou, A.; Bogacz, A.; Cross, J.H.; Elger, C.E.; Engel, J., Jr.; Forsgren, L.; French, J.A.; Glynn, M.; et al. ILAE official report: A practical clinical definition of epilepsy. Epilepsia 2014, 55, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Popkirov, S.; Asadi-Pooya, A.A.; Duncan, R.; Gigineishvili, D.; Hingray, C.; Kanner, A.M.; LaFrance, W.C.; Pretorius, C.; Reuber, M.; on behalf of the ILAE PNES Task Force. The aetiology of psychogenic non-epileptic seizures: Risk factors and comorbidities. Epileptic Disord. 2019, 21, 529–547. [Google Scholar] [CrossRef]
- Tsyglakova, M.; McDaniel, D.; Hodes, G.E. Immune mechanisms of stress susceptibility and resilience: Lessons from animal models. Front. Neuroendocrinol. 2019, 54, 100771. [Google Scholar] [CrossRef]
- Segerstrom, S.C.; Miller, G.E. Psychological Stress and the Human Immune System: A Meta-Analytic Study of 30 Years of Inquiry. Psychol. Bull. 2004, 130, 601–630. [Google Scholar] [CrossRef]
- Morkavuk, G.; Koc, G.; Leventoglu, A. Is the differential diagnosis of epilepsy and psychogenic nonepileptic seizures possible by assessing the neutrophil/lymphocyte ratio? Epilepsy Behav. 2021, 116, 107736. [Google Scholar] [CrossRef] [PubMed]
- Bandelow, B.; Baldwin, D.; Abelli, M.; Bolea-Alamanac, B.; Bourin, M.; Chamberlain, S.R.; Cinosi, E.; Davies, S.; Domschke, K.; Fineberg, N.; et al. Biological markers for anxiety disorders, OCD and PTSD: A consensus statement. Part II: Neurochemistry, neurophysiology and neurocognition. World. J. Biol. Psychiatry 2017, 18, 162–214. [Google Scholar] [CrossRef] [PubMed]
- Mula, M.; Kanner, A.M.; Jetté, N.; Sander, J.W. Psychiatric Comorbidities in People with Epilepsy. Neurol. Clin. Pract. 2021, 11, e112–e120. [Google Scholar] [CrossRef]
- Mula, M.; Coleman, H.; Wilson, S.J. Neuropsychiatric and Cognitive Comorbidities in Epilepsy. Continuum (Minneap. Minn.) 2022, 28, 457–482. [Google Scholar] [CrossRef] [PubMed]
- Kustov, G.V.; Zinchuk, M.S.; Rider, F.K.; Pashnin, E.V.; Voinova, N.I.; Avedisova, A.S.; Guekht, A.B. Comorbidity of psychogenic non-epileptic seizures with mental disorders. Neurosci. Behav. Physiol. 2022, 52, 871–877. [Google Scholar] [CrossRef]
- Druzhkova, T.A.; Yakovlev, A.A.; Rider, F.K.; Zinchuk, M.S.; Guekht, A.B.; Gulyaeva, N.V. Elevated Serum Cortisol Levels in Patients with Focal Epilepsy, Depression, and Comorbid Epilepsy and Depression. Int. J. Mol. Sci. 2022, 23, 10414. [Google Scholar] [CrossRef] [PubMed]
- Fries, E.; Hesse, J.; Hellhammer, J.; Hellhammer, D.H. A new view on hypocortisolism. Psychoneuroendocrinology 2005, 30, 1010–1016. [Google Scholar] [CrossRef]
- Bernard, V.; Young, J.; Binart, N. Prolactin—A pleiotropic factor in health and disease. Nat. Rev. Endocrinol. 2019, 15, 356–365. [Google Scholar] [CrossRef]
- Faron-Górecka, A.; Latocha, K.; Pabian, P.; Kolasa, M.; Sobczyk-Krupiarz, I.; Dziedzicka-Wasylewska, M. The Involvement of Prolactin in Stress-Related Disorders. Int. J. Environ. Res. Public Health. 2023, 20, 3257. [Google Scholar] [CrossRef]
- Bauer, J. Epilepsy and prolactin in adults: A clinical review. Epilepsy Res. 1996, 24, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J. Interactions between hormones and epilepsy in female patients. Epilepsia. 2001, 42 (Suppl. S3), 20–22. [Google Scholar] [CrossRef] [PubMed]
- Luef, G. Hormonal alterations following seizures. Epilepsy Behav. 2010, 19, 131–133. [Google Scholar] [CrossRef] [PubMed]
