Description of a Non-Canonical AsPt Blue Species Originating from the Aerobic Oxidation of AP-1 in Aqueous Solution
Abstract
1. Introduction
2. Results and Discussion
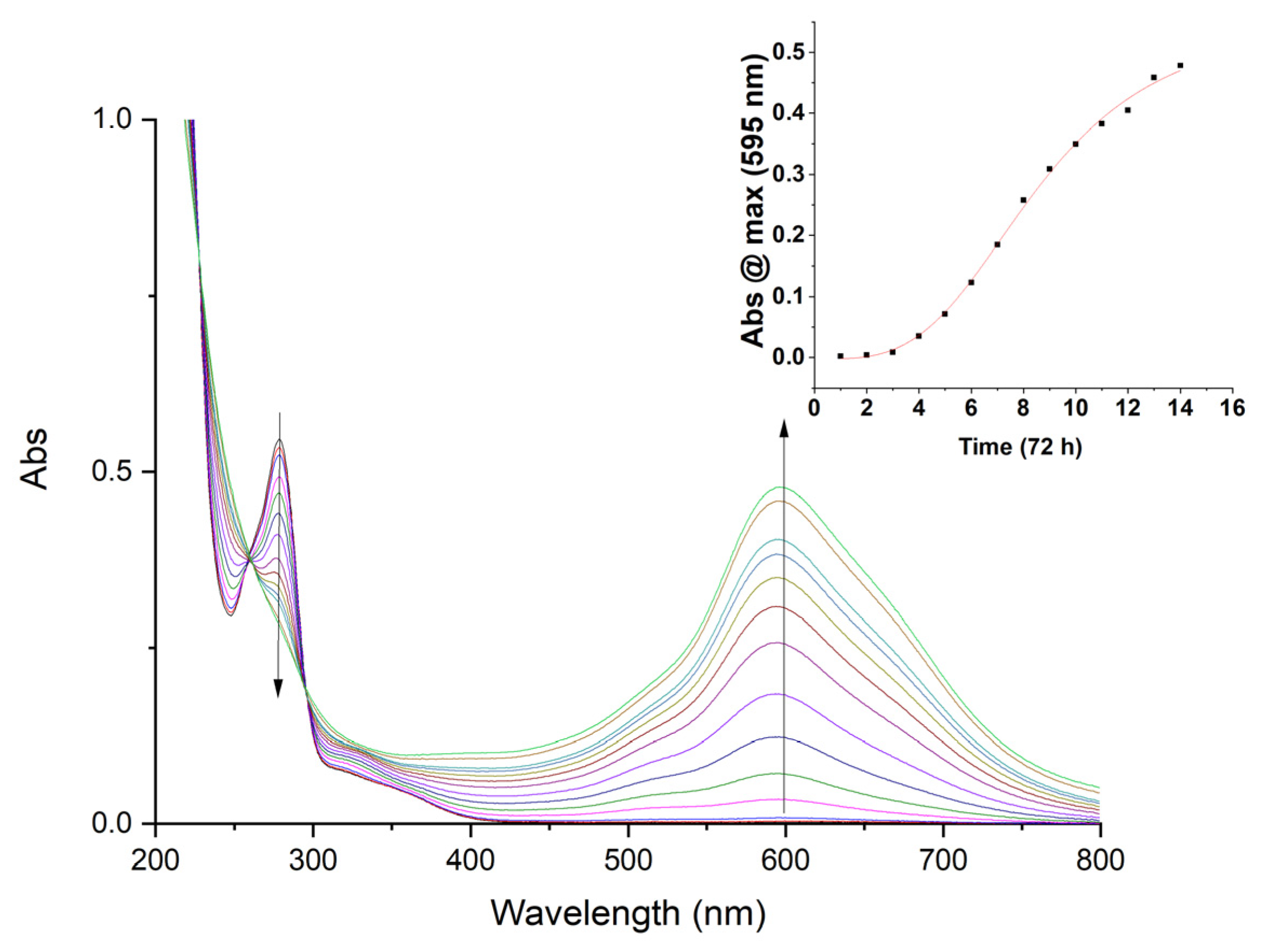
2.1. Formation of AsPt Blue in Solution
2.2. Pt White: A More Advanced Characterization
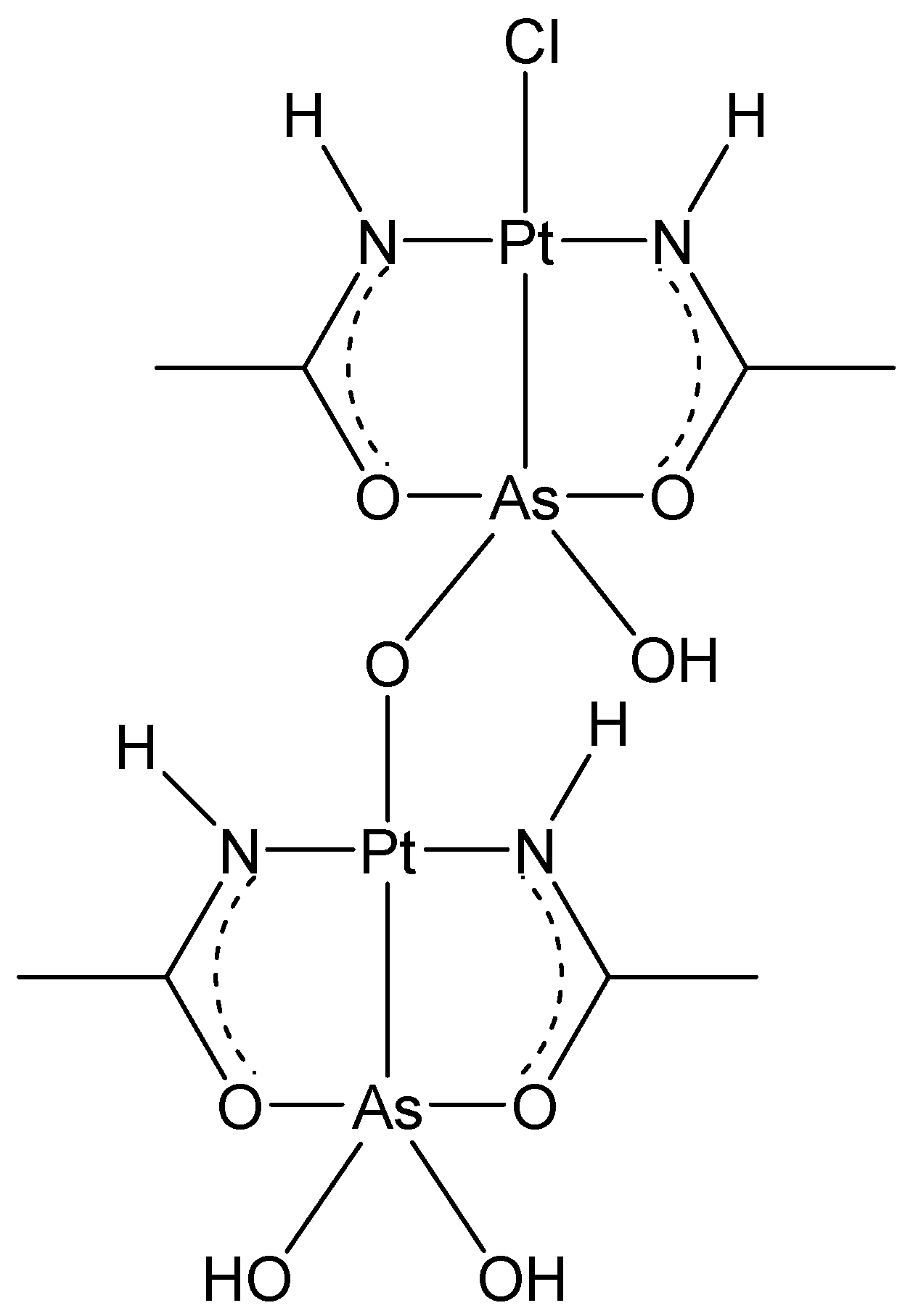
2.3. AsPt Blue: Preparation, Analytical Results, and Absorption Spectra
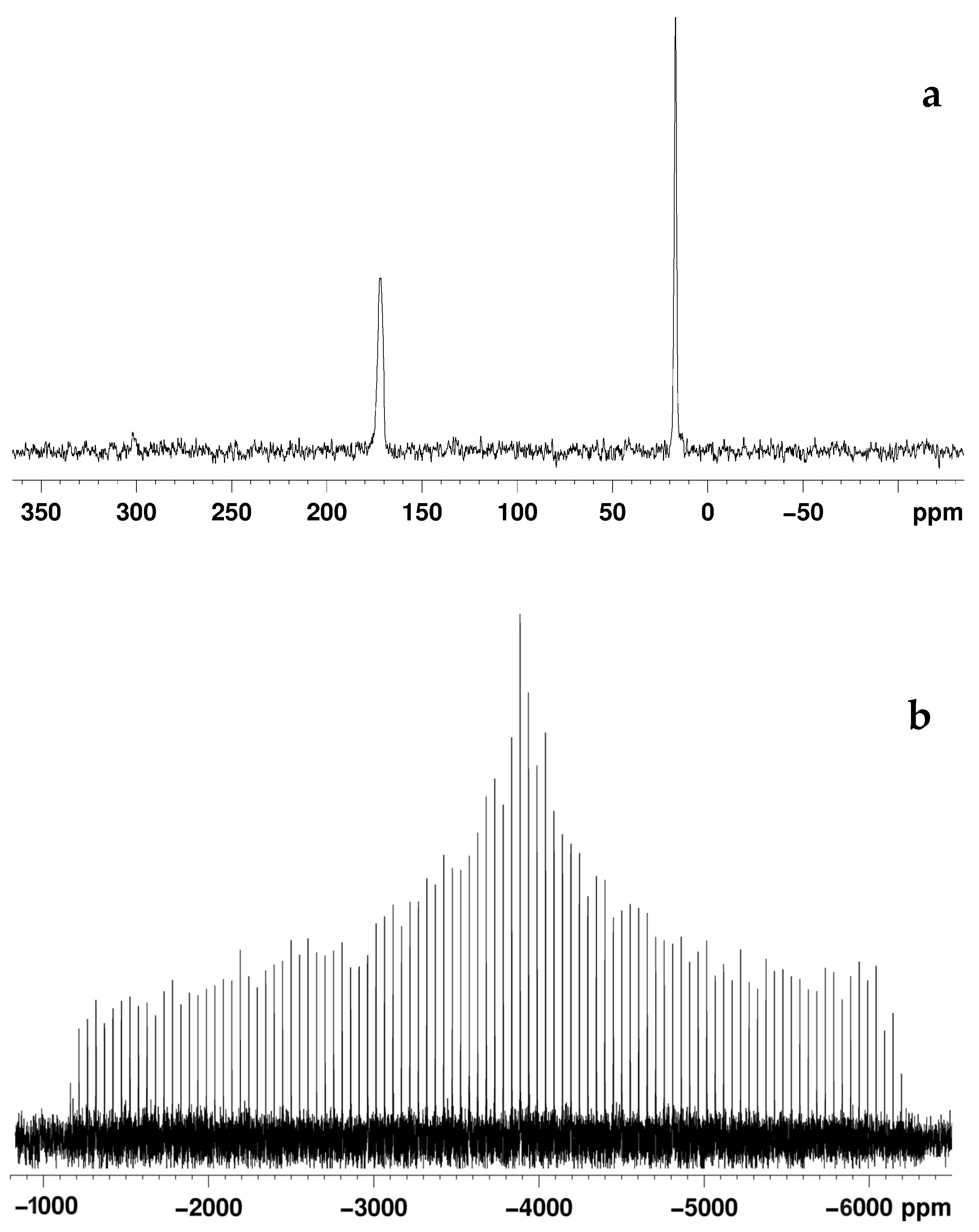
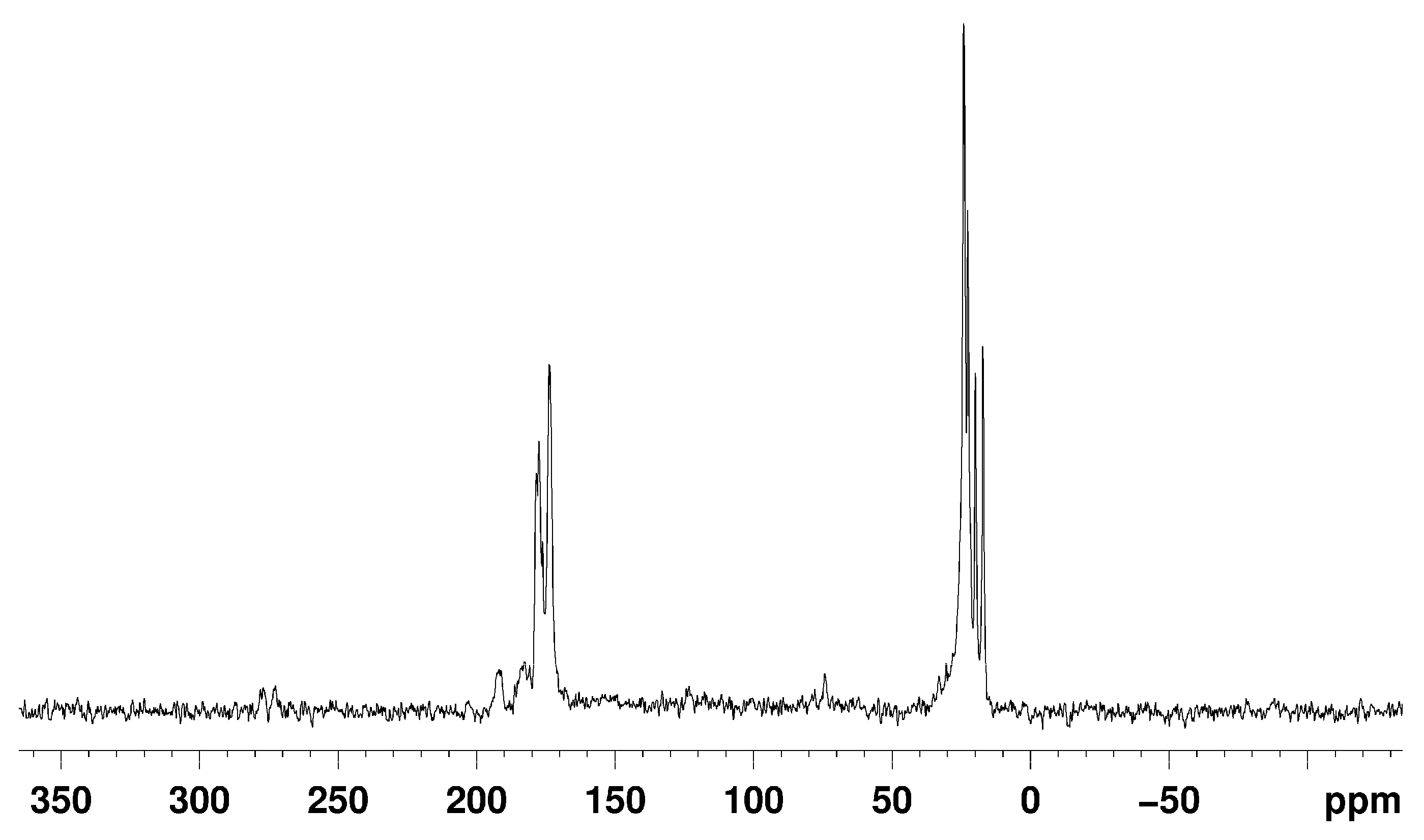
2.4. AsPt Blue: NMR Studies
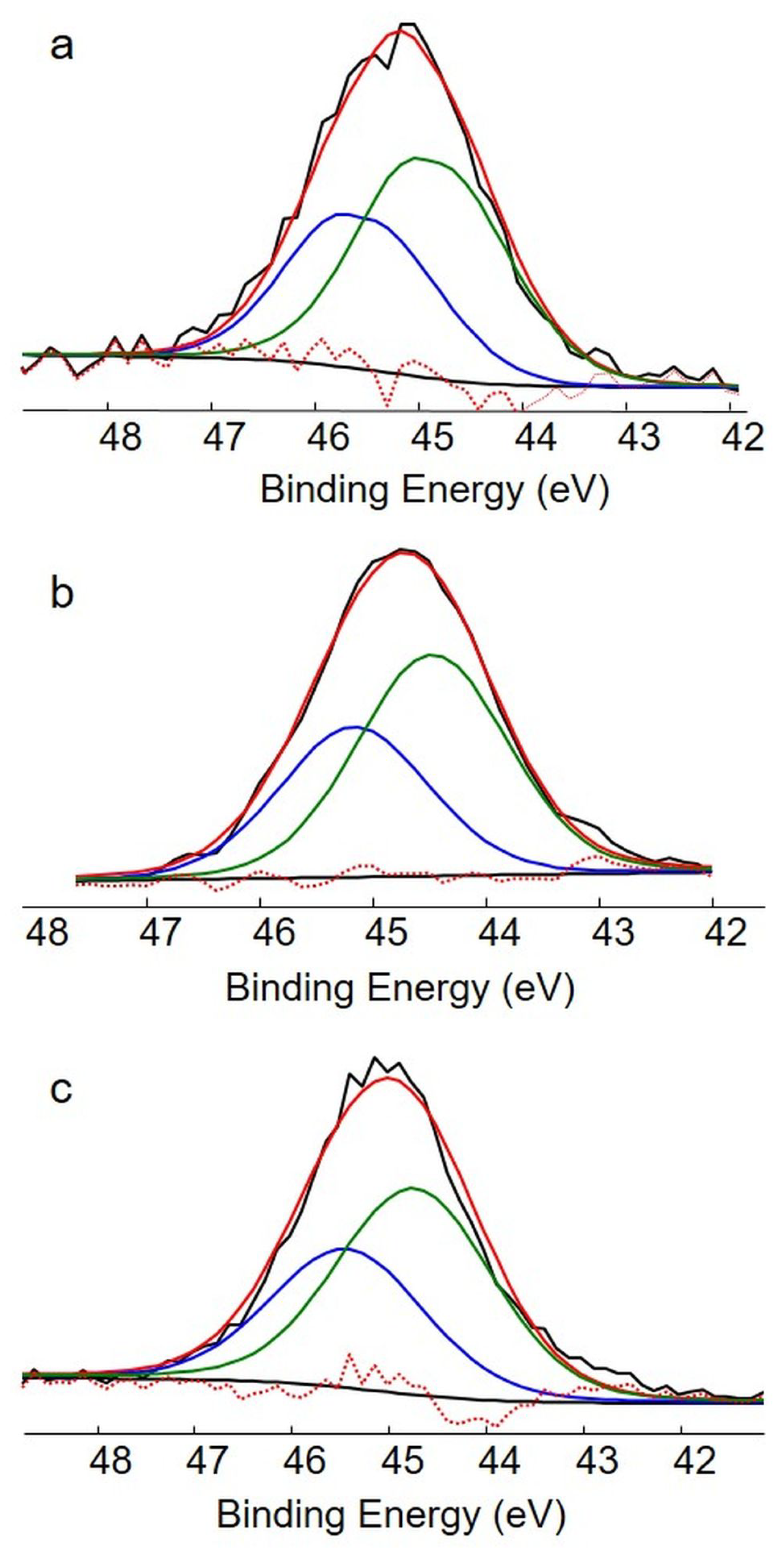
2.5. AsPt Blue: XPS Measurements
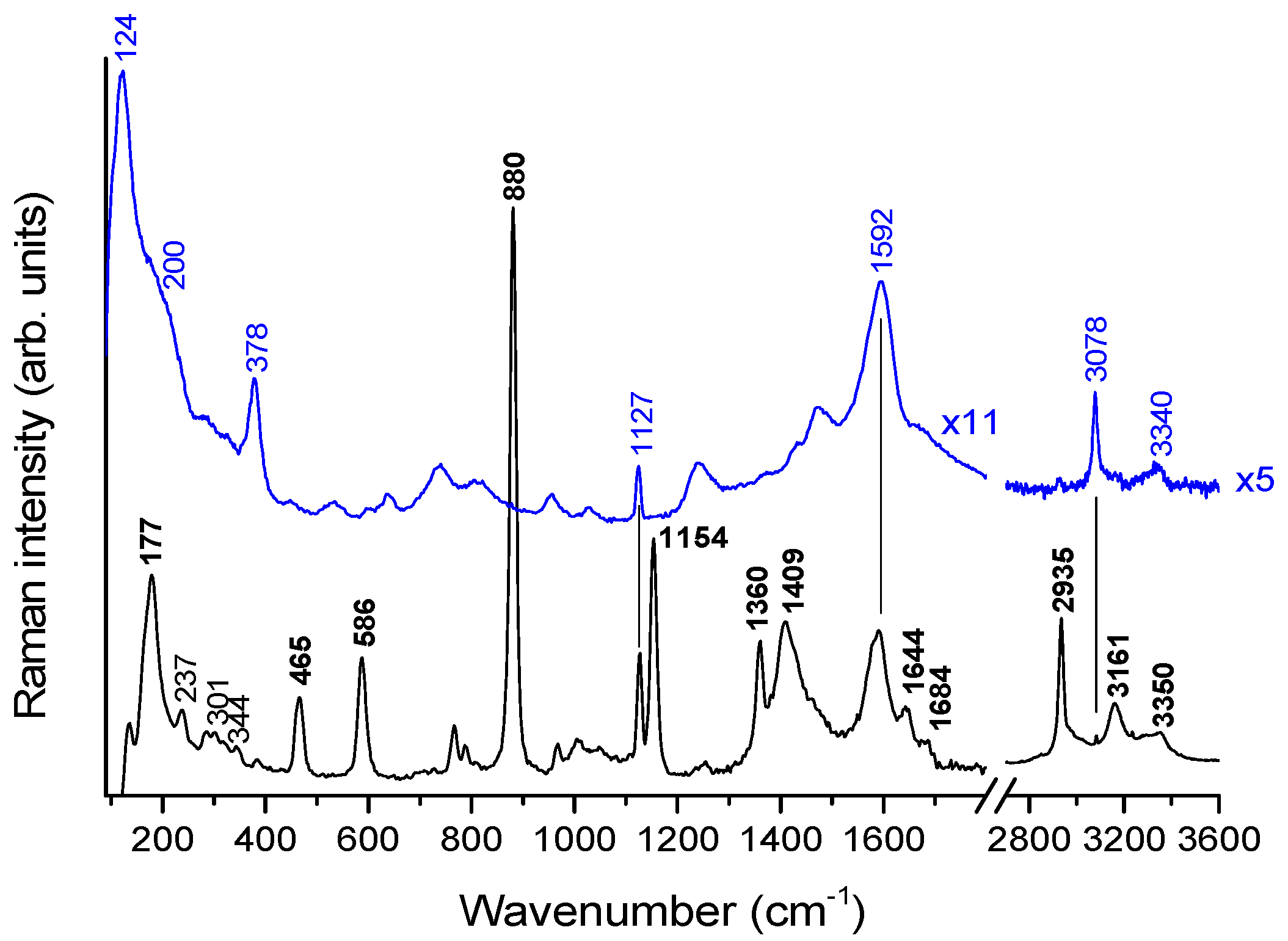
2.6. Pt Blue: Vibrational Spectroscopy
3. Materials and Methods
3.1. UV-Vis Experiments
3.2. NMR Experiments
3.3. XPS Experiments
3.4. ICP-OES Measurements for Pt and As
3.5. Raman Measurements
3.6. EPR Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miodragović, Ð.U.; Quentzel, J.A.; Kurutz, J.W.; Stern, C.L.; Ahn, R.W.; Kandela, I.; Mazar, A.; O’Halloran, T.V. Robust Structure and Reactivity of Aqueous Arsenous Acid–Platinum(II) Anticancer Complexes. Angew. Chem. Int. Ed. 2013, 52, 10749–10752. [Google Scholar] [CrossRef] [PubMed]