- Tharyan, P.; Kuruvilla, K.; Prabhakar, S. Serum prolactin changes in epilepsy and hysteria. Indian J. Psychiatry 1988, 30, 145–152. [Google Scholar] [PubMed]
- Rao, M.L.; Stefan, H.; Bauer, J. Epileptic but not psychogenic seizures are accompanied by simultaneous elevation of serum pituitary hormones and cortisol levels. Neuroendocrinology 1989, 49, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Malekpour, M.; Jafari, A.; Kashkooli, M.; Salarikia, S.R.; Negahdaripour, M. A systems biology approach for discovering the cellular and molecular aspects of psychogenic non-epileptic seizure. Front. Psychiatry 2023, 14, 1116892. [Google Scholar] [CrossRef]
- van der Kruijs, S.J.; Bodde, N.M.; Vaessen, M.J.; Lazeron, R.H.; Vonck, K.; Boon, P.; Hofman, P.A.M.; Backes, W.H.; Aldenkamp, A.P.; Jacobus, F. A Jansen Functional connectivity of dissociation in patients with psychogenic non-epileptic seizures. J. Neurol. Neurosurg. Psychiatry 2012, 83, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Diprose, W.; Sundram, F.; Menkes, D.B. Psychiatric comorbidity in psychogenic nonepileptic seizures compared with epilepsy. Epilepsy Behav. 2016, 56, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Green, B.; Norman, P.; Reuber, M. Attachment style, relationship quality, and psychological distress in patients with psychogenic non-epileptic seizures versus epilepsy. Epilepsy Behav. 2017, 66, 120–126. [Google Scholar] [CrossRef]
- Roberts, N.A.; Burleson, M.H.; Weber, D.J.; Larson, A.; Sergeant, K.; Devine, M.J.; Vincelette, T.M.; Wang, N.C. Emotion in psychogenic nonepileptic seizures: Responses to affective pictures. Epilepsy Behav. 2012, 24, 107–115. [Google Scholar] [CrossRef]
- Walsh, S.; Levita, L.; Reuber, M. Comorbid depression and associated factors in PNES versus epilepsy: Systematic review and meta-analysis. Seizure 2018, 60, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Indranada, A.M.; Mullen, S.A.; Duncan, R.; Berlowitz, D.J.; Kanaan, R.A.A. The association of panic and hyperventilation with psychogenic non-epileptic seizures: A systematic review and meta-analysis. Seizure 2018, 59, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Khawaja, I. Structural Changes in Brain Magnetic Resonance Imaging Associated with Psychogenic Non-epileptic Seizures: An Analytical Cross-Sectional Study. Cureus 2022, 14, e32144. [Google Scholar] [CrossRef] [PubMed]
- Allendorfer, J.B.; Nenert, R.; Hernando, K.A.; DeWolfe, J.L.; Pati, S.; Thomas, A.E.; Billeaud, N.; Martin, R.C.; Szaflarski, J.P. FMRI response to acute psychological stress differentiates patients with psychogenic non-epileptic seizures from healthy controls—A biochemical and neuroimaging biomarker study. Neuroimage Clin. 2019, 24, 101967. [Google Scholar] [CrossRef] [PubMed]
- Asadi-Pooya, A.A. Psychogenic nonepileptic seizures are predominantly seen in women: Potential neurobiological reasons. Neurol. Sci. 2016, 37, 851–855. [Google Scholar] [CrossRef] [PubMed]
- Hinchliffe, C.; Yogarajah, M.; Tang, L.; Abasolo, D. Electroencephalogram Connectivity for the Diagnosis of Psychogenic Non-epileptic Seizures. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2022, 2022, 301–304. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, M.; Winton-Brown, T.T.; Hew, A.; Rayner, G.; Foster, E.; Rychkova, M.; Ali, R.; Velakoulis, D.; O’Brien, T.J.; Kwan, P.; et al. Multidimensional psychopathological profile differences between patients with psychogenic nonepileptic seizures and epileptic seizure disorders. Epilepsy Behav. 2022, 135, 108878. [Google Scholar] [CrossRef] [PubMed]
- Schneider, G.; Levin, L.; Herskovitz, M. Psychogenic nonepileptic seizures: Are they a freeze reaction? Epilepsy Behav. 2022, 129, 108655. [Google Scholar] [CrossRef] [PubMed]
- Nass, R.D.; Sassen, R.; Elger, C.E.; Surges, R. The role of postictal laboratory blood analyses in the diagnosis and prognosis of seizures. Seizure 2017, 47, 51–65. [Google Scholar] [CrossRef]
- Patel, J.; Tran, Q.K.; Martinez, S.; Wright, H.; Pourmand, A. Utility of serum lactate on differential diagnosis of seizure-like activity: A systematic review and meta-analysis. Seizure 2022, 102, 134–142. [Google Scholar] [CrossRef]
- Abdelnaby, R.; Elgenidy, A.; Heckelmann, J.; Bedewy, M.M.; Shabib, A.S.; Ebrahim, M.A.; Elmenawi, K.A.; Maallem, I.; Youssef, M.W.; Attia, A.M.; et al. The role of creatine kinase in distinguishing generalized tonic-clonic seizures from psychogenic non-epileptic seizures (PNES) and syncope: A retrospective study and meta-analysis of 1300 patients. Neurol. Res. Pract. 2023, 5, 56. [Google Scholar] [CrossRef]
- Kalkan, A.; Demirel, A.; Atiş, Ş.E.; Karaaslan, E.B.; Ferhatlar, M.E.; Senturk, M. A new biomarker in the differential diagnosis of epileptic seizure: Neurogranin. Am. J. Emerg. Med. 2022, 54, 147–150. [Google Scholar] [CrossRef]
- Ahl, M.; Taylor, M.K.; Avdic, U.; Lundin, A.; Andersson, M.; Amandusson, Å.; Kumlien, E.; Strandberg, M.C.; Ekdahl, C.T. Immune response in blood before and after epileptic and psychogenic non-epileptic seizures. Heliyon 2023, 9, e13938. [Google Scholar] [CrossRef]
- Tan, T.H.L.; Sanfilippo, P.; Colman, B.; Perucca, P.; Kwan, P.; O’Brien, T.J.; Monif, M. Development and validation of a peripheral cell ratio and lactate score for differentiating status epilepticus from prolonged psychogenic nonepileptic seizures. Epilepsia Open 2023, 8, 1460–1473. [Google Scholar] [CrossRef]
- Winterdahl, M.; Miani, A.; Vercoe, M.J.H.; Ciovica, A.; Uber-Zak, L.; Rask, C.U.; Zak, P.J. Vulnerability to psychogenic non-epileptic seizures is linked to low neuropeptide Y levels. Stress 2017, 20, 589–597. [Google Scholar] [CrossRef]
- Miani, A.; Pedersen, A.S.; Rask, C.U.; Uber-Zak, L.; Zak, P.J.; Winterdahl, M. Predicting psychogenic non-epileptic seizures from serum levels of neuropeptide Y and adrenocorticotropic hormone. Acta Neuropsychiatr. 2019, 31, 167–171. [Google Scholar] [CrossRef]
- Wulsin, A.C.; Solomon, M.B.; Privitera, M.D.; Danzer, S.C.; Herman, J.P. Hypothalamic-pituitary-adrenocortical axis dysfunction in epilepsy. Physiol. Behav. 2016, 166, 22–31. [Google Scholar] [CrossRef]
- Gulyaeva, N.V. Stress-Associated Molecular and Cellular Hippocampal Mechanisms Common for Epilepsy and Comorbid Depressive Disorders. Biochemistry 2021, 86, 641–656. [Google Scholar] [CrossRef]
- Maguire, J.; Salpekar, J.A. Stress, seizures, and hypothalamic-pituitary-adrenal axis targets for the treatment of epilepsy. Epilepsy Behav. 2013, 26, 352–362. [Google Scholar] [CrossRef]
- Galimberti, C.A.; Magri, F.; Copello, F.; Arbasino, C.; Cravello, L.; Casu, M.; Patrone, V.; Murialdo, G. Seizure frequency and cortisol and dehydroepiandrosterone sulfate (DHEAS) levels in women with epilepsy receiving antiepileptic drug treatment. Epilepsia 2005, 46, 517–523. [Google Scholar] [CrossRef]
- den Heijer, J.M.; Otte, W.M.; van Diessen, E.; van Campen, J.S.; Lorraine Hompe, E.; Jansen, F.E.; Joels, M.; Braun, K.P.J.; Sander, J.W.; Zijlmans, M. The relation between cortisol and functional connectivity in people with and with out stress-sensitive epilepsy. Epilepsia 2018, 59, 179–189. [Google Scholar] [CrossRef] [PubMed]