- Miodragović, D.; Merlino, A.; Swindell, E.P.; Bogachkov, A.; Ahn, R.W.; Abuhadba, S.; Ferraro, G.; Marzo, T.; Mazar, A.P.; Messori, L.; et al. Arsenoplatin-1 Is a Dual Pharmacophore Anticancer Agent. J. Am. Chem. Soc. 2019, 141, 6453–6457. [Google Scholar] [CrossRef] [PubMed]
- Tolbatov, I.; Cirri, D.; Tarchi, M.; Marzo, T.; Coletti, C.; Marrone, A.; Messori, L.; Re, N.; Massai, L. Reactions of Arsenoplatin-1 with Protein Targets: A Combined Experimental and Theoretical Study. Inorg. Chem. 2022, 61, 3240–3248. [Google Scholar] [CrossRef]
- Hofmann, K.A.; Bugge, G. Platinblau. Berichte Dtsch. Chem. Ges. 1908, 41, 312–314. [Google Scholar] [CrossRef]
- Ginsberg, A.P.; O’Halloran, T.V.; Fanwick, P.E.; Hollis, L.S.; Lippard, S.J. Electronic Structure and Optical Spectrum of Cis-Diammineplatinum.alpha.-pyridone Blue: Metal-Metal Bonding and Charge Transfer in a Four-Atom Pt(2.25) Chain. J. Am. Chem. Soc. 1984, 106, 5430–5439. [Google Scholar] [CrossRef]
- Lippert, B.; Schoellhorn, H.; Thewalt, U. Formation of Platinum [Pt2.25]4-1-Methyluracil Blue through Silver(I) Oxidation of [Pt2.0]2 and Isolation of a Heteronuclear (Pt2,Ag2) Precursor. Inorg. Chem. 1987, 26, 1736–1741. [Google Scholar] [CrossRef]
- Matsumoto, K.; Sakai, K. Structures and Reactivities of Platinum-Blues and the Related Amidate-Bridged Platinumiii Compounds. In Advances in Inorganic Chemistry; Sykes, A.G., Ed.; Academic Press: Cambridge, MA, USA, 1999; Volume 49, pp. 375–427. [Google Scholar]
- Usón, R.; Forniés, J.; Tomás, M.; Menjón, B.; Sünkel, K.; Bau, R. The First Mononuclear PtIII Complex. Molecular Structures of (NBu4)[PtIII(C6Cl5)4] and of Its Parent Compound {NBu4}2[PtII(C6Cl5)4]·2CH2Cl2. J. Chem. Soc. Chem. Commun. 1984, 12, 751–752. [Google Scholar] [CrossRef]
- Rivada-Wheelaghan, O.; Ortuño, M.A.; García-Garrido, S.E.; Díez, J.; Alonso, P.J.; Lledós, A.; Conejero, S. A Stable, Mononuclear, Cationic Pt(III) Complex Stabilised by Bulky N-Heterocyclic Carbenes. Chem. Commun. 2014, 50, 1299–1301. [Google Scholar] [CrossRef] [PubMed]
- Newman, R.A.; Martin, D.S.; Dallinger, R.F.; Woodruff, W.H.; Stiegman, A.E.; Che, C.M.; Schaefer, W.P.; Miskowski, V.M.; Gray, H.B. Vibrational and Electronic Spectra of Tetrasulfatodiplatinate(2-) Complexes. Inorg. Chem. 1991, 30, 4647–4654. [Google Scholar] [CrossRef]
- Laurent, M.P.; Tewksbury, J.C.; Krogh-Jespersen, M.-B.; Patterson, H. Trimethylacetamide Platinum Blue. Inorg. Chem. 1980, 19, 1656–1662. [Google Scholar] [CrossRef]