- van Campen, J.S.; Hompe, E.L.; Jansen, F.E.; Velis, D.N.; Otte, W.M.; van de Berg, F.; Braun, K.P.J.; Visser, G.H.; Sander, J.W.; Joels, M.; et al. Cortisol fluctuations relate to interictal epileptiform discharges in stress sensitive epilepsy. Brain 2016, 139, 1673–1679. [Google Scholar] [CrossRef]
- van Campen, J.S.; Valentijn, F.A.; Jansen, F.E.; Joëls, M.; Braun, K.P. Seizure occurrence and the circadian rhythm of cortisol: A systematic review. Epilepsy Behav. 2015, 47, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.W.; Liu, Y.X. Changes of serum adrenocorticotropic hormone and cortisol levels during sleep seizures. Neurosci. Bull. 2008, 24, 84–88. [Google Scholar] [CrossRef]
- Marinelli, I.; Walker, J.J.; Seneviratne, U.; D’Souza, W.; Cook, M.J.; Anderson, C.; Bagshaw, A.P.; Lightman, S.L.; Woldman, W.; John, R. TerryCircadian distribution of epileptiform discharges in epilepsy: Candidate mechanisms of variability. PLoS Comput. Biol. 2023, 19, e1010508. [Google Scholar] [CrossRef]
- Novakova, B.; Harris, P.R.; Rawlings, G.H.; Reuber, M. Coping with stress: A pilot study of a self-help stress management intervention for patients with epileptic or psychogenic nonepileptic seizures. Epilepsy Behav. 2019, 94, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Cano-López, I.; González-Bono, E. Cortisol levels and seizures in adults with epilepsy: A systematic review. Neurosci. Biobehav. Rev. 2019, 103, 216–229. [Google Scholar] [CrossRef]
- Bakvis, P.; Spinhoven, P.; Giltay, E.J.; Kuyk, J.; Edelbroek, P.M.; Zitman, F.G.; Roelofs, K. Basal hypercortisolism and trauma in patients with psychogenic nonepileptic seizures. Epilepsia 2010, 51, 752–759. [Google Scholar] [CrossRef]
- Bolshakov, A.P.; Tret’yakova, L.V.; Kvichansky, A.A.; Gulyaeva, N.V. Glucocorticoids: Dr. Jekyll and Mr. Hyde of Hippocampal Neuroinflammation. Biochemistry (Moscow) 2021, 86, 156–167. [Google Scholar] [CrossRef]
- Gulyaeva, N.V. Glucocorticoids Orchestrate Adult Hippocampal Plasticity: Growth Points and Translational Aspects. Biochemistry (Moscow) 2023, 88, 565–589. [Google Scholar] [CrossRef]
- Firouzabadi, N.; Asadi-Pooya, A.A.; Alimoradi, N.; Simani, L.; Asadollahi, M. Polymorphism of glucocorticoid receptor gene (rs41423247) in functional seizures (psychogenic nonepileptic seizures/attacks). Epilepsia Open 2023, 8, 1425–1431. [Google Scholar] [CrossRef] [PubMed]
- Molaie, M.; Culebras, A.; Miller, M. Nocturnal plasma prolactin and cortisol levels in epileptics with complex partial seizures and primary generalized seizures. Arch. Neurol. 1987, 44, 699–702. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.K.; So, Y.T.; Fisher, R.S. Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Use of serum prolactin in diagnosing epileptic seizures: Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2005, 65, 668–675. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [CrossRef] [PubMed]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- LaFrance, W.C., Jr.; Baker, G.A.; Duncan, R.; Goldstein, L.H.; Reuber, M. Minimum require-ments for the diagnosis of psychogenic nonepileptic seizures: A staged approach: A report from the International League Against Epilepsy Nonepileptic Seizures Task Force. Epilepsia 2013, 54, 2005–2018. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.; Debono, M. Replication of cortisol circadian rhythm: New advances in hydrocortisone replacement therapy. Ther. Adv. Endocrinol. Metab. 2010, 1, 129–138. [Google Scholar] [CrossRef]