- Young, J.F.; Osborn, J.A.; Jardine, F.H.; Wilkinson, G. Hydride Intermediates in Homogeneous Hydrogenation Reactions of Olefins and Acetylenes Using Rhodium Catalysts. Chem. Commun. Lond. 1965, 7, 131–132. [Google Scholar] [CrossRef]
- Ferraro, G.; Cirri, D.; Marzo, T.; Pratesi, A.; Messori, L.; Merlino, A. The First Step of Arsenoplatin-1 Aggregation in Solution Unveiled by Solving the Crystal Structure of Its Protein Adduct. Dalton Trans. 2021, 50, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Kuveke, R.E.H.; Barwise, L.; van Ingen, Y.; Vashisth, K.; Roberts, N.; Chitnis, S.S.; Dutton, J.L.; Martin, C.D.; Melen, R.L. An International Study Evaluating Elemental Analysis. ACS Cent. Sci. 2022, 8, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Appleton, T.G.; Byriel, K.A.; Garrett, J.M.; Hall, J.R.; Kennard, C.H.L.; Mathieson, M.T.; Stranger, R. Preparation, Crystal Structures, and Properties of the Acetate-Bridged Platinum(III) Compounds [{(H2O)Pt(.Mu.-CH3CO2)2}2]A2 (A− = ClO4−, CF3SO3−). Inorg. Chem. 1995, 34, 5646–5655. [Google Scholar] [CrossRef]
- Wilson, J.J.; Lippard, S.J. Acetate-Bridged Platinum(III) Complexes Derived from Cisplatin. Inorg. Chem. 2012, 51, 9852–9864. [Google Scholar] [CrossRef] [PubMed]
- Kirakosyan, G.A.; Fedotova, T.N.; Kuznetsova, G.N. Structure of Binuclear Platinum(III) Acetamidate Complexes in Solutions as Probed by 195Pt, 13C, and 1H NMR Spectroscopy. Russ. Chem. Bull. 2015, 64, 2408–2414. [Google Scholar] [CrossRef]
- Stadnichenko, A.; Svintsitskiy, D.; Kibis, L.; Fedorova, E.; Stonkus, O.; Slavinskaya, E.; Lapin, I.; Fakhrutdinova, E.; Svetlichnyi, V.; Romanenko, A.; et al. Influence of Titania Synthesized by Pulsed Laser Ablation on the State of Platinum during Ammonia Oxidation. Appl. Sci. 2020, 10, 4699. [Google Scholar] [CrossRef]
- Marić, I.; Dražić, G.; Radin, E.; Peter, R.; Škrabić, M.; Jurkin, T.; Pustak, A.; Baran, N.; Mikac, L.; Ivanda, M.; et al. Impact of Platinum Loading and Dispersion on the Catalytic Activity of Pt/SnO2 and Pt/α-Fe2O3. Appl. Surf. Sci. 2023, 607, 155073. [Google Scholar] [CrossRef]
- Fantauzzi, M.; Atzei, D.; Elsener, B.; Lattanzi, P.; Rossi, A. XPS and XAES Analysis of Copper, Arsenic and Sulfur Chemical State in Enargites. Surf. Interface Anal. 2006, 38, 922–930. [Google Scholar] [CrossRef]