- Sundararajan, T.; Tesar, G.E.; Jimenez, X.F. Biomarkers in the diagnosis and study of psychogenic nonepileptic seizures: A systematic review. Seizure 2016, 35, 11–22. [Google Scholar] [CrossRef]

| Parameter/Group | ES (n = 29) | PNES (n = 45) | p-Value |
|---|---|---|---|
| Socio-demographic data | |||
| Age, years, M ± SD | 41.6 ± 13.8 | 36 ± 13.8 | 0.08 2 |
| Females, n (% of patients in the group) | 18 (62%) | 33 (73%) | 0.3 3 |
| Education, n (%) Secondary Higher | 21 (72%) 8 (28%) | 34 (76%) 11 (24%) | 0.76 3 |
| Employment, n (%) Student/employed Unemployed | 9 (31%) 20 (69%) | 8 (18%) 37 (82%) | 0.19 3 |
| Marital status, n (%) Single/widowed/divorced Married/in a relationship | 19 (66%) 10 (34%) | 35 (78%) 10 (22%) | 0.25 3 |
| Clinical data | |||
| Current depressive episode, n (%) | 15 (52%) | 24 (39%) | 0.89 3 |
| Anxiety disorders, n (%) | 14 (48%) | 42 (92%) | <0.001 3 |
| MMSE, M ± SD | 28 ± 2.9 | 28 ± 1.9 | 1.0 1 |
| Period of paroxysmal events registration, years, Me (IQR) | 16 (10–28) | 3 (1.8–6) | <0.001 2 |
| Age of paroxysmal events onset, years, Me (IQR) | 18 (11–37) | 27 (18–40) | <0.001 2 |
| Frequency of paroxysmal events, n (%) 1–2/year 3–11/year More than 12/year | 3 (10%) 8 (28%) 18 (62%) | 0 8 (18%) 37 (82%) | 0.041 3 (3 × 2 χ2 test) |
| Hemogram | |||
| While blood cells, WBCs, 103 µL | 5.46 ± 1.5 | 6.18 ± 1.41 | 0.04 1 |
| Lymphocytes, %, Me (IQR) 103 µL, Me (IQR) | 34.8 (28.7–41) 1.6 (1.4–2.0) | 38.2 (29.5–44)/ 2.0 (1.6–2.5) | 0.01 2 |
| Neutrophils, NEI, %, M ± SD 103 µL, M ± SD | 53.43 ± 9.06 2.93 ± 0.93 | 53.02 ± 10.43 3.47 ± 1.43 | 0.08 1 |
| Monocytes, MO, %, M ± SD 103 µL, M ± SD | 8.86 ± 2.74/ 0.47 ± 0.14 | 8.31 ± 2/ 0.49 ± 0.13 | 0.32 1 |
| Hemoglobin, Hb, g/L | 133.5 ± 17.16 | 136.41 ± 13.4 | 0.42 1 |
| Platelets, PLT, 103 µL | 255.29 ± 58.66 | 249.52 ± 58.17 | 0.68 1 |
| Hormones | |||
| Cortisol, nmol/L, mean ± SD | 410 ± 143.2 | 417.2 ± 137.1 | 0.8 1 |
| Prolactin, ng/mL, Me (IQR) | 17.2 (9.0–21.3) | 15.9 (12.2–23.2) | 0.4 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rider, F.; Turchinets, A.; Druzhkova, T.; Kustov, G.; Guekht, A.; Gulyaeva, N. Dissimilar Changes in Serum Cortisol after Epileptic and Psychogenic Non-Epileptic Seizures: A Promising Biomarker in the Differential Diagnosis of Paroxysmal Events? Int. J. Mol. Sci. 2024, 25, 7387. https://doi.org/10.3390/ijms25137387
Rider F, Turchinets A, Druzhkova T, Kustov G, Guekht A, Gulyaeva N. Dissimilar Changes in Serum Cortisol after Epileptic and Psychogenic Non-Epileptic Seizures: A Promising Biomarker in the Differential Diagnosis of Paroxysmal Events? International Journal of Molecular Sciences. 2024; 25(13):7387. https://doi.org/10.3390/ijms25137387
Chicago/Turabian StyleRider, Flora, Alexander Turchinets, Tatyana Druzhkova, Georgii Kustov, Alla Guekht, and Natalia Gulyaeva. 2024. "Dissimilar Changes in Serum Cortisol after Epileptic and Psychogenic Non-Epileptic Seizures: A Promising Biomarker in the Differential Diagnosis of Paroxysmal Events?" International Journal of Molecular Sciences 25, no. 13: 7387. https://doi.org/10.3390/ijms25137387
APA StyleRider, F., Turchinets, A., Druzhkova, T., Kustov, G., Guekht, A., & Gulyaeva, N. (2024). Dissimilar Changes in Serum Cortisol after Epileptic and Psychogenic Non-Epileptic Seizures: A Promising Biomarker in the Differential Diagnosis of Paroxysmal Events? International Journal of Molecular Sciences, 25(13), 7387. https://doi.org/10.3390/ijms25137387