- Vernooij, R.R.; Joshi, T.; Shaili, E.; Kubeil, M.; Appadoo, D.R.T.; Izgorodina, E.I.; Graham, B.; Sadler, P.J.; Wood, B.R.; Spiccia, L. Comprehensive Vibrational Spectroscopic Investigation of trans,trans,trans-[Pt(N3)2(OH)2(Py)2], a Pt(IV) Diazido Anticancer Prodrug Candidate. Inorg. Chem. 2016, 55, 5983–5992. [Google Scholar] [CrossRef]
- Kocsis, L.; Mink, J.; Jalilehvand, F.; Laffin, L.J.; Berkesi, O.; Hajba, L. Vibrational Spectroscopic Study of the Hydrated Platinum(II), Palladium(II) and Cis-Diammineplatinum(II) Ions in Acidic Aqueous Solutions. J. Raman Spectrosc. 2009, 40, 481–490. [Google Scholar] [CrossRef]
- Maliarik, M.; Glaser, J.; Tóth, I. Novel Pentacyano Complexes of Tri- and Tetravalent Platinum. Inorg. Chem. 1998, 37, 5452–5459. [Google Scholar] [CrossRef] [PubMed]
- Baxter, L.A.M.; Heath, G.A.; Raptis, R.G.; Willis, A.C. Synthesis and Characterization of a Diplatinum(III)-Tetrakis(.Alpha.-Dioximato) Complex Containing an Unsupported Metal-Metal Bond. J. Am. Chem. Soc. 1992, 114, 6944–6946. [Google Scholar] [CrossRef]
- Stein, P.; Dickson, M.K.; Roundhill, D.M. Raman and Infrared Spectra of Binuclear Platinum(II) and Platinum(III) Octaphosphite Complexes. A Characterization of the Intermetallic Bonding. J. Am. Chem. Soc. 1983, 105, 3489–3494. [Google Scholar] [CrossRef]
- Quinson, J.; Jensen, K.M.Ø. From Platinum Atoms in Molecules to Colloidal Nanoparticles: A Review on Reduction, Nucleation and Growth Mechanisms. Adv. Colloid Interface Sci. 2020, 286, 102300. [Google Scholar] [CrossRef] [PubMed]
- Hung, I.; Rossini, A.J.; Schurko, R.W. Application of the Carr–Purcell Meiboom–Gill Pulse Sequence for the Acquisition of Solid-State NMR Spectra of Spin-1/2 Nuclei. J. Phys. Chem. A 2004, 108, 7112–7120. [Google Scholar] [CrossRef]
- Siegel, R.; Nakashima, T.T.; Wasylishen, R.E. Application of Multiple-Pulse Experiments to Characterize Broad NMR Chemical-Shift Powder Patterns from Spin-1/2 Nuclei in the Solid State. J. Phys. Chem. B 2004, 108, 2218–2226. [Google Scholar] [CrossRef]



| C | H | N | O | Cl | Pt | As | |
|---|---|---|---|---|---|---|---|
| AsPt blue | 10.67 | 2.49 | 5.15 | 25.00 | 7.01 | 36.70 | 10.80 |
| Pt white | 11.09 | 2.38 | 5.94 | 22.21 | - | 43.66 | 17.43 |
| AP-1 * | 10.55 | 2.21 | 6.15 | 14.05 | 7.78 | 42.82 | 16.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cirri, D.; Marzo, T.; Mastrorilli, P.; Petrelli, V.; Todisco, S.; De Giglio, E.; Gellini, C.; Ricci, M.; Pratesi, A.; Messori, L. Description of a Non-Canonical AsPt Blue Species Originating from the Aerobic Oxidation of AP-1 in Aqueous Solution. Int. J. Mol. Sci. 2024, 25, 7408. https://doi.org/10.3390/ijms25137408
Cirri D, Marzo T, Mastrorilli P, Petrelli V, Todisco S, De Giglio E, Gellini C, Ricci M, Pratesi A, Messori L. Description of a Non-Canonical AsPt Blue Species Originating from the Aerobic Oxidation of AP-1 in Aqueous Solution. International Journal of Molecular Sciences. 2024; 25(13):7408. https://doi.org/10.3390/ijms25137408
Chicago/Turabian StyleCirri, Damiano, Tiziano Marzo, Piero Mastrorilli, Valentina Petrelli, Stefano Todisco, Elvira De Giglio, Cristina Gellini, Marilena Ricci, Alessandro Pratesi, and Luigi Messori. 2024. "Description of a Non-Canonical AsPt Blue Species Originating from the Aerobic Oxidation of AP-1 in Aqueous Solution" International Journal of Molecular Sciences 25, no. 13: 7408. https://doi.org/10.3390/ijms25137408
APA StyleCirri, D., Marzo, T., Mastrorilli, P., Petrelli, V., Todisco, S., De Giglio, E., Gellini, C., Ricci, M., Pratesi, A., & Messori, L. (2024). Description of a Non-Canonical AsPt Blue Species Originating from the Aerobic Oxidation of AP-1 in Aqueous Solution. International Journal of Molecular Sciences, 25(13), 7408. https://doi.org/10.3390/ijms25137408












